Abstract
As a chronic degenerative joint disease, osteoarthritis is among the most common diseases all over the world. In osteoarthritis, inflammation plays an important role in the generation of joint symptoms and the development of disease. When the programmed cell death-1 (PD-1)/programmed death-ligand 1 (PD-L1) immune checkpoint is blocked, the antitumor immunity will be enhanced. We aim to illustrate the function of PD-L1 in osteoarthritis. Osteoarthritis in mice was induced by the injection of collagenase or anterior cruciate ligament transection (ACLT). Anti-PD-L1 was employed to block the signal of PD-L1. Knee joints histological sections were stained by Safranin-O. The level of cytokine was checked by enzyme-linked immunosorbent assay (ELISA) and mRNA level was shown by quantitative reverse transcriptase polymerase chain reaction. The blockade of PD-L1 signal up-regulated inflammatory response and promoted the development of osteoarthritis in mice. The inflammatory cytokine interleukin-6 and tumor necrosis factor-α expression were promoted by PD-L1 blocking in macrophages. Osteoarthritis was aggravated when the expression of inflammatory cytokine is elevated in macrophages. Our results indicated that the blockade of PD-L1 signal in macrophages elevates the expression of inflammatory cytokine and promotes the development of osteoarthritis in mice, which could be utilized as a potential diagnostic and therapeutic target for osteoarthritis patients.
Keywords: inflammatory cytokine, macrophages, osteoarthritis, programmed death-ligand 1
Introduction
As one of the most frequent chronic diseases all over the world, osteoarthritis has higher incidence and prevalence as a consequence of the longer life expectancy.1 The progression of osteoarthritis results in the decline of joint function and causes the reduction of life quality.2 The clinical characters of osteoarthritis include tenderness, joint pain, movement limitation, effusion, and inflammation.3 Among these characters, inflammation is variable in the development process of osteoarthritis and contributes to several different joint symptoms.4 During the development of osteoarthritis, inflammation in joint tissue includes the production of cytokine, low-grade infiltration of cells, and inflammatory activation of synoviocytes, articular chondrocytes, and other cells in joint.5 More and more studies in human or animal models have demonstrated the details of the molecular mechanisms in the process of inflammatory pathway initiation, inflammatory mediator generation, and cellular infiltration. But there is still incomplete understanding of molecular pathways in the process of inflammatory osteoarthritis.
The interaction of programmed cell death-1 (PD-1) and programmed death-ligand 1 (PD-L1) attenuates the phosphorylation signaling and suppresses the activation of immune cells.6 The expression of PD-1 and PD-L1 can be tested in both hematopoietic cells and non-hematopoietic cells. PD-1/PD-L1 axis is an immune checkpoint which plays a critical role in immune tolerance and suppression by the regulation of T-cell function.7 Based on the previous studies, the high levels of PD-1 and PD-L1 in carcinoma cells and tumor-infiltrating lymphocytes have a strong correlation with the highly suppressive microenvironment and promote the development of carcinoma.7
Even though the function of PD-L1 axis in cancer has been reported, it is still unknown whether this immune checkpoint participates in the regulation of inflammatory process in osteoarthritis. We aim to examine the association between PD-1 pathway and inflammation and understand the potential role of PD-1/PD-L1 axis in osteoarthritis.
Methods
Osteoarthritis animal model
To induce osteoarthritis mouse model, mice were treated with one unit of collagenase type VII (Sigma-Aldrich, St. Louis, MO, USA) by intra-articular injection on week 0. All mice were sacrificed 4 weeks post collagenase injection and knee joints were collected for histology. Animal experiments were strictly complied with protocol approved by the Institutional Committee of Animal Care and Use of the Second Hospital of Shandong University.
Anterior cruciate ligament transection (ACLT) was also performed in the induction of osteoarthritis in mice. Before the surgery, mice were anesthetized by ketamine hydrochloride (60 mg/kg). After making a parapatellar incision in the knee joint, the lateral displacement of the patella was done to generate an access to anterior cruciate ligament (ACL). The ACL was transected and the surgical incision was closed.
Histology and Osteoarthritis Research Society International score
The articular cartilage damage was examined by histological staining. After the induction of osteoarthritis, mice knee joints were collected, fixed, decalcified, and embedded by paraffin. Safranin-O staining showed the loss of proteoglycans in knee joint tissue sections.
