Abstract
Objective: To determine the prevalence of cognitive (memory or confusion) complaints in older adults with visual impairment (VI). Method: We assessed the relationship between VI (corrected visual acuity [VA] < 20/40) and self-reported confusion or problems with memory among participants aged 60 years to 85 years in the 1999-2006 cycles of National Health and Nutrition Examination Survey (n = 5,795). Prevalence estimates of cognitive complaints were calculated using Current Population Surveys. Results: Memory/confusion complaints were reported in 22% of the VI group and 11% of the no VI group (p < .001). In individuals aged ≥ 80 years, 30% of those with VI reported cognitive complaints, as compared with 19% with no VI (p = .003). In fully adjusted models, individuals with VI were more likely (OR = 1.3, p = .049) to report cognitive complaints as compared with those without VI. Conclusion: Subjective reports of memory or confusion are highly prevalent in older individuals with VI.
Keywords: cognition, epidemiology, quality of life, visual impairment, aging
Introduction
Subjective complaints of confusion or memory loss are troublesome for patients and their families and have been posited to be early indicators of cognitive decline, although the evidence for this association is mixed (L. Wang et al., 2004). Estimates of these complaints range from 25% to 50% of older adults, and the prevalence increases with age, with 24% in older adults aged 65 years to 69 years to 57% in those aged 90 years and above (Jonker, Geerlings, & Schmand, 2000; Montejo, Montenegro, Fernández, & Maestú, 2011). Previous studies have examined the prevalence of memory and confusion complaints in various populations categorized by age group, sex, race, level of education, employment status, and living status (Taylor, Bouldin, & McGuire, 2018). There is limited research, however, on the prevalence of these complaints in specific populations (i.e., those with visual impairment [VI]) and on whether there is an association between impaired functioning across domains such as vision and cognitive function. The importance of identifying subjective memory or confusion complaints is threefold: (a) subjective memory complaints may indicate risk of progression to dementia in some individuals, (b) self-perceived problems with cognition have been associated with quality of life, well-being, and ability for self-care (McHorney, Ware, & Raczek, 1993; Verhaeghen, Geraerts, & Marcoen, 2000), and (c) characterizing specific older populations with expressed concerns about memory or confusion allows for the identification of patients who may benefit advanced care planning and health care coordination (Taylor et al., 2018). Here, we investigate the prevalence of memory and confusion complaints in visually impaired older adults.
Literature Review
Association of Subjective Complaints With Objectively Assessed Cognitive Function
Studies on the association between subjective memory or confusion complaints and objectively assessed cognitive function have yielded mixed results. Cross-sectionally, the association between subjective memory complaints and current cognitive function is inconsistent (Mitchell, 2008; Reid & MacLullich, 2006). For example, Thompson et al. found no association between self-report of memory function as measured by the propsepctive and retrospective memory questionnaire and cognitive scores on the mini mental status exam (MMSE) in individuals with normal cognition, mild cognitive impairment, and dementia (Thompson, Henry, Rendell, Withall, & Brodaty, 2015). Some longitudinal studies have found that subjective memory complaints in older adults are associated with subsequent cognitive decline and incident dementia (Glodzik-Sobanska et al., 2007; Kryscio et al., 2014; Mitchell, Beaumont, Ferguson, Yadegarfar, & Stubbs, 2014; Reisberg, Shulman, Torossian, Leng, & Zhu, 2010; L. Wang et al., 2004). Notably, subjective memory complaints assessed by a single question was associated with higher progression to dementia and a shorter dementia-free survival time in older adults (75+ years; Luck et al., 2015). However, other longitudinal studies have found that subjective memory complaints were not predictive of future cognitive decline or incident dementia (Flicker, Ferris, & Reisberg, 1993; Jorm et al., 1997; P.-N. Wang et al., 2000). One of these studies extensively assessed for memory complaints using a series of questions on global cognitive complaints as well as specific areas of everyday memory. This study found that cognitive complaints were not associated with MMSE scores, or scores on an epidosdic memory test or a test of mental speed (Jorm et al., 1997). Studies examining brain pathology have demonstrated that memory complaints are associated with pathology consistent with Alzheimer’s disease (AD; Barnes, Schneider, Boyle, Bienias, & Bennett, 2006; Kryscio et al., 2014; Saykin et al., 2006; Schultz et al., 2015). However, some have questioned the usefulness of assessing these complaints to identify individuals at risk of cognitive impairment given the high prevalence of these complaints (L. Wang et al., 2004) and the unclear association with objectively assessed cognitive function (Edmonds, Delano-Wood, Galasko, Salmon, & Bondi, 2014).
