Abstract
Introduction:
Islet transplantation can treat the most severe cases of type 1 diabetes (T1D) but it currently requires deceased donor pancreata as an islet source and chronic immunosuppression to prevent rejection and recurrence of autoimmunity. Stem cell-derived insulin-producing cells may address the shortage of organ donors while cell encapsulation may reduce or eliminate the requirement for immunosuppression, minimizing the risks associated with the islet transplantation procedure, and potentially prolonging graft survival.
Areas covered:
This review focuses on the design principles for immunoisolation devices and on stem cell differentiation into insulin-producing cell products. The reader will gain understanding of the different types of immunoisolation devices and the key parameters that affect the outcome of the encapsulated graft. Progresses in stem cell differentiation towards mature endocrine islet cells, including the most recent clinical trials and the challenges associated with the application of immunoisolation devices designed for primary islets to stem-cell products are also discussed.
Expert opinion:
Recent advancements in the field of stem cell-derived islet cell products and immunoisolation strategies hold great promise for T1D. However, a combination product including both cells and an immunoisolation strategy still needs to be optimized and tested for safety and efficacy.
Keywords: islet transplantation, macroencapsulation, microencapsulation, hydrogels, vascular supply, scaffold
1. Introduction
Three hundred eighty-seven million people are estimated to have diabetes, with 4.9 million deaths attributed to the disease conditions in 2014. In 2013, more than 79,000 children developed type-1 diabetes (T1D)1. T1D is an autoimmune disease in which beta cells within pancreatic islets are selectively destroyed by autoimmune responses against beta cell autoantigens2. The pathophysiology of beta cell destruction in T1D has been previously reviewed3–6. Beta cells are responsible for secreting insulin, which regulates glucose metabolism and homeostasis. Currently, patients with T1D depend on exogenous insulin injections but insulin injections do not prevent acute and chronic complications of T1D, which can be life-threatening4. Further, severe hypoglycemia (hypo) is commonly experienced by patients as a consequence of exogenous insulin injections. Hypo unawareness accounts for 6 to 10% of all deaths in T1D. Despite the widespread use of novel insulin analogues, pump therapy, and glucose sensors, hypo unawareness persists7. Restoring normoglycemia without increasing the risk of severe hypo would have substantial implications for the well being of individuals with T1D. Sensor-augmented pump therapy with automated insulin suspension reduced the combined rate of severe and moderate hypo in patients with T1D8. A wearable, automated, bi-hormonal, bionic pancreas improved mean glycemic levels, with less frequent hypo episodes, among both adults and adolescents with T1D, as compared with an insulin pump9. However, such devices do not represent a cure for T1D and they are susceptible to wearing out and to breakage.
Replacement of β cell function through transplantation of whole pancreas represents a possible biological cure. The endocrine component of the pancreas constitutes only ~1% of the pancreas. Therefore, in order to restore euglycemia, transplanting just the endocrine cells, rather than the whole pancreas may represent a simpler but equally efficacious procedure and it may decrease the complications arising from whole pancreas transplantation. Since Ricordi et al. first described an automated method that allowed isolation of human pancreatic islets with high yield, purity and with preserved response to glucose stimulation10, 11, clinical trials on transplantation of human islets initiated12.
Currently, adult islets are isolated from the pancreas of deceased donors and infused into the liver following percutaneous transhepatic catheterization of the portal vein of recipients with T1D. The minimally invasive procedure is generally performed under local anesthesia by interventional radiology. Currently, chronic immunosuppression of the recipients is required to prevent allograft rejection and recurrence of autoimmunity. Further, availability of organ donors is far inferior to the number of patients than may benefit from islet transplantation in the future13. The results of clinical islet transplantation have improved considerably in the past two decades11, 14–16. Annual reports of the CITR (collaborative islet transplant registry) concluded that islet transplantation resulted in improved long-term insulin independence and associated benefits, including normal or near normal HbA1C levels, sustained decrease in severe hypoglycemic episodes and the return of hypoglycemia awareness17.
Despite progresses in the islet isolation, transplantation and immunosuppression regimes, islet engraftment and long-term function is far from being ideal11. Additionally, side effects associated with chronic immunosuppression include the increased susceptibility to infection, renal dysfunction, hyperlipidemia, anemia, mouth ulcers, and increased risk of cancer. Due to the risks associated with the procedure and the chronic immunosuppression, islet transplantation is currently indicated only for a small percentage of T1D patients and only for adults. The main challenges that remain to be addressed in order to make islet transplantation available for a larger number of subjects with T1D are:
Avoidance of chronic immunosuppression, either by tolerance induction or by immunoisolation strategies
Identification of an unlimited source of insulin producing cells
Identification of a suitable transplantation site that optimizes islet engraftment and long-term function
This review will focus mainly on islet immunoisolation through encapsulation as a strategy to decrease and potentially eliminate immunosuppression, on stem cells as a potentially unlimited source of insulin-producing cells, and on the selection and engineering of most appropriate site.
2. Cell immunoisolation through encapsulation
Currently, islet graft survival is limited by several factors, including autoimmune and allogeneic responses that are not completely prevented by immunosuppression. Furthermore, immunosuppression is the main cause of adverse events in islet transplantation18. Eliminating chronic immunosuppression in islet transplantation may increase the safety of the islet transplantation procedure and make it a therapeutic option for a larger number of T1D patients.
Immunoisolation of pancreatic islets through encapsulation may allow transplantation without immunosuppression19. However, this concept has yet to be translated into reality despite three decades of research20. In encapsulation devices, immunoisolation is achieved by enclosing pancreatic islets within biocompatible and permeable capsules. The permeability of such capsules should allow exchange of nutrients, electrolytes, oxygen, waste products, bio-therapeutic agents, and smaller molecules like insulin through the capsules, while blocking the passage of high-molecular weight substances, such as large complement complexes and cytotoxic cells of the immune response21. Immunoisolation devices can be classified into macrodevices and microcapsules depending on whether multiple islets or single islets, respectively, are enclosed within the same capsule and on the size of the device, millimeters vs. microns, respectively.
2.1. Macrodevices
Macroencapsulation consists in entrapping multiple pancreatic islets (up to the full dose) within a device that has macroscopic sizes (larger than 1 mm). In macrodevices, the immunoisolation membrane encapsulates the entire islet graft, rather than single islets22. To reverse diabetes, adequate numbers of islets need to be implanted within the device. Because of the device immunoisolation feature, the host vasculature, which carries oxygen and nutrients and allows insulin secretion in response to hyperglycemia, is allowed to grow up to the immunoisolation membrane. As a result, the distance between the islet core and the closest blood vessel equals d/2 (d=device thickness). Because solute transport through macrodevices relies mainly on diffusion, in order to maximize the transport, d needs to be minimized. Additionally, because islets inside the device consume oxygen and nutrients at a rate that is proportional to the islet density, there is an inverse correlation between the islet loading density and d. To accommodate curative numbers of islets without exceeding the recommended islet loading density, while minimizing d, the surface area of the device exceeds what is reasonable for implantation in a patient. To address such size limitations, several modification have been implemented to improve transport of critical molecules like oxygen to the islets entrapped in the device23.
One approach to address the limitations associated with oxygen transport, is to supply the device with oxygen. In the work by Barkai et al., the device was supplied with exogenous oxygen at such a pressure that guarantees optimal (oxygen supply equals oxygen consumption by islets) oxygen partial pressure in the islet space24. The macrodevice was a disk-shaped polyether ether ketone (PEEK) with 31.3 mm diameter × 7 mm height dimensions. Inside the device, a 11.5 mm diameter islet module containing 2000IEQ islets was fabricated by suspending the islets within ultrapure, high guluronic acid (68%) alginate, UP-MVG hydrogels. Next to the islet module, the oxygen gas module was placed. A custom-fabricated 25-μm-thick Silon interpenetrating network of an oxygen-permeable polydimethylsiloxane and polyetherfluoroethylene membrane was placed to separate the gas module from the islet module. A hydrophylized PTFE membrane with a pore size of 0.2 μm was used as immunoisolation membrane and it was placed between the device and the host. To provide mechanical support to the composite device, a metal grid was placed on each side of the islet module. Based on successfully studies in preclinical models, a pilot trial was performed in one patient by Ludwig et al.25, showing c-peptide response to glucose challenges up to 6-months and decrease in HbA1c, despite insulin independency was not achieved. Building on these promising results, a phase I safety/efficacy study has been started In October 2014 by BetaO2 Technologies to evaluate the safety and efficacy of implanting the ßAir macro-encapsulation with human islets.
Another macrodevice, the Theracyte device was designed with an outer membrane that facilitates neovascularization and an inner immunoprotective membrane that provided protection against alloimmunity, even in allosensitized recipients in rodent preclinical models of islet transplantation. However, the curative dose of transplanted macroencapsulated islets to reverse diabetes in preclinical models was 10 times higher than the curative dose of non-encapsulated islets, mainly due to the lack of sufficient vascularization of the freshly implanted device and to transport limitations26. In addition to transport limitations, macrodevices like the Theracyte device generally induced heavy fibrotic responses at the device-host interface27, 28. To minimize host responses, the Theracyte device has been modified by Viacyte into the Encaptra® Drug Delivery System. The Encaptra device was designed and manufactured to guarantee long-term biocompatibility and biostability in the subcutaneous (SC) space while providing 100% encapsulation of βeta cell progenitor cells (to prevent cell escape and teratoma formation), protecting against alloimmunity and autoimmunity. The SC site was chosen as transplant site for the Encaptra device because it can be easily accessed for graft monitoring and for retrieval. The EN250 device, which contains an approximate volume of 250μl and has a size of 3×8 cm, is currently being tested in a Phase I/II trial only in conjunction with islet progenitor cells (as discussed in section 4).