Osteoarthritis Research Society International (OARSI) osteoarthritis cartilage histopathology assessment system was used to shown the quantification of cartilage damage. The OARSI score was influenced by both the grade and the stage of cartilage damage. The score was between 0 and 24. The higher score shows a more sever cartilage damage.
In vivo antibody treatment
Mice were injected with Anti-PD-L1 (ab80276; Abcam, Cambridge, MA, USA) or Anti-Rat-IgG2a (ab106783; Abcam) 1–3 weeks after the induction of osteoarthritis. Antibodies were injected at a dose of 200 μg per mouse.
Enzyme-linked immunosorbent assay
Tumor necrosis factor alpha (TNF-α) and interleukin (IL)-6 levels in the synovial fluid, serum, and supernatant were tested by enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) as per manufacturer’s instruction. A microplate reader (Bio-Rad, Hercules, CA, USA) was employed to detect the 450 nm absorbance.
Real-time quantitative polymerase chain reaction
TRIzol reagent (Invitrogen, Waltham, MA, USA) was employed to isolate total RNA. cDNA was reverse-transcribed from 1 μg total RNA by superscript III First-Strand Synthesis System (Invitrogen). Polymerase chain reactions (PCRs) were performed with Talent qPCR PreMix (Tiangen) in LightCycler480 (Roche, New York, USA). Primers used in quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) were shown as follows:
IL-6: 5′-gctaccaaactggatataatcagga-3′, 5′-ccaggtagctatggtactccagaa-3′
TNF-α: 5′-ccgatgggttgtaccttgtc-3′, 5′-gggctgggtagagaatggat-3′
IL-10: 5′-ccccaggcagagaagcatggc-3′, 5′-ggggagaaatcgatgacagcgcc-3′
Transforming growth factor beta (TGF-β): 5′-caccggagagccctggata-3′, 5′-tgtacagctgccgcacaca-3′
β-actin: 5′-aaggccaaccgtgaaaagat-3′, 5′-gtggtacgaccagaggcatac-3′
Flow cytometry
Cells were incubated with relevant antibodies (F4/80, CD3, CD11b, and CD45) for 30 min on ice. Flow cytometry was performed on Cyan Flowcytometer (Dako Cytomation) and data were analyzed with the FlowJo software (TreeStar, Ashland, OR, USA). 4′,6-diamidino-2-phenylindole (DAPI) staining was employed to exclude dead cells.
Bone marrow–derived macrophage activation
Bone marrow–derived macrophages (BMDMs) were isolated and cultured using the method described.8 BMDMs were treated with 1 ng/mL Pam3CSK4 (Pam; InvivoGen) or medium for 6 h. IL-6 and TNF-α concentration in media supernatants were detected by ELISA. After the Pam3CSK4 treatment, 2 × 105 BMDMs were injected intra-articularly to mice.
Statistical analyses
SPSS software version 19.0 was used in the data analyses. All data in the experiment were shown as mean ± standard error of mean (SEM). Student’s t-test or one-way analysis of variance (ANOVA) analysis were used to calculate the differences between each group, *P < 0.05, **P < 0.01, ns = not significant.