Association of Subjective Memory/Confusion Complaints With Personality, Mood, and Quality of Life
Beyond the relationship with objective measures of cognitive function, subjective memory and other cognitive complaints are consistently associated with mood and personality. Longitudinal data suggest that memory complaints are affected by depression (Bassett & Folstein, 1993; Christensen, Griffiths, MacKinnon, & Jacomb, 1997; Kahn, Zarit, Hilbert, & Niederehe, 1975; Kim, Stewart, Shin, Choi, & Yoon, 2003; Kliegel, Zimprich, & Eschen, 2005), concerns about aging (Pearman & Storandt, 2004), and neuroticism (Comijs, Deeg, Dik, Twisk, & Jonker, 2002; Merema, Speelman, Foster, & Kaczmarek, 2013; Pearman, Hertzog, & Gerstorf, 2014; Snitz et al., 2015), and these complaints are hypothesized to be mediators of the relationship between mood or personality and actual cognition (Yates, Clare, Woods, & Matthews, 2015).
More recently, there has been a focus on the relationship between memory and other cognitive complaints with quality of life and overall functioning. Health-related quality of life has been inversely associated with memory complaints (Bazargan & Bazargan, 1997; Commissaris, Ponds, & Jolles, 1998; Derouesné et al., 1989; Derouesné, Lacomblez, Thibault, & Leponcin, 1999; Verhaeghen et al., 2000), particularly among aspects related to limitations in daily living (McHorney et al., 1993). A correlation between memory complaints and domains of the 36 item Short Form Health Survey (SF-36) have also been found, including pain, mental health, social performance, energy-fatigue, physical health, and limitations due to emotional problems (McHorney et al., 1993; Waldorff, Rishoj, & Waldemar, 2008). Thus, cognitive complaints may be an important metric regardless of its association with current or future cognition.
Association of Subjective Complaints With Other Domains of Function
Prior work identifying cognitive complaints as a consequence of other aspects of health and well-being suggests that the presence of cognitive complaints may be reflective of decreased functioning in a number of domains, not solely limited to cognitive function. Comijs et al. (2002) suggest that complaints about memory in the absence of actual cognitive impairment may reflect psychoaffective or health problems. The authors posit that memory complaints may result from reduced overall functioning and well-being due to a number of diseases and conditions as well as an individual’s psychological state or personality. Montejo, Montenegro, Fernández, and Maestú (2012) hypothesize that impairment in one area of functioning (i.e., impaired vision) affects other types of functioning and leads to a global decline in well-being, and this negative perception of overall health status may result in complaints about memory and cognitive function. Therefore, memory or confusion complaints may provide additional insight on health-related well-being and functional ability in other domains. Research in this area has moved toward examining the relationship between cognitive complaints and its association with overall health (Cosentino, Devanand, & Gurland, 2018). However, there is limited research testing these hypothesized relationships between cognitive complaints within specific patient populations, and it remains unknown if impaired functioning outside of cognitive functioning (i.e., impaired vision) is associated with a greater likelihood of having memory or confusion complaints.
The aim of this study is to compare the prevalence of memory and confusion complaints in older adults with and without VI using National Health and Nutrition Examination Survey (NHANES) data. In NHANES, subjective complaints about memory and confusion were assessed with a single question. Our a priori hypothesis was that visually impaired older adults would be more likely to report memory and confusion complaints. This research is the first to use subjective memory and confusion complaints in characterizing the perceived cognitive functioning of visually impaired older adults.
Research Design
Survey Data
Analyses used data from the 1999-2006 cycles of NHANES, a national study probability sample representative of the noninstitutionalized U.S. civilian population (Centers for Disease Control and Prevention [CDC], 2013a), as these cycle years collected both vision examination data and assessed memory and confusion complaints (CDC, 1999). Our analyses were further limited to adults ≥60 years of age with complete vision testing and the physical function questionnaire data (n = 5,795). The study was approved by the CDC/NCHS (National Center for Health Statistics) ethics review board (ERB) and all survey participants gave written informed consent.
Vision Assessment
Presenting visual acuity (VA) was assessed unilaterally for each eye with participants wearing their corrective lenses if present. VA was assessed using the built-in chart in an auto-refractor (NIDEK ARK-760; Nidek Co Ltd, Tokyo, Japan). The chart had the following lines, each consisting of a combination of five items (letters and numbers): 20/20, 20/25, 20/30, 20/40, 20/50, 20/60, 20/80, and 20/200. Participants were first asked to read the 20/50 line and were allowed to proceed to the next line if they answered at least four out of the five items in the previous line. If they stopped reading in the middle of a line, they were prompted to guess and continue. If participants were unable to read the 20/50 line, the 20/200 line was presented. The test ended once the participant read the 20/20 line correctly or missed more than one item on a line for two lines in a row (CDC, 2008). For eyes with a presenting VA worse than 20/25, VA was reassessed after autorefraction. VI was defined as autorefractor corrected VA worse than 20/40 in the better-seeing eye.
Memory or confusion complaints were defined as present if participants responded “yes” to the question—“(Are you/is survey participant) limited in any way because of difficulty remembering or because (you/s/he) experience(s) periods of confusion?”
