Abstract
Objectives. To evaluate infantile spasms in children with Down syndrome including assessment of efficacy of treatments, presence of treatment lag, and to identify risk factors that may predict the occurrence of infantile spasms in this population. Methods. Medical charts, electroencephalograms, and brain magnetic resonance images were evaluated in 37 children treated for infantile spasms at a single institution from 2005 to 2015. Results. Mean age at diagnosis was 9.16 months, with an average 1.38-month lag from spasms onset to start of medication. Prevalence of heart defects and pulmonary hypertension were significantly higher in those with infantile spams compared with those without. Eighty-one percent receiving adrenocorticotropic hormone as initial treatment experienced remission within 2 weeks, 94.1% had remission at 3 months compared with 18.8% at 2 weeks and 35.3% at 3 months for other first-line treatments. Type of treatment was the only predictor of good outcome. Conclusions. Results stress the importance of early recognition and adrenocorticotropic hormone treatment for this seizure disorder in children with Down syndrome.
Keywords: Down syndrome, genetics, West syndrome, developmental disability, anti-seizure drugs
Introduction
West syndrome is a constellation of symptoms characterized by epileptic spasms, an abnormal electroencephalogram (EEG) pattern called hypsarrhythmia, and intellectual disability. Epileptic spasms are often called infantile spasms in children under the age of 2 years.1 The clinical seizures present as a sudden flexion, extension, or mixed extension-flexion of predominantly proximal and truncal muscles.2 Infantile spasms is the most common seizure type occurring in children with Down syndrome with an overall prevalence of 2.5% to 3.1%.3,4 The prevalence of this age-specific epilepsy is significantly higher in the Down syndrome population compared with the general population (0.016% to 0.042%) and can be associated with poor long-term developmental outcomes and further seizure types if not treated early and appropriately.5-9 This can be especially detrimental to children with Down syndrome who already have existing developmental delay.
It is unclear why infantile spasms occur more frequently in children with Down syndrome. Researchers have suggested that inherent structural brain anomalies in Down syndrome may be a factor due to decreased neuronal density with frontal and temporal hypoplasia, persistence of dendrites, abnormal neuronal lamination, fewer inhibitory interneurons, and other metabolic adaptations related to genetic overexpression.10-12 Other potential influences include the comorbidities found in Down syndrome such as cardiovascular anomalies requiring surgeries that could potentially be associated with hypoxia, stroke, and infection.10,13-15
Despite the known prevalence, the time from symptom onset to evaluation, diagnosis, and treatment has been reported to be longer for children with Down syndrome compared with typical children, thus putting the child with Down syndrome at risk for further developmental impact and future long-term intractable seizures.16,17 Symptoms of infantile spasms in children with Down syndrome can be mistakenly attributed to underlying comorbidities such as developmental delay, gastroesophageal reflux, or hypotonia. Developmental regression may be relatively subtle in this population due to the presence of existing developmental delays and, therefore, not immediately recognized. However, infants with Down syndrome may have an improved response to treatment and have a lower rate of persistent seizures in comparison to the general population when seizures are identified early and treated appropriately.11,16,18,19
Prior research investigating treatments for children with Down syndrome and infantile spasms demonstrate the lack of uniformity in drug choice, dose, and duration.5,20-22 These investigations, typically with only 5 to 17 cases, suggest children with Down syndrome may have a different response compared with typical children, including reduced recurrence and earlier resolution of seizures and hypsarrhythmia on EEG.11,14,17,19,23-25 With the high comorbidity of infantile spasms in this population and rise in cost of available treatments licensed for use, understanding the difference in the course, treatment, and outcome of infantile spasms in children with Down syndrome is imperative.
The objective of this study was to evaluate infantile spasms in children with Down syndrome to assess for efficacy of treatments, presence of treatment lag, and to identify clinical presentation and comorbidities likely to contribute to infantile spasms outcomes in this population.
