Abstract
The role of viral infection in developing cancer was determined in the start of 20th century. Until now, 8 different virus-associated cancers have been discovered and most of them progressed in immunosuppressed individuals. The aim of the present study is to look into the benefits of natural products in treating virally infected cancers. The study focuses on bioactive compounds derived from natural sources. Numerous pharmaceutical agents have been identified from plants (vincristine, vinblastine, stilbenes, combretastatin, and silymarin), marine organisms (bryostatins, cephalostatin, ecteinascidins, didemnin, and dolastatin), insects (cantharidin, mastoparan, parectadial, and cecropins), and microorganisms (vancomycin, rhizoxin, ansamitocins, mitomycin, and rapamycin). Beside these, various compounds have been observed from fruits and vegetables which can be utilized in anticancer therapy. These include curcumin in turmeric, resveratrol in red grapes, S-allyl cysteine in allium, allicin in garlic, catechins in green tea, and β-carotene in carrots. The present study addresses various types of virally infected cancers, their mechanism of action, and the role of different cell surface molecules elicited during viral binding and entry into the target cell along with the anticancer drugs derived from natural products by targeting screening of bioactive compounds from natural sources.
Keywords: virus, cancer, cell surface molecules, natural sources
Introduction
An unusual growth of cells that propagates through unlimited cell division is termed as cancer. These cells also attack different tissues and then spread to other parts of the body. About 11 million people worldwide have been detected with cancer, which may approximately increase to 16 million by 2020.1 International Agency in Cancer Research estimated that viruses are the cause of almost 20% of aggregate cancers.2 Viral infection leads to chronic inflammation which proliferates growth of cancer cells. Nearly all of the tumor cells are at the mercy of virus for survival. Virally infected cancer is in fact major issue in low-income populations, developing countries, and immunosuppressed individuals from developed nations.
Generally, people of all ages may be affected by cancer, but risk tends to increase with age because DNA damages more easily with aging because of the mutations that occur at the cellular level and cause alterations in protein function and regulation.3,4 Besides vaccination, surgery, radiotherapy, and drug therapy are used for the treatment of virus-associated human malignances. Dietary supplements and medicinal plants are proposed to prevent cancer development because of their long history of human utilization. This review elaborates different types of virally infected cancers and highlights the naturally derived products used for its treatment.
Historical Background
Discovery of Associated Cancer Viruses
Viruses’ role as cancer-causing agents emerged in the beginning of 20th century.5 The tumor growth takes place by the cooperation of several events. To date, 8 cancer viruses have been discovered and are classified into 2 groups, that is, DNA and RNA tumor viruses on the basis of their genetic makeup (Table 1).
Table 1.
Names of Viruses Along With Their Associated Malignancies.
| Serial Number | Viruses | Genome | Cancers | References |
|---|---|---|---|---|
| 1. | Epstein-Barr virus (EBV) (or HHV-4) | Double-stranded DNA herpesvirus (∼172 kb) | Burkitt lymphoma, nasopharyngeal carcinoma | 6 ,7 |
| 2. | Kaposi sarcoma herpesvirus (KSHV) (or HHV-8) | Double-stranded DNA herpesvirus (∼160 kb) | Primary effusion lymphoma, Kaposi sarcoma | 6,8,9 |
| 3. | Human papilloma virus (HPV) | Double-stranded DNA papilloma virus (∼8 kb) | Cervical, neck, head, and anogenital tract carcinoma | 6,10 |
| 4. | Merkel cell polyomavirus (MCPV) | Double-stranded DNA polyomavirus (∼5.4 kb) | Merkel cell carcinoma | 11 |
| 5. | Hepatitis B virus (HBV) | Partially double-stranded DNA hepadenovirus (∼3.2 kb) | Hepatocellular carcinoma | 6,12 |
| 6. | Hepatitis C virus (HCV) | Single-stranded RNA flavivirus (∼9.6 kb) | Hepatocellular carcinoma, lymphomas | 13 |
| 7. | Human T-cell leukemia virus-1 (HTLV-1) | Single-stranded RNA retrovirus (∼9.0 kb) | Adult T-cell leukemia | 6,14 |
| 8. | Human immunodeficiency virus (HIV) | Double-stranded RNA retroviridae (∼9.7 kb) | Enhances immunosuppression-mediated cancers by other viruses | 6,15 |
Abbreviation: HHV, human herpesvirus.
DNA tumor viruses
Epstein-Barr virus
In 1964, Anthony Epstein, Bert Achong, and Yvonne Barr deduce Epstein-Barr virus (EBV) particles, also known as human herpesvirus 4 (HHV-4), in cell lines from African patients with Burkitt lymphoma.16 The EBV possesses linear double-stranded DNA (dsDNA) genome and favorably infect epithelial cells and B lymphocytes.17 The EBV is ubiquitous affecting more than 90% of adults.6 Oral and blood are its principal route of transmission.18 During childhood, primary infection with EBV is mostly asymptomatic; nevertheless, the infected person is rendered a carrier for whole life. However, EBV infection during adolescence results in a disease called infectious mononucleosis.7,19 In some cases, EBV infection leads to the development of carcinomas and lymphomas and non-Hodgkin lymphoma.6 The virally encoded latent membrane protein 1 activates STAT and nuclear factor κB (NF-κB) transcription factors in B cells and PI3K in epithelial cells.20 It enhances the growth and survival of the infected cell.
Kaposi sarcoma-associated herpesvirus
Kaposi sarcoma-associated herpesvirus (KSHV) have linear dsDNA and remains in the nucleus as episomes even after infecting the cell.21 The KSHV, also known as HHV-8, is epidemic in sub-Saharan Africa (>50%), moderately spread in the Mediterranean region (10%-30%), and less in northern Europe, United States, and Asia (<10%).6 Primary routes of KSHV transmission include blood transfusion, sexual contact, and organ transplant. The KSHV is the causative agent of lymphomas and sarcomas.22 The latency-associated nuclear antigen (LANA) plays a significant role in KSHV-associated tumorigenesis.8, 9
Human papilloma virus
Human papilloma virus (HPV), which belongs to the Papillomaviridae family, is principally transmitted by skin contact even during sexual intercourse. Infection is controlled by the immune system, while, in some cases, HPV persists which further leads to the development of epithelial lesions.22 The HPV is highly prevalent in Africa and America (20%-30%).6 It is classified into low-risk or high-risk groups depending on their cancer-causing potential. Moreover, HPV has greater tendency to develop skin cancers in immunosuppressed patients and other anogenital cancers.23
Merkel cell polyomavirus
Polyomaviruses have a circular, dsDNA of approximately 5000 bp. Merkel cell polyomavirus (MCPyV) is cosmopolitan and is found in about 80% of the tumors. Evidence revealed that oncogenic transformation of MCPyV occurs due to loss of immune surveillance, because Merkel cell carcinoma (MCC) was detected only in immunocompromised people.24
Hepatitis B virus
Hepatitis B virus (HBV) belongs to Hepadnaviridae family and has a potential to provoke liver disease in animals, including humans. The HBV is an enveloped virus comprising of a dsDNA chain along with a single-stranded fragment. The HBV infection may initiate in early childhood or during later stages of life.25 Long-lasting infections by HBV result in hepatocellular carcinoma (HCC). Both direct and indirect methods are involved in carcinogenesis process induced by HBV.26
RNA tumor viruses
Hepatitis C virus
Hepatitis C virus (HCV) is a positive-strand RNA virus which shows RNA polymerase activity and its genome includes roughly 9600 nucleotides.13 The HCV infects liver cells causing severe infection that may become chronic in immunosuppressed individuals, leading to hepatitis and HCC.27 All inclusive, about 170 million people are affected by HCV globally.28 The HCV transmission chiefly occurs through infected blood products. Nonstructural proteins (such as NS5) of HCV can derange signal transduction pathways, leading to cellular proliferation followed by cancerous development. Core protein of HCV also performs various functions including altered cellular gene transcription and cell death.29
Human T-lymphotropic virus type 1
Human T-lymphotropic virus type 1 (HTLV-1) is associated with a range of lymphoproliferative diseases such as adult T-cell lymphoma (ATL).14 Currently, about 15 to 25 million people have been affected with HTLV-1 worldwide. Vertical, sexual, and parenteral are 3 fundamental routes of HTLV-1 transmission. The viral-encoded Tax protein acts as a major oncogenic determinant of HTLV-1 by directly interacting with several signaling cascades and DNA repair pathways, increasing cell survival and transformation, instead of immunomodulatory response mediating inflammation as involved in case of other cancers.30
Human immunodeficiency virus
HIV is a retrovirus that induces immunodeficiency, which in turn assists the cancer cell progression caused by other viruses. HIV does not directly cause cancer.2 Presently, 33.3 million people are infected with HIV.6 Principal routes of HIV-1 infection include sexual intercourse and via blood contact.15
Cancer Development in Immunocompromised Individuals
International Agency for Research on Cancer determined that 1 of 5 cancer victims are due to infection and nearly all of them are caused by viruses.2 These are notably universal health problems for underprivileged as well as immunosuppressed individuals. Up till now, evidences confirmed that the evasion of inherent immunity plays a vital role in viral oncogenesis. Inborn immune surveillance signaling along with cancer suppressor signaling processes initiates apoptotic pathways.31 Hence, targeting of tumor suppressor pathways by viruses depicts an immune evasion response that impairs antiviral pathways but keeps the affected cell at danger for cancerous growth (known as anti-antivirus hypothesis). The 2-fold nature of inborn immune signaling in antivirus and anticancer functions is characterized by interferon regulatory factors.32
The KSHV encodes 4 interferon regulatory factor homologues,33 which inhibit interferon signaling and initiate cell transformation. Other established KSHV oncoproteins, namely, interleukin-6 (IL-6),34 latent nuclear antigen-1 (LANA1), and LANA2,35,36 also clearly describe the inherent immunomodulatory roles. Of EBV-associated cancers, non-Hodgkin lymphoma and Hodgkin lymphoma are chronic in immunosuppressed individuals compared to common population.2
Merkel cell carcinoma is more recurrent in patients with AIDS than in general people.37 Evidences revealed that the replication of several polyomaviruses increases in immunosuppressed individuals, thereby illustrating the role of immune system in MCC development. Similarly, HPV and squamous intraepithelial lesions diagnosis in HIV-infected women is greatly linked to high viral load.38 So, the initial stages of HPV replication leading to cervical cancer may be affected by HIV-associated immune suppression.39 Moreover, ATL, also known as human T-lymphotropic virus type 1 (HTLV-I), is also more frequent in immunodeficient individuals than the general people.40 Hence, viral replication is modulated by immune system and these virus-mediated cancers increases in immune suppression cases due to the host inability to curb viral replication and the development of infected cells. Immune effector cells recognize viral proteins and direct tumor cell growth.