2.2. Microcapsules
In microencapsulation, each islet or cell cluster is individually encapsulated, offering several advantages over macroencapsulation. The concept of immunoisolation through microencapsulation and the key capsule design parameters are illustrated in Fig. 1. Unlike macrodevices, microcapsules (because of their spherical geometry) allow maximizing the surface area/volume ratio, maximizing the transport of critical solutes like oxygen and nutrients through the capsule29. Additionally, because sharp surfaces (corners) on biomaterials worsen the host inflammatory reactions30, the spherical geometry of the microcapsules minimizes foreign body reactions. Since Chang first described the concept in 196431, microencapsulation has been used with a variety of cell types, including PC12 cells for the treatment of Parkinson’s disease32, hepatocytes for the treatment of liver diseases33, cells genetically modified to secrete factor IX for the treatment of haemophilia B34 and to secrete growth hormones for the treatment of dwarfism35. Lim and Sun in 1980 first microencapsulated pancreatic islets in alginate beads for the treatment of T1D36. Since then, alginate microencapsulation has been evaluated in several pre-clinical trials in rodents, dogs, pigs, non-human primates, and in few human pilot trials20. Despite three decades of research, encapsulation systems haven’t been clinically successful and the reasons for failure are unknown20, 42, 43.
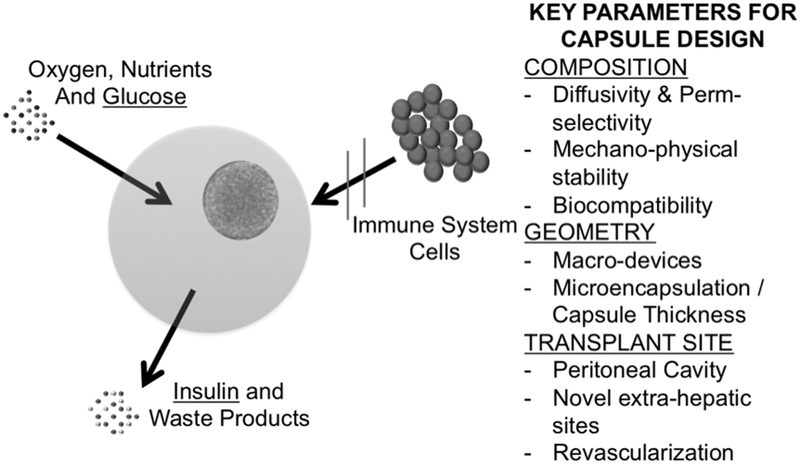
Fig. 1.
Schematic of cell immunoisolation through microencapsulation for transplantation without immunosuppression. Before transplantation isolated islets are completely enclosed by a capsule. The capsule material allows transport of oxygen, nutrients, cytokines, glucose, insulin and waste products through the capsule. The capsule prevents contact between the enclosed cells and the host immune cells (immunoisolation), in turn preventing immunorejection. Key parameters that directly affect capsule performances are indicated.
Capsule composition, geometry and transplantation site are three of the main parameters for capsule design. It is likely that these design parameters are critical determinants of the in vitro and in vivo performance of encapsulated islets and non-optimal combinations of these key capsule parameters may have contributed to failure of encapsulated grafts19, 44–48. A better understanding of the influence of capsule composition, geometry, and transplantation site on capsule performance and underlying mechanisms may help identify the parameters that lead to successful outcomes of the encapsulated graft. Capsule design parameters are reviewed below.
2.3. Capsule design criteria
2.3.1. Influence of Capsule Composition on biocompatibility, permselectivity and stability
Due to their high water content and three-dimensional structure, hydrogels formed by crosslinking natural or synthetic monomers are commonly used for microencapsulation. The hydrophilic nature of hydrogels prevents damage to surrounding tissues after implantation. Additionally, transparency of hydrogels allows easy visualization of the encapsulated cells49. A variety of hydrogel monomers have been investigated during the past thirty years22, including polyethylene glycol (PEG), polyurethane, polyacrylates, chitosan, cellulose, xanthan gum, and alginate (ALG).
Polyacrylates such as hydroxyethyl methacrylate (HEMA) and methyl methacrylate (MMA) form stable capsules, but diffusion of water-soluble nutrients and long-term viability of enclosed cells is limited50.
Alginate (ALG) is a natural anionic polysaccharide, isolated from algae22. ALG is constituted by linear copolymers of (1–4)-linked β-D-mannuronate (M) and C-5 epimer α-L-guluronate (G) residues, covalently linked together in different blocks. The ratio and sequence of the uronic acid groups depends on the source of the ALG51 and affects the properties of the ALG hydrogels52. During gelation, the G-blocks of one polymeric chain form junctions with the G-blocks of the adjacent polymer chain (egg-box model of crosslinking). Ion binding and affinity is selective and it depends on the ALG composition (Ba2+ ions bind to G-G and M-M blocks, whereas Ca2+ binds to G-G and M-G blocks, and Sr2+ binds only to G-G blocks). Higher affinity of cations for the ALG residues is associated with stronger gels. ALG is by far the most common hydrogel for encapsulation because it is thermo-stable and can form hydrogels rapidly and under physiological conditions54. Moreover, no ALG-degrading enzymes have been reported in humans so far, supporting long-term implantation. Long-term survival of allogeneic60–64 and xenogeneic65–67 islets encapsulated in ALG devices without immunosuppression has been achieved in preclinical models. The first human trial with ALG microcapsules and immunosuppression demonstrated that encapsulated islets could provide a glycemic control similar to non-encapsulated islets transplanted in the portal vein in a T1D patient68. Almost 12 years later, Calafiore carried out a phase I trial using human islets encapsulated in ALG-PLO and transplanted in the peritoneal cavity (IP) without immunosuppression69. Although this study proved that allografting of encapsulated islets is safe, only a minor clinical benefit was observed. In another phase I trial with human islets encapsulated within Ba2+ ALG microcapsules and transplanted IP without immunosuppression, neither insulin requirement nor glycemic control was improved. A biopsy confirmed that the loss of graft function was due to a combination of islet central necrosis and inflammation70. The large discrepancies in preclinical studies with ALG-encapsulated islets from different groups prevents investigators from understanding the reasons for clinical failure of ALG encapsulation. The lack of consistency in alginate encapsulation may be attributed to (i) the absence of standardization between laboratories71, (ii) the intrinsic variability in pancreatic islets, (iii) the variability in the ALG composition37, (iv) the lack of extensive capsule characterization (composition, molecular weight, purity, permselectivity, mechanical stability, surface properties, biocompatibility), and (v) the variability in the transplantation site.
PEG is a polyether composed of repeating units of ethylene glycol. PEG has been used for the encapsulation of a broad range of cell types, including pancreatic islets72–75, chondrocytes76, osteoblasts77, and mesenchymal stem cells78. For islet encapsulation, PEG hydrogels have been used in the layer-by-layer, pegylation, and conformal coating technologies, with promising preclinical results79–82. PEG have some advantages over other synthetic molecules that also form hydrogels because PEG molecules can be easily coupled to functional peptides to mimic the extracellular matrix and to improve survival and function of encapsulated cells83 and to provide local immunomodulation. Another major advantage of PEG hydrogels is the low protein adsorption on PEG surfaces84. Addition of PEG to the encapsulation material can increase the durability and the mechanical properties, and decrease the permeability of the capsules85, 86. Finally, pilot clinical studies showed that PEG capsules are safe20.
Capsule biocompatibility depends on the capsule chemical composition, surface charge, porosity, surface roughness, implant site and shape, among all87. Porosity of hydrogels (like ALG and PEG) is determined by the microarchitecture of the hydrogel network that represents physical impediments to transport of solutes. Poor biocompatibility results in a host reaction to the biomaterials leading to formation of a fibrotic capsule. The inflammatory response towards a poorly biocompatible material starts with the adsorption of cell adhesion proteins, immunoglobulins, complement components, and growth factors on the surface of the capsules. Macrophages recognizes adsorbed proteins, adhere to the capsule surface of the biomaterials and secrete inflammatory cytokines, such as interleukin-1β (IL-1β), tumor necrosis factor (TNF−α) and transforming growth factor (TGF-β), further activating macrophages and fibroblasts88. The activation of macrophages and fibroblasts leads to the cellular overgrowth on the capsule89. Such fibrotic deposition decreases transport through the capsule and interferes with adequate nutrition of the encapsulated cells, leading to necrosis of the enclosed cells. Further, the fibroblasts accumulating on the capsule surface compete with islets for oxygen and nutrient supplies. Finally, the complement system may also be activated by the chemical characteristics of the capsule surface, and by inadequate permselectivity, further activating immune cells87.
Biocompatibility of capsule compositions should be evaluated in parallel to safety and efficacy studies in preclinical models. In vivo methods to evaluate biocompatibility include the implantation of up to five times the curative dose of biomaterials in the same site where capsules are placed in efficacy studies. Experienced pathologists should score the biocompatibility of each composition by histological evaluation of explanted cell-free grafts at early time points, to evaluate acute reactions and after long-term implantation, to evaluate chronic reactions. Alternative in vitro assays are being evaluated and include quantifying macrophage adhesion and activation92, fibrinogen adsorption, fibroblast adhesion and proliferation, and granulocyte activation after exposure to cell-free biomaterials93.