Methods
We retrospectively reviewed the records of 40 children with Down syndrome who presented for treatment of infantile spasms at Children’s Hospital Colorado from 2005 to 2015. Thirty-seven children met study criteria and were included in the analyses. Written informed consent was waived for the collection of retrospective data. Inclusion criteria included treatment for infantile spasms at Children’s Hospital Colorado prior to 3 years of age and Down syndrome diagnosis. Patients with infantile spasms were identified through an electronic medical chart query using the International Classification of Diseases, Ninth Revision, codes and were included without regard to prior comorbidities. To ensure data accuracy, EEGs, brain magnetic resonance imaging (MRI), and outcomes were reviewed by a board-certified pediatric epileptologist. Children were defined as having infantile spasms if a clinical diagnosis was made by the provider and hypsarrhythmia or modified hypsarrhythmia were documented on EEG. Of the 37 participants, EEGs were completed in 31 prior to treatment and the remaining 6 patients were completed 1 to 23 days after start of treatment.
Data were collected on sex, comorbidities, age at onset of spasms, seizure frequency, developmental progress, EEG, MRI, and information regarding patient and family history. Each antiepileptic therapy was recorded including start date of treatment, initial dose, targeted duration of treatment, and maximum dose achieved. Specific interventions included the following: adrenocorticotropic hormone (ACTH), prednisone, prednisolone, vigabatrin, topiramate, clonazepam, zonisamide, valproate, or ketogenic diet. Initial dose of ACTH was classified as either high dosage (≥150 IU/m2) or low dosage (<150 IU/m2).
Our primary outcome measure was freedom from spasms defined as being without seizures for 7 days under the same treatment and a resolution of hypsarrhythmia on EEG. Study outcomes were determined by the data documented at the 3-month follow-up visit. Impact on spasms at 2 weeks, outcome at 3 months, and hypsarrhythmia on EEG, as well as assessment of drug dosage and impact, were recorded when available. Spasms outcome at 3 months was classified using a dichotomous approach: cessation of clinical spasms and resolution of hypsarrhythmia on EEG versus all other outcomes, which were considered treatment failure. Patients were considered spasms-free if they experienced no seizures for at least 7 days on an unchanged treatment. Disposition with regard to treatment was classified using the following categories: (1) stopped for side effects, (2) stopped for behavior, (3) stopped for therapeutic failure, (4) stopped for other reason, (5) stop planned due to success as per protocol, (6) still on due to therapeutic failure, (7) still on due to therapeutic failure but successfully used with other treatment, (8) still on due to success but on concomitant treatments, (9) still on due to therapeutic success on own, and (10) still on for other reasons. Finally, 3-month follow-up medication dosage, skill regression, and seizure frequency were documented.
Descriptive statistics were performed on demographic and clinical characteristics. Results are presented as mean ± standard deviation (SD), median (Mdn), interquartile range (IQR), range, or percentage. Chi-square tests for association were conducted between study patients with Down syndrome and infantile spasms compared with patients with no infantile spasms with regard to the prevalence of heart defects requiring surgical repair before spasms onset, pulmonary hypertension, prematurity, and thyroid abnormalities. Patients with Down syndrome receiving care at the Sie Center for Down Syndrome (SCDS) at Children’s Hospital Colorado without infantile spasms (n = 1260) were used as the comparison group. Data from the Colorado Department of Public Health and Environment of all children born with Down syndrome in the state from 2000 to 2013 were compared with the patient population at the SCDS. Results indicate that SCDS clinic patients capture approximately 50.3% of the state of Colorado’s population of children with Down syndrome and provides support for a population-based representation.26 Because we tested infantile spasms diagnosis differences separately for each of the 5 medical comorbidities and complications, we used the Benjamini and Hochberg false discovery rate to control for potential Type I errors.27 Fisher’s exact tests were conducted between ACTH, vigabatrin, oral steroids, and other treatments with (1) regression of skills at spasms onset, (2) cessation of spasms at 2 weeks (n = 32), and (3) cessation of spasms at 3 months (n = 34). A one-way analysis of variance was performed to determine if the time from spasms onset to start of treatment was different for the 4 treatment groups. Chi-square tests for association were also run between spasms outcome at 3 months and (1) first treatment type, (2) presence of hypsarrhythmia versus modified hypsarrhythmia on EEG, (3) abnormal MRI, (4) preterm status, (5) perinatal hypoxia-ischemia, (6) heart defect, (7) pulmonary hypertension, (8) thyroid abnormality, and (9) prior surgery requiring general anesthesia. Data were analyzed by the statistical package SPSS 24.0. All analyses were conducted at an α-level .05 significance. Children were omitted in an analysis if the variable of investigation had an “unknown” or “missing data” value.