Results
Blocking of PD-L1 signal promotes the development of osteoarthritis in mice
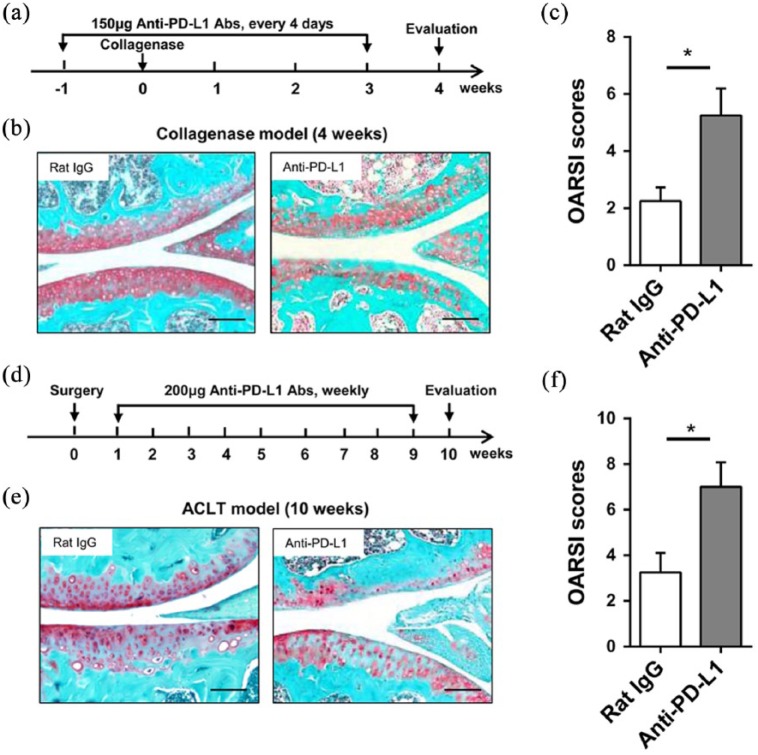
To investigate PD-L1 function in mice osteoarthritis, collagenase or ACLT was used in the induction of osteoarthritis. Mice were administered with anti-PD-L1 or Rat IgG after the treatment of collagenase from week 1 to week 3 and then sacrificed at week 4 (Figure 1(a)). Safranin-O staining of knee joints showed more severe articular cartilage damage after PD-L1 blocking than the control group (Figure 1(b)). The higher OARSI scores also indicated a worse cartilage condition in PD-L1 blocked mice knee joint (Figure 1(c)). In ACLT mice, anti-PD-L1 antibody or Rat IgG was injected for 8 weeks and tissues were collected at week 10 (Figure 1(d)). Both Safranin-O staining and OARSI scores indicted a more severe articular cartilage damage after PD-L1 blocking in osteoarthritis mice knee joint induced by ACLT (Figure 1(e) and (f)). These data showed that the development of osteoarthritis in mice can be promoted by PD-L1 blockade.
Figure 1.
Blocking of PD-L1 signal promotes the development of osteoarthritis in mice. (a–c) Procedure of PD-L1 blocking in collagenase-induced osteoarthritis: mice were intra-articularly injected with collagenase at week 0 and administered with anti-PD-L1 from week 1 to week 3. (a) Collagenase treated mice were injected with Rat IgG as control. All mice were sacrificed at week 4. (b) Representative images of Safranin-O staining of knee joints from two groups were shown. Bar = 100 μm. (c) Quantification of cartilage damage is shown as OARSI scores. (d–f) Procedure of PD-L1 blocking in ACLT-induced osteoarthritis. (d) Briefly, mice undergoing ACLT were injected with anti-PD-L1 antibody for 8 weeks. Mice undergoing ACLT were injected with Rat IgG as control. All mice were sacrificed at week 10 after surgery. (e) Representative images of Safranin-O staining of knee joints from two groups were shown. Bar = 100 μm. (f) Quantification of cartilage damage was shown as OARSI scores. Data are representative of three or more independent experiments with ⩾6 mice per group. Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01, ns = not significant.
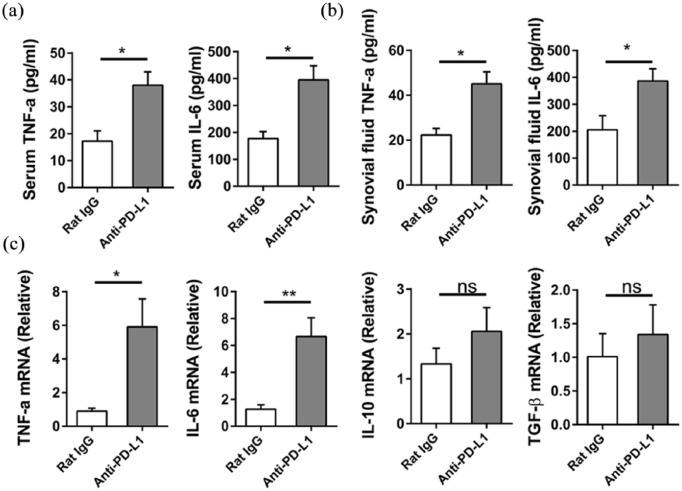
Blocking of PD-L1 up-regulates inflammatory response in osteoarthritis
To examine the connection between PD-L1 and inflammatory response in osteoarthritis, levels of proinflammatory cytokine were examined. ELISA data demonstrated the increases in IL-6 and TNF-α level in both serum and synovial fluid after the PD-L1 blockade in mice treated with collagenase (Figure 2(a) and (b)). The mRNA levels of IL-6, TNF-α, IL-10, and TGF-β in synovial tissues were detected by qRT-PCR. Figure 2(c) shows that the PD-L1 blocking in collagenase-induced osteoarthritis significantly enhances the expression of IL-6, TNF-α but does not influence the mRNA levels of IL-10 and TGF-β. It is shown that the PD-L1 blockade has a positive relationship with the inflammatory response in osteoarthritis induced by collagenase in mice.