Capsule permselectivity should maintain cell viability and function while protecting against the host cytotoxic cells. While immune cells can be excluded from the capsule, cytokines secreted by activated immune cells, which are deleterious to islets, have a low molecular weight (comparable to critical nutrients) and they can diffuse through the capsules and reach the islets48. The permselectivity of the capsules depends on the balance between the mass transport and the molecular weight cutoff (MWCO) of the immunoisolation membrane94. It was suggested for the ideal MWCO to be in the 50–150kDa range49. Permeability of ALG capsules is determined by a combination of type and concentration of ALG and the type of cations53. It is known that ALG is permeable to Immunoglobulin G (IgG, 150 kDa) and to complement molecules. To reduce ALG permeability controlling its permselectivity, polyanion-polycation membranes have been integrated within ALG capsules. As ALG are negatively charged, ALG polymers form strong complexes with polycations such as polysaccharides (e.g. chitosan55), polypeptides (e.g. poly-L-lysine, PLL56 and poly-L-ornithine, PLO57) or synthetic polymers (e.g. polymethylene-co-guanidine58 and polyethylene-imine59). As these complexes are stable in the presence of non-gelling cations or calcium chelators, unlike ALG, they have been extensively used to reduce the permselectivity of ALG gels. However, the composite capsules made of polyanion-polycation membranes resulted in worse biocompatibility96. While to prevent allorejection microcapsules do not need to prevent diffusion of antibodies and cytokines97, higher permselectivity may be required to prevent xenorejection62. Because capsule permselectivity, and in particular the permeability to immunological products are the main determinants of capsule immunoisolation, immunoisolation membranes should be thoroughly characterized and permselectivity should be tailored to achieve the desired amount of immunoprotection. Unlike ALG, synthetic materials like PEG-based hydrogels offer better control of permselectivity because they can be modified to match the desired permselectivity values79.
For long-term implantation capsules need to display optimal mechanical stability98. For ALG capsules, stability depends on the type of ALG (relative content of G-blocks), its concentration, and the type and concentration of gelling cations99. Failure of conventional ALG microcapsules after transplantation has been associated with their poor stability. ALG capsules swell during long-term exposure to physiological conditions. The swelling is caused by chelation of the gel network by phosphate, citrate and lactate, or non-gelling cations, such as sodium and magnesium. While capsule swelling leads to increased porosity (Fig. 2C.1)101, capsule disruption results in exposure of the transplanted cells to the host immune cells (Fig. 2C.2)102. Additionally, shear forces associated with the implantation procedure and the mechanical environment of the transplant site may further damage the microcapsules103.
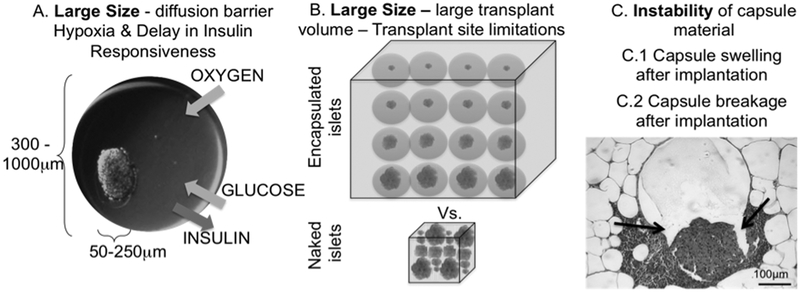
Fig. 2.
Schematic of limitations associated with traditional alginate (ALG) microencapsulation. A. Diffusion limitations imposed by large capsule size (600–1000μm) that results in core hypoxia, central necrosis and delayed insulin secretion in response to glucose; B. Large volumes of encapsulated cells that do not allow implantation in sites that might be more favorable to islet cell engraftment but that can accommodate only small volumes. 3. Instability of capsules that causes change in permselectivity and breakage after implantation, leading to rejection of enclosed cells.
Capsule stability properties have been determined through bulk measurements, which do not reflect the properties of single capsules45. Experimental and computational tools for measuring mechanical properties of single capsules at the microscopic level (i.e. the atomic force microscopy and computational models) are available and they can be exploited to compare capsules of different composition and geometries.
2.3.2. Influence of capsule geometry on molecular transport
Capsule geometry refers to the combination of shape (irregular vs. spherical), thickness, overall size, distance of enclosed cells from the capsule surface and the volume of cell-free space within the capsule. Traditional microcapsules are made of fixed-diameter spheres of hydrogel materials. Since islets have variable sizes (50 to 400μm in diameter) and microcapsules need to be large enough to include even the largest islets, traditional microcapsules range in size from 600 to 1000 μm; most of the volume is actually islet-free and biologically non-functional material44. Transport of oxygen, nutrients and waste products from and to the tissue surrounding the capsules is critical for the function and survival of encapsulated islets. Since transport through the capsule is mainly regulated by passive diffusion, such large amounts of islet-free bulk capsule material are a barrier for diffusion of critical solutes, leading to core hypoxia and necrosis (Fig. 2A)104, 105. More importantly, large diffusion barriers will hamper the transport of glucose and insulin, leading to a delay in glucose sensing and insulin responsiveness of the encapsulated islets106. Large diffusion barriers resulting from the large size of traditional capsules may help explain why traditional islet encapsulation has failed in maintaining glucose homeostasis in patients transplanted with microencapsulated islets69, 107–109.
2.3.3. Influence of capsule geometry on choice of transplant site
The large size of traditional capsules increases the volume of transplantation materials up to 100 times over the volume of naked islets (Fig. 2B)75. To reverse diabetes in patients, up to one million islets need to be ideally transplanted, which would amount to volumes in the hundreds of milliliters20. Such large volumes can be transplanted only IP. The peritoneal cavity is not an islet-friendly environment110. After IP transplant, capsules fall by gravity and aggregate in the lower abdomen. Additionally, foreign body reactions to poorly biocompatible capsule materials cause the formation of a thick layer of fibrotic tissue. Both packing of the capsules and the fibrosis further impair transport of critical molecules111. Consequently, higher numbers of islets are required to reverse diabetes when islets are transplanted IP versus other sites, such as the intrahepatic site in patients and the kidney capsule in mice and rats. Currently, islets for transplantation are isolated from cadaveric pancreata. Since organ availability is limited, minimizing the islet dose per patient would make islet transplantation available to a larger number of patients105. However, this is not possible with large traditional capsules implanted in the IP site.
3. Choice of Islet Transplantation site
Currently, islets are transplanted intraportally. Hepatic embolization of islets exposes them to a relatively hypoxic environment because the liver has a parenchymal oxygen tension that is inferior to that of the pancreas53. More importantly, due to the ‘instant blood-mediated inflammatory reaction’ (IBMIR) 50–75% of the transplanted islet mass is lost right after transplantation because of the suboptimal environment provided by the liver transplant site9. Among the influences that can cause early graft loss is the poor re-vascularization (that causes lack of glucose homeostasis and islet hypoxia) and the higher concentration of immunosuppressive toxic drugs in the portal vein, which can impair angiogenesis and islet proliferation113.
Alternative transplant sites have been long sought to improve islet engraftment and long-term function. Design criteria for alternative sites are aimed at minimizing early inflammatory reactions, promoting a well-vascularized microenvironment (to guarantee exchange of oxygen and nutrients, pH homeostasis and cell waste removal), gaining easy-access to the transplant site (for minimally invasive surgery and follow-up) and protecting the islet graft from immune responses (inflammation and immune rejection)113. Among the sites that have been explored are the skin, the pancreas, the submandibular gland, the muscle, the omentum, the bone marrow, the gastric submucosa, the genitourinary tract, the kidney capsule, the anterior eye chamber, the testis, the spleen, the brain, and the thymus13.
Among those alternative sites, the SC site is easily accessible and it minimizes the invasiveness of both the transplant and the follow-up monitoring procedures. Additionally, the SC site offers an extensive surface area for transplantation. However, results in the SC site have been disappointing, for reasons that may include poor oxygen tension and slow re-vascularization13.
Another alternative site, the omental pouch (OP) site is well vascularized and provides portal drainage, which is desirable for physiological effects of insulin on the liver, and it can accommodate large graft volumes114. The omentum is a thin and highly vascularized membrane. Islets can be placed on top of the omentum in close contact with blood vessels. Also, the omentum can be wrapped after placing the islets, forming a pouch, which provides mechanical protection to the islets. At the Diabetes Research Institute we are currently exploring the omentum as an alternative islet transplantation site to the liver in a phase I/II trial of allogeneic islet transplantation with systemic immunosuppression. In such trial, a biodegradable scaffold is generated by adding recombinant human thrombin to islets that have been previously resuspended in autologous plasma, forming a plasma clot. The biodegradable scaffold is utilized to immobilize the islets to the omentum, in close contact with host vascular beds, and to prevent aggregation. Our preclinical data in a surrogate pouch in mice, the epididymal fat pad, support the beneficial effect of immobilizing islets to vascularized membrane and confining the graft in a pouch115.
The gastric submucosal space (GSMS) has also been explored because it allows localized implantation, portal venous drainage and it can be easily accessed for monitoring, allowing localized imaging and graft biopsy116. In a preclinical trial in pigs, minimal immediate loss of islets following transplantation in the GSMS vs. the portal vein and significant reductions in mean blood glucose and mean exogenous insulin requirement were observed.
Poor islet revascularization after transplantation is one of the major impediments to islet engraftment and long-term function117. Native islets in the pancreas are highly vascularized by the fenestrated endothelium that is found throughout the islet core and that receives 15–20% of the total pancreatic blood supply while comprising only 1–2% of the total pancreatic mass118. The high degree of native islet vascularization is rarely recapitulated in isolated and re-transplanted islets23. To augment islet vascularization several approaches have been undertaken. Pro-angiogenic gene transduction or protein delivery has shown benefits in preclinical models119, but cannot be easily translated because of many of the poor efficacy of protein delivery and the safety concerns associated with exogenous gene expression. Co-delivery of progenitor or endothelial cells has shown promises in augmenting islet vascularization120.
Devices and scaffolds have been designed to provide mechanical support and promote angiogenesis in the transplantation site. Engineering the islet transplant microenvironment has improved islet engraftment and long-term function in extrahepatic sites121, 122. However, biocompatible non-degradable biomaterials can generate foreign body responses and fibrotic capsules that reduce diffusion of oxygen and nutrients, impairing glucose homeostasis. Further, synthetic devices can activate the innate and adaptive immune response and trigger graft rejection. Alternatively, fibrin matrices are natural scaffolds and completely degrade days after implantation through cell-mediated degradation and they are gradually replaced by autologous tissues. In addition, fibrin matrices have been shown to be beneficial for islet culture123.