Mechanism of Virus-Mediated Carcinogenesis
Virus-mediated carcinogenesis involves initiation, promotion, and progression in order to change an ordinary cell into oncogenic cell. Initiation involves a reaction between carcinogen and the DNA of tissue cells. The second stage is promotion during which cell proliferation occurs and it happens gradually from few months to years. Change in eating routine along with way of life can have a helpful impact at this stage so that the person may not develop cancer. The last stage involves progression and spread of the tumor. Diet may have less impact at this stage.
Cancer causes the death of the host, and in this way, it ceases the infection. Incorrect diet, genetic susceptibility, and the environmental factors (air and water pollution, radiation, etc) predominantly cause cancer. About 35% of cancers are caused by inappropriate diet and 20% of cancers are because of genetic predisposition. In this manner, remaining cancer cases are related to ecological cancer-causing agents.41
Direct Mechanism of Viral Carcinogenesis
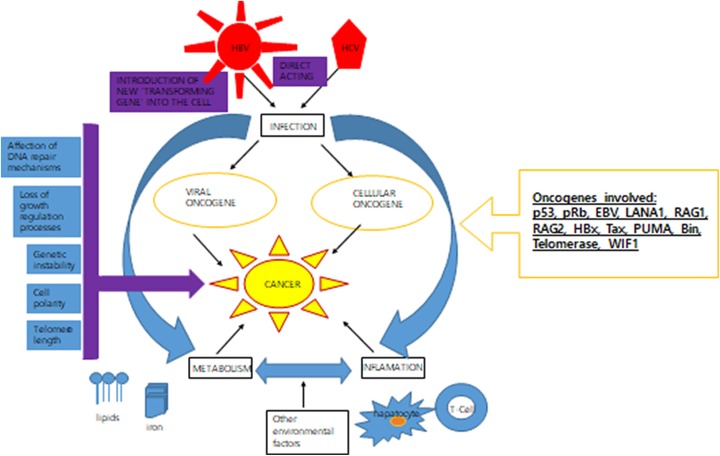
For direct carcinogenesis, viruses have developed a plethora of events to hijack different cellular processes (Figure 1).
Figure 1.
Direct mechanism of viral carcinogenesis. After infecting cells, tumor infections are kept as hereditary components; viral genomes can form episomes or integrate into the host genomic DNA.
Virus-encoded genes that activate growth
Viral or cellular oncogenes are direct-acting agents found in the form of a single clone inside the tumor cells. Expression of such genes enhances the resistance to apoptosis, which ultimately results in modifications in DNA repair mechanisms. For example, inactivation of p53 and pRb tumor suppressor genes mostly occurs during viral oncogenesis.42 When DNA is damage, p53, that is, product of the TP53 gene, inhibits cell cycle until the damage is restored. Otherwise, p53 induces cell senescence if it does not occur. The viral inactivation of p53 has been observed in E6 protein from HPV and in LANA1 expressed by KSHV.43
Genomic instability
The tendency of genome is increased to acquire mutations. For example, EBV Epstein–Barr nuclear antigen (EBNA)-1 may cause genomic instability by activating recombinase-activating genes (RAG1 and RAG2).5 Similarly, HBx alters the centrosome function by forming complex with HBx interacting protein.44
Interfering with telomere shortening
Infinite cell proliferation normally results in telomere shortening and eventually leads to cell death. Oncogenic viruses also maintain telomere with the help of enzyme telomerase along with various proteins.45 Telomerase expression is observed only in cells with stem properties, such as germinal cells. To date, HPV E6, KSHV LANA, HBV HBx, and HTLV1 Tax have been shown to cause the telomerase expression.46
Interfering with cell polarity
Viral oncoproteins also inactivate proteins related to cell polarity, which ultimately leads to oncogenesis by impairing morphogenesis and differentiation programs. For instance, postsynaptic density protein and zonula occludens-1 protein domains, which play a significant role in cell–cell contact, generally interact with target proteins via PDZ domain-binding motif. A class I PBM was first identified in adenovirus 9 and later on observed in HPV E6 and HTLV1 Tax.47
Viral microRNAs
MicroRNAs (miRNAs) are about 22 nucleotides long that usually inhibit messenger RNA translation.48 Recent studies revealed that all cancers almost show altered expression of cellular miRNAs.49 The first 5 viral miRNAs with oncogenic capabilities were observed in EBV-positive B95 cell line. Till now, 40 miRNAs have been identified from EBV BARTs and BHFR1 transcripts.50 These miRNAs have tendency to inhibit apoptosis, while some target cellular tumor suppressor genes, such as PUMA, Bin, and WIF1.51
Indirect mechanism of viral carcinogenesis
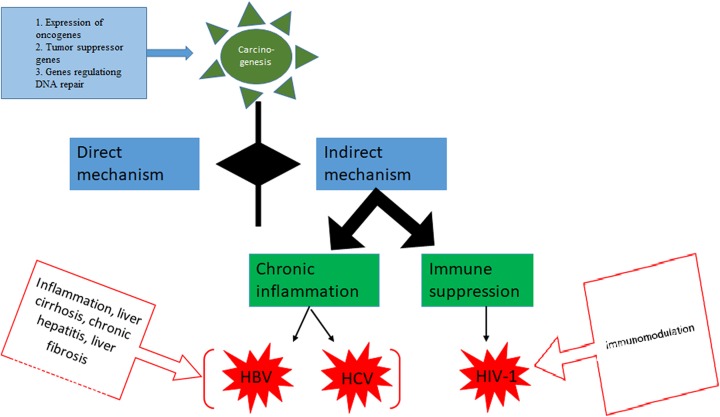
The indirect transforming viruses do not reside inside the tumor cells and generally act through following mechanisms (Figure 2).
Figure 2.
Indirect mechanisms of viral carcinogenesis. A, Chronic inflammation. Affected cells produce cytokines, chemokines attracting insusceptible cells, which harm the local tissue. Cancer develops inside this cycle of infection and causes inflammation. B, Immunosuppression. The model operator for immunosuppression is HIV. Epstein-Barr virus (EBV) infection is mostly controlled by cytotoxic CD8T cells; as HIV disease progresses and immune system is suppressed, people get to be at increased danger of having EBV-related lymphomas.
Chronic inflammation
Cells of the immune system proliferate as a result of infection and induce local damage to tissues identified by the formation of pro-inflammatory cytokines, chemokines, and antiapoptotic genes that stimulate endothelial cells to divide along with recasting tissues and neovascularization.52 Common viral agents that act through chronic inflammation are HBV and HCV.
Immunosuppression
Viruses cause immunosuppression which indirectly reduces antitumor immune surveillance mechanisms. For instance, immunosuppression generated by HIV infection leads to the cancer development.53
Oncomodulation
Viruses also take part in carcinogenesis by curbing the usual process of an already established cancer. The term “oncomodulation” was proposed by Martin Michaelis to express the function of human cytomegalovirus (HCMV) in tumor growth.54
Antiviral Defense Pathways Induced by Cells and Molecules Elicited During Viral Infections
Viruses, a class of intracellular pathogens, form different factors for invulnerability against these pathogens. Particular viral infections differ from others in magnitude and composition as well as in response to various cells and molecules. Studies demonstrated that many cell surface molecules are produced amid viral binding and entry into the host cell which actuates different but overlapping antiviral defense pathways. Foremost among these are discussed below.
Interferons α and β
Interferons α and β (IFN-α/β) are also known as type 1 interferons. Amid certain viral diseases, high levels of IFN-α/β are evoked in the host and intercede the immunoregulatory effects.55 The IFN-α/β-induced cellular antiviral response is the primary defense against a viral disease. The ultimate changes caused by IFN-α/β are control of cytokine receptor gene expression,56 activation of natural killer (NK) cell cytotoxic activity, and memory CD8 T-cell proliferation.57 Many viruses including both DNA and RNA viruses are sensitive to interferons-mediated antiviral effects.58
Tumor Necrosis Factors and IFN-γ
Both IFN-γ and tumor necrosis factor (TNF) are elicited at early times amid certain viral diseases.59 The pathways evoked by TNF and IFN coincide with those prompted by IFN-α/β. The TNF is weaker, yet collaborates with IFN-α/β for these effects. Whereas IFN-γ is a strong activator, TNF combines with IFN-α/β to activate macrophages that can mediate resistance through inducible nitric oxide synthase–dependent pathways successful in damaging other pathogens. The nitric oxide synthase (NOS2) enzyme catalyzes nitric oxide NO, which reacts with oxygen and its intermediates to yield various molecules capable of altering vital components for virus replication.60
Cytotoxic T Lymphocytes
The HBV-specific cytotoxic T lymphocytes (CTLs) inhibit viral gene replication in the liver by mechanism that is interceded by IFN-γ and TNF-α. Hence, CTLs have ability to kill infected hepatocytes and cause liver infection or suppress viral replication and alleviate the infection.61
Interleukin 12
Although IFN-α/β cytokines canal on negatively controlled IL-12 expression, IL-12 is produced in particular viral diseases and activates NK cell IFN-γ and creates normal Th1 responses. The TNF stimulates IL-12 induction of NK cell IFN-γ production. Besides promoting defense mechanisms, IL-12 also mediates IFN-γ-independent mechanisms.62
Double-Stranded RNA
Although double-stranded RNA (dsRNA) is absent in normal cells, it is often produced in cells as a consequence of viral replication. In the presence of dsRNA, (2′-9′)-oligoadenylate synthetase and the dsRNA-dependent protein kinase (PKR) stimulate inhibition of host cell protein synthesis and thus hinder viral replication.58
Heparin-Derived Molecules
Sometimes heparin-derived molecules are generated as a result of viral infection. For example, herpesvirus and HHV-8 utilize HS molecules for viral binding and entry. Heparin can effectively inhibit HCVcc, HCV-LP, and HCVpp binding to hepatoma cells.63
Scavenger Receptor Class B Type I and Serum Amyloid
In some cases, scavenger receptor class B type I binds and internalizes serum amyloid A (SAA) protein that mediates the pro-inflammatory cellular responses. The SAA has the ability to inhibit HCV entry and thus reduces its infectivity.64
Lectin Cyanovirin-N
Recently, another active compound, that is, lectin cyanovirin-N, has been discovered against HIV and showed potent antiviral activity against other viruses.65 Lectin cyanovirin-N and high-mannose oligosaccharides amalgamate present on viral envelope glycoproteins inhibit the viral entry. However, in HCV, mostly the glycosylation sites are so conserved that drugs targeting glycoproteins are unable to escape from virus as rapidly as in case of HIV does.66
Phosphorothioate Oligonucleotides and Other Carbohydrate-Binding Agents
Antiviral compounds directing viral passage sometimes target particular cell surface molecules. Studies revealed that amphipathic DNA polymer, namely, phosphorothioate oligonucleotides, exhibits significant activity against HIV by blocking virus–cell fusion.67 Other carbohydrate-binding agents including plant lectins and the mannose-specific antibiotic pradimicin A prevent HIV infection.68 Such compounds may also be effective against other viruses that require a glycosylated envelope for entry into host cells.