Figure 2.
Blocking PD-L1 up-regulates inflammatory response in osteoarthritis. (a, b) PD-L1 blocking in collagenase-induced osteoarthritis and the mice were sacrificed at week 4. (a) The levels of IL-6 and TNF-α in serum were analyzed by ELISA. (b) The levels of IL-6 and TNF-α in synovial fluid were analyzed by ELISA. (c) The synovial tissue mRNA was extracted and IL-6, TNF-α, IL-10, and TGF-β expression was analyzed by qRT-PCR. Data are representative of three or more independent experiments with ⩾6 mice per group. Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01, ns = not significant.
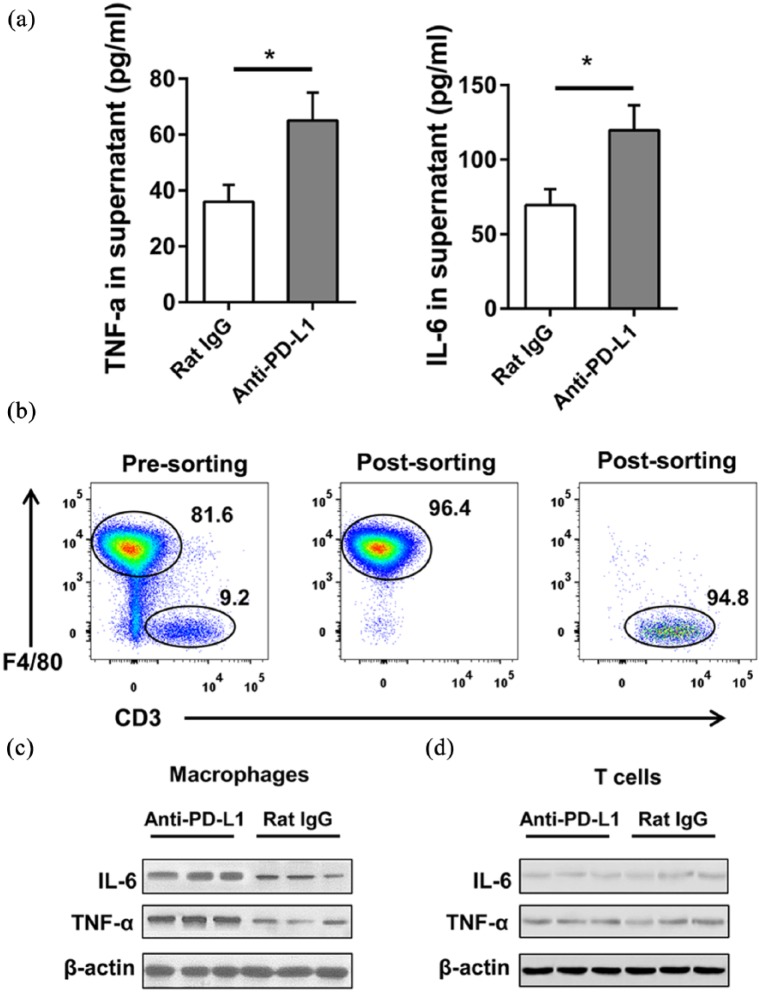
PD-L1 blocking promotes IL-6 and TNF-α expression in macrophages
We aimed to examine the mechanism in the elevation of IL-6 and TNF-α level by PD-L1 blockade. Joint-infiltrating immune cells were isolated from mice knee joint with collagenase-induced osteoarthritis and co-cultured with anti-PD-L1 or Rat IgG. The elevation of IL-6 and TNF-α concentration in the supernatant after the treatment of anti-PD-L1 was shown by ELISA (Figure 3(a)). Flow cytometry analysis demonstrated that joint-infiltrating immune cells are mainly composed of macrophages and T cells (Figure 3(b)). Macrophages and T cells were both co-cultured with anti-PD-L1 and the protein levels of IL-6 and TNF-α were tested by western blot. In Figure 3(c) and (d), the protein levels of IL-6 and TNF-α were only elevated in macrophages but not in T cells. Therefore, the PD-L1 blockade mainly controlled the expression of IL-6 and TNF-α in macrophages in mice knee joint with collagenase-induced osteoarthritis.