Encapsulated islets cannot get fully revascularized after transplantation because of the immunoisolation membrane between the islets and the host. The lack of direct vascular access of encapsulated islets limits the exchange of glucose / insulin and the exchange of nutrients / metabolic waste to passive diffusion through the immunoisolation membrane. Such diffusion limitations are worsened when encapsulated islets are transplanted in sites that do not get revascularized after transplantation. Therefore, especially for transplantation of encapsulated islets, the choice of transplantation site is a critical determinant of the outcome of the encapsulated graft.
4. Stem cells as sources of insulin-producing cells
Pancreatic islets isolated from cadaveric donor pancreata are the current source for islet transplantation. Donors shortage requires seeking alternative and inexhaustible sources of insulin-producing cells. Xenotransplantation of islets from pigs is a valuable alternative source of primary islets. However, xenorejection will require a combination of immunoisolation devices and chronic immunosuppression.
Human islet-like cell clusters have been generated from
stem cells isolated from the cord blood, the amniotic fluid, the adipose tissue, the endometrial and menstrual blood
embryonic pancreatic precursors
fetal and neonatal progenitor cells;
differentiated stromal tissue, either by 4.1 transdifferentiation and tissue reprogramming, or by 4.2 epigenetic conversion124.
The challenges associated with differentiation of stem cells into fully mature beta cells125 and of regenerating beta cells126 have been previously reviewed.
Embryonic stem (ES) cells are self-renewing and pluripotent cells that can differentiate into any cell type. Differentiation of ES cells into beta cells has been poorly successful, due to the intrinsic nature of beta cells and the natural resistance of ES cells to differentiate into beta cells.
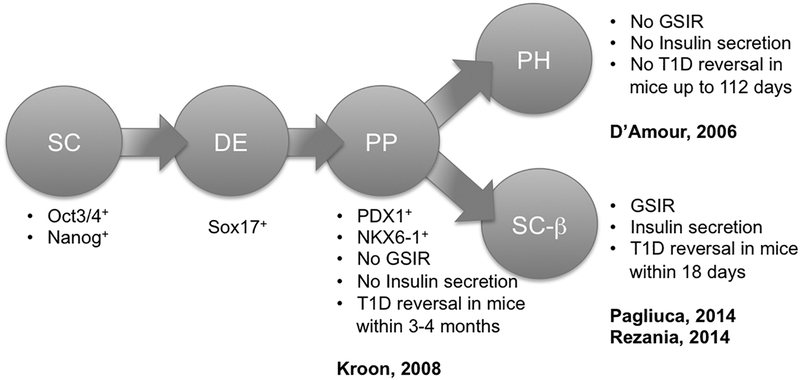
In 2006, Novocell (currently Viacyte, San Diego, CA, USA) first proved that differentiation of human ES cells into beta cells is feasible, but only 5–7% of the total cell population could be fully transformed and, despite displaying beta cell phenotype, the islet-like clusters derived with their protocol did not secrete insulin in response to glucose stimulation (no GSIR) and did not reverse hyperglycemia after transplantation in diabetic mice127. Viacyte protocol was based on sequential differentiation of human ES cells into mesoderm, definite endoderm, primitive gut, posterior foregut, pancreatic endoderm, followed by final differentiation into endocrine precursors and β cells (polyhormonal, PH, Fig. 3). On the other hand, a different strategy that aimed at transplanting only partially differentiated human ES cells, at the pancreatic endoderm stage, rather than fully matured into beta cells, efficiently generated glucose-responsive (GSIR) endocrine cells 3 to 4 months after implantation into diabetic mice. ES cells differentiated into pancreatic endoderm (PE, Fig. 3) and transplanted into mice protected against streptozotocin (STZ)-induced hyperglycemia and showed serum C-peptide that was comparable to 3000 IEQ human islets128. At graft retrieval, ES-derived beta cells demonstrated expression of critical beta cell transcription factors and presence of pro-insulin and mature endocrine secretory granules. These important studies suggest that functional beta cells that reverse hyperglycemia and maintain glucose homeostasis can be efficiently generated by a 1st differentiation phase in vitro and a 2nd final maturation phase in vivo where the in vivo microenvironment is critical for promoting full maturation of beta cells. The question remains on whether the human microenvironment is as conductive to beta cells maturation as the murine microenvironment. Viacyte has performed extensive preclinical testing on this particular cell product based on differentiation of human ES cells in the pancreatic endoderm to assure reliable production of a safe and effective product. Following successful results of the preclinical testing, in August 2014 ViaCyte received approval from the U.S. Food and Drug Administration (FDA) to begin evaluation in a Phase I/II trial of their stem cell-derived cells in combination with the Encaptra macroencapsulation device.
Fig. 3.
Schematic summarizing the most recent efforts in differentiating stem cells into beta cells. SC: stem cell; DE: definitive endoderm; PP: pancreatic progenitors; PH: polyhormonal; SC-b: stem cell-derived functional beta cells; GSIR: glucose-stimulated insulin release; T1D: type-1 diabetes
Following the first generation of ES cell-derived beta cells that require 3–4 months maturation in vivo to express functional characteristics of bona fide β cells and maintain glucose homeostasis in vivo, several groups have attempted to optimize in vitro maturation of stem cell-derived beta cells to higher degrees of maturation. The overall goal of these strategies was to generate a stem cell-based product that could reverse hyperglycemia right after transplantation in diabetic recipients. Additional goal was to generate a cell product that could be fully characterized before implantation, addressing safety and efficacy concerns of what happens to the cells after implantation. Almost at the same time the Melton’s group at Harvard129 and the Kieffer’s group at the University of British Columbia in partnership with BetaLogics Venture (Janssen R&D LLC)130 succeeded in developing protocols to differentiate stem cells into functional beta cells with higher maturity than the ES-derived cells from Viacyte.
The Melton group used a scalable suspension-based culture system that can generate up to 108 human pluripotent stem cells and that can be differentiated into hundreds of millions of glucose-responsive beta cells in vitro (SC-β, Fig. 3). SC-β are able to secrete quantities of insulin comparable to adult β cells (GSIR). Furthermore, SC-β prevented the development of hyperglycemia when they were transplanted in pre-diabetic and immunodeficient Akita mice, which develop progressive hyperglycemia as a result of a defective insulin gene.
The Kieffer’s group described a seven-stage protocol that efficiently converts human ES cells into insulin-producing cells. Differentiated cells expressed key markers of mature pancreatic β cells, including MafA, and displayed GSIR similar to that of human islets during static incubations in vitro. Despite single-cell imaging and dynamic glucose stimulation assays revealed some differences with primary human β cells, converted cells reversed diabetes in mice four times faster than pancreatic progenitors (PP, Fig. 3).
5. Expert opinion
Encapsulation may eliminate immunosuppression in allogeneic cell transplantation, increasing the safety and the efficacy of the procedure. Clinical failure of cell encapsulation in the past three decades may be attributed to the limited understanding of the mechanisms of graft failure. A summary of current clinical trials has been presented by Scharp D. et al.20 and more recently by Yang H.K. et al.41 We suggest that a different approach to islet encapsulation needs to be undertaken with the aim to identify more successful encapsulation strategies. Our recent data suggest that the choice of capsule composition, geometry, and transplantation sites are the main key factors in determining the performance of encapsulated islets75. An integrated evaluation of the specific contributions of these three capsule parameters defining success vs. failure of encapsulated pancreatic islets needs to be performed to provide insight into the mechanisms of graft failure that may occur for specific combinations of capsule composition, geometry and transplantation site and to identify the specific role of these three key capsule parameters independently analyzed. More importantly, such a study will help identify the most promising combinations of capsule parameters to reduce graft failure rates and increase the likelihood of success of future clinical studies with primary islets and with stem cell-derived insulin-secreting cell products.
The optimal combination of cell source and immunoisolation device needs to be carefully selected based on safety concerns and the metabolic requirements of the selected cell product, which may vary after implantation. Islet cell precursors with the potential to proliferate, evading the immune surveillance and possibly forming tumors, should be transplanted in a device and in a site that can guarantee stable and complete confinement of the transplanted cells. Additionally, non-invasive monitoring of the graft is desirable to monitor graft size and prevent possible breakages of the device in case of undesired growth. Because immature islet-precursors are expected to mature in vivo, an increased metabolic activity is expected to develop over time, paralleling an increased requirement for oxygen and nutrients by the progressively differentiating transplanted cells. The current clinical trials will be of assistance to define the relevance of these challenges, which are difficult to be fully evaluated in preclinical model systems. Ideally, instead of transplanting beta cell precursors, whose fate is unknown, we should aim at transplanting mature beta cells to the end of increasing safety of the procedure and enhancing efficacy.
A multi-functional platform may be necessary to support encapsulated cell-based products. Such platform should comprise:
A three-dimensional scaffold that provides mechanical support to the cells and prevents cell clumping. Such scaffold should allow blood vessels to grow around immunoisolated cells, minimizing the distance between the core of islet-like cell clusters and the blood supply. Such scaffolds could be either resorbable (e.g. plasma clot) or permanent (e.g., silicone-based)
In situ oxygen generation to support the transplanted cells before re-vascularization occurs. Additionally, in case of a macrodevice, a permanent oxygen supply (like the Beta-O2 design) could be necessary for cells that are farther away from the blood supply. Ideally, such platform should allow for modulation of oxygen delivery proportionally to the increasing metabolic requirements of the transplanted cell products (i.e., undifferentiated vs. differentiated cells)
Local delivery of anti-inflammatory and immunomodulatory agents, to dampen any acute foreign body response to biomaterials and prevent formation of a fibrotic capsule around the device. Additionally, local immunomodulation may be desirable to minimize indirect pathways of immune activation, following antigen shedding which cannot be blocked by immunoisolation strategies and that could lead to allosensitization131. A promising approach may be targeting PDL-1, as prviously shown by Guleria132. Alternatively, helper cells, like mesenchymal stem cells (MSC)133 or cord blood-derived134 or hematopoietic135 stem cells could be co-transplanted to provide local immunomodulation and pro-angiogenic effects
A suitable transplantation site, that would allow for physiological delivery of insulin (intraportal) and for retrievability of the implanted cell products, in case of adverse events. Additionally, the capsule can be functionalized with islet extracellular matrix-mimetic additives to provide further protection against autoimmunity136 and for recapitulating signaling interactions between islet cells and extracellular matrix, which are known regulators of islet survival, proliferation, and insulin secretion.