Ethics and Informed Consent
Written informed consent was waived for the collection of retrospective data of patients receiving care prior to study’s April 2013 approval by the Institutional Review Board of Children’s Hospital Colorado, University of Colorado School of Medicine. Investigators received written consent for all patients enrolled prospectively beyond this date. This study did not involve any personal contact or interviews with patients or their caregivers other than prospective consent.
Results
Identification and Testing
There were 40 records identified for inclusion in the study. Three children were excluded due to not meeting study criteria of infantile spasms diagnosis. A total of 37 children (male = 22; female = 15; mean age at initial contact: 2.02 years, SD ± 3.05) were included in the final study cohort. Baseline characteristics of our sample at infantile spasms diagnosis are presented in Table 1. Average age of onset of infantile spasms (n = 37) was 7.71 months (SD = 4.32, Mdn = 6.41, IQR = 5.06-8.50, and range = 2.73-22.26 months) and treatment was initiated (n = 34) at a mean of 9.16 months (SD = 4.52, Mdn = 7.61, IQR = 5.92-11.99, and range = 4.47-24.13 months). Regression of skills was documented in 40.5% children and was frequently reported as less interaction, eye contact, and smiling; loss of gross motor skills; and poor head control and tone. No child had seizures prior to the onset of infantile spasms. Prior surgeries or procedures requiring general anesthesia before the onset of spasms included the following: cardiac surgery (n = 5), bronchoscopy (n = 3), tracheotomy (n = 2), gastrostomy tube (n = 2), tympanotomy (n = 2), and other surgeries (n = 4).
Table 1.
Descriptive Statistics for Characterizing IS/DS Comorbidities.
| Characteristics | Study IS/DS Patients |
Total DS Clinic Patients With No IS |
χ2 Significance P | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Total patients | 37 | 100.0% | 1260 | 100.0% | |
| Sex | |||||
| Female | 15 | 40.5% | 576 | 45.7% | |
| Race/ethnicity | |||||
| White, non-Hispanic | 19 | 51.4% | 724 | 57.5% | |
| Hispanic/Latino | 8 | 21.6% | 356 | 28.3% | |
| More than one race, non-Hispanic | 3 | 8.1% | 19 | 1.5% | |
| Black or African American, non-Hispanic | 2 | 5.4% | 46 | 3.7% | |
| Other race, non-Hispanic | 2 | 5.4% | 28 | 2.2% | |
| Asian | 0 | 0.0% | 20 | 1.6% | |
| American Indian/Alaska native | 0 | 0.0% | 6 | 0.5% | |
| Native Hawaiian/Pacific Islander | 0 | 0.0% | 0 | 0.0% | |
| Unknown | 3 | 8.1% | 61 | 4.8% | |
| Comorbidities | |||||
| Heart defects | 32 | 86.5% | 768 | 61.0% | .002 |
| Heart defects requiring surgical repair | 5 | 13.5% | 225 | 17.9% | .495 |
| Before spasms onseta | |||||
| Pulmonary hypertension | 14 | 38.9% | 269 | 21.3% | .017 |
| Perinatal hypoxia-ischemia | 12 | 32.4% | —b | —b | |
| Prematurity | 11 | 29.7% | 284 | 22.5% | .304 |
| Thyroid abnormalities | 8 | 21.6% | 394 | 31.3% | .211 |
| Surgery/procedure requiring use of general | 12 | 32.4% | —b | —b | |
| Anesthesia prior to IS onset | |||||
Abbreviations: IS, infantile spasms; DS, Down syndrome.
Total DS clinic patients with no IS were included if surgery date was at age 234 days or less.
Missing specific details in participant records.
There was a statistically significant association between infantile spasms diagnosis and heart defects (χ2[1] = 9.915, P = .002) and pulmonary hypertension (χ2[1] = 5.729, P = .017). There were no other statistically significant associations (P < .05) between infantile spasms diagnosis and the other potential medical comorbidity and complication risk factors analyzed when performing the Benjamini and Hochberg false discovery rate control model at an α-level of 5%. Pulmonary hypertension and heart defects were statistically independent in regard to risk of infantile spasms (P = .678) indicating that these are separate diagnoses without significant redundancy (see Table 1).