Figure 3.
PD-L1 blocking promotes IL-6 and TNF-α expression in macrophages. (a) Joint-infiltrating immune cells were isolated from mice with collagenase-induced osteoarthritis. The total cells were co-cultured with 20 μg/mL anti-PD-L1 or Rat IgG in vitro for 2 days. The concentration of IL-6 and TNF-α in the supernatant was analyzed by ELISA. (b–d) PD-L1 blocking in collagenase-induced osteoarthritis and the mice were sacrificed at week 4. Joint-infiltrating immune cells were isolated and the macrophages and T cells were sorted. (b) FACS analysis of purity is shown after sorting (gating on CD45+). The level of IL-6 and TNF-α expression in macrophages (c) and T cells (d) was analyzed by western blot. Data were representative of three or more independent experiments. Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01, ns = not significant.
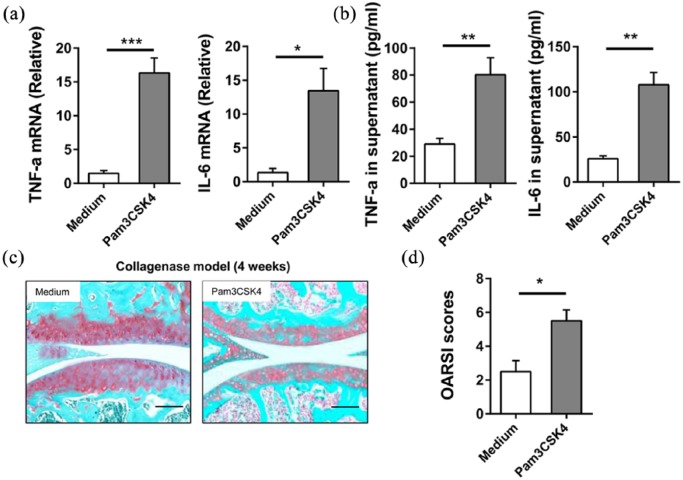
Increased expression of inflammatory cytokine by macrophages aggravates collagenase-induced osteoarthritis
To further confirm the relationship between macrophages and osteoarthritis, BMDMs were isolated and cultured. BMDMs were treated with 1 ng/mL Pam3CSK4 or medium for 6 h. The mRNA levels of TNF-α and IL-6 in BMDMs were significantly up-regulated by the treatment of Pam3CSK4 (Figure 4(a)). ELISA data also illustrated that there are more levels of TNF-α and IL-6 in supernatant after the treatment of Pam3CSK4 (Figure 4(b)). BMDMs were intra-articularly injected into mice with collagenase-induced osteoarthritis. Both the Safranin-O staining and the OARSI scores indicted that BMDM in the Pam3CSK4-treated group generates a more severe articular cartilage damage than that treated with medium (Figure 4(c) and (d)). These data confirmed that the TNF-α and IL-6 secreted from macrophages promotes the development of osteoarthritis in mice.
Figure 4.
The increased expression of inflammatory cytokines by macrophages aggravates collagenase-induced osteoarthritis. (a, b) BMDMs were treated with 1 ng/mL Pam3CSK4 or medium for 6 h. (a) The mRNA was extracted and IL-6; TNF-α expression was analyzed by qRT-PCR. (b) The concentration of IL-6 and TNF-α in the supernatant was analyzed by ELISA. (c, d) BMDMs were treated with 1 ng/mL Pam3CSK4 or medium for 6 h. 2 × 105 BMDMs were intra-articularly injected into mice with collagenase-induced osteoarthritis from week 1 to week 3, once a week. All mice were sacrificed at week 4. (c) Representative images of Safranin-O staining of knee joints from two groups are shown. Bar = 100 μm. (d) Quantification of cartilage damage was shown as OARSI scores. Data are representative of three or more independent experiments. Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01, ns = not significant.