We have developed a conformal coating technology that allows for modulation of capsule composition, geometry and transplant site through microfluidics75. Unlike traditional ALG microencapsulation based on generation of fixed-size capsules with the electrostatic droplet generator, this method allows to control capsule size by adjusting microfluidic parameters, including minimizing the capsule size to a few tens of microns (vs. hundreds of microns of traditional capsules, Fig. 3). Further, the conformal coating method can be adapted for most coating hydrogels, including ALG, to obtain capsules with different physical and biological properties, determined by the specific hydrogels utilized for coating. Compared to other conformal coating technologies, including layer by layer81 and pegylation-based encapsulation80, the conformal coating technology does not require direct binding of the capsule to the islet surface that may compromise the integrity of the islet membrane, which is critical for promoting proper function of the islet and for protecting against autoimmunity136. Since conformal capsules are not covalently bound to the cells, the conformal coating technology does not impair critical cellular functions and survival. We believe that the conformal coating technology is a valuable platform for designing immunoisolation of different sources of insulin-secreting cells.
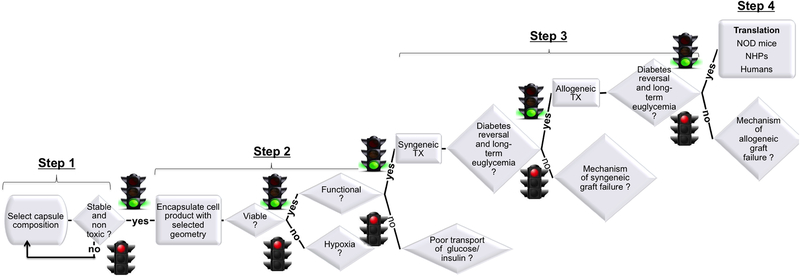
The necessary steps that need to be undertaken to evaluate safety and efficacy of an encapsulated stem cell product for beta cell replacement in type-1 diabetes are summarized in the flow chart depicted in Fig. 4. As we learnt from preclinical development of primary islet encapsulation products, in step 1 the composition of acellular capsules should be defined and its non-toxicity and its stability should be confirmed. In step 2 viability and functionality of encapsulated insulin-secreting cell products should be confirmed and mechanisms of poor viability (central hypoxia?) and poor functionality (poor transport of glucose/insulin?), if any, should be investigated and properly addressed by modifying the combination of capsule composition and geometry. In step 3 the efficacy of the selected combination of capsule composition and geometry should be evaluated in syngeneic and allogeneic cell transplantation models in chemically induced diabetic mice; mechanism of syngeneic graft failure, if any, should be investigated by histological comparison (inflammation? vascularization?) of functioning and non-functioning grafts and confirmed by studies in transgenic mice (or with compounds that target the specific pathways); mechanism of allogeneic graft failure should be investigated by histological comparison (immunoisolation?) of functioning and non-functioning grafts and confirmed by ex vivo studies that quantify antigen shedding through the capsules and capsule mechanical stability before and after implantation. In step 4 the safety and efficacy of the selected capsule combinations should be evaluated in the allo and autoimmune NOD mouse model of T1D (to confirm capsule protection from recurrence of autoimmunity) and in the alloimmune model in non-human primates (NHPs) before performing clinical trials in humans. Alternative strategies to the ones discussed in this review include genetic engineering of human pluripotent stem cells to match the human leukocyte antigen genes and prevent allorejection138, so that capsule role would focus just on preventing recurrence of autoimmunity after transplantation.
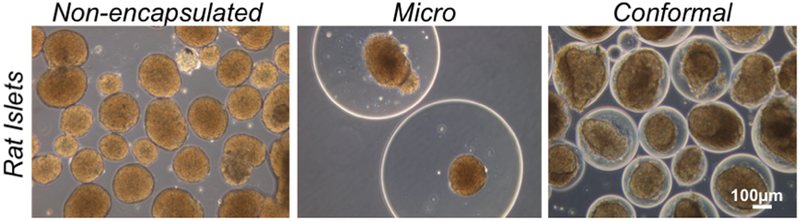
Fig. 4.
Phase contrast microscope images of rat islets encapsulated with conformal capsules vs. microcapsules vs. non-encapsulated.
Fig. 5.
Flowchart illustrating the step-wise strategy that we suggest should be undertaken for evaluating safety and efficacy of a new encapsulated stem cell product for T1D. TX: transplantation; NOD: non-obese diabetic mice; NHP: non-human primates.
Highlight box:
Islet transplantation may restore beta cell function in type-1 diabetic patients but islet sourcing and safety concerns about chronic immunosuppression are currently limiting the procedure to the most severe cases of type-1 diabetes
Immunoisolation of insulin-secreting cells can reduce and ideally eliminate chronic immunosuppression but additional studies are required to identify the reasons of previous failures of the encapsulated islet grafts in clinical trials and to design more effective immunoisolation devices for future clinical trials
We suggest that understanding the specific effects of the composition, geometry and transplantation site for the immunoisolation devices on the outcome of the encapsulated islet graft will allow designing more effective immunoisolation devices
Recent advances in generating mature insulin-secreting cell products from stem cells that rapidly reverse diabetes after transplantation in preclinical models represent a great leap forward in the field of beta cell sourcing
Combining immunoisolation devices for primary islets with stem cell-derived insulin secreting cell products will require tailoring the properties of such devices (composition, geometry, transplantation site) to the new cell source to guarantee safety, in addition to efficacy of the final combination product.
Acknowledgments
We apologize to those colleagues whose work we could not cited because of the 100 references limit.
Financial and competing interests disclsoure
Dr. Tomei and Dr. Ricordi are co-inventors of Intellectual Property discussed in this review and licensed to Converge Biotech. Dr. Tomei and Dr. Ricordi have a financial interest and stand to gain royalties from the commercialization of the Intellectual Property. Dr. Ricordi is a member of the scientific advisory board and an equity owner in Converge Biotech, licensee of some of the intellectual property used in some of the described studies. Funding was provided by philanthropic funds from the Diabetes Research Institute Foundation, grants from the Juvenile Diabetes Research Foundation (grant # 17-2001-268, 17-2010-5 and 17-2012-361), Converge Biotech, Inc. (Miami, FL, USA), BioRep Technologies, the Fondazione Tronchetti Provera, the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico and the National Institute of Health (grant # 5U01DK070460–08 and 5U01DK070431–10). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Bibliography
- 1.Federation ID. IDF Diabetes ATLAS, 2014.