Other Measurements
Information on age, sex, race, body mass index (BMI), education, smoking status, and total number of comorbidities was also collected. Smoking status was categorized into never, current, and former smokers based on survey responses to smoking (cigarette or tobacco use) at least 100 cigarettes (no responses categorized as never smokers) and smoking currently. Diabetes was defined as present based on a self-reported physician diagnosis of diabetes, or currently taking insulin or oral medication to lower blood sugar. Hypertension was defined as a mean of the second and third blood pressure measurements, with a systolic blood pressure ≥ 130, or diastolic blood pressure ≥ 80, or self-report of a physician diagnosis of hypertension, or currently taking prescription medication for high blood pressure. Other comorbidities including arthritis, cancer, coronary heart disease, liver disease, and stroke were based on self-report of a physician diagnosis.
Statistical Methods
Estimates were weighted to account for planned oversampling of specific subgroups, survey nonresponse and poststratification (CDC, 2013b). We used Chi-square and t tests to compare categorical and continuous sociodemographic characteristics, respectively, between individuals with and without VI. Age-stratified prevalence estimates of memory or confusion complaints in the groups with and without VI were calculated using the relevant population totals from the Current Population Surveys (CPS) for the years 1999-2002, 2003-2004, and 2005-2006 (CDC & NCHS, 2009). Logistic regression models were used to determine odds ratios (ORs) and associated 95% confidence intervals (CI) to determine the relationship between covariates and the presence of complaints about memory or confusion, and effect modification by age was examined by stratifying by age group (60-<70 and 70-85 years). Taylor linearization was used to derive variance estimation. Covariates in the final models were included if significant in the univariate analysis and/or clinically relevant. The covariates included were VI status, age, sex, race, education, diabetes, and total number of comorbidities. A p value <.05 was considered statistically significant. All analyses were conducted in STATA 14 (StataCorp, College Station, TX).
Results
Sample Characteristics
As compared to the non-VI group, those with VI were more likely to be older (77.8 ± 0.5 vs. 70.3 ± 0.2), have a lower level of education (51% with less than a high school education vs. 27%), have a lower BMI (27.3 ± 0.3 vs. 28.4 ± 0.1), and have diabetes (21% vs. 16%; Table 1).
Table 1.
Participant Characteristics by Vision Impairment Status, NHANES, 1999-2006 (n = 5,795).
| Nonvisually impaired n = 5,439 (95%) |
Visually impaireda
n = 396 (5%) |
p value | |
|---|---|---|---|
| Age, M ± SD | 70.3 ± 7.2 | 77.8 ± 8.2 | < .001 |
| Female, n (%) | 2,698 (55) | 186 (61) | .053 |
| Race, n (%) | .211 | ||
| Non-Hispanic White | 3,227 (83) | 219 (79) | |
| Non-Hispanic Black | 915 (8) | 53 (9) | |
| Mexican American | 1,021 (3) | 63 (4) | |
| Other Hispanics and other race | 276 (6) | 21 (9) | |
| Body mass index, M ± SD | 28.4 ± 0.1 | 27.3 ± 0.3 | .004 |
| Education, n (%) | < .001 | ||
| Less than high school | 2,103 (27) | 199 (51) | |
| High school or equivalent | 1,341 (30) | 81 (24) | |
| More than high school | 1,985 (43) | 74 (25) | |
| Smoking, n (%) | .141 | ||
| Never | 2,468 (78) | 180 (83) | |
| Former | 545 (17) | 32 (13) | |
| Current | 172 (6) | 10 (3) | |
| Diabetes, n (%) | 996 (16) | 91 (21) | .017 |
| Total comorbid conditionsb, M ± SD | 1.7 ± 0.9 | 1.8 ± 1.1 | .137 |
Note. All results reported as n (weighted%). NHANES = National Health and Nutrition Examination Survey; n = sample size.
Visual impairment defined as autorefractor-corrected visual acuity worse than 20/40.
Total comorbid conditions included arthritis, cancer, coronary heart disease, hypertension, liver disease, and stroke.