Blocking of PD-L1 aggravates osteoarthritis through up-regulation of IL-6 and TNF-α in macrophage-dependent manner
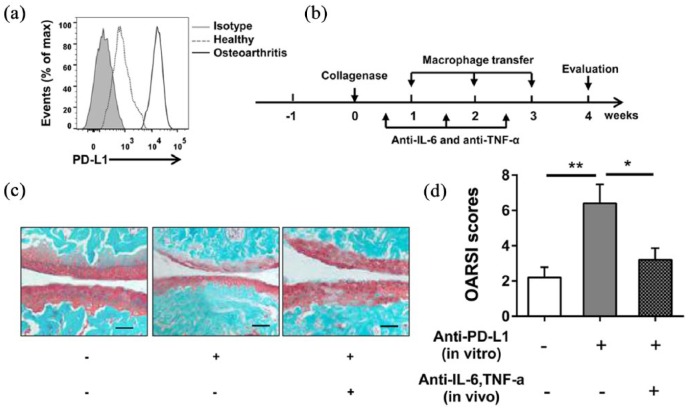
To detect whether the up-regulation of IL-6 and TNF-α in macrophage has relationship with PD-L1 blockade in mice osteoarthritis, the expression of PD-L1 in macrophages isolated from joint-infiltrating immune cells was tested. Figure 5(a) shows that macrophages from mice with collagenase-induced osteoarthritis have a higher PD-L1 level than macrophages from healthy control. The high PD-L1 expression macrophages were isolated and co-cultured with anti-PD-L1 or Rat IgG before intra-articular injection (Figure 5(b)). It is shown by Safranin-O staining and the OARSI scores that a more severe articular cartilage damage is generated after the injection of macrophages co-cultured with anti-PD-L1 and the damage aggravated by PD-L1 blockade in macrophages is decreased by the blocking of IL-6 and TNF-α (Figure 5(c) and (d)). The PD-L1 blockade in macrophages promoted the secretion of IL-6 and TNF-α which enhances the development of osteoarthritis in mice.
Figure 5.
Blocking PD-L1 aggravates osteoarthritis through up-regulation of IL-6 and TNF-α in macrophage-dependent manner. (a) Macrophages from mice with collagenase-induced osteoarthritis and healthy control were obtained and PD-L1 expression was determined by FACS. (b–d) Macrophages from mice with collagenase-induced osteoarthritis were sorted and co-cultured with 20 μg/mL anti-PD-L1 or Rat IgG in vitro for 2 days. (b) 1 × 105 macrophages were intra-articularly injected into mice with collagenase-induced osteoarthritis from week 1 to week 3, once a week. Anti-IL-6 and anti-TNF-α antibodies were injected once a week, 150 μg/mouse, for three times. All mice were sacrificed at week 4. (c) Representative images of Safranin-O staining of knee joints from each group are shown. Bar = 100 μm. (d) Quantification of cartilage damage is shown as OARSI scores. Data were representative of three or more independent experiments. Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01, ns = not significant.
Discussion
Osteoarthritis is a musculoskeletal disease with a high incidence all over the world and it is becoming a critical public health risk. The development of osteoarthritis causes the decline in the joint function and the loss of life quality. The incidence of osteoarthritis is low before age 40, increases rapidly between 40 and 60, and has a linear increase after age 60.9 As an irreversible chronic disease, osteoarthritis has two main characteristics in pathophysiology, one is the damage of articular cartilage and the other is the sclerosis of subchondral bones.10 The degeneration of entire joint, including the articular cartilage, ligaments, subchondral bone, and peri-articular muscles, is caused by a combination of mechanical force, structure, and signaling pathways.11 The clinical characters of osteoarthritis include tenderness, joint pain, movement limitation, effusion, and inflammation.3 Osteoarthritis has been identified as an immunopathological disease in recent years. It is demonstrated that various immune cells, proinflammatory cytokines, complements, and other parts of immune system play a crucial role in osteoarthritis pathogenesis.12 During the development of osteoarthritis, inflammation in joint tissue includes the production of cytokine, low-grade infiltration of cells, and inflammatory activation of synoviocytes, articular chondrocytes, and other cells in joint.5 During the development of osteoarthritis, the innate immune system is first activated in the early osteoarthritis. The activation of innate immune system results in the activation of adaptive immune system, which promotes the inflammation and the development of osteoarthritis. It has been reported that IL-1β and TNF-α are two dominant cytokines involved in osteoarthritis.13 The activation of innate immune and adaptive immune system also causes the increase of vascular endothelial growth factor, cyclooxygenase-2, and CD4+ T-cell and macrophage infiltration.14 In the synovium region of osteoarthritis patients, T cells and macrophages are the dominant cell types.13 In this article, we also checked the cell types in the joint-infiltrating immune cells isolated from mice with collagenase-induced osteoarthritis. Flow cytometry analysis demonstrated that joint-infiltrating immune cells are mainly composed of macrophages and T cells. It is reported that the secretion of IL-6 and TNF-α in macrophages can be promoted by toll-like receptor 2 (TLR2).15 We treated the BMDMs with TLR2 ligand Pam3CSK4 to enhance the production of IL-6 and TNF-α. The injection of BMDMs in mice knee joint proved that the IL-6 and TNF-α generated from macrophages play a crucial role in the damage to articular cartilage. The development of osteoarthritis is promoted by cytokines secreted from macrophages.