- 2.Pugliese A Advances in the etiology and mechanisms of type 1 diabetes. Discovery medicine 2014. September;18(98):141–50.* recent review on pathogenesis of type-1 diabetes
- 3.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 2006. February 14;103(7):2334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiological reviews 2011. January;91(1):79–118.** comprehensive review on type-1 diabetes
- 5.Pugliese A The multiple origins of Type 1 diabetes. Diabetic medicine: a journal of the British Diabetic Association 2013. February;30(2):135–46. [DOI] [PubMed] [Google Scholar]
- 6.Bogdani M, Korpos E, Simeonovic CJ, Parish CR, Sorokin L, Wight TN. Extracellular matrix components in the pathogenesis of type 1 diabetes. Current diabetes reports 2014. December;14(12):552.* Importance of islet extracellular matrix in type-1 diabetes pathogenesis
- 7.Tanenberg RJ, Newton CA, Drake AJ. Confirmation of hypoglycemia in the “dead-in-bed” syndrome, as captured by a retrospective continuous glucose monitoring system. Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists 2010. Mar-Apr;16(2):244–8. [DOI] [PubMed] [Google Scholar]
- 8.Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. Jama 2013. September 25;310(12):1240–7. [DOI] [PubMed] [Google Scholar]
- 9.Russell SJ, El-Khatib FH, Sinha M, Magyar KL, McKeon K, Goergen LG, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. The New England journal of medicine 2014. July 24;371(4):313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes 1988. April;37(4):413–20.** The first report describing the Ricordi chamber
- 11.Piemonti L, Pileggi A. 25 Years of the Ricordi Automated Method for Islet Isolation. CellR4 2013;1(1):e128. [PMC free article] [PubMed] [Google Scholar]
- 12.Tzakis AG, Ricordi C, Alejandro R, Zeng Y, Fung JJ, Todo S, et al. Pancreatic islet transplantation after upper abdominal exenteration and liver replacement. Lancet 1990. August 18;336(8712):402–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibly RF, Graham JG, Luo X, Lowe WL Jr., Hering BJ, Shea LD. Advancing islet transplantation: from engraftment to the immune response. Diabetologia 2011. October;54(10):2494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricordi C Islet transplantation: a brave new world. Diabetes 2003. July;52(7):1595–603. [DOI] [PubMed] [Google Scholar]
- 15.Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nature reviews Immunology 2004. April;4(4):259–68. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. The New England journal of medicine 2000. July 27;343(4):230–8.** The first report on the Edmonton protocol of clinical islet transplantation
- 17.Collaborative Islet Transplant Registry Coordinating Center - The EMMES Coproration R, MD. Seventh Annual Report. 2010. [Google Scholar]
- 18.Bruni A, Gala-Lopez B, Pepper AR, Abualhassan NS, Shapiro AJ. Islet cell transplantation for the treatment of type 1 diabetes: recent advances and future challenges. Diabetes, metabolic syndrome and obesity : targets and therapy 2014;7:211–23.** the most recent report on clinical islet transplantation
- 19.Basta G, Calafiore R. Immunoisolation of pancreatic islet grafts with no recipient’s immunosuppression: actual and future perspectives. Current diabetes reports 2011. October;11(5):384–91.** comprehensive review of islet encapsulation
- 20.Scharp DW, Marchetti P. Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution. Advanced drug delivery reviews 2014. April;67–68:35–73.** comprehensive review of islet encapsulation, including preclinical and clinical trials of polyethylene glcyol(PEG)-based conformal coatings
- 21.Ryan EA, Bigam D, Shapiro AM. Current indications for pancreas or islet transplant. Diabetes, obesity & metabolism 2006. January;8(1):1–7. [DOI] [PubMed] [Google Scholar]
- 22.de Vos P, Lazarjani HA, Poncelet D, Faas MM. Polymers in cell encapsulation from an enveloped cell perspective. Advanced drug delivery reviews 2014;67–68:15–34. [DOI] [PubMed] [Google Scholar]
- 23.Van Schilfgaarde R, De Vos P. Factors influencing the properties and performance of microcapsules for immunoprotection of pancreatic islets. J Mol Med 1999;77:199–205. [DOI] [PubMed] [Google Scholar]
- 24.Barkai U, Weir GC, Colton CK, Ludwig B, Bornstein SR, Brendel MD, et al. Enhanced oxygen supply improves islet viability in a new bioartificial pancreas. Cell transplantation 2013;22(8):1463–76. [DOI] [PubMed] [Google Scholar]
- 25.Ludwig B, Reichel A, Steffen A, Zimerman B, Schally AV, Block NL, et al. Transplantation of human islets without immunosuppression. Proc Natl Acad Sci U S A 2013. November 19;110(47):19054–8.** First human trial with Beta-O2 device
- 26.Kumagai-Braesch M, Jacobson S, Mori H, Jia X, Takahashi T, Wernerson A, et al. The TheraCyte device protects against islet allograft rejection in immunized hosts. Cell transplantation 2013;22(7):1137–46.** Preclinical study confirming alloprotection of the Theracyte macrodevice even in allosensitized hosts
- 27.Rafael E, Wernerson A, Arner P, Tibell A. In vivo studies on insulin permeability of an immunoisolation device intended for islet transplantation using the microdialysis technique. European surgical research Europaische chirurgische Forschung Recherches chirurgicales europeennes 1999;31(3):249–58. [DOI] [PubMed] [Google Scholar]
- 28.Uludag H, De Vos P, Tresco PA. Technology of mammalian cell encapsulation. Advanced drug delivery reviews 2000. August 20;42(1–2):29–64. [DOI] [PubMed] [Google Scholar]
- 29.Brendel MH B; Schulz A; Bretzel R. International Islet Transplant Registry report.: Giessen, Germany: University of Giessen; 1999. [Google Scholar]
- 30.Spector M, Cease C, Tong-Li X. The local tissue response to biomaterials. Crit Rev Biocomp 1989;5:269–95. [Google Scholar]
- 31.Chang TM. Semipermeable Microcapsules. Science (New York, NY) 1964. October 23;146(3643):524–5.** this was the first publication discussing the potential of cell encapsulation
- 32.Winn SR, Tresco PA, Zielinski B, Greene LA, Jaeger CB, Aebischer P. Behavioral recovery following intrastriatal implantation of microencapsulated PC12 cells. Exp Neurol 1991;113:322–29. [DOI] [PubMed] [Google Scholar]
- 33.Legallais C, Doré DE. Bioartificial livers (BAL): Current technological aspects and future developments. J Membrane Sci 2001;181(181):81–54. [Google Scholar]
- 34.Hortelano G, Xu N, Vandenberg A, Solera J, Chang PL, Ofosu FA. Persistent delivery of factor IX in mice: gene therapy for hemophilia using implantable microcapsules. Hum Gene Ther 1999;10:1281–88. [DOI] [PubMed] [Google Scholar]
- 35.Cheng WT, Chen BC, Chiou ST, Chen CM Use of nonautologous microencapsulated fibroblasts in growth hormone gene therapy to improve growth of midget swine. Hum Gene Ther 1998(9):1995–2003. [DOI] [PubMed] [Google Scholar]
- 36.Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science (New York, NY) 1980. November 21;210(4472):908–10.** This was the first report on alginate microencapsulation of pancreatic islets for type-1 diabetes
- 37.Jacobs-Tulleneers-Thevissen D, Chintinne M, Ling Z, Gillard P, Schoonjans L, Delvaux G, et al. Sustained function of alginate-encapsulated human islet cell implants in the peritoneal cavity of mice leading to a pilot study in a type 1 diabetic patient. Diabetologia 2013. July;56(7):1605–14. [DOI] [PubMed] [Google Scholar]
- 38.Opara EC, McQuilling JP, Farney AC. Microencapsulation of pancreatic islets for use in a bioartificial pancreas. Methods in molecular biology 2013;1001:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dufrane D, Gianello P. Macro- or microencapsulation of pig islets to cure type 1 diabetes. World journal of gastroenterology: WJG 2012. December 21;18(47):6885–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santos E, Pedraz JL, Hernandez RM, Orive G. Therapeutic cell encapsulation: ten steps towards clinical translation. Journal of controlled release: official journal of the Controlled Release Society 2013. August 28;170(1):1–14. [DOI] [PubMed] [Google Scholar]
- 41.Yang HK, Yoon KH. Current status of encapsulated islet transplantation. Journal of diabetes and its complications 2015. April 6. [DOI] [PubMed] [Google Scholar]
- 42.Calafiore R, Basta G. Clinical application of microencapsulated islets: Actual prospectives on progress and challenges. Advanced drug delivery reviews 2013. November 1.** recent review on clinical islet encapsulation
- 43.Rabanel JM, Banquy X, Zouaoui H, Mokhtar M, Hildgen P. Progress technology in microencapsulation methods for cell therapy. Biotechnology progress 2009. Jul-Aug;25(4):946–63. [DOI] [PubMed] [Google Scholar]
- 44.Paredes Juarez GA, Spasojevic M, Faas MM, de Vos P. Immunological and technical considerations in application of alginate-based microencapsulation systems. Frontiers in bioengineering and biotechnology 2014;2:26.** recent review on alginate microencapsulation
- 45.Rokstad AM, Lacik I, de Vos P, Strand BL. Advances in biocompatibility and physico-chemical characterization of microspheres for cell encapsulation. Advanced drug delivery reviews 2013. July 20.** comprehensive review on characterization of immunosiolation devices
- 46.Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Current diabetes reports 2011. October;11(5):364–74.** recent review of extrahepatic sites for islet transplantation
- 47.Schweicher J, Nyitray C, Desai TA. Membranes to achieve immunoprotection of transplanted islets. Frontiers in bioscience (Landmark edition) 2014;19:49–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colton CK. Implantable biohybrid artificial organs. Cell transplantation 1995. Jul-Aug;4(4):415–36. [DOI] [PubMed] [Google Scholar]
- 49.Li RH. Materials for immunoisolated cell transplantation. Advanced drug delivery reviews 1998. August 3;33(1–2):87–109. [DOI] [PubMed] [Google Scholar]
- 50.Wang RN, Rosenberg L. Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. The Journal of endocrinology 1999. November;163(2):181–90. [DOI] [PubMed] [Google Scholar]
- 51.Carlsson PO, Palm F, Andersson A, Liss P. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes 2001. March;50(3):489–95. [DOI] [PubMed] [Google Scholar]
- 52.Strand BL, Mörch YA, Skjåk-Bræk G. Alginate as immobilization matrix for cells. Minerva Biotecnologica 2001;12:223–33. [Google Scholar]