Cyclin-Dependent Kinases
Human immunodeficiency virus type 1 (HIV-1) is the causative agent of AIDS. Cyclin-dependent kinases (cdks) are required for HIV-1 transcription, and thus, particular cdk inhibitors act as antiviral agents in the treatment of AIDS. Likewise, HCMV is a herpesvirus that activates cdk upon infection, which controls cell cycle in G1 and S phases. Consequently, the inhibition of cdk activity blocks HCMV replication.69
Cellular Proteins Prohibitin 1 and 2
Cellular protein prohibitin-1 (PHB1) exhibits antiproliferative activity, while PHB2, also known as repressor of estrogen receptor activity, curbs the estrogen receptor–dependent gene activation.70 Both PHB1 and PHB2 are generally present on the plasma membrane, cytosol, and nucleus. They mediate HCV entry at a postbinding step via interacting with signaling molecule CRaf. However, removal of the entire C-terminal domains of PHB1 and PHB2 stops the interaction between PHB and CRaf. Such PHB-CRaf interaction can be jammed using natural products, such as rocaglamide (Roc-A), that will inhibit subsequent HCV entry into target cell. Henceforth, PHB1 and PHB2 are pan-genomic HCV entry factors.71
Integrin-Mediated Activation of Focal Adhesion Kinase
Integrin 31 is a cellular receptor for KSHV entry into the target cells. Human herpesvirus 8 is involved in the pathogenesis of Kaposi sarcoma. The HHV-8 envelope glycoprotein has arginylglycylaspartic acid (RGD) motif that synergizes with integrin molecules, and HHV-8 infectivity is blocked by RGD peptides and antibodies against RGD-dependent α3 and β1 integrins. Moreover, HHV-8 infection leads to the integrin-mediated activation of focal adhesion kinase (FAK). Hence, HHV-8 utilizes integrin and the associated signaling pathways to enter the cells. Activation of FAK and integrin-linked kinases is involved in signaling by integrins, actin assembly, and endocytosis.72 So, FAK activation has a central role in HHV-8 infection.
2B4 Molecules
2B4 is a surface molecule that activates the NK-cell-mediated cytotoxicity. It binds Src homology 2 domain-containing protein (SH2D1A) or signaling lymphocyte activation molecule-associated protein (SAP), which regulates 2B4-associated signal transduction pathway. So, 2B4 molecules also play significant role in infected cells such as in EBV.73
Bay 11-7082, an Inhibitor of NF-κB
T-cell lines infected with HTLV-I and leukemic cells show high activity of transcription factor NF-κB. Bay 11-7082, an inhibitor of NF-κB, induces apoptosis of HTLV-I-infected T-cell lines. Bay 11-7082 reduces the DNA binding of NF-κB in HTLV-I-infected T-cell lines and downregulates the expression of antiapoptotic gene, Bcl-xL, that is regulated by NF-κB. In short, NF-κB is an appropriate target for the treatment of ATL.74
Tax
Tax initiates viral gene expression and activates the expression of many genes through various transcription factors, such as NF-κB/Rel, AP-1, and serum response factor.75 Normally, genes induced by Tax include cytokines/chemokines (IL-1a, IL-6, IL-8, TNF-β, and granulocyte colony-stimulating factor), their receptors, cell adhesion molecule (OX40), apoptosis inhibitor (Bcl-xL), and G1 cyclins.76 The transcriptional activation of genes deregulates the growth of HTLV-I-infected cells.
Influenza Virus NS1 Protein
Influenza virus NS1 protein prevents the PKR-mediated antiviral response. The PKR upon activation forms a dimer and autophosphorylates. The PKR blocks the protein synthesis, which ultimately prevents viral replication. Some viruses have set up certain approaches to block the PKR activity to mitigate the effects of PKR activation. For instance, influenza A virus can curb PKR activity by recruiting cellular protein P58IPK, which binds directly to kinase.77
Interferon Gamma Inducing Factor and Transforming Growth Factors
Interferon gamma inducing factor and transforming growth factor can be elicited by cells of the innate immune system as well as by other nonimmune cells. They also play a vital role in the clearance of many pathogens.78
Other Molecules
Other molecules involved in immunoregulatory system during chronic viral infections include claudin-1, occludin, epidermal growth factor receptor, low-density lipoprotein receptor, and DC-SIGN (dendritic cell-specific intercellular adhesion molecule 3 grabbing nonintegrin)/L-SIGN (DC-SIGNr, liver and lymph node specific).79-83
Natural Products as a Robust Source for Cancer Chemoprevention
In the most obvious way, a “natural product” is a minute particle made by a biological source. In this manner, a natural product is a biologically dynamic chemical substance present in nature and generated by a living creature. For the most part, the expression “natural product” is viewed as being synonymous with “secondary metabolite.” Natural products are actually extremely small particles with a molecular weight below 3000 Da.84
Natural products, isolated from microorganisms, insects, dietary products, and medicinal plants, have been the greatest wellspring of hostile to malignancy drugs.85 From the earliest starting point, people are relying on nature for their essential requirements for the creation of sustenance stuffs, composts, and drugs, too. Respective conventional sources for secondary metabolites or natural products are elaborated in the following sections.
Natural Products From Microorganisms
Billions of microorganisms are available ashore, in freshwater, and all territories of the ocean and the biosphere activities rely on this microbial world.86 The microbial world is an immense untapped asset for drug discovery. Truly, microorganisms have assumed an imperative part in giving new structures, similar to anti-infection agents for medication disclosure and advancement. Actinomycetes, for case, include numerous unrecognized secondary metabolites. Cyanophytes, also known as blue-green algae, are probably the most productive makers of medications utilized for cancer treatment. Illustrations incorporate coibamide A65 segregated from basic extraction of wild collects or fermentation of purified living beings.87 Extremophilic microbes are those living in extreme environments, including acidophiles, alkalophiles, hyperthermophiles, and psychrophiles.88-90 Examples of natural compounds isolated from various microorganisms that are effectively used as anticancer agents are given in Table 2.
Table 2.
Natural Compounds From Microorganisms Used as a Source of Anticancer Agents.
| Serial Number | Microorganism Name | Bioactive Compounds | References |
|---|---|---|---|
| 1. | Amycolatopsis orientalis | Vancomycin | 91 |
| 2. | Actinosynnema pretiosum | Ansamitocins | 92 |
| 3. | Gymnodinium species | GA3P, a d-galactan sulfate | 93 |
| 4. | Streptomyces pneuceticus | Doxorubicin, daunorubicin | 94 |
| 5. | Talaromyces wortmanni | Wortmannin | 95 |
| 6. | Chromobacterium violaceum | Depsipeptide | 96 |
| 7. | Burkholderia rhizoxina | Rhizoxin | 97 |
Natural Products From Marine Organisms
Marine environment is an uncommon repository of bioactive natural products, a considerable number which displays basic features not found in terrestrial natural products. The seas cover more than 70% of earth’s surface. Marine life forms contain roughly 50% of the aggregate biodiversity on earth and the marine biological system is the best source to find valuable drugs. By and large, 3000 new chemical substances have been recognized from marine creatures.98 Marine has a place with extremely differing basic classes, including steroids and peptides. The life forms yielding these bioactive marine mixes include invertebrate creatures, algae, fungi, and bacteria.99 Sessile marine spineless creatures, for example, sponges and tunicates, generally missing morphological resistance structures, have built up the biggest number of marine inferred secondary metabolites, including the absolute most fascinating drug applicants. During the 21st century, bigger rates of bioactive non chemical entitites (NCEs) were accounted for marine life forms in contrast with terrestrial creatures.
The primary anticancer product didemnin B, secluded from the tunicate Trididemnum solidum from marine source, showed activity against non-Hodgkin lymphoma.100 Some ecteinascidins have been obtained from the tunicate Ecteinascidia turbinate possessing antitumor effects. Similarly, dolastatins obtained from Dolabella auricularia and bryostatins, isolated from Bugula neritina and other marine bryozoans, have demonstrated noteworthy activity against lymphocytic leukemia cell line.101,102 Beside these, there are many other compounds isolated from marine life forms as potential antitumor agents, as given in Table 3.
Table 3.
Natural Compounds From Marine Organisms Used as a Source of Anticancer Agents.
| Serial Number | Marine Organisms | Bioactive compounds | References |
|---|---|---|---|
| 1. | Salinispora arenicola | Saliniketals | 102 |
| 2. | Marinispora | Marinomycins | 103 |
| 3. | Cacospongia mycofijiensis | Laulimalide | 104 |
| 4. | Crambe crambe | Crambescidin 800 | 105 |
| 5. | Dactylospongia elegans | Smenospongine | 105 |
| 6. | Discodermia dissolute | Discodermolide | 104 |
| 7. | Erythropodium caribaeorum | Eleutherobin (diterpene glycoside) | 104 |
| 8. | Mycale hentscheli | Peloruside A | 106 |
| 9. | Poecillastra species | Psammaplin A | 107 |
| 10. | Amphimedon species | Ascididemin | 108 |
| 11. | Cephalodiscus gilchrist | Cephalostatin 1 | 109 |
| 12. | Cryptotheca crypta | Cytarabine | 110 |
| 13. | Dolabella auricularia | Dolastatins | 111 |
| 14. | Bugula neritina | Bryostatins | 112 |
| 15. | Halichondria okadai | Halichondrin B | 113 |
Natural Products From Insects
Insects make up around 75% of all species. Insects have provided profitable natural products, including honey (beeswax, pollen, and Royal Jelly) and silk, and have highlighted in pharmaceuticals for treating throat infection, tuberculosis, cancer, and numerous different maladies and afflictions for many years. Other than China, the utilization of insects in medicine is likewise basic in numerous different areas, including India and South Korea. Usually, the medicines are extricated from the stings of honeybees and wasps, or from their secretions, and after that teas made for drinking or treatments for external use.114
Stinging insects mostly produce venom containing an intricate mixture of various chemicals possessing various pharmacological functions. The entire body extracts of many wasps, butterflies, cockroaches, and beetles can also be used as anticancer agents. Moreover, insect antimicrobial peptides have been isolated from insects which kill microscopic organisms including bacteria, fungi, protozoans, and virally infected cancer cells.115 For example, cecropins can kill bacteria, fungi, protozoans, and cancer cells.116 Other compounds isolated from marine organisms that are successfully used as anticancer agents are given in Table 4.