A relevant biomarker in osteoarthritis could be helpful in disease diagnosis and prognosis and may in addition shed light on potential therapeutic target(s). These markers will indicate the activation of crucial molecular pathways, the degree of cartilage damage, and the stage of disease progression.16 Till now, there is still no reliable biomarker which can be quantified to reflect the development stage of osteoarthritis.
PD-1 is a protein involved in the murine immune cell lines programmed cell death induced by stimulation.17 PD-1 binds to PD-L1 to suppress the activation of immune cells by blocking the phosphorylation signal.6 The expression of PD-1 and PD-L1 can be tested in both hematopoietic cells and non-hematopoietic cells. Most importantly, PD-1/PD-L1 signal pathway attenuates the activation of immune cells. It has been reported that the immune suppressive microenvironment in tumors has a strong correlation with the activation of PD-1/PD-L1 signal pathway.18 The up-regulation of PD-1 expression in tumor-infiltrating lymphocytes is observed in several different carcinomas, such as lung cancer, breast cancer, and ovarian cancer.7 As a consequence of the function of PD-1/PD-L1 signal pathway in the maintenance of microenvironment in tumors, PD-L1 is an immunotherapy target for carcinoma patients. The blockade of PD-1/PD-L1 enhances the function of effector T cells and promotes the immune cell accumulation in tumor region.19 In clinical trials, the blockade of PD-1/PD-L1 is performed by PD-1 or PD-L1 antibodies which can inhibit the interaction between ligand and receptor. Since the blockade of PD-1/PD-L1 has been proved to enhance the anticancer T-cell immunity and increase the overall survival in several human cancers, it is possible that PD-1/PD-L1 signal pathway participates in the inflammation process in the development of osteoarthritis.
In this article, we examine the function of PD-L1 in the collagenase-induced or ACLT-induced osteoarthritis in mice. After the blocking of PD-L1, the cartilage damage is promoted in both collagenase-induced and ACLT-induced osteoarthritis mice knee joints. In both synovial fluid and serum, the levels of IL-6 and TNF-α are elevated by PD-L1 blockade. Therefore, the blockade of PD-L1 promotes the cartilage damage in mice osteoarthritis by the elevation of IL-6 and TNF-α levels. Flow cytometry analysis demonstrates that joint-infiltrating immune cells are mainly composed of macrophages and T cells. After the blockade of PD-L1, IL-6 and TNF-α levels are only elevated in macrophages in synovial fluid. It has been shown that both the Pam3CSK4 treated macrophages and PD-L1 blockade macrophages enhanced the cartilage damage after being injected into knee joint of osteoarthritis mice. Meanwhile, the blocked of IL-6 and TNF-α by antibodies can rescue the phenotype generated by PD-L1 blockade in macrophages. Therefore, PD-L1 blockade specifically in macrophages regulates inflammation directly through IL-6 and TNF-α.
In this research, we have illustrated that the blockade of PD-L1 in macrophages can promote the inflammation in osteoarthritis. However, there are still some unanswered questions. In the development of osteoarthritis, the degree of local inflammation is variable. What is the downstream signaling pathway of PD-1/PD-L1? What condition controls the activation of the PD-1/PD-L1 signaling pathway? Is PD-1/PD-L1 the dominant signal in the regulation of inflammation or does it work in concert with other signaling pathways? The answer of all these questions will help us in understanding the process of inflammation during the development of osteoarthritis.