Prevalence of Complaints About Memory or Confusion
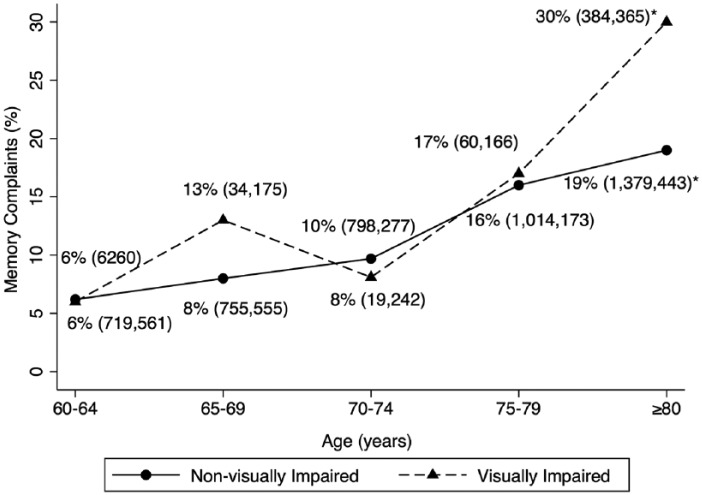
Overall, memory or confusion complaints were reported in 22% of the VI group and 11% of the non-VI group (p < .001). Figure 1 represents the weighted percentages of individuals ≥60 years in 5-year age bands reporting memory or confusion complaints by VI status. Of those aged 60 years to 64 years, memory or confusion complaints was reported in 6% of the VI group and 6% of the non-VI group. In each subsequent age category, there were similar percentages of memory or confusion complaints reported in the VI and non-VI groups (65-69: 13% vs. 8%; 70-74: 8% vs. 10%; 75-79: 17% vs. 16%; respectively, p > .05 for all).
Figure 1.
Weighted percentages and population estimates of individuals ≥ 60 with memory or confusion complaints by visual impairment status, NHANES, 1999-2006.
Note. Weighted% (population estimate). NHANES = National Health and Nutrition Examination Survey.
*Statistically significant difference (p < .05).
The relevant U.S. population estimates are reported in Figure 1. Overall, an average of 396 sampled participants, representing an estimated 2.2 million U.S. adults, or an average of 4.7% of the U.S. population over the years 1999-2006, had VI. Of those visually impaired, 22% of individuals aged ≥60 years reported complaints about memory or confusion, representing an estimated 504,149 people in the U.S. population, as compared with 11% (an estimated 4,667,008 people) of the non-VI group. Within each age category, the percentage reporting memory or confusion complaints were similar in both VI and non-VI groups, except in the oldest age category of ≥80 years, where 30% of the VI group had memory or confusion complaints as compared with 19% of the non-VI group (p = .003). This represents 384,365 individuals with VI reporting memory or confusion complaints and 1.38 million individuals without VI reporting complaints about memory or confusion.
Factors Associated With Complaints About Memory or Confusion
In fully adjusted models, individuals with VI were 1.30 times more likely to report memory or confusion complaints as compared with individuals without VI (OR = 1.30, 95% CI = [1.00, 1.70], p = .049, Table 2). When stratified by age, there was no association between memory or confusion complaints and VI in the younger age group 60-69 years (OR = 0.94, 95% CI = [0.33, 2.62], p = .897, Table 3). In the older age group (70+ years), individuals with VI were 1.43 times more likely to report memory or confusion complaints as compared with individuals without VI (OR = 1.43, 95% CI = [1.04, 1.96], p = .027, Table 4).
Table 2.
Multivariable-Adjusted Relationship of Visual Impairment With Self-Report of Complaints About Memory or Confusion, NHANES, 1999-2006 (n = 5,778).
| Odds ratio (OR) | 95% CI | p value | |
|---|---|---|---|
| Visual impairment statusa | |||
| No visual impairment | Reference | — | — |
| Yes visual impairment | 1.30 | [1.00, 1.70] | .049 |
Note. NHANES = National Health and Nutrition Examination Survey; n = sample size.
Visual impairment defined as auto refractor corrected visual acuity worse than 20/40.
Table 3.
Multivariable-Adjusted Relationship of Visual Impairment With Self-Report of Memory or Confusion Complaints Limited to 60-to-69-Year Olds, NHANES, 1999-2006 (n = 2,635).
| Odds ratio (OR) |
95% CI | p value | |
|---|---|---|---|
| Visual impairment statusa | |||
| No visual impairment | Reference | — | — |
| Yes visual impairment | 0.94 | [0.33, 2.62] | .897 |
Note. NHANES = National Health and Nutrition Examination Survey; n = sample size.
Visual impairment defined as auto refractor corrected visual acuity worse than 20/40.
Table 4.
Multivariable-Adjusted Relationship of Visual Impairment With Self-Report of Memory or Confusion Complaints Limited to 70+-Year Olds, NHANES, 1999-2006 (n = 3143).
| Odds ratio (OR) | 95% CI | p value | |
|---|---|---|---|
| Visual impairment statusa | |||
| No visual impairment | Reference | — | — |
| Yes visual impairment | 1.43 | [1.04, 1.96] | .027 |
Note. NHANES = National Health and Nutrition Examination Survey; n = sample size.
Visual impairment defined as auto refractor corrected visual acuity worse than 20/40.