EEG demonstrated hypsarrhythmia (n = 16, 51.6%) and modified hypsarrhythmia (n = 9, 29.0%) in 31 patients receiving EEG prior to treatment. Of the remaining 6 patients, EEGs were completed during treatment documenting hypsarrhythmia. Overall, 17 patients completed an MRI, 6 (35.3%) of which had abnormal results, all described as atrophy. First-line medications for these 6 patients included the following: ACTH (n = 2), topiramate (n = 1), vigabatrin (n = 1), valproate (n = 1), and other (n = 1).
Response to Treatment
Average time from spasms onset to start of medication was 1.38 months (SD = 1.72) with ACTH most often administered initially (n = 18, 48.6%). Initial dose of ACTH varied from 104.97 IU/m2 to 226.58 IU/m2 (Mdn = 145.89 IU/m2, IQR = 118.79-149.85 IU/m2). Other first-line treatments included the following: topiramate (n = 5; dosage range = 1.8-11.5 mg/kg), vigabatrin (n = 4; dosage range = 56.8-137.0 mg/kg), zonisamide (n = 2; dosage range = 9.5 mg/kg), valproate (n = 2; dosage range = 15.0-30.0 mg/kg), and others (n = 4). Higher dosage for these other first treatments did not result in a more favorable response. Comparison between patients with ACTH (n = 18); topiramate, zonisamide, and valproate (n = 9); vigabatrin (n = 4); and other treatments (n = 4) as a first-line treatment indicated no statistically significant difference between the 4 groups for regression of skills at spasms onset as assessed by Fisher’s exact test (P = .385). Differences between these groups in the time from onset of spasms to the start of treatment were not statistically significant (F[3, 30] = .939, P = .434).
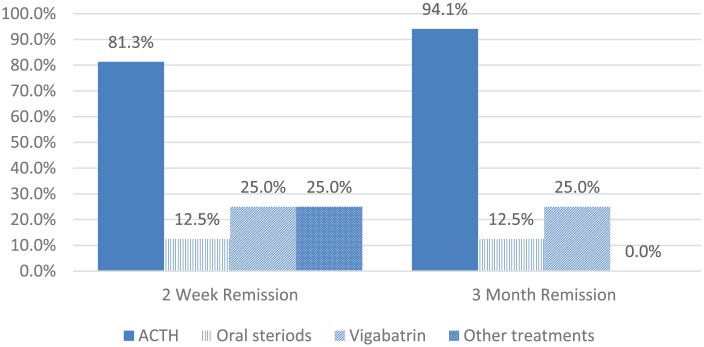
There was a statistically significant association between treatment type and cessation of spasms at 2 weeks (P = .003) and 3 months (P = .001; Fisher’s exact test). ACTH was the most effective first-line treatment in patients with Down syndrome with 81.3% experiencing remission of spasms at 2 weeks and 94.1% at 3 months compared with topiramate, zonisamide, and valproate (12.5%; 12.5%); vigabatrin (25.0%; 25.0%); and other treatments (25.0%; 0.0%), respectively (Figure 1).
Figure 1.
Response to first treatment.
Difference in outcome between patients receiving high (≥150 IU/m2) and low doses (<150 IU/m2) of ACTH was calculated for those patients with dosage data available (n = 13). All high-dose ACTH users (n = 3/3) experienced initial remission of spasms at 2 weeks but decreased to 33.3% (n = 1/3) at 3 months in comparison to low-dose users at 2 weeks (80.0%, n = 8/10) and 3 months (90.0%, n = 9/10). Statistical significance was not established due to limited power.
Outcomes
By the 3-month follow-up, 64.7% (n = 22/34) of patients with Down syndrome had cessation of spasms with no other form of seizures. At this time point, 32.3% (n = 10/31) of the patients had concluded medication treatment. Of the 22 patients with resolution of spasms within 3 months, 94.7% (n = 18/19) no longer had regression in their skills by clinical observation. Only treatment type was a predictor of continued spasms at 3 months (see Table 2). Presence of hypsarrhythmia versus modified hypsarrhythmia on EEG, abnormal MRI, preterm status, perinatal hypoxia-ischemia, heart defect, pulmonary hypertension, thyroid abnormality, and prior surgery requiring general anesthesia were not associated with spasms outcome at 3 months.