- 53.Carlsson PO, Palm F, Andersson A, Liss P. Chronically decreased oxygen tension in rat pancreatic islets transplanted under the kidney capsule. Transplantation 2000. March 15;69(5):761–6. [DOI] [PubMed] [Google Scholar]
- 54.Draget KI, Skjak-Braek G, Smidsrod O. Alginate based new materials. International journal of biological macromolecules 1997. August;21(1–2):47–55. [DOI] [PubMed] [Google Scholar]
- 55.Gaserod O, Smidsrod O, Skjak-Braek G. Microcapsules of alginate-chitosan--I. A quantitative study of the interaction between alginate and chitosan. Biomaterials 1998. October;19(20):1815–25. [DOI] [PubMed] [Google Scholar]
- 56.Thu B, Bruheim P, Espevik T, Smidsrod O, Soon-Shiong P, Skjak-Braek G. Alginate polycation microcapsules. II. Some functional properties. Biomaterials 1996. June;17(11):1069–79. [DOI] [PubMed] [Google Scholar]
- 57.Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C, Luca G, et al. Preparation and in vitro and in vivo characterization of composite microcapsules for cell encapsulation. International journal of pharmaceutics 2006. October 31;324(1):27–36. [DOI] [PubMed] [Google Scholar]
- 58.Lacik I, Brissova M, Anilkumar AV, Powers AC, Wang T. New capsule with tailored properties for the encapsulation of living cells. Journal of biomedical materials research 1998. January;39(1):52–60. [DOI] [PubMed] [Google Scholar]
- 59.Orive G, Hernandez RM, Gascon AR, Igartua M, Pedraz JL. Survival of different cell lines in alginate-agarose microcapsules. European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences 2003. January;18(1):23–30. [DOI] [PubMed] [Google Scholar]
- 60.Soon-Shiong P, Desai NP, Sanford PA, Heitz R, Sojomihardjo S. Crosslinkable polysaccharides, polycations and lipids useful for encapsulation and drug release. Patent PCT/US92/09364 World International Property Organization; 1993:1–52. [Google Scholar]
- 61.Mazaheri R, Atkison P, Stiller C, Dupre J, Vose J, O’Shea G. Transplantation of encapsulated allogeneic islets into diabetic BB/W rats. Effects of immunosuppression. Transplantation 1991. April;51(4):750–4. [DOI] [PubMed] [Google Scholar]
- 62.Duvivier-Kali VF, Omer A, Parent RJ, O’Neil JJ, Weir GC. Complete protection of islets against allorejection and autoimmunity by a simple barium-alginate membrane. Diabetes 2001. August;50(8):1698–705.** remarkable report on complete immunoisolation against allo and autoimmunity in mice by alginate microencapsulation
- 63.Omer A, Duvivier-Kali V, Fernandes J, Tchipashvili V, Colton CK, Weir GC. Long-term normoglycemia in rats receiving transplants with encapsulated islets. Transplantation 2005. January 15;79(1):52–8. [DOI] [PubMed] [Google Scholar]
- 64.Wang T, Adcock J, Kuhtreiber W, Qiang D, Salleng KJ, Trenary I, et al. Successful allotransplantation of encapsulated islets in pancreatectomized canines for diabetic management without the use of immunosuppression. Transplantation 2008. February 15;85(3):331–7. [DOI] [PubMed] [Google Scholar]
- 65.Lanza RP, Jackson R, Sullivan A, Ringeling J, McGrath C, Kuhtreiber W, et al. Xenotransplantation of cells using biodegradable microcapsules. Transplantation 1999. April 27;67(8):1105–11. [DOI] [PubMed] [Google Scholar]
- 66.Lum ZP, Krestow M, Tai IT, Vacek I, Sun AM. Xenografts of rat islets into diabetic mice. An evaluation of new smaller capsules. Transplantation 1992. June;53(6):1180–3. [DOI] [PubMed] [Google Scholar]
- 67.Sun Y, Ma X, Zhou D, Vacek I, Sun AM. Normalization of diabetes in spontaneously diabetic cynomologus monkeys by xenografts of microencapsulated porcine islets without immunosuppression. The Journal of clinical investigation 1996. September 15;98(6):1417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soon-Shiong P, Heintz RE, Merideth N, Yao QX, Yao Z, Zheng T, et al. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet 1994. April 16;343(8903):950–1.* clinical trial with encapsulated islets
- 69.Calafiore R, Basta G, Luca G, Lemmi A, Montanucci MP, Calabrese G, et al. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: first two cases. Diabetes care 2006. January;29(1):137–8.* clinical trial with encapsulated islets
- 70.Bracho-Blanchet AC, Ramírez-González B, López-Santos MG, León-Mancilla BH, and Dorantes LM. Long-term follow-up of patients with type 1 diabetes trans-planted with neonatal pig islets. Clin Exp Immunol 2010;62:537–42.* clinical trial with encapsulated islets
- 71.Gala-Lopez B, Pepper AR, Shapiro JAM. Biologic agents in islet transplantation. Curr Diab Rep 2013;13(5):713–22. [DOI] [PubMed] [Google Scholar]
- 72.Cruise GM, Hegre OD, Scharp DS, Hubbell JA. A sensitivity study of the key parameters in the interfacial photopolymerization of poly(ethylene glycol) diacrylate upon porcine islets. Biotechnology and bioengineering 1998. March 20;57(6):655–65. [DOI] [PubMed] [Google Scholar]
- 73.Cruise GM, Scharp DS, Hubbell JA. Characterization of permeability and network structure of interfacially photopolymerized poly(ethylene glycol) diacrylate hydrogels. Biomaterials 1998. July;19(14):1287–94.* First report of polyethlyene glycol (PEG) conformal coating based on interfacial photopolymerization
- 74.Hill RS, Cruise GM, Hager SR, Lamberti FV, Yu X, Garufis CL, et al. Immunoisolation of adult porcine islets for the treatment of diabetes mellitus. The use of photopolymerizable polyethylene glycol in the conformal coating of mass-isolated porcine islets. Annals of the New York Academy of Sciences 1997. December 31;831:332–43. [DOI] [PubMed] [Google Scholar]
- 75.Tomei AA, Manzoli V, Fraker CA, Giraldo J, Velluto D, Najjar M, et al. Device design and materials optimization of conformal coating for islets of Langerhans. Proc Natl Acad Sci U S A 2014. June 30.* First report of polyethlyene glycol (PEG) conformal coating based on microfluidics
- 76.Bryant SJ, Bender RJ, Durand KL, Anseth KS. Encapsulating chondrocytes in degrading PEG hydrogels with high modulus: engineering gel structural changes to facilitate cartilaginous tissue production. Biotechnology and bioengineering 2004. June 30;86(7):747–55. [DOI] [PubMed] [Google Scholar]
- 77.Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials 2002. November;23(22):4315–23. [DOI] [PubMed] [Google Scholar]
- 78.Nuttelman CR, Tripodi MC, Anseth KS. In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in PEG hydrogels. Journal of biomedical materials research Part A 2004. March 15;68(4):773–82. [DOI] [PubMed] [Google Scholar]
- 79.Tomei AA, Manzoli V, Fraker CA, Giraldo J, Velluto D, Najjar M, et al. Device design and materials optimization of conformal coating for islets of Langerhans. Proc Natl Acad Sci U S A 2014. July 22;111(29):10514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee DY, Park SJ, Nam JH, Byun Y. A new strategy toward improving immunoprotection in cell therapy for diabetes mellitus: long-functioning PEGylated islets in vivo. Tissue engineering 2006. March;12(3):615–23. [DOI] [PubMed] [Google Scholar]
- 81.Gattas-Asfura KM, Stabler CL. Bioorthogonal layer-by-layer encapsulation of pancreatic islets via hyperbranched polymers. ACS applied materials & interfaces 2013. October 23;5(20):9964–74.* Layer by layer nanocoatings
- 82.Rengifo HR, Giraldo JA, Labrada I, Stabler CL. Long-term survival of allograft murine islets coated via covalently stabilized polymers. Advanced healthcare materials 2014. July;3(7):1061–70.PEGylation nanocoatings
- 83.Stendahl JC, Kaufman DB, Stupp SI. Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation. Cell transplantation 2009;18(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sawhney AS, Pathak CP, Hubbell JA. Interfacial photopolymerization of poly(ethylene glycol)-based hydrogels upon alginate-poly(l-lysine) microcapsules for enhanced biocompatibility. Biomaterials 1993. October;14(13):1008–16. [DOI] [PubMed] [Google Scholar]
- 85.Chen P, Chu IU, Shiao SY, Fu J. Microencapsulation of islets in PEG-amine modified alginate–poly(l-lysine)–alginate microcapsules for constructing bioartificial pancreas. Ferment Bioeng 1998;86:185–90. [Google Scholar]
- 86.O’Shea GM, Sun AM. Encapsulation of rat islets of langerhans prolongs xenograft survival in diabetic mice Diabetes 1986;35:943–46. [DOI] [PubMed] [Google Scholar]
- 87.Mikos AG, McIntire LV, Anderson JM, Babensee JE. Host response to tissue engineered devices. Advanced drug delivery reviews 1998. August 3;33(1–2):111–39. [DOI] [PubMed] [Google Scholar]
- 88.Rihova B. Immunocompatibility and biocompatibility of cell delivery systems. Advanced drug delivery reviews 2000. August 20;42(1–2):65–80. [DOI] [PubMed] [Google Scholar]
- 89.Narang AS, Mahato RI. Biological and biomaterial approaches for improved islet transplantation. Pharmacological reviews 2006. June;58(2):194–243. [DOI] [PubMed] [Google Scholar]
- 90.de Vos P, Faas MM, Strand B, Calafiore R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials 2006. November;27(32):5603–17. [DOI] [PubMed] [Google Scholar]
- 91.De Vos P, De Haan BJ, Wolters GH, Strubbe JH, Van Schilfgaarde R. Improved biocompatibility but limited graft survival after purification of alginate for microencapsulation of pancreatic islets. Diabetologia 1997. March;40(3):262–70. [DOI] [PubMed] [Google Scholar]
- 92.Charissoux JL, Najid A, Moreau JC, Setton D, Rigaud M. Development of in vitro biocompatibility assays for surgical material. Clinical orthopaedics and related research 1996. May(326):259–69. [DOI] [PubMed] [Google Scholar]
- 93.Lloyd AW, Dropcova S, Faragher RG, Gard PR, Hanlon GW, Mikhalovsky SV, et al. The development of in vitro biocompatibility tests for the evaluation of intraocular biomaterials. Journal of materials science Materials in medicine 1999. Oct-Nov;10(10/11):621–7. [DOI] [PubMed] [Google Scholar]
- 94.Brissova M, Petro M, Lacik I, Powers AC, Wang T. Evaluation of microcapsule permeability via inverse size exclusion chromatography. Analytical biochemistry 1996. November 1;242(1):104–11. [DOI] [PubMed] [Google Scholar]
- 95.Lacik I Polymer chemistry in diabetes treatment by encapsulated islets of Langerhans: review to 2006. Aust J Chem 59 2006;59(8):508–24. [Google Scholar]