Discussion
In this study of a large, nationally representative sample, we found a high prevalence of memory or confusion complaints in older adults with VI. Overall, there was an increasing proportion of older adults with memory or confusion complaints in both the VI and non-VI groups with increasing age, and a significantly higher prevalence of memory or confusion complaints in the VI group as compared with the non-VI group in adults ≥80 years old. This nonlinear increase of memory or confusion complaints with age is similar to that found in other studies (Caracciolo et al., 2012). The slight decrease in prevalence of memory or confusion complaints noted in our 70 to 74 year age group with VI may be due to the increased use of services and reliance on regular help after recognizing the need for such services following retirement (62-64 years), (Munnell, 2015) and thus less perceived limitations due to problems with cognition.(J. J. Wang, Mitchell, Smith, Cumming, & Attebo, 1999)
In addition, we explored age-stratified analyses, as only the older age bands 70+ were associated with memory or confusion complaints in the full model. In fully adjusted age-stratified models, VI was associated with memory or confusion complaints in the older age group 70+, but not in the younger age group 60 to 69 years. This may, in part, be due to the high prevalence of VI and memory or confusion complaints in older age groups, and may indicate that it may be important to inquire about memory or confusion complaints in older adults ≥70 years of age, but that assessment for memory or confusion complaints may not be as important a consideration in older adults between the ages of 60 and 69 years unless there is a clear indication to inquire about memory complaints (Jonker et al., 2000; Klaver, Wolfs, Vingerling, Hofman, & de Jong, 1998).
The results from this study may have implications for ophthalmic care settings, as many older patients with VI may have complaints about memory or confusion. For ophthalmologists, this is an important consideration as the majority of patients in the ophthalmologic setting are older and are at increased risk of memory impairment (American Academy of Ophthalmology, 2015). In patients with age-related eye disease, self-monitoring or medication adherence may be an important aspect of care, which may be complicated by issues with memory and cognition (Sleath et al., 2011). Ophthalmologists may consider inquiring about memory or confusion complaints to better address the underlying cause of medication nonadherence or difficulty with self-monitoring. Assessing for memory and confusion complaints may also provide insight into a patient’s awareness and perception of cognitive health, psychological status, and overall well-being, allowing ophthalmologists and other providers to give more individualized advice regarding health promotion and medication adherence. Future research is needed to determine whether visually impaired individuals who report memory or confusion complaints are at increased risk of cognitive impairment, decreased quality of life, and whether these individuals may benefit from advanced planning/health care coordination.
To our knowledge, this is the first study to investigate the prevalence of memory or confusion complaints in individuals with VI. Other strengths of this study include the use of a nationally representative sample that allowed us to generate population estimates and prevalence rates. In addition, we used objective measures of vision to define VI rather than subjective measures in association with complaints about memory or confusion.
Limitations
Limitations of the study include the absence of objective measures of cognition that may be examined in conjunction with reported complaints about memory or confusion. NHANES collected cognitive data from only the digital symbol substitution test (DSST), a test highly dependent on vision, over the time period when vision was objectively measured, but strikingly, we found that over 40% of DSST scores were missing from individuals with VI. This could indicate a possible bias in the assessment of cognitive function in individuals with VI using this test score. We did not have depression data available for our study population for the years 1999-2004, thus we could not account for this potential confounding or mediating variable in our model. In addition, in NHANES, the question about memory or confusion was worded as “Are you limited in any way because of difficulty remembering or because you experience periods of confusion?,” which is a “double-barreled” question, as it asks about two different things but only allows one answer, and we are unable to examine memory and confusion complaints separately. In addition, it is possible that VI in itself may have caused limitations in function, which may lead to confusion, potentially leading to positive responses to the questionnaire. Furthermore, the NHANES survey assesses specifically for limitations experienced by memory problems and confusion. This categorization may have excluded individuals who felt that their memory problem or confusion did not result in any restriction of activities. A limitation inherent with all NHANES analyses is the exclusion of institutionalized populations, and thus a lack of data on older U.S. adults in facilities such as nursing homes. This may have led to an underestimation of prevalence estimates, as older adults in nursing homes and assisted living facilities may disproportionately experience problems with memory (Waldorff, Siersma, & Waldemar, 2009a, 2009b). Finally, we did not have vision data or reports of memory or confusion complaints for individuals >85 years of age due to the truncation of age at 85 years in NHANES to reduce the risk of disclosure, but CPS population totals used to estimate prevalence rates included all those aged ≥80 years (CDC, 1999). This may also have contributed to lower prevalence estimates of memory or confusion complaints and VI in the oldest age groups.
In conclusion, our study found a high prevalence of memory or confusion complaints in older adults with VI and an increased likelihood of reporting memory or confusion complaints in individuals with VI as compared with individuals without VI. This study adds to the increasing body of literature on the aging profiles of visually impaired older adults. Further studies are needed to investigate the implications of memory or confusion complaints in individuals with VI to determine if these complaints are associated with medication adherence, dementia, other patient outcomes and health-related well-being. In addition, research into potential mechanisms underlying this association is warranted, as these complaints may represent an understudied dimension of quality of life among older adults with VI.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH and NIA (grant numbers K01AG052640 to B.S.K, R01AG043438 to H.E.W, P30 AG028716 to H.E.W, K01AG054693 to J.A.D); and funds from Dr. Jane Kroger to B.K.S.