Table 2.
Chi-Square Tests: 3-Month Spasms Outcome.
| Clinical Presentation/Comorbidities | Results |
|---|---|
| ACTH compared to other treatment | χ2(1) = 12.879, P < .001 |
| Clinical presentation | |
| Hypsarrhythmia on EEGa | χ2(1) = 1.867, P = .172 |
| Abnormal MRI | χ2(1) = 1.377, P = .241 |
| Comorbidities | |
| Preterm status | χ2(1) = 0.137, P = .711 |
| Perinatal hypoxia-ischemia | χ2(1) = 0.458, P = .498 |
| Heart defect | χ2(1) = 0.429, P = .512 |
| Pulmonary hypertension | χ2(1) = 2.344, P = .126 |
| Thyroid | χ2(1) = 0.012, P = .912 |
| Prior surgery requiring general anesthesia | χ2(1) = 0.008, P = .928 |
Abbreviations: ACTH, adrenocorticotropic hormone; EEG, electroencephalogram; MRI, magnetic resonance imaging.
Hypsarrhythmia (yes) versus modified hypsarrhythmia (no).
Discussion
We found that ACTH is more effective than other treatments for infantile spasms in children with Down syndrome. While there was an overall resolution of spasms by 3 months after treatment, those treated with ACTH had better outcomes. This adds to our understanding of infantile spasms in children with Down syndrome by incorporating a larger single population sample reflecting recent improved treatment data from a single pediatric hospital. The previous studies on infantile spasms in children with Down syndrome were limited to a small number of investigations with no more than 17 patients.11,14,17,19,23-25 Average time from spasms onset to treatment of infantile spasms was longer for children with Down syndrome in our investigation (1.38 months) than prior studies reporting a treatment lag of 7 to 25 days for all children presenting with infantile spasms.28-32 Children with Down syndrome experienced a high rate of spasms resolution (81.3%) at the 2-week and 3-month intervals when ACTH was utilized as the first line of treatment. This response was significantly more effective than the use of other treatments for infantile spasms. Our patients experienced a high rate of positive response to ACTH similar to previous studies, which report an 88% to 96% effective response.3,11,17,19,24,32 A prior review of the literature reported a 96% effectiveness to hormonal (ACTH) treatment across 7 studies.17
Children with Down syndrome and infantile spasms had a higher prevalence of heart defects and pulmonary hypertension compared with children with Down syndrome without infantile spasms. A recent study also reported this increased occurrence of heart defects in 9 out of 11 (81.8%) patients with Down syndrome and infantile spasms.33 Our results suggest cardiac and pulmonary issues may be risk factors for infantile spasms in children with Down syndrome. Families and providers may need additional instruction and vigilance to monitor for infantile spasms once heart defects and pulmonary hypertension have been identified. Prospective studies would be helpful in clarifying this association.
Our data suggest a positive outcome in children with Down syndrome with early initiation of low-dose ACTH and the need for standardizing medication type and dosage when treating this population. Prior studies examining Down syndrome and leukemia have documented the higher sensitivity of pharmacodynamic effects of children with Down syndrome.34-37 One investigation on children with Down syndrome and acute lymphocytic leukemia suggested that altered pharmacokinetics may be a contributing factor in this patient population.35 Our results indicate that further investigation is needed to determine whether children with Down syndrome are also highly sensitive to ACTH. Reducing the dose of ACTH could lead to fewer side effects and significantly less cost of treatment as long as outcomes are unchanged.38,39 Of the patients who were included in this study, it appears that the majority of children recovered their previous developmental baseline within 3 months based on parent report and clinical observation.
Our results are consistent with the existing literature regarding treatment delay in children with Down syndrome, suggesting the need for further work focusing on children with Down syndrome and infantile spasms to improve identification, treatment, and outcomes. Data presented in this article and prior literature stress the importance of early recognition and treatment, especially when considering this population has an underlying preexisting developmental delay. Treatment lag can be associated with delayed time to cessation of spasms, further developmental impact, higher rate of autistic features, and later seizure types.17-19,30
Our results also support the need to provide further education and outreach to primary care providers, as well as families, to increase awareness of the various presentations of infantile spasms in the young child with Down syndrome. This is consistent with the efforts made by the United Kingdom Infantile Spasms Study.30 Resources should include, but not be limited to, educational offerings, handouts, video demonstration of symptoms/presentation, and utilization of phone/home video for early diagnosis and treatment. Prolonged video EEG may also be necessary. By providing this education and outreach we can potentially decrease the risk of further developmental impact and intractable seizures and, thereby, improve a child’s overall quality of life.