Table 4.
Natural Compounds From Insects Used as a Source of Anticancer Agents.
| Serial Number | Insects | Bioactive Compounds | References |
|---|---|---|---|
| 1. | Parectatosoma mocquerysi | Parectadial | 114 |
| 2. | Drosophila melanogaster, Anopheles gambiae | Cecropins (antimicrobial peptides) | 116 |
| 3. | Ants, bee, and wasp venom | Melittin, phospholipases and hyaluronidase | 117 |
| 4. | Paederus subspecies (rove beetle) | Pederin | 118 |
| 5. | Honey bee | Fatty acid 10-hydroxy-2-decenoic acid | 119 |
| 6. | S. peregrine | Lectin | 115 |
| 7. | Polybia polista | Mastoparan | 120 |
| 8. | Solenopsis invicta | Solenopsin A (alkaloid) | 121 |
| 9. | Mylabris phalerata and Mylabris cichorii | Cantharidin (terpenoid) | 122 |
| 10. | Pieris subspecies (butterfly) | Pierisin-1 | 123 |
Dietary Natural Products
Undoubtedly, dietary sources play an essential role in cancers including fruits, vegetables, and spices, which yield bioactive components, namely, curcumin, isoflavones, saponins, and lycopene. Mounted evidences proposed that regular intake of a high-fiber, low-fat diet altogether with different fruits, legumes, and vegetables effectively reduces the cancer risks, as they possess various antioxidants which provide protection against harmful effects of free radicals that lead to the cancer development.124 However, saturated fats, salt, and sugar intake should be avoided or reduced in order to prevent cancer development. Hence, dietary natural products act as valuable tonic for different types of cancer. Studies also revealed that dietary natural products have potential to inhibit cancer by underlying various mechanisms such as inhibiting growth of cancer cells and metastasis and protecting against carcinogens.125 The anticancer potential of fruits, vegetables, spices, cereals, and edible macro-fungi and their bioactive constituents studied in various bioassay systems are given in Table 5.
Table 5.
Natural Compounds From Dietary Sources Used as a Source of Anticancer Agents.
| Serial Number | Dietary Natural Products | Bioactive Compounds | References |
|---|---|---|---|
| Fruits | |||
| 1. | Apples | Polyphenols | 126 |
| 2. | Mangosteen | γ-Mangostin | 127 |
| 3. | Pomegranate | Alkaloids, anthocyanidins | 128 |
| 4. | Sweetsop | Annonaceous acetogenins | 129 |
| 5. | Berries | Resveratrol | 130 |
| 6. | Indian gooseberry | Tannins, flavonoids | 131 |
| 7. | Plum | Polyphenols | 132 |
| 8. | Grapes | Procyanidins | 133 |
| 9. | Apricot | Carotenoids | 134 |
| Vegetables | |||
| 1. | Purple perilla | Rosmarinic acid, caffeic acid, apigenin, and isoegomake-tone | 135 |
| 2. | Bitter gourd | Lectin | 136 |
| 3. | Asparagus | Polysaccharides | 137 |
| 4. | Tomato | Lycopene and tomatine | 138 |
| 5. | Mungbean sprouts | NA | 139 |
| 6. | French beans | Lectins | 140 |
| 7. | Potato | Glycoalkaloids | 141 |
| 8. | Celery | Pigenin, linamarose, vitamins A/C | 142 |
| 9. | Cruciferous vegetables (radish, cauliflower, and broccoli) | Glucosinolates and isothiocyanates | 143 |
| Spices | |||
| 1. | Turmeric | Curcumin, curcuma oil, aromatic tumerone, and sesquiterpenoids | 144 |
| 2. | Garlic | Organosulphur compounds (OSC), such as alliin, diallyl disulfide, allicin, and sodium 2-propenyl thiosulfate | 145,146 |
| 3. | Cinnamon | Isoobtusilactone A | 129 |
| 4. | Chili pepper | Capsaicin | 147 |
| 5. | Ginger | Geraniol, pinostrobin, clavatol, gingeols, zingerone | 148 |
| 6. | Saffron | Carotenoids | 149 |
| Coffee | Caffeic acid (3,4-dihydroxycinnamic acid) and ferulic acid (4-hydroxy-3-methoxycinnamic acid) | 150 | |
| Meat products | Conjugated linoleic acid (CLA) | 151 | |
| Green and black teas (Camellia sinensis) | Catechin and theaflavins | 152 | |
| Cereals | |||
| 1. | Rice bran | Peptide hydrolysates and phytic acid | 153 |
| 2. | Corn silk | Polysaccharides | 154 |
| 3. | Oats, barley | β-Glucan | 151 |
| Edible macro-fungi | |||
| 1. | Pleurotus pulmonarius | NA | 155 |
| 2. | Lentinula edodes | Polysaccharide | 156 |
| 3. | Agrocybe aegerita | Lectin | 157 |
| 4. | Flammulina velutipes | Glycoprotein | 145 |
| 5. | Agaricus blazei | β-glucan, blazeispirols A and C | 158 |
| 6. | Grifola frondosa | O-orsellinaldehyde | 159 |
| 7. | Schizophyllum commune | Schizophyllum | 160 |
| 8. | Grifola frondosa | Grifon D | 160 |
Abbreviation: NA, not available.
Natural Products From Plants
Plants have been used in the treatment of cancer since many years. Natural products from plants specifically play an essential part in the improvement of treating various virally infected cancers (Table 6). It is assessed that plant-derived compounds constitute more than half of anticancer agents.182
Table 6.
Natural Compounds From Plants Used as a Source of Anticancer Agents.
| Serial Number | Plant Name | Family | Active Constituents | References |
|---|---|---|---|---|
| 1. | Aegle marmelos | Rutaceae | Lupeol (triterpene) | 131 |
| 2. | Aglaia sylvestris | Meliaceae | Silvestrol | 161 |
| 3. | Allium cepa | Liliaceae | Allicin alliin, quercetin, flavonoids, vitamins C and E | 133 |
| 4. | Aloe barbadensis | Liliaceae | Aloe-emodin, emodin, aloin acemannan | 162 |
| 5. | Alpinia galangagalangal | Zingeberaceae | Acetoxy chavicol acetate, pinocembrin, galangin | 131 |
| 6. | Angelica sinensis | Umbelliferae | Carbohydrates | 128 |
| 7. | Apium graveolens | Umbelliferae | Apigenin (flavonoid) | 142 |
| 8. | Artemisia monosperma | Asteraceae | Capillin (1-phenyl-2,4-pentadiyne) | 163 |
| 9. | Azadirecta indica | Meliaceae | Triterpenoid | 131 |
| 10. | Bauhinia variegata | Caesalpinaceae | Glycosides, essential oil | 131 |
| 11. | Berberis vulgaris | Berberidaceae | Berberine (an isoquinoline alkaloid) | 131 |
| 12. | Broussonetia papyrifera | Urticaceae | Isolicoflavonol | 164 |
| 13. | Brucea antidysenterica | Simaroubaceae | Bruceantin | 165 |
| 14. | Camellia sinensis | Theaceae | Catechin | 128 |
| 15. | Campotheca acuminate | Nyssaceae | Campothecin (alkaloid) | 166 |
| 16. | Catharanthus roseus | Apocynaceae | Vinblastine, vincristine, and reserpine | 166 |
| 17. | Centaurea montata | Asteraceae | Montamine (alkaloid) | 128 |
| 18. | Cephalotaxus harringtonia | Cephalotaxaceae | Homoharringtonine (alkaloid) | 166 |
| 19. | Combretum caffrum | Cobretaceae | Stilbenes, combretastatin | 167 |
| 20. | Croton lechleri | Euphorbiaceae | Taspine (alkaloid) | 168 |
| 21. | Erythroxylum pervillei | Erythroxylaceae | Pervilleine A | 169 |
| 22. | Fagopyrum esculentum | Polygonaceae | Tanins, flavonoids | 128 |
| 23. | Fragaria vesca | Rosaceae | Tanins, flavonoids | 131 |
| 24. | Ginkgo biloba | Ginkgoaceae | Ginkgolides B, A, C, and J | 170 |
| 25. | Glycyrrhiza glabra | Leguminosae | Glycyrrhizin | 171 |
| 26. | Gossypium barbadense | Malvaceae | Gossypol | 172 |
| 27. | Indigofera tinctoria | Leguminosae | Indigoids | 166 |
| 28. | Larrea tridentate | Zygophyllaceae | Lignan | 173 |
| 29. | Lentinus edodes | Agaricaceae | Lentinan | 174 |
| 30. | Nigella sativa | Ranunculaceae | Thymoquinone, dithymoquinone | 131 |
| 31. | Ocimum sanctum | Lamiaceae | Eugenol, orientin, and vicenin | 131 |
| 32. | Panax ginseng | Aralaceae | Ginsenosides, panaxosides | 175 |
| 33. | Podophyllum peltatum | Berberidaceae | Epipodophyllotoxin | 176 |
| 34. | Prunella vulgaris | Lamiaceae | Ursolic acid, oleanolic acid | 131 |
| 35. | Psoralea corylifolia | Fabaceae | Bavachinin and psoralen, psoralidin | 131 |
| 36. | Pteris multifidi | Pteridaceae | Terpenoid | 177 |
| 37. | Saussurea lappa | Compositae | Sesquiterpenes | 131 |
| 38. | Silybum marianum | Asteraceae | Silymarin | 178 |
| 39. | Solanum nigrum | Solanaceae | Solamargine, solasonine, solanin, quercetin | 128 |
| 40. | Taxus brevifolia | Taxaceae | Taxanes, taxol cepholomannine | 179 |
| 41. | Tinospora cardifolia | Menispermaceae | Sesquiterpenes, diterpenes | 131 |
| 42. | Vitex rotundifolia | Verbenaceae | Flavonoid | 180 |
| 43. | Ziziphus mauritiana | Rhamnaceae | Betulinic acid | 181 |
Future Perspective
Tumor virology in humans has achieved great attention since past 100 years. Viruses have adopted a lot of approaches for hijacking host cellular pathways that are mandatory for carcinogenesis. Infection-associated tumors remained an eminent problem globally. Immune system of body normally inhibits the growth of tumor cells that require the expression of viral proteins for their survival, or sometimes curb the viral replication, thus reducing the likelihood of a “oncogenic hit.” So, all human cancers, mostly progresses in case of immunosuppression and the protective effect of immune system, play a central role in carcinogenesis.