References
- American Academy of Ophthalmology. (2015). Eye health statistics—American academy of ophthalmology. Retrieved from https://www.aao.org/newsroom/eye-health-statistics
- Barnes L. L., Schneider J. A., Boyle P. A., Bienias J. L., Bennett D. A. (2006). Memory complaints are related to Alzheimer disease pathology in older persons. Neurology, 67, 1581-1585. doi: 10.1212/01.wnl.0000242734.16663.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett S. S., Folstein M. F. (1993). Memory complaint, memory performance, and psychiatric diagnosis: A community study. Journal of Geriatric Psychiatry and Neurology, 6, 105-111. doi: 10.1177/089198879300600207 [DOI] [PubMed] [Google Scholar]
- Bazargan M., Bazargan S. (1997). Self-reported memory function and psychological well-being among elderly African American persons. Journal of Black Psychology, 23, 103-119. doi: 10.1177/00957984970232002 [DOI] [Google Scholar]
- Caracciolo B., Gatz M., Xu W., Pedersen N. L., Fratiglioni L. (2012). Differential distribution of subjective and objective cognitive impairment in the population: A nation-wide twin-study. Journal of Alzheimer’s Disease, 29, 393-403. doi: 10.3233/JAD-2011-111904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (1999). National Center for Health Statistics (NCHS): National health and nutrition examination survey data. Retrieved from https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=1999
- Centers for Disease Control and Prevention. (2008). Vision procedures manual. Retrieved from https://wwwn.cdc.gov/nchs/data/nhanes/2007-2008/manuals/manual_vi.pdf
- Centers for Disease Control and Prevention. (2013. a). NHANES–Continuous NHANES web tutorial–Sample design. Retrieved from https://www.cdc.gov/nchs/tutorials/nhanes/SurveyDesign/SampleDesign/intro.htm
- Center for Disease Control and Prevention. (2013. b). NHANES–Continuous NHANES web tutorial–Specifying weighting parameters. Retrieved from https://www.cdc.gov/nchs/tutorials/NHANES/SurveyDesign/Weighting/intro.htm
- Centers of Disease Control Prevention, & National Center for Health Statistics. (2009). NHANES response rates and population totals. Retrieved from https://wwwn.cdc.gov/nchs/nhanes/ResponseRates.aspx
- Christensen H., Griffiths K., MacKinnon A., Jacomb P. (1997). A quantitative review of cognitive deficits in depression and Alzheimer-type dementia. Journal of the International Neuropsychological Society, 3, 631-651. Retrieved from https://www.cambridge.org/core/terms [PubMed] [Google Scholar]
- Comijs H., Deeg D. J., Dik M., Twisk J. W., Jonker C. (2002). Memory complaints; the association with psycho-affective and health problems and the role of personality characteristics: A 6-year follow-up study. Journal of Affective Disorders, 72, 157-165. doi: 10.1016/S0165-0327(01)00453-0 [DOI] [PubMed] [Google Scholar]
- Commissaris C. J. A., Ponds R. W. H., Jolles J. (1998). Subjective forgetfulness in a normal Dutch population: Possibilities for health education and other interventions. Patient Education & Counseling, 34(1), 25-32. doi: 10.1016/S0738-3991(98)00040-8 [DOI] [PubMed] [Google Scholar]
- Cosentino S., Devanand D., Gurland B. (2018). A link between subjective perceptions of memory and physical function: Implications for subjective cognitive decline. Journal of Alzheimer’s Disease, 61, 1387-1398. doi: 10.3233/JAD-170495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouesné C., Alperovitch A., Arvay N., Migeon P., Moulin F., Vollant M., . . . Le Poncin M. (1989). Memory complaints in the elderly: A study of 367 community-dwelling individuals from 50 to 80 years old. Archives of Gerontology and Geriatrics Supplement, 1, 151-163. [PubMed] [Google Scholar]
- Derouesné C., Lacomblez L., Thibault S., Leponcin M. (1999). Memory complaints in young and elderly subjects. International Journal of Geriatric Psychiatry, 14, 291-301. doi: [DOI] [PubMed] [Google Scholar]
- Edmonds E. C., Delano-Wood L., Galasko D. R., Salmon D. P., Bondi M. W. (2014). Subjective cognitive complaints contribute to misdiagnosis of mild cognitive impairment. Journal of the International Neuropsychological Society, 20, 836-847. doi: 10.1017/S135561771400068X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flicker C., Ferris S. H., Reisberg B. (1993). A longitudinal study of cognitive function in elderly persons with subjective memory complaints. Journal of the American Geriatrics Society, 41, 1029-1032. doi: 10.1111/j.1532-5415.1993.tb06448.x [DOI] [PubMed] [Google Scholar]