There were limitations to our study that are inherent to a retrospective review including a lack of control of treatment parameters that may have affected outcomes and variability in testing evaluation reports available. Although all neurologic testing and outcomes were reviewed by a board-certified epileptologist with expertise treating and working with children with Down syndrome, some inconsistencies in the available data details were inevitable. One might also speculate that children receiving services from a single pediatric hospital can potentially create a population bias. This study was designed to reduce limitations in the existing literature through one of the largest investigations of infantile spasms in children with Down syndrome based at a single institution and incorporating recent improved treatment data. All children received medical care between 2005 and 2015 at a tertiary care pediatric hospital with the majority of participants also seen in a dedicated Down syndrome focused clinic in coordination with hospital neurologists, allowing for close follow-up and identification of comorbidities and outcomes.
Considering that at least 3% of children with Down syndrome may have infantile spasms, improvements in early identification and targeted ACTH treatment and testing are necessary. Cardiac and pulmonary comorbidities may be risk factors in this population. This study further clarifies that ACTH is effective as an early first-line treatment for infantile spasms in children with Down syndrome when compared with other initial treatments. Pediatricians, family practice physicians, and families need to be aware of the increased risk of infantile spasms in children with Down syndrome and the importance of early identification and referral of all children with Down syndrome who present with clinical symptoms of infantile spasms.
Footnotes
Author Contributions: DD: Contributed to conception and design; contributed to acquisition and interpretation; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
KK: Contributed to conception and design; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
TB: Contributed to conception and design; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
KWW: Contributed to conception and design; contributed to analysis and interpretation; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
MM: Contributed to acquisition and analysis; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
FH: Contributed to conception and design; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We would like to thank the Global Down Syndrome Foundation and the Anna and John J. Sie Foundation for their financial support of research conducted at the Sie Center for Down Syndrome at Children’s Hospital Colorado. Dr. Benke was supported by the Ponzio Family Chair in Neurology Research.
ORCID iD: Dee Daniels  https://orcid.org/0000-0001-5638-5999
https://orcid.org/0000-0001-5638-5999
References
- 1. International League Against Epilepsy. Definition and classification. https://www.ilae.org/guidelines/definition-and-classification. Accessed September 2, 2017.
- 2. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522-530. doi: 10.1111/epi.13670 [DOI] [PubMed] [Google Scholar]
- 3. Goldberg-Stern H, Strawsburg RH, Patterson B, et al. Seizure frequency and characteristics in children with Down syndrome. Brain Dev. 2001;23:375-378. doi: 10.1016/S0387-7604(01)00239-X [DOI] [PubMed] [Google Scholar]
- 4. Escofet C, Póo P, Valbuena O, Gassió R, Sanmartí F, Campistol J. Infantile spasms in children with Down’s syndrome [in Spanish]. Rev Neurol. 1995;23:315-317. [PubMed] [Google Scholar]
- 5. Arya R, Kabra M, Gulati S. Epilepsy in children with Down syndrome. Epileptic Disord. 2011;13:1-7. doi: 10.1684/epd.2011.0415 [DOI] [PubMed] [Google Scholar]