With the advent of technologies, it is important to find out different techniques for cancer treatment and its prevention. Nature has remained a prime source of medicinal products for centuries. Secondary metabolites present in the microbes and marine organisms serve as a new frontier for discovery of natural products used for the treatment of virally infected cancers. The culturing of the respective microorganism may for the most part be a practical way to increase quantities. Moreover, with the incredible advancements of pharmaceutical industries, medicinal plants offer major resource for the development of potential novel agents. Compounds isolated from the plants have potential to effectively target the tumor cells and avoid their harmful effects on normal healthy tissues. Besides all, tobacco smoking and imperfect diet also result in carcinogenesis. So, cancer development can also be mitigated by consumption of proper and adequate diet.
Overall studies revealed that these natural products isolated from various insects, marine, and microorganisms as well as medicinal plants are useful in protecting virally infected cancer. Moreover, consuming a diet rich in antioxidant fruits, vegetables, herbs, and so on, also provides health-protective effects. Cancer is one of major problem in both developing and developed countries. Chemotherapy and radiation therapy causes various side effects; therefore, there is requirement of an alternative medicine to treat cancer. Nature possesses great chemical diversity that provides one of the most efficient sources of inspiration for medicinal and drug discovery chemists. Natural products can be recommended to the rural and poor people to treat effectively the cancers as they are cheaper; beside this, they can be used in developing drugs as they have no side effects. So, the data analyzed in this study have provided us with information that can provide basis for the future pharmacological screening, leading to natural drugs discovery development. Various bioactive compounds can be isolated and identified easily from natural products extracts via advance techniques. Moreover, in future, attention should be paid on the role of various molecules involved in carcinogenesis and further detailed pharmacological and in vivo studies are required for large-scale implementation of drugs for cancer chemoprevention.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. World Health Organization. National Cancer Control Programmes: Policies and Managerial Guidelines. WHO: Geneva; 2002. [Google Scholar]
- 2. Bouvard V, Baan R, Straif K, et al. ; WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens—part B: biological agents. Lancet Oncol. 2009;10(4):321–322. [DOI] [PubMed] [Google Scholar]
- 3. Soares JP, Cortinhas A, Bento T, et al. Aging and DNA damage in humans: a meta-analysis study. Aging. 2014;6(6):432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW. DNA repair, genome stability and aging. Cell. 2005;120(4):497–512. [DOI] [PubMed] [Google Scholar]
- 5. Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer. 2010;10(12):878–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Biological Agents Vol. 100B. World Health Organization. 2012 http://monographs.iarc.fr/ENG/Monographs/vol100B/mono100B.pdf.
- 7. Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4(10):757–768. [DOI] [PubMed] [Google Scholar]
- 8. Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10(10):707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woodman CB, Collins SI, Young LS. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. 2007;7(1):11–22. [DOI] [PubMed] [Google Scholar]
- 10. Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond). 2006;110(5):525–541. [DOI] [PubMed] [Google Scholar]
- 11. Chang Y, Moore PS. Merkel cell carcinoma: a virus induced human cancer. Annu Rev Pathol. 2012;7:123–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64(1):51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scheel TK, Rice CM. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat Med. 2013;19(7):837–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kannian P, Green PL. Human T lymphotropic virus type 1 (HTLV-1): molecular biology and oncogenesis. Viruses. 2010;2(9):2037–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stevenson M. HIV-1 pathogenesis. Nat Med. 2003;9(7):853–860. [DOI] [PubMed] [Google Scholar]
- 16. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet. 1964;1(7335):702–703. [DOI] [PubMed] [Google Scholar]
- 17. Klein G, Klein E, Kashuba E. Interaction of Epstein-Barr virus (EBV) with human B lymphocytes. Biochem Biophy Res Commun. 2010;396(1):67–73. [DOI] [PubMed] [Google Scholar]
- 18. Gerber P, Walsh JH, Rosenblum EN, et al. Association of EB-virus infection with the post-perfusion syndrome. Lancet. 1969;1(7595):593–595. [DOI] [PubMed] [Google Scholar]
- 19. Stock I. Infectious mononucleosis—a “childhood disease” of great medical concern. Med Monatsschr Pharm. 2013;36(10):364–368. [PubMed] [Google Scholar]
- 20. Shair KH, Schnegg CI, Raab-Traub N. EBV latent membrane protein 1 effects on plakoglobin, cell growth, and migration. Cancer Res. 2008;68(17):6997–7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rickinson AB, Kieff E. Epstein-Barr Virus. In Fields Virology. Vol. 2 Philadelphia, PA: Lippincott-Raven Publishers; 1996:2397–2446. [Google Scholar]
- 22. Cai Q, Verma SC, Lu J, Robertson ES. Molecular biology of Kaposi’s sarcoma-associated herpesvirus and related oncogenesis. Adv Virus Res. 2010;78:87–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370(9580):890–907. [DOI] [PubMed] [Google Scholar]
- 24. Moens U, Van Ghelue M, Johannessen M. Oncogenic potentials of the human polyomavirus regulatory proteins. Cell Mol Life Sci. 2007;64(13):1656–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kew MC. Epidemiology of chronic hepatitis B virus infection, hepatocellular carcinoma, and hepatitis B virus-induced hepatocellular carcinoma. Pathol Biol. 2010;58(4):273–277. [DOI] [PubMed] [Google Scholar]
- 26. Tarocchi M, Polvani S, Marroncini G, et al. Molecular mechanism of hepatitis B virus induced hepatocarcinogenesis. World J Gastroenterol. 2014;20(33):11630–11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tang H, Grise H. Cellular and molecular biology of HCV infection and hepatitis. Clin Sci. 2009;117(2):49–65. [DOI] [PubMed] [Google Scholar]
- 28. Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 pt 1):329–337. [DOI] [PubMed] [Google Scholar]
- 29. Moriya K, Yotsuyanagi H, Shintani Y, et al. Hepatitis C virus core protein induces hepatic steatosis in transgenic mice. J Gen Virol. 1997;78(pt 7):1527–1531. [DOI] [PubMed] [Google Scholar]
- 30. Boxus M, Willems L. Mechanisms of HTLV-1 persistence and transformation. Br J Cancer. 2009;101(9):1497–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takaoka A, Hayakawa S, Yanai H, et al. Integration of interferon-α/β signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424(6948):516–523. [DOI] [PubMed] [Google Scholar]
- 32. Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8(7):559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee HR, Kim MH, Lee JS, Liang C, Jung JU. Viral interferon regulatory factors. J Interferon Cytokine Res. 2009;29(9):621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chatterjee M, Osborne J, Bestetti G, Chang Y, Moore PS. Viral IL-6-induced cell proliferation and immune evasion of interferon activity. Science. 2002;298(5597):1432–1435. [DOI] [PubMed] [Google Scholar]
- 35. Cloutier N, Flamand L. Kaposi sarcoma-associated herpesvirus latency-associated nuclear antigen inhibits interferon (IFN) β expression by competing with IFN regulatory factor-3 for binding to IFNB promoter. J Biol Chem. 2010;285(10):7208–7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wies E, Hahn AS, Schmidt K, et al. The Kaposi’s sarcoma-associated herpesvirus-encoded vIRF-3 inhibits cellular IRF-5. J Biol Chem. 2009;284(13):8525–8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. Merkel cell carcinoma and HIV infection. Lancet. 2002;359(9305):497–498. [DOI] [PubMed] [Google Scholar]
- 38. Harris TG, Burk RD, Palefsky JM, et al. Incidence of cervical squamous intraepithelial lesions associated with HIV serostatus, CD4 cell counts, and human papillomavirus test results. JAMA. 2005;293(12):1471–1476. [DOI] [PubMed] [Google Scholar]
- 39. Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst. 2000;92:1500–1510. [DOI] [PubMed] [Google Scholar]
- 40. Kawano N, Shimoda K, Ishikawa F, et al. Adult T-cell leukemia development from a human T-cell leukemia virus type I carrier after a living-donor liver transplantation. Transplantation. 2006;82(6):840–843. [DOI] [PubMed] [Google Scholar]
- 41. Reddy L, Odhava B, Bhool KD. Natural products for cancer prevention: a global perspective. Pharmacol Ther. 2003;99(1):1–13. [DOI] [PubMed] [Google Scholar]
- 42. Khidr L, Chen PL. RB, the conductor that orchestrates life, death and differentiation. Oncogene. 2006;25(38):5210–5219. [DOI] [PubMed] [Google Scholar]
- 43. Friborg J, Kong W, Hottiger MO, et al. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature. 1999;402(6764):889–894. [DOI] [PubMed] [Google Scholar]