In conclusion, the secretion of IL-6 and TNF-α is enhanced by the blockade of PD-L1 in macrophages, which enhances the development of osteoarthritis in mice. The relationship between the high level of macrophages PD-L1 and the enhanced inflammation in mice osteoarthritis indicates that PD-L1 can be used as a potential diagnostic and therapeutic target for the osteoarthritis patients.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Key Research and Development Plan of Shandong Province (no. 2015GSF118107).
ORCID iD: Chunzheng Gao  https://orcid.org/0000-0002-2290-8410
https://orcid.org/0000-0002-2290-8410
References
- 1. Pereira D, Ramos E, Branco J. (2015) Osteoarthritis. Acta Médica Portuguesa 28(1): 99–106. [DOI] [PubMed] [Google Scholar]
- 2. Chu CR, Millis MB, Olson SA. (2014) Osteoarthritis: From palliation to prevention: AOA critical issues. The Journal of Bone and Joint Surgery 96(15): e130 DOI: 10.2106/JBJS.M.01209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Felson DT. (2009) Developments in the clinical understanding of osteoarthritis. Arthritis Research & Therapy 11(1): 203 DOI: 10.1186/ar2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lieberthal J, Sambamurthy N, Scanzello CR. (2015) Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis and Cartilage 23(11): 1825–1834. DOI: 10.1016/j.joca.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang X, Hunter D, Xu J, et al. (2015) Metabolic triggered inflammation in osteoarthritis. Osteoarthritis and Cartilage 23(1): 22–30. DOI: 10.1016/j.joca.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 6. Keir ME, Butte MJ, Freeman GJ, et al. (2008) PD-1 and its ligands in tolerance and immunity. Annual Review of Immunology 26: 677–704. DOI: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zheng P, Zhou Z. (2015) Human cancer immunotherapy with PD-1/PD-L1 blockade. Biomarkers in Cancer 7(Suppl. 2): 15–18. DOI: 10.4137/BIC.S29325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang X, Goncalves R, Mosser DM. (2008) The isolation and characterization of murine macrophages. Current Protocols in Immunology Chapter 14:Unit 14 1. DOI: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Altman RD. (2010) Early management of osteoarthritis. The American Journal of Managed Care 16: S41–S47. [PubMed] [Google Scholar]
- 10. Van der Kraan PM. (2012) Osteoarthritis year 2012 in review: Biology. Osteoarthritis and Cartilage 20(12): 1447–1450. DOI: 10.1016/j.joca.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 11. Andrianakos AA, Kontelis LK, Karamitsos DG, et al. (2006) Prevalence of symptomatic knee, hand, and hip osteoarthritis in Greece: The ESORDIG study. The Journal of Rheumatology 33(12): 2507–2513. [PubMed] [Google Scholar]
- 12. Kandahari AM, Yang X, Dighe AS, et al. (2015) Recognition of immune response for the early diagnosis and treatment of osteoarthritis. Journal of Immunology Research 2015: 192415 DOI: 10.1155/2015/192415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Lange-Brokaar BJ, Ioan-Facsinay A, van Osch GJ, et al. (2012) Synovial inflammation, immune cells and their cytokines in osteoarthritis: A review. Osteoarthritis and Cartilage 20(12): 1484–1499. DOI: 10.1016/j.joca.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 14. Benito MJ, Veale DJ, FitzGerald O, et al. (2005) Synovial tissue inflammation in early and late osteoarthritis. Annals of the Rheumatic Diseases 64(9): 1263–1267. DOI: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qadri M, Almadani S, Jay GD, et al. (2018) Role of CD44 in regulating TLR2 activation of human macrophages and downstream expression of proinflammatory cytokines. Journal of Immunology 200(2): 758–767. DOI: 10.4049/jimmunol.1700713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mobasheri A. (2012) Osteoarthritis year 2012 in review: Biomarkers. Osteoarthritis and Cartilage 20(12): 1451–1464. DOI: 10.1016/j.joca.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 17. Ishida Y, Agata Y, Shibahara K, et al. (1992) Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. The EMBO Journal 11(11): 3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hirano F, Kaneko K, Tamura H, et al. (2005) Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Research 65(3): 1089–1096. [PubMed] [Google Scholar]
- 19. Wong RM, Scotland RR, Lau RL, et al. (2007) Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. International Immunology 19(10): 1223–1234. DOI: 10.1093/intimm/dxm091. [DOI] [PubMed] [Google Scholar]