- 6. Tapp S, Anderson T, Visootsak J. Neurological outcomes in children with Down syndrome and infantile spasms. J Pediatr Neurol. 2015;13:74-77. doi: 10.1055/s-0035-1556768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shields WD. Infantile spasms: little seizures, BIG consequences. Epilepsy Curr. 2006;6:63-69. doi: 10.1111/j.1535-7511.2006.00100.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cowan LD, Hudson LS. The epidemiology and natural history of infantile spasms. J Child Neurol. 1991;6:335-364. doi: 10.1177/088307389100600412 [DOI] [PubMed] [Google Scholar]
- 9. Rikonen R. A long-term follow-up study of 214 children with the syndrome of infantile spasms. Neuropediatrics. 1982;13:14-23. doi: 10.1055/s-2008-1059590 [DOI] [PubMed] [Google Scholar]
- 10. Smigielska-Kuzia J, Sobaniec W, Kułak W, Boćkowski L. Clinical and EEG features of epilepsy in children and adolescents in Down syndrome. J Child Neurol. 2009;24:416-420. doi: 10.1177/0883073808324542 [DOI] [PubMed] [Google Scholar]
- 11. Verrotti A, Cusmai R, Nicita F, et al. Electroclinical features and long-term outcome of cryptogenic epilepsy in children with Down syndrome. J Pediatr. 2013;163:1754-1758. doi: 10.1016/j.jpeds.2013.07.022 [DOI] [PubMed] [Google Scholar]
- 12. Mikati M, Lepejian G, Holmes G. Medical treatment of patients with infantile spasms. Clin Neuropharmacol. 2002;25:61-70. [DOI] [PubMed] [Google Scholar]
- 13. Stafstrom CE, Patxot OF, Gilmore HE, Wisniewski KE. Seizures in children with Down syndrome: etiology, characteristics and outcome. Dev Med Child Neurol. 1991;33:191-200. doi: 10.1111/j.1469-8749.1991.tb05108.x [DOI] [PubMed] [Google Scholar]
- 14. Stafstrom CE, Konkol RJ. Infantile spasms in children with Down syndrome. Dev Med Child Neurol. 1994;36:576-585. doi: 10.1111/j.1469-8749.1994.tb11894.x [DOI] [PubMed] [Google Scholar]
- 15. Sobey CG, Judkins CP, Sundararajan V, Phan TG, Drummond GR, Srikanth VK. Risk of major cardiovascular events in people with Down syndrome. PLoS One. 2015;10:e0137093. doi: 10.1371/journal.pone.0137093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buterbaugh A, Visootsak J. Implications of delayed diagnosis of infantile spasm in a child with Down syndrome. J Pediatr Neurol. 2014;12:105-107. [PMC free article] [PubMed] [Google Scholar]
- 17. Sanmaneechai O, Sogawa Y, Silver W, Ballaban-Gil K, Moshe SL, Shinnar S. Treatment outcomes of West syndrome in infants with Down syndrome. Pediatr Neurol. 2013;48:42-47. doi: 10.1016/j.pediatrneurol.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 18. Lott IT, Dierssen M. Cognitive deficits and associated neurological complications in individuals with Down’s syndrome. Lancet Neurol. 2010;9:623-633. doi: 10.1016/S1474-4422(10)70112-5 [DOI] [PubMed] [Google Scholar]
- 19. Eisermann MM, DeLaRaillere A, Dellatolas G, et al. Infantile spasms in Down syndrome—effects of delayed anticonvulsive treatment. Epilepsy Res. 2003;55:21-27. doi: 10.1016/S0920-1211(03)00088-3 [DOI] [PubMed] [Google Scholar]
- 20. Wilmshurst JM, Gaillard WD, Vinayan KP, et al. Summary of recommendations for the management of infantile seizures: Task Force Report for the ILAE Commission of Pediatrics. Epilepsia. 2015;56:1185-1197. doi: 10.1111/epi.13057 [DOI] [PubMed] [Google Scholar]
- 21. Go CY, Mackay MT, Weiss SK, et al. Evidence-based guideline update: medical treatment of infantile spasms. Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2012;78:1974-1980. doi: 10.1212/WNL.0b013e318259e2cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mackay MT, Weiss SK, Adams-Weber T, et al. Practice parameter: medical treatment of infantile spasms: report of the American Academy of Neurology and the Child Neurology Society. Neurology. 2004;62:1668-1681. doi: 10.1212/01.WNL.0000127773.72699.C8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beatty CW, Wrede JE, Blume HK. Diagnosis, treatment, and outcomes of infantile spasms in the Trisomy 21 population. Seizure. 2016;45:184-188. doi: 10.1016/j.seizure.2016.12.016 [DOI] [PubMed] [Google Scholar]