- 96.de Vos P, Spasojevic M, de Haan BJ, Faas MM. The association between in vivo physicochemical changes and inflammatory responses against alginate based microcapsules. Biomaterials 2012. August;33(22):5552–9. [DOI] [PubMed] [Google Scholar]
- 97.Soon-Shiong PFE, Nelson R, Heintz R, Merideth N, Sandford P, Zheng T, Komtebedde J. Long-term reversal of diabetes in the large animal model by encapsulated islet transplantation. Transplant Proc 1992;24(6):2946–47. [PubMed] [Google Scholar]
- 98.Schmidt JJ, Rowley J, Kong HJ. Hydrogels used for cell-based drug delivery. Journal of biomedical materials research Part A 2008. December 15;87(4):1113–22. [DOI] [PubMed] [Google Scholar]
- 99.Morch YA, Donati I, Strand BL, Skjak-Braek G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 2006. May;7(5):1471–80. [DOI] [PubMed] [Google Scholar]
- 100.Darrabie MD, Kendall WF, Opara EC. Effect of alginate composition and gelling cation on microbead swelling. Journal of microencapsulation 2006. September;23(6):613–21. [DOI] [PubMed] [Google Scholar]
- 101.O’Sullivan ES, Vegas A, Anderson DG, Weir GC. Islets transplanted in immunoisolation devices: a review of the progress and the challenges that remain. Endocrine reviews 2011. December;32(6):827–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Vos P, Bucko M, Gemeiner P, Navratil M, Svitel J, Faas M, et al. Multiscale requirements for bioencapsulation in medicine and biotechnology. Biomaterials 2009. May;30(13):2559–70. [DOI] [PubMed] [Google Scholar]
- 103.Thanos CG, Calafiore R, Basta G, Bintz BE, Bell WJ, Hudak J, et al. Formulating the alginate-polyornithine biocapsule for prolonged stability: evaluation of composition and manufacturing technique. Journal of biomedical materials research Part A 2007. October;83(1):216–24. [DOI] [PubMed] [Google Scholar]
- 104.Williams SJ, Huang HH, Kover K, Moore W, Berkland C, Singh M, et al. Reduction of diffusion barriers in isolated rat islets improves survival, but not insulin secretion or transplantation outcome. Organogenesis 2010. Apr-Jun;6(2):115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Buchwald P FEM-based oxygen consumption and cell viability models for avascular pancreatic islets. Theoretical biology & medical modelling 2009;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Buchwald P A local glucose-and oxygen concentration-based insulin secretion model for pancreatic islets. Theoretical biology & medical modelling 2011;8:20.* clinical islet encapsulation
- 107.Elliott RB, Escobar L, Tan PL, Muzina M, Zwain S, Buchanan C. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation 2007. March;14(2):157–61.* clinical islet encapsulation
- 108.Tuch BE, Keogh GW, Williams LJ, Wu W, Foster JL, Vaithilingam V, et al. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes care 2009. October;32(10):1887–9.* clinical islet encapsulation
- 109.De Vos P, Vegter D, De Haan BJ, Strubbe JH, Bruggink JE, Van Schilfgaarde R. Kinetics of intraperitoneally infused insulin in rats. Functional implications for the bioartificial pancreas. Diabetes 1996. August;45(8):1102–7. [DOI] [PubMed] [Google Scholar]
- 110.Colton CK, Avgoustiniatos ES. Bioengineering in development of the hybrid artificial pancreas. Journal of biomechanical engineering 1991. May;113(2):152–70. [DOI] [PubMed] [Google Scholar]
- 111.de Vos P, Vegter D, Strubbe JH, de Haan BJ, van Schilfgaarde R. Impaired glucose tolerance in recipients of an intraperitoneally implanted microencapsulated islet allograft is caused by the slow diffusion of insulin through the peritoneal membrane. Transplantation proceedings 1997. Feb-Mar;29(1–2):756–7.* considerations about the intraperitoneal transplantation site
- 112.Bennet W, Groth CG, Larsson R, Nilsson B, Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Upsala journal of medical sciences 2000;105(2):125–33. [DOI] [PubMed] [Google Scholar]
- 113.Gill RG, Bishop NH. Clinical islet transplantation: where immunity and metabolism intersect? Current opinion in endocrinology, diabetes, and obesity 2012. August;19(4):249–54. [DOI] [PubMed] [Google Scholar]
- 114.Berman DM, O’Neil JJ, Coffey LC, Chaffanjon PC, Kenyon NM, Ruiz P Jr., et al. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2009. January;9(1):91–104.* preclinical studies of the omental pouch for islet transplantation
- 115.Najjar M, Manzoli V, Villa C, Martino MM, Molano RD, Torrente Y, et al. Fibrin Gels Engineered with Pro-Angiogenic Growth Factors Promote Engraftment of Pancreatic Islets in Extrahepatic Sites in Mice. Biotechnology and bioengineering 2015. March 18.* preclinical study showing the benefits of engineering the transplantation site with proangiogenic hydrogels
- 116.Echeverri GJ, McGrath K, Bottino R, Hara H, Dons EM, van der Windt DJ, et al. Endoscopic gastric submucosal transplantation of islets (ENDO-STI): technique and initial results in diabetic pigs. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2009. November;9(11):2485–96. [DOI] [PubMed] [Google Scholar]
- 117.Jansson L, Carlsson PO. Graft vascular function after transplantation of pancreatic islets. Diabetologia 2002. June;45(6):749–63. [DOI] [PubMed] [Google Scholar]
- 118.Brissova M, Powers AC. Revascularization of transplanted islets: can it be improved? Diabetes 2008. September;57(9):2269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Narang AS, Cheng K, Henry J, Zhang C, Sabek O, Fraga D, et al. Vascular endothelial growth factor gene delivery for revascularization in transplanted human islets. Pharmaceutical research 2004. January;21(1):15–25. [DOI] [PubMed] [Google Scholar]
- 120.Stendahl JC, Wang LJ, Chow LW, Kaufman DB, Stupp SI. Growth factor delivery from self-assembling nanofibers to facilitate islet transplantation. Transplantation 2008. August 15;86(3):478–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc Natl Acad Sci U S A 2012. March 13;109(11):4245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marzorati S, Bocca N, Molano RD, Hogan AR, Doni M, Cobianchi L, et al. Effects of systemic immunosuppression on islet engraftment and function into a subcutaneous biocompatible device. Transplantation proceedings 2009. Jan-Feb;41(1):352–3. [DOI] [PubMed] [Google Scholar]
- 123.Beattie GM, Montgomery AM, Lopez AD, Hao E, Perez B, Just ML, et al. A novel approach to increase human islet cell mass while preserving beta-cell function. Diabetes 2002. December;51(12):3435–9. [DOI] [PubMed] [Google Scholar]
- 124.Orlando G, Gianello P, Salvatori M, Stratta RJ, Soker S, Ricordi C, et al. Cell replacement strategies aimed at reconstitution of the beta-cell compartment in type 1 diabetes. Diabetes 2014. May;63(5):1433–44. [DOI] [PubMed] [Google Scholar]
- 125.Abdelalim EM, Emara MM. Advances and challenges in the differentiation of pluripotent stem cells into pancreatic beta cells. World journal of stem cells 2015. January 26;7(1):174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lysy PA, Weir GC, Bonner-Weir S. Concise review: pancreas regeneration: recent advances and perspectives. Stem cells translational medicine 2012. February;1(2):150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nature biotechnology 2006. November;24(11):1392–401.** Viacyte protocol for generating stem cell-derived insulin-secreting cell products
- 128.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nature biotechnology 2008. April;26(4):443–52. [DOI] [PubMed] [Google Scholar]
- 129.Pagliuca FW, Millman JR, Gurtler M, Segel M, Van Dervort A, Ryu JH, et al. Generation of functional human pancreatic beta cells in vitro. Cell 2014. October 9;159(2):428–39.** Melton’s protocol for generating stem cell-derived insulin-secreting cell products
- 130.Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nature biotechnology 2014. November;32(11):1121–33.** BetaLogics protocol for generating stem cell-derived insulin-secreting cell products
- 131.Bellin MD, Barton FB, Heitman A, Harmon JV, Kandaswamy R, Balamurugan AN, et al. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2012. June;12(6):1576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guleria I, Gubbels Bupp M, Dada S, Fife B, Tang Q, Ansari MJ, et al. Mechanisms of PDL1-mediated regulation of autoimmune diabetes. Clinical immunology (Orlando, Fla) 2007. October;125(1):16–25. [DOI] [PubMed] [Google Scholar]
- 133.Pileggi A, Xu X, Tan J, Ricordi C. Mesenchymal stromal (stem) cells to improve solid organ transplant outcome: lessons from the initial clinical trials. Current opinion in organ transplantation 2013. December;18(6):672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Francese R, Fiorina P. Immunological and regenerative properties of cord blood stem cells. Clinical immunology (Orlando, Fla) 2010. September;136(3):309–22. [DOI] [PubMed] [Google Scholar]
- 135.Fiorina P, Jurewicz M, Vergani A, Petrelli A, Carvello M, D’Addio F, et al. Targeting the CXCR4-CXCL12 axis mobilizes autologous hematopoietic stem cells and prolongs islet allograft survival via programmed death ligand 1. Journal of immunology (Baltimore, Md: 1950) 2011. January 1;186(1):121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Korpos E, Kadri N, Kappelhoff R, Wegner J, Overall CM, Weber E, et al. The peri-islet basement membrane, a barrier to infiltrating leukocytes in type 1 diabetes in mouse and human. Diabetes 2013. February;62(2):531–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Verhoef JJ, Carpenter JF, Anchordoquy TJ, Schellekens H. Potential induction of anti-PEG antibodies and complement activation toward PEGylated therapeutics. Drug discovery today 2014. September 7. [DOI] [PubMed] [Google Scholar]
- 138.Riolobos L, Hirata RK, Turtle CJ, Wang PR, Gornalusse GG, Zavajlevski M, et al. HLA engineering of human pluripotent stem cells. Molecular therapy: the journal of the American Society of Gene Therapy 2013. June;21(6):1232–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ricordi C Towards a constructive debate and collaborative efforts to resolve current challenges in the delivery of novel cell based therapeutic strategies. CellR4 2013;1(1):e110. [PMC free article] [PubMed] [Google Scholar]
- 140.Goodman KV. Getting it Goldilocks Just Right: Science, Regulation and Ethics. CellR4 2013;1(2):e387. [Google Scholar]