- Glodzik-Sobanska L., Reisberg B., De Santi S., Babb J. S., Pirraglia E., Rich K. E., . . . De Leon M. J. (2007). Subjective memory complaints: Presence, severity and future outcome in normal older subjects. Dementia and Geriatric Cognitive Disorders, 24, 177-184. doi: 10.1159/000105604 [DOI] [PubMed] [Google Scholar]
- Jonker C., Geerlings M. I., Schmand B. (2000). Are memory complaints predictive for dementia? A review of clinical and population-based studies. International Journal of Geriatric Psychiatry, 15, 983-991. doi: [DOI] [PubMed] [Google Scholar]
- Jorm A. F., Christensen H., Korten A. E., Henderson A. S., Jacomb P. A., Mackinnon A. (1997). Do cognitive complaints either predict future cognitive decline or reflect past cognitive decline? A longitudinal study of an elderly community sample. Psychological Medicine, 27, 91-98. Retrieved from https://www.cambridge.org/core/product/56C6FA93C5CC5BA20106CB692436EA14 [DOI] [PubMed] [Google Scholar]
- Kahn R. L., Zarit S. H., Hilbert N. M., Niederehe G. (1975). Memory complaint and impairment in the aged: The effect of depression and altered brain function. Archives of General Psychiatry, 32, 1569-1573. doi: 10.1001/archpsyc.1975.01760300107009 [DOI] [PubMed] [Google Scholar]
- Kim J.-M., Stewart R., Shin I.-S., Choi S.-K., Yoon J.-S. (2003). Subjective memory impairment, cognitive function and depression—A community study in older Koreans. Dementia and Geriatric Cognitive Disorders, 15, 218-225. doi: 10.1159/000068783 [DOI] [PubMed] [Google Scholar]
- Klaver C. C. W., Wolfs R. C. W., Vingerling J. R., Hofman A., de Jong P. T. (1998). Age-specific prevalence and causes of blindness and visual impairment in an older population. The Archives of Ophthalmology, 116, 653-658. doi: 10.1001/archopht.116.5.653 [DOI] [PubMed] [Google Scholar]
- Kliegel M., Zimprich D., Eschen A. (2005). What do subjective cognitive complaints in persons with aging-associated cognitive decline reflect? International Psychogeriatrics, 17, 499-512. doi: 10.1017/S1041610205001638 [DOI] [PubMed] [Google Scholar]
- Kryscio R. J., Abner E. L., Cooper G. E., Fardo D. W., Jicha G. A., Nelson P. T., . . . Schmitt F. A. (2014). Self-reported memory complaints: Implications from a longitudinal cohort with autopsies. Neurology, 83, 1359-1365. doi: 10.1212/WNL.0000000000000856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck T., Luppa M., Matschinger H., Jessen F., Angermeyer M. C., Riedel-Heller S. G. (2015). Incident subjective memory complaints and the risk of subsequent dementia. Acta Psychiatrica Scandinavica, 131, 290-296. doi: 10.1111/acps.12328 [DOI] [PubMed] [Google Scholar]
- McHorney C. A., Ware J. E., Raczek A. E., Jr. (1993). The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care, 31, 247-263. [DOI] [PubMed] [Google Scholar]
- Merema M. R., Speelman C. P., Foster J. K., Kaczmarek E. A. (2013). Neuroticism (not depressive symptoms) predicts memory complaints in some community-dwelling older adults. The American Journal of Geriatric Psychiatry, 21, 729-736. doi: 10.1016/J.JAGP.2013.01.059 [DOI] [PubMed] [Google Scholar]
- Mitchell A. J. (2008). The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: A meta-analysis. International Journal of Geriatric Psychiatry, 23, 1191-1202. doi: 10.1002/gps.2053 [DOI] [PubMed] [Google Scholar]
- Mitchell A. J., Beaumont H., Ferguson D., Yadegarfar M., Stubbs B. (2014). Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis. Acta Psychiatrica Scandinavica, 130, 439-451. doi: 10.1111/acps.12336 [DOI] [PubMed] [Google Scholar]
- Montejo P., Montenegro M., Fernández M. A., Maestú F. (2011). Subjective memory complaints in the elderly: Prevalence and influence of temporal orientation, depression and quality of life in a population-based study in the city of Madrid. Aging & Mental Health, 15(1), 85-96. doi: 10.1080/13607863.2010.501062 [DOI] [PubMed] [Google Scholar]
- Montejo P., Montenegro M., Fernández M. A., Maestú F. (2012). Memory complaints in the elderly: Quality of life and daily living activities. A population based study. Archives of Gerontology and Geriatrics, 54, 298-304. doi: 10.1016/J.ARCHGER.2011.05.021 [DOI] [PubMed] [Google Scholar]
- Munnell A. H. (2015). The average retirement age—An update (IB#15–4). Retrieved from http://crr.bc.edu/briefs/the-average-retirement-age-an-update/
- Pearman A., Hertzog C., Gerstorf D. (2014). Little evidence for links between memory complaints and memory performance in very old age: Longitudinal analyses from the Berlin aging study. Psychology and Aging, 29, 828-842. doi: 10.1037/a0037141 [DOI] [PubMed] [Google Scholar]