- 24. Nabbout R, Melki I, Gerbaka B, Dulac O, Akatcherian C. Infantile spasms in Down syndrome: good response to a short course of vigabatrin. Epilepsia. 2001;42:1580-1583. doi: 10.1046/j.1528-1157.2001.13501.x [DOI] [PubMed] [Google Scholar]
- 25. Pollack MA, Golden GS, Schmidt R, Davis JA, Leeds N. Infantile spasms in Down syndrome: a report of 5 cases and review of the literature. Ann Neurol. 1978;3:406-408. doi: 10.1002/ana.410030508 [DOI] [PubMed] [Google Scholar]
- 26. Hickey F, Wolter-Warmerdam K, Hickey E, Yoon J, Daniels D. Pediatric comorbidities and medical complications identified in children with Down syndrome. J Down Syndr Chr Abnorm. 2017;3:124. doi: 10.4172/2472-1115.1000124 [DOI] [Google Scholar]
- 27. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodological). 1995;57:289-300. [Google Scholar]
- 28. Knupp KG, Coryell J, Nickels KC, et al. Response to treatment in a prospective national infantile spasms cohort. Ann Neurol. 2016;79:475-484. doi: 10.1002/ana.24594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cohen-Sadan S, Kramer U, Ben-Zeev B, et al. Multicenter long-term follow-up of children with idiopathic West syndrome: ACTH versus vigabatrin. Eur J Neurol. 2009;16:482-487. doi: 10.1111/j.1468-1331.2008.02498.x [DOI] [PubMed] [Google Scholar]
- 30. O’Callaghan F, Lux A, Darke K, et al. The effect of lead time to treatment and age of onset on developmental outcome at 4 years in infantile spasms: evidence from the United Kingdom Infantile Spasms Study. Epilepsia. 2011;52:1359-1364. doi: 10.1111/j.1528-1167.2011.03127.x [DOI] [PubMed] [Google Scholar]
- 31. O’Callaghan F, Edwards SW, Alber FD, et al. Safety and effectiveness of hormonal treatment versus hormonal treatment with vigabatrin for infantile spasms (ICISS): a randomised, multicentre, open-label trial. Lancet Neurol. 2017;16:33-42. doi: 10.1016/S1474-4422(16)30294-0 [DOI] [PubMed] [Google Scholar]
- 32. Mohamed BP, Scott RC, Desai N, Gutta P, Patil S. Seizure outcome in infantile spasms—a retrospective study. Epilepsia. 2011;52:746-752. doi: 10.1111/j.1528-1167.2010.02963.x [DOI] [PubMed] [Google Scholar]
- 33. Lujic L, Bosnjak VM, Delin S, Duranovic V, Krakar G. Infantile spasms in children with Down syndrome. Coll Antropol. 2011;35(suppl 1):213-218. [PubMed] [Google Scholar]
- 34. Buitenkamp TD, Mathot RA, de Haas V, Pieters R, Zwaan CM. Methotrexate-induced side effects are not due to differences in pharmacokinetics in children with Down syndrome and acute lymphoblastic leukemia. Haematologica. 2010;95:1106-1113. doi: 10.3324/haematol.2009.019778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garré ML, Relling MV, Kalwinsky D, et al. Pharmacokinetics and toxicity of methotrexate in children with Down syndrome and acute lymphocytic leukemia. J Pediatr. 1987;111:606-612. doi: 10.1016/S0022-3476(87)80131-2 [DOI] [PubMed] [Google Scholar]
- 36. Peeters M, Poon A. Down syndrome and leukemia: unusual clinical aspects and unexpected methotrexate sensitivity. Eur J Pediatr. 1987;146:416-422. [DOI] [PubMed] [Google Scholar]
- 37. Zwaan CM, Kaspers GJ, Pieters R, et al. Different drug sensitivity profiles of acute myeloid and lymphoblastic leukemia and normal peripheral blood mononuclear cells in children with and without Down syndrome. Blood. 2002;99:245-251. doi: 10.1182/blood.V99.1.245 [DOI] [PubMed] [Google Scholar]
- 38. Ito M, Aiba H, Hashimoto K, et al. Low-dose ACTH therapy for West syndrome: initial effects and long-term outcome. Neurology. 2002;58:110-114. doi: 10.1212/WNL.58.1.110 [DOI] [PubMed] [Google Scholar]
- 39. Yin J, Lu Q, Yin F, et al. Effectiveness and safety of different once-daily doses of adrenocorticotropic hormone for infantile spasms. Paediatr Drugs. 2017;19:357-365 doi: 10.1007/s40272-017-0225-5 [DOI] [PubMed] [Google Scholar]