- 44. Wen Y, Golubkov VS, Strongin AY, et al. Interaction of hepatitis B viral oncoprotein with cellular target HBXIP dysregulates centrosome dynamics and mitotic spindle formation. J Biol Chem. 2008;283(5):2793–2803. [DOI] [PubMed] [Google Scholar]
- 45. Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315(5820):1850–1853. [DOI] [PubMed] [Google Scholar]
- 46. Terrin L, Dal Col J, Rampazzo E, et al. Latent membrane protein 1 of Epstein-Barr virus activates the HTERT promoter and enhances telomerase activity in B lymphocytes. J Virol. 2008;82(20):10175–10187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ohashi M, Sakurai M, Higuchi M, et al. Human T-cell leukemia virus type 1 Tax oncoprotein induces and interacts with a multi-PDZ domain protein, MAGI-3. Virology. 2004;320(1):52–62. [DOI] [PubMed] [Google Scholar]
- 48. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. [DOI] [PubMed] [Google Scholar]
- 49. Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–866. [DOI] [PubMed] [Google Scholar]
- 50. Cai X, Schafer A, Lu S, et al. Epstein-Barr virus microRNAs are evolutionarily conserved and differentially expressed. PLoS Pathog. 2006;2(3):e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marquitz AR, Raab-Traub N. The role of miRNAs and EBV Barts in NPC. Semin Cancer Biol. 2012;22(2):166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chimal-Ramirez GK, Espinoza-Sanchez NA, Fuentes-Panana EM. Protumor activities of the immune response: insights in the mechanisms of immunological shift, oncotraining, and oncopromotion. J Oncol. 2013:835–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chadburn A, Abdul-Nabi AM, Teruya BS, Lo AA. Lymphoid proliferations associated with human immunodeficiency virus infection. Arch Pathol Lab Med. 2013;137(3):360–370. [DOI] [PubMed] [Google Scholar]
- 54. Michaelis M, Doerr HW, Cinatl J. The story of human cytomegalovirus and cancer: increasing evidence and open questions. Neoplasia. 2009;11(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Orange JS, Biron CA. Characterization of early IL-12, IFN-α/β, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J Immunol. 1996;156(12):4746–4756. [PubMed] [Google Scholar]
- 56. Diefenbach A, Schindler H, Donhauser N, et al. Type 1 interferon IFN-α/β and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity. 1998;8(1):77–87. [DOI] [PubMed] [Google Scholar]
- 57. Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272(5270):1947–1950. [DOI] [PubMed] [Google Scholar]
- 58. Vilcek J, Sen GC. Interferons and other cytokines In: Bernard N, Knipe DM, Howley PM, eds. Fundamental Virology Fields. 3rd ed New York, NY: Lippincott-Raven Publishers; 1996:341–365. [Google Scholar]
- 59. Ruzek MC, Miller AH, Opal SM, Pearce BD, Biron CA. Characterization of early cytokine responses and an interleukin-6-dependent pathway of endogenous glucocorticoid induction during murine cytomegalovirus infection. J Exp Med. 1997;185(7):1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. [DOI] [PubMed] [Google Scholar]
- 61. Guidotti LG, Chisari FV. To kill or to cure: options in host defense against viral infection In: Zinkernagel R, Bloom B, eds. Current Opinion in Immunology. London, England: Current Biology, Ltd; 1996:478–483. [DOI] [PubMed] [Google Scholar]
- 62. Victoria JC, Luca GG, Francis VC. Interleukin-12 inhibits hepatitis B virus replication in transgenic mice. J Virol. 1997;71(4):3236–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Koutsoudakis G, Kaul A, Steinmann E, et al. Characterization of the early steps of hepatitis C virus infection by using luciferase reporter viruses. J Virol. 2006;80(11):5308–5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lavie M, Voisset C, Vu-Dac N, et al. Serum amyloid A has antiviral activity against hepatitis C virus by inhibiting virus entry in a cell culture system. Hepatology. 2006;44(6):1626–34. [DOI] [PubMed] [Google Scholar]
- 65. O’Keefe BR, Smee DF, Turpin JA, et al. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob Agents Chemother. 2003;47(8):2518–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wei X, Decker JM, Wang S, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422(6929):307–312. [DOI] [PubMed] [Google Scholar]
- 67. Vaillant A, Juteau JM, Lu H, et al. Phosphorothioate oligonucleotides inhibit human immunodeficiency virus type 1 fusion by blocking gp41 core formation. Antimicrob Agents Chemother. 2006;50(4):1393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Balzarini J. Carbohydrate-binding agents: a potential future cornerstone for the chemotherapy of enveloped viruses? Antivir Chem Chemother. 2007;18(1):1–11. [DOI] [PubMed] [Google Scholar]
- 69. Wang D, De La Fuente C, Deng L, et al. Inhibition of human immunodeficiency virus type 1 transcription by chemical cyclin-dependent kinase inhibitors. J Virol. 2001;75(16):7266–7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Montano MM, Ekena K, Delage-Mourroux R, Chang W, Martini P, Katzenellenbogen BS. An estrogen receptor-selective coregulator that potentiates the effectiveness of antiestrogens and represses the activity of estrogens. Proc Natl Acad Sci U S A 1999;96(12):6947–6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu S, Wang W, Brown LE, et al. A novel class of small molecule compounds that inhibit hepatitis C virus infection by targeting the prohibitin–CRaf pathway. EBioMedicine. 2015;2(11):1600–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Calderwood DA, Shattil SJ, Ginsberg MH. Integrins and actin filaments: reciprocal regulation of cell adhesion and signaling. J Biol Chem. 2000;275(30):22607–22610. [DOI] [PubMed] [Google Scholar]
- 73. Parolini S, Bottino C, Falco M, et al. X-linked lymphoproliferative disease: 2B4 molecules displaying inhibitory rather than activating function are responsible for the inability of natural killer cells to kill Epstein-Barr virus-infected cells. J Exp Med. 2000;192(3):337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mori N, Yamada Y, Ikeda S, et al. Bay 11-7082 inhibits transcription factor NF-κB and induces apoptosis of HTLV-I-infected T-cell lines and primary adult T-cell leukemia cells. Blood. 2002;100(5):1828–1834. [DOI] [PubMed] [Google Scholar]
- 75. Fujii M, Tsuchiya H, Chuhjo T, Akizawa T, Seiki M. Interaction of HTLV-1 Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 1992;6(11):2066–2076. [DOI] [PubMed] [Google Scholar]
- 76. Huang Y, Ohtani K, Iwanaga R, Matsumura Y, Nakamura M. Direct transactivation of the human cyclin D2 gene by the oncogene product Tax of human T-cell leukemia virus type I. Oncogene. 2001;20(9):1094–1102. [DOI] [PubMed] [Google Scholar]
- 77. Melville MW, Tan SL, Wambach M, Song J, Morimoto RI, Katze MG. The cellular inhibitor of the PKR protein kinase, P58 (IPK), is an influenza virus-activated co-chaperone that modulates heat shock protein 70 activity. J Biol Chem. 1999;274(6):3797–3803. [DOI] [PubMed] [Google Scholar]
- 78. Biron CA. Role of early cytokines, including alpha and beta interferons IFN-(α/β), in innate and adaptive immune responses to viral infections. Semin Immunol. 1998;10:383–390. [DOI] [PubMed] [Google Scholar]
- 79. Evans MJ, Von Hahn T, Tscherne DM, et al. Claudin-1 is a hepatitis c virus coreceptor required for a late step in entry. Nature 2007;446(7137):801–805. [DOI] [PubMed] [Google Scholar]
- 80. Ploss A, Evans MJ, Gaysinskaya VA, et al. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457(7231):882–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lupberger J, Zeisel MB, Xiao F, et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med. 2011;17(5):589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Agnello V, Abel G, Elfahal M, Knight GB, Zhang QX. Hepatitis C virus and other Flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1999;96(22):12766–12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lozach PY, Amara A, Bartosch B, et al. 2004. C-type lectins L-SIGN and DC-SIGN capture and transmit infectious hepatitis C virus pseudotype particles. J Biol Chem. 2004;279(31):32035–32045. [DOI] [PubMed] [Google Scholar]
- 84. Kinghorn AD, Chin YW, Swanson SM. Discovery of natural product anticancer agents from biodiverse organisms. Curr Opin Drug Discov Dev. 2009;12(2):189–196. [PMC free article] [PubMed] [Google Scholar]
- 85. Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75(3):311–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Madigan MT, Martinko J, Dunlap PV, et al. Brock Biology of Microorganisms. 12th ed Upper Saddle River, NJ:Benjamin Cummings; 2008. [Google Scholar]
- 87. Tan LT. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry. 2007;68(7):954–979. [DOI] [PubMed] [Google Scholar]
- 88. Amato A. Microbes live near undersea CO2 lake. Chem Eng News. 2006;84(38):14. [Google Scholar]
- 89. Short PL. New Zealand plays to its strengths. Chem Eng News. 2007;85(4):20–21. [Google Scholar]
- 90. Cavicchioli R, Siddiqui KS, Andrews D, Sowers KR. Low-temperature extremophiles and their applications. Curr Opin Biotechnol. 2002;13(3):253–261. [DOI] [PubMed] [Google Scholar]
- 91. Banskota AH, McAlpine JB, Sorensen D, et al. Genomic analyses lead to novel secondary metabolites. Part 3 ECO-0501, a novel antibacterial of a new class. Antibiotics. 2006;59(9):533–542. [DOI] [PubMed] [Google Scholar]