- Pearman A., Storandt M. (2004). Predictors of subjective memory in older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 59(1), P4-P6. doi: 10.1093/geronb/59.1.P4 [DOI] [PubMed] [Google Scholar]
- Reid L. M., MacLullich A. M. J. (2006). Subjective memory complaints and cognitive impairment in older people. Dementia and Geriatric Cognitive Disorders, 22, 471-485. doi: 10.1159/000096295 [DOI] [PubMed] [Google Scholar]
- Reisberg B., Shulman M. B., Torossian C., Leng L., Zhu W. (2010). Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimer’s & Dementia, 6(1), 11-24. doi: 10.1016/j.jalz.2009.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin A. J., Wishart H. A., Rabin L. A., Santulli R. B., Flashman L. A., West J. D., . . . Mmourian A. (2006). Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology, 67, 834-842. doi: 10.1212/01.wnl.0000234032.77541.a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. A., Oh J. M., Koscik R. L., Dowling N. M., Gallagher C. L., Carlsson C. M., . . . Okonkwo O. C. (2015). Subjective memory complaints, cortical thinning, and cognitive dysfunction in middle-age adults at risk of AD. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 1(1), 33-40. doi: 10.1016/j.dadm.2014.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleath B., Blalock S., Covert D., Stone J. L., Skinner A. C., Muir K., Robin A. L. (2011). The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmology, 118, 2398-2402. doi: 10.1016/J.OPHTHA.2011.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz B. E., Weissfeld L. A., Cohen A. D., Lopez O. L., Nebes R. D., Aizenstein H. J., . . . Klunk W. E. (2015). Subjective cognitive complaints, personality and brain Amyloid-beta in cognitively normal older adults. The American Journal of Geriatric Psychiatry, 23, 985-993. doi: 10.1016/J.JAGP.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. A., Bouldin E. D., McGuire L. C. (2018). Subjective cognitive decline among adults aged ≥45 Years—United States, 2015–2016. Morbidity and Mortality Weekly Report (MMWR), 67, 753-757. doi: 10.15585/mmwr.mm6727a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. L., Henry J. D., Rendell P. G., Withall A., Brodaty H. (2015). How valid are subjective ratings of prospective memory in mild cognitive impairment and early dementia? Gerontology, 61, 251-257. doi: 10.1159/000371347 [DOI] [PubMed] [Google Scholar]
- Verhaeghen P., Geraerts N., Marcoen A. (2000). Memory complaints, coping, and well-being in old age: A systemic approach. The Gerontologist, 40, 540-548. doi: 10.1093/geront/40.5.540 [DOI] [PubMed] [Google Scholar]
- Waldorff F. B., Rishoj S., Waldemar G. (2008). If you don’t ask (about memory), they probably won’t tell. The Journal of Family Practice, 57(1), 41-44. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18171569 [PubMed] [Google Scholar]
- Waldorff F. B., Siersma V., Waldemar G. (2009. a). Association between subjective memory complaints and health care utilisation: A three-year follow up. BMC Geriatrics, 9(1), Article 43. doi: 10.1186/1471-2318-9-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldorff F. B., Siersma V., Waldemar G. (2009. b). Association between subjective memory complaints and nursing home placement: A four-year follow-up. International Journal of Geriatric Psychiatry, 24, 602-609. doi: 10.1002/gps.2163 [DOI] [PubMed] [Google Scholar]
- Wang J. J., Mitchell P., Smith W., Cumming R. G., Attebo K. (1999). Impact of visual impairment on use of community support services by elderly persons: The Blue Mountains Eye Study. Investigative Ophthalmology & Visual Science, 40, 12-19. [PubMed] [Google Scholar]
- Wang L., Van Belle G., Crane P. K., Kukull W. A., Bowen J. D., McCormick W. C., Larson E. B. (2004). Subjective memory deterioration and future dementia in people aged 65 and older. Journal of the American Geriatrics Society, 52, 2045-2051. doi: 10.1111/j.1532-5415.2004.52568.x [DOI] [PubMed] [Google Scholar]
- Wang P.-N., Wang S.-J., Fuh J.-L., Teng E. L., Liu C.-Y., Lin C.-H., . . . Liu H.-C. (2000). Subjective memory complaint in relation to cognitive performance and depression: A longitudinal study of a rural chinese population. Journal of the American Geriatrics Society, 48, 295-299. doi: 10.1111/j.1532-5415.2000.tb02649.x [DOI] [PubMed] [Google Scholar]
- Yates J. A., Clare L., Woods R. T., Matthews F. E. (2015). Subjective memory complaints are involved in the relationship between mood and mild cognitive impairment. Journal of Alzheimer’s Disease, 48(s1), S115-S123. doi: 10.3233/JAD-150371 [DOI] [PubMed] [Google Scholar]