- 92. Yu TW, Floss H. The ansamitocins In: Cragg GM, Kingston DGI, Newman DJ, eds. Anticancer Agents from Natural Products. Boca Raton, FL: Brunner-Routledge Psychology Press, Taylor & Francis Group; 2005;321–338. Chap 17. [Google Scholar]
- 93. Umemura K, Yanase K, Suzuki M, Okutani K, Yamori T, Andoh T. Inhibition of DNA topoisomerases I and II, and growth inhibition of human cancer cell lines by a marine microalgal polysaccharide. Biochem Pharmacol. 2003;66(3):481–487. [DOI] [PubMed] [Google Scholar]
- 94. Zeng S, Chen YZ, Fu L, Johnson KR, Fan W. In vitro evaluation of schedule-dependent interactions between docetaxel and doxorubicin against human breast and ovarian cancer cells. Clin Cancer Res. 2000;6(9):3766–3773. [PubMed] [Google Scholar]
- 95. Cadenas ME, Sandfrison A, Cutler NS, Heitman J. Signal transduction cascades as targets for therapeutic intervention by natural products. Trends Biotechnol. 1998;16(10):427–433. [DOI] [PubMed] [Google Scholar]
- 96. Ueda H, Nakajima H, Hori Y, Goto T, Okuhara M. Action of FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968 on Ha-Ras transformed NIH3T3 cells. Biosci Biotechnol Biochem. 1994;58(9):1579–1583. [DOI] [PubMed] [Google Scholar]
- 97. Tsuruo T, Oh-hara T, Iida H, et al. Rhizoxin, a macrocyclic lactone antibiotic, as a new antitumor agent against human and murine tumor cells and their vincristine-resistant sublines. Cancer Res. 1986;46(1):381–385. [PubMed] [Google Scholar]
- 98. Schweitzer J, Handley FG, Edwards J, et al. Summary of the workshop on drug development, biological diversity, and economic growth. J Natl Cancer Inst. 1991;83(18):1294–1298. [DOI] [PubMed] [Google Scholar]
- 99. Rinehart KL. Antitumor compounds from tunicates. Med Res Rev. 2000;20(1):1–27. [DOI] [PubMed] [Google Scholar]
- 100. Chun HG, Davies B, Hoth D, et al. Didemnin B—the first marine compound entering clinical trials as an antineoplastic agent. Invest New Drugs. 1986;4(3):279–284. [DOI] [PubMed] [Google Scholar]
- 101. Pathak S, Multani AS, Ozen M, Richardson MA, Newman RA. Dolastatin10 induces polyploidy, telomeric associations and apoptosis in a murine melanoma cell line. Oncol Rep. 1998;5(2):373–376. [DOI] [PubMed] [Google Scholar]
- 102. Williams PG, Asolkar RN, Kondratyuk T, Pezzuto JM, Jensen PR, Fenical W. Saliniketals A and B, bicyclic polyketides from the marine actinomycete Salinispora arenicola. J Nat Prod. 2007;70(1):83–88. [DOI] [PubMed] [Google Scholar]
- 103. Kwon HC, Kauffman CA, Jensen PR, Fenical W. Marinomycins A-D, antitumor-antibiotics of a new structure class from a marine actinomycete of the recently discovered genus “Marinispora”. J Am Chem Soc. 2006;128(5):1622–1632. [DOI] [PubMed] [Google Scholar]
- 104. Newman DJ, Cragg GM. Marine natural products and related compounds in clinical and advanced preclinical trials. J Nat Prod. 2004;67(8):1216–1238. [DOI] [PubMed] [Google Scholar]
- 105. Aoki S, Kong D, Matsui K, Kobayashi M. Erythroid differentiation in K562 chronic myelogenous cells induced by crambescidin 800, a pentacyclic guanidine alkaloid. Anticancer Res. 2004;24(4):2325–2330. [PubMed] [Google Scholar]
- 106. Gaitanos TN, Buey RM, Diaz JF, et al. Peloruside A does not bind to the taxoid site on beta-tubulin and retains its activity in multidrug-resistant cell lines. Cancer Res. 2004;64(15):5063–5067. [DOI] [PubMed] [Google Scholar]
- 107. Shim JS, Lee HS, Shin J, Kwon HJ. Psammaplin A, a marine natural product, inhibits aminopeptidase N and suppresses angiogenesis in vitro. Cancer Lett. 2004;203(2):163–169. [DOI] [PubMed] [Google Scholar]
- 108. Matsumoto SS, Biggs J, Copp BR, Holden JA, Barrows LR. Mechanism of ascididemin-induced cytotoxicity. Chem Res Toxicol. 2003;16(2):113–122. [DOI] [PubMed] [Google Scholar]
- 109. Dirsch VM, Muller IM, Eichhorst ST, et al. Cephalostatin 1 selectively triggers the release of Smac/DIABLO and subsequent apoptosis that is characterized by an increased density of the mitochondrial matrix. Cancer Res. 2003;63(24):8869–8876. [PubMed] [Google Scholar]
- 110. Schwartsmann G. Marine organisms and other novel natural sources of new cancer drugs. Ann Oncol. 2000;11(suppl 3):235–243. [DOI] [PubMed] [Google Scholar]
- 111. Poncet J. The dolastatins, a family of promising antineoplastic agents. Curr Pharm Des. 1999;5(3):139–162. [PubMed] [Google Scholar]
- 112. Pettit GR. The bryostatins. Fortschritte Chem Organ Nat. 1991;57:153–195. [DOI] [PubMed] [Google Scholar]
- 113. Kitagawa I, Kobayashi M. Antitumor marine natural products. Gan To Kagaku Ryoho. 1990;17(3 pt 1):322–329. [PubMed] [Google Scholar]
- 114. Dossey AT. Insects and their chemical weaponry: new potential for drug discovery. Nat Prod Rep. 2010;27(12):1737–1757. [DOI] [PubMed] [Google Scholar]
- 115. Slocinska M, Marciniak P, Rosinski G. Insect’s antiviral and anticancer peptides: new leads for the future? Protein Peptide Lett. 2008;15(6):578–585. [DOI] [PubMed] [Google Scholar]
- 116. Kokoza V, Ahmed A, Woon Shin S, Okafor N, Zou Z, Raikhel AS. Blocking of Plasmodium transmission by cooperative action of cecropin A and defensin A in transgenic Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A. 2010;107(18):8111–8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Park JH, Jeong YJ, Park KK, et al. Melittin suppresses PMA-induced tumor cell invasion by inhibiting NF-κB and AP-1-dependent MMP-9 expression. Mol Cells. 2010;29(2):209–215. [DOI] [PubMed] [Google Scholar]
- 118. Liu Z, Wu X, Wang J, et al. Molecular evidences for the biosynthesis of pederin by endosymbiont. Agr Sci China. 2009;8:1339–1350. [Google Scholar]
- 119. Izuta H, Chikaraishi Y, Shimazawa M, Mishima S, Hara H. 10-Hydroxy-2-decenoic acid, a major fatty acid from Royal Jelly, inhibits VEGF-induced angiogenesis in human umbilical vein endothelial cells. Evid Complement Altern Med. 2009;6(4):489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wang KR, Zhang BZ, Zhang W, Yan JX, Li J, Wang R. Antitumor effects, cell selectivity and structure activity relationship of a novel antimicrobial peptide polybia-MPI. Peptides. 2008;29(6):963–968. [DOI] [PubMed] [Google Scholar]
- 121. Arbiser JL, Kau T, Konar M, et al. Solenopsin, the alkaloidal component of the fire ant (Solenopsis invicta), is a naturally occurring inhibitor of phosphatidylinositol-3-kinase signaling and angiogenesis. Blood. 2007;109(2):560–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Rauh R, Kahl S, Boechzelt H, Bauer R, Kaina B, Efferth T. Molecular biology of cantharidin in cancer cells. Chin Med. 2007;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Shiga A, Kakamu S, Sugiyama Y, Shibata M, Makino E, Enomoto M. Acute toxicity of pierisin-1, a cytotoxic protein from Pieris rapae, in mouse and rat. J Toxicol Sci. 2006;31(2):123–137. [DOI] [PubMed] [Google Scholar]
- 124. Turati F, Rossi M, Pelucchi C, Levi F, La Vecchia C. Fruit and vegetables and cancer risk: a review of southern European studies. Br J Nutr. 2015;113(suppl 3):102–110. [DOI] [PubMed] [Google Scholar]
- 125. Ren M, Ye L, Hao X, et al. Polysaccharides from Tricholoma matsutake and Lentinus edodes enhance 5-fluorouracil-mediated H22 cell growth inhibition. J Trad Chin Med. 2014;34(3):309–316. [DOI] [PubMed] [Google Scholar]
- 126. Sudan S, Rupasinghe HP. Flavonoid-enriched apple fraction AF4 induces cell cycle arrest, DNA topoisomerase II inhibition, and apoptosis in human liver cancer HepG2 cells. Nutr Cancer. 2014;66(7):1237–1246. [DOI] [PubMed] [Google Scholar]
- 127. Chang HF, Wu CH, Yang LL. Antitumour and free radical scavenging effects of γ-mangostin isolated from Garcinia mangostana pericarps against hepatocellular carcinoma cell. J Pharm Pharmacol. 2013;65(9):1419–1428. [DOI] [PubMed] [Google Scholar]
- 128. Chavan SS, Manoj GD, Prashant BS, et al. Traditional medicinal plants for anticancer activity. Int J Curr Pharm Res. 2013;5(4):50–54. [Google Scholar]
- 129. Chen Y, Xu SS, Chen JW, et al. Anti-tumor activity of Annona squamosa seeds extract containing annonaceous acetogenin compounds. J Ethnopharmacol. 2012;142(2):462–466. [DOI] [PubMed] [Google Scholar]
- 130. Smoliga JM, Baur JA, Hausenblas HA. Resveratrol and health—a comprehensive review of human clinical trials. Mol Nutr Food Res. 2011;55(8):1129–1141. [DOI] [PubMed] [Google Scholar]
- 131. Govind P. Some important anticancer herbs: a review. Int Res J Pharm. 2011;2(7):45–52. [Google Scholar]
- 132. Yu MH, Im HG, Kim HI, Lee IS. Induction of apoptosis by immature plum in human hepatocellular carcinoma. J Med Food. 2009;12(3):518–527. [DOI] [PubMed] [Google Scholar]
- 133. Jo JY, De Mejia EG, Lila MA. Cytotoxicity of bioactive polymeric fractions from grape cell culture on human hepatocellular carcinoma, murine leukemia and non-cancerous PK15 kidney cells. Food Chem Toxicol. 2006;44(10):1758–1767. [DOI] [PubMed] [Google Scholar]
- 134. Ruiz D, Egea J, Tomas-Barberan FA, Gil MI. Carotenoids from new apricot (Prunus armeniaca L.) varieties and their relationship with flesh and skin color. J Agri Food Chem. 2005;53(16):6368–6374. [DOI] [PubMed] [Google Scholar]
- 135. Zhou Y, Ya L, Zhou T, Zheng J, Li S, Li HB. Dietary natural products for prevention and treatment of liver cancer. Nutrients. 2016;8(3):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Zhang CZ, Fang EF, Zhang HT, Liu LL, Yun JP. Momordica charantia lectin exhibits antitumor activity towards hepatocellular carcinoma. Invest New Drugs. 2015;33(1):1–11. [DOI] [PubMed] [Google Scholar]
- 137. Xiang J, Xiang Y, Lin S, et al. Anticancer effects of deproteinized asparagus polysaccharide on hepatocellular carcinoma in vitro and in vivo. Tumour Biol. 2014;35(4):3517–3524. [DOI] [PubMed] [Google Scholar]
- 138. Gupta P, Bansal MP, Koul A. Evaluating the effect of lycopene from Lycopersicum esculentum on apoptosis during NDEA induced hepatocarcinogenesis. Biochem Biophys Res Commun. 2013;434(4):479–485. [DOI] [PubMed] [Google Scholar]
- 139. Hafidh RR, Abdulamir AS, Bakar FA, Jalilian FA, Abas F, Sekawi Z. Novel molecular, cytotoxical, and immunological study on promising and selective anticancer activity of mung bean sprouts. BMC Complement Altern Med. 2012;12:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Fang EF, Pan WL, Wong JH, Chan YS, Ye XJ, Ng TB. A new Phaseolus vulgaris lectin induces selective toxicity on human liver carcinoma Hep G2 cells. Arch Toxicol. 2011;85(12):1551–1563. [DOI] [PubMed] [Google Scholar]
- 141. Friedman M, Lee KR, Kim HJ, Lee IS, Kozukue N. Anticarcinogenic effects of glycoalkaloids from potatoes against human cervical, liver, lymphoma, and stomach cancer cells. J Agric Food Chem. 2005;5 3(15):6162–6169. [DOI] [PubMed] [Google Scholar]
- 142. Sultana S, Ahmed S, Jahangir T, Sharma S. Inhibitory effect of celery seeds extract on chemically induced hepatocarcinogenesis: modulation of cell proliferation, metabolism and altered hepatic foci development. Cancer Lett. 2005;221(1):11–20. [DOI] [PubMed] [Google Scholar]
- 143. Kleijnen J, Knipschild P. Gingko biloba for cerebral insufficiency. Br J Clin Pharmacol. 1992;34(4):352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Li Y, Shi X, Zhang J, Zhang X, Martin RC. Hepatic protection and anticancer activity of curcuma: a potential chemopreventive strategy against hepatocellular carcinoma. Int J Oncol. 2014;44(2):505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Chang HS, Ko M, Ishizuka M, et al. Sodium 2-propenyl thiosulfate derived from garlic induces phase II detoxification enzymes in rat hepatoma H4IIE cells. Nutr Res. 2010;30(6):435–440. [DOI] [PubMed] [Google Scholar]
- 146. Chu YL, Ho CT, Chung JG, Raghu R, Lo YC, Sheen LY. Allicin induces anti-human liver cancer cells through the p53 gene modulating apoptosis and autophagy. J Agric Food Chem. 2013;61(41):9839–9848. [DOI] [PubMed] [Google Scholar]
- 147. Huang SP, Chen JC, Wu CC, et al. Capsaicin-induced apoptosis in human hepatoma HepG2 cells. Anticancer Res. 2009;29(1):165–174. [PubMed] [Google Scholar]
- 148. Vijaya PV, Arul DCS, Ramkuma KM. Induction of apoptosis by ginger in HEp-2 cell line is mediated by reactive oxygen species. Basic Clin Pharmacol Toxicol. 2007;100(5):302–307. [DOI] [PubMed] [Google Scholar]
- 149. Abdullaev FI. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.). Exp Biol Med. 2002;227(1):20–25. [DOI] [PubMed] [Google Scholar]
- 150. Lee YS. Role of NADPH oxidase-mediated generation of reactive oxygen species in the mechanism of apoptosis induced by phenolic acids in HepG2 human hepatoma cells. Arch Pharmacol Res. 2005;28(10):1183–1189. [DOI] [PubMed] [Google Scholar]
- 151. Tripathi YB, Tripathi B, Arjmandi BH. Nutraceuticals and cancer management. Front Biosci. 2005;10:1607–1618. [DOI] [PubMed] [Google Scholar]
- 152. Yang F, Oz HS, Barve S, de Villiers WJ, McClain CJ, Varilek GW. The green tea polyphenol (-)-epigallocatechin-3-gallate blocks nuclear factor-kappa B activation by inhibiting I kappa B kinase activity in the intestinal epithelial cell line IEC-6. Mol Pharmacol. 2001;60(3):528–33. [PubMed] [Google Scholar]
- 153. Al-Fatlawi AA, Irshad M, Zafaryab M, Zafaryab M, Rizvi MM, Ahmad A. Rice bran phytic acid induced apoptosis through regulation of Bcl-2/Bax and p53 genes in HepG2 human hepatocellular carcinoma cells. Asian Pac J Cancer Prev. 2014;15(8):3731–3736. [DOI] [PubMed] [Google Scholar]
- 154. Yang J, Li X, Xue Y, Wang N, Liu W. Anti-hepatoma activity and mechanism of corn silk polysaccharides in H22 tumor-bearing mice. Int J Biol Macromol. 2014;64:276–280. [DOI] [PubMed] [Google Scholar]
- 155. Xu WW, Li B, Lai ET, et al. Water extract from Pleurotus pulmonarius with antioxidant activity exerts in vivo chemoprophylaxis and chemosensitization for liver cancer. Nutr Cancer. 2014;66(6):989–998. [DOI] [PubMed] [Google Scholar]
- 156. Yukawa H, Ishikawa S, Kawanishi T, Tamesada M, Tomi H. Direct cytotoxicity of Lentinula edodes mycelia extract on human hepatocellular carcinoma cell line. Biol Pharm Bull. 2012;35(7):1014–1021. [DOI] [PubMed] [Google Scholar]
- 157. Jiang S, Chen Y, Wang M, et al. A novel lectin from Agrocybe aegerita shows high binding selectivity for terminal N-acetylglucosamine. Biochem J. 2012;443(2):369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Angeli JP, Ribeiro LR, Bellini MF, Mantovani MS. . β-glucan extracted from the medicinal mushroom Agaricus blazei prevents the genotoxic effects of benzo(α)pyrene in the human hepatoma cell line HepG2. Arch Toxicol. 2009;83(1):81–86. [DOI] [PubMed] [Google Scholar]
- 159. Lin JT, Liu WH. o-Orsellinaldehyde from the submerged culture of the edible mushroom Grifola frondosa exhibits selective cytotoxic effect against Hep 3B cells through apoptosis. J Agric Food Chem. 2006;54(20):7564–7569. [DOI] [PubMed] [Google Scholar]
- 160. Smith JE, Rowan NK, Sullivan R. Medicinal Mushrooms: Their Therapeutic Properties and Current Medical Usage with Special Emphasis on Cancer Treatments. London, United Kingdom: Cancer Research UK; 2000:220. [Google Scholar]
- 161. Hwang BY, Su BN, Chai H, et al. Silvestrol and episilvestrol, potential anticancer rocaglate derivatives from Aglaia silvestris. J Org Chem. 2004;69(10):3350–3358. [DOI] [PubMed] [Google Scholar]
- 162. Pecere T, Gazzola MV, Micignat C, et al. Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Res. 2000;60(11):2800–2804. [PubMed] [Google Scholar]
- 163. Whelan LC, Ryan MF. Effects of the polyacetylene capillin on human tumour cell lines. Anticancer Res. 2004;24(4):2281–2286. [PubMed] [Google Scholar]
- 164. Lee D, Bhat KPL, Fong HH, Farnsworth NR, Pezzuto JM, Kinghorn AD. Aromatase inhibitors from Broussonetia papyrifera. J Nat Prod. 2001;64(10):1286–1293. [DOI] [PubMed] [Google Scholar]
- 165. Cuendet M, Pezzuto JM. Antitumor activity of bruceantin. An old drug with new promise. J Nat Prod. 2004;67(2):269–272. [DOI] [PubMed] [Google Scholar]
- 166. Cragg GM, Newman DJ. Plants as a source of anticancer agents. J Ethnopharmacol. 2005;100(1-2):72–79. [DOI] [PubMed] [Google Scholar]
- 167. Banerjee S, Wang Z, Mohammad M, Sarkar FH, Mohammad RM. Efficacy of selected natural products as therapeutic agents against cancer. J Nat Prod. 2008;71(3):492–496. [DOI] [PubMed] [Google Scholar]
- 168. Hartwell JL. Plants used against cancer. Lloydia. 1969;32:158–176. [PubMed] [Google Scholar]
- 169. Silva GL, Cui B, Chavez D, et al. Modulation of the multidrug-resistance phenotype by new tropane alkaloid aromatic esters from Erythroxylum pervillei. J Nat Prod. 2001;64(12):1514–1520. [DOI] [PubMed] [Google Scholar]
- 170. Tyler V. Herbs of Choice. The Therapeutic Use of Phytomedicinals. New York, NY: Haworth Press; 1994:32–33. [Google Scholar]
- 171. Ambasta SP. The Useful Plant of India. 4th ed Delhi, India: National Institution of Science Communication; 2000:239. [Google Scholar]
- 172. Ambasta SP. The Useful Plant of India. 4th ed Delhi, India: National Institution of Science Communication; 2000:243. [Google Scholar]
- 173. Prajapati ND, Purohit S, Sharma AK, et al. Handbook of Medicinal Plants. 1st ed India: Agrobios; 2003. [Google Scholar]
- 174. Mizuno T, Shiitake (Lentinus edodes): functional properties for medicinal and food purposes. Food Rev Int. 1995;11:111–128. [Google Scholar]
- 175. Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58(11):1685–1693. [DOI] [PubMed] [Google Scholar]
- 176. Lee KH, Xiao Z. Podophyllotoxins and analogs In: Cragg GM, Kingston DGI, Newman DJ, eds. Anticancer Agents from Natural Products. Boca Raton, FL: Brunner-Routledge Psychology Press, Taylor & Francis Group; 2005:71–88. Chap 5. [Google Scholar]
- 177. Han D, Xu R. Progress in the research of blood activation and hemostasis removal. Abstr Chin Med. 1988;2:466–483. [Google Scholar]
- 178. Rašković A, Stilinović N, Kolarović J, Vasović V, Vukmirović S, Mikov M. The protective effects of silymarin against doxorubicin-induced cardiotoxicity and hepatotoxicity in rats. Molecules. 2011;16(10):8601–8613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Devi PU, Akagi K, Ostapenko V, Tanaka Y, Sugahara T. Withaferin A: a new radiosensitizer from the Indian medicinal plant Withania somnifera. Int J Rad Biol. 1996;69(2):193–197. [DOI] [PubMed] [Google Scholar]
- 180. Hecht S, Kenney PM, Wang P, et al. Evaluation of butylated hydroxyanisole, myo-inositol, curcumin, esculetin, resveratrol and lycopene as inhibitors of benzo[a]pyrene plus 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Lett. 1999;137(2):123–130. [DOI] [PubMed] [Google Scholar]
- 181. Pisha E, Chai H, Lee IS, et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med. 1995;1(10):1046–1051. [DOI] [PubMed] [Google Scholar]
- 182. Nipun D, Vijay S, Jaykumar B, et al. Antitumor activity of Dendrophthoe falcata against Ehrlich ascites carcinoma in Swiss albino mice. Pharm Crops. 2011;2:1–7. [Google Scholar]