Abstract
BACKGROUND:
Identifying preclinical Alzheimer’s dementia is an important step towards developing approaches to early treatment and dementia prevention.
METHODS:
We applied latent class analysis (LCA) to 10 baseline neuropsychological assessments for 1,345 participants from Einstein Aging Study. Time-to-event models for all-cause dementia and AD were run examining events in 4-year intervals.
RESULTS:
Five classes were identified: Mixed-Domain Impairment (n = 107), Memory-Specific Impairment (n = 457), Average (n = 539), Frontal Impairment (n = 118), and Superior Cognition (n = 124). Compared to the Average class, the Mixed-Domain Impairment and Memory-Specific Impairment classes were at higher risk of incident all-cause dementia and AD in the first 4 years from baseline, while the Frontal Impairment class was associated with higher risk between 4 and 8 years of follow-up.
CONCLUSION:
LCA identified classes which differ in cross-sectional cognitive patterns and in risk of dementia over specific follow-up intervals.
Keywords: Alzheimer’s disease, all-cause dementia, neuropsychology, cognitive aging, cognitive subtypes, individual differences, heterogeneity
1. Introduction
Identifying individuals at high risk of developing dementia is an important step towards developing strategies which prevent or delay the onset of dementia [1–3]. Many clinical trials for the prevention and treatment of Alzheimer’s dementia have failed. We highlight two of the many explanations offered for these therapeutic failures. First, the participants enrolled in prevention and treatment trials differ biologically; if treatment works only for a subset of the heterogeneous patient population the effects in that subgroup may be undetectable unless the subgroup is very large [4–6]. Second, treatment may fail because it is being given very late in a neurodegenerative process [3]. Our approach addresses these problems by using a statistical method, latent class analysis, to improve the early detection and identify more homogeneous groups of patients likely to develop Alzheimer’s disease (AD) or other dementias.
In previous work [7], we applied latent class analysis (LCA) to baseline neuropsychological assessment of 1,345 community-dwelling older adults from the Einstein Aging Study (EAS). The 9-class solution fit slightly better than the 5-class solution and we developed the model to characterize the patterns of neuropsychological performance within and across the classes (See Zammit et al. [7] for details). Briefly, in the 9-class solution we identified classes with dimensional patterns of cognitive function (which we labelled as “High Average”, “Average”, and “Low Average”), classes which clustered at the high end or the lower end of the cognitive spectrum (which we labelled as “Elite” and “Disadvantaged”), and classes which displayed discontinuity of scores across neuropsychological measures (“Poor Language”, “Poor Episodic”, “Poor Processing Speed”, “Poor Executive and Poor Memory”). However, a smaller number of subgroups might be more parsimonious for clinical applicability. The aim of this study was to investigate whether latent class assignment based on the 5-class model predicted time to all-cause dementia and AD over up to 19 years of follow-up using longitudinal data from the EAS. We hypothesized that the 5-class model represents a parsimonious solution; that the model will have clinical implications in predicting all-cause dementia and AD; and that dementia and AD would develop in these groups at different rates.
2. Materials and Methods
We used the EAS cohort for our analyses [8]. Participants are 70 years and older, community-dwelling, English-speaking and reside in the Bronx, New York. Participants were systematically recruited from the Health Care Financing Administration/Centers for Medicaid and Medicare Services rosters for Medicare-eligible between 1993 and 2004, and from New York City Board of Elections voter registration lists from 2004 onwards. Written informed consent was obtained on their first clinical visit. The study protocol was approved by the local institutional review board. Between 1993 and 2015, 2,262 participants had baseline evaluations, of those, 1,395 had follow-up data at the time this study was conducted. Among participants with follow-up, 50 had dementia at baseline, and were excluded from these analyses. Therefore 1,345 participants who had at least one wave of follow up data and were non-demented at baseline, were selected for the purpose of this study. Follow-ups are done annually, and are consistent across participants.
2.1. Statistical analysis
2.1.1. Latent Class Analysis
Our four-step methodological approach has been described previously [7]. Briefly, i) we selected our study population (described above); ii) we selected core neuropsychological measures representing domains of episodic memory (Free and Cued Selective Reminding Test (FCSRT) free recall test [9–11]; Logical Memory (LM) [12]), language/semantic fluency (Categories (CAT) [13], and the Boston Naming Test (BNT) [14]), attention/working memory (Digit Span [15] and Trail Making Tests A (TMTA), visual and spatial functions (Digit Symbol Coding [15], , Block Design [15]), and executive function (Controlled Oral Word Fluency Test (FAS) [16], and B (TMTB) [17]), and demographic covariates (age, sex, and education) and we fitted the LCA model with increasing number of classes (between 2 and 10) to determine an optimal class solution; iii) we applied two-fold cross-validation split-half procedures for replication and validation purposes; and iv) we characterized and validated our model using pre-existing characteristics to determine if the classes are distinguishable on core neuropsychological characteristics and external validators. For simplicity and for illustrative purposes, we later summarized the individual neuropsychological measures into domains, as described above by averaging and z-scoring results within domains. This approach has been done previously in other cohorts (e.g. [18, 19].
External validators.
We present the Wide Range Achievement Test (WRAT) to represent premorbid IQ [20], the Blessed Information Memory Concentration test [21] as a marker of global cognition, and race/ethnicity. Since this model is aimed for a clinical audience we also included variables that constitute the Framingham 10-year cardiovascular risk [22] and vascular burden as a means to further characterize and validate the subgroups. Apart from sex, age and education, which were included in our model, and are part of the Framingham Risk Score, these variables included: systolic blood pressure, hypertension medication use, HDL and total cholesterol, current smoking, and diabetes; for cumulative vascular disease we included: a history of any of the following conditions: claudication, stroke, myocardial infarction, angina, and heart failure. All vascular variables were self-reported during the clinical interview, except for SBP, HDL and total cholesterol which were part of annual routine during in-house assessment.
Model selection.
Supplementary Table 1 shows a comparison between the previously developed 9-class model and the 5-class model we are investigating in this paper. Cross-validation showed that the 5-class model fit the data almost as well the 9-class solution (Supplementary Table 2). The two cross-validated subsamples in the 5-class solution showed that for subsample 1 the BIC was 91409.15 and entropy was >0.8, and for subsample 2 the BIC was 91221.61 and the entropy was 0.9. When mapped onto the trained solutions participants in the five-class solution generally fell into similar classes, with Kappa > .95 and >.87 (Supplementary Table 3).
2.1.2. Time-to-Event Models
Cox proportional hazards regression models were used to determine the adjusted hazard ratio of incident all-cause dementia and AD. The mean time to a dementia diagnosis from baseline assessment in this sample was 4.4 years, thus we further stratified time-to-event models by <4 and 4 – 8 years and ≥ 8 of follow-up to determine if specific profiles are at risk of developing dementia earlier than the sample’s average. The proportional hazards assumption was met overall, thus we proceeded in testing our hypotheses that dementia will develop at different rates in the classes as shown by stratifying the models into specific time intervals. Since the classes were already adjusted for age, sex, and education we did not add further adjustments to the models to study the predictive validity of the classes per se. The Average class was used as reference.
Dementia diagnosis:
The diagnosis of dementia in EAS was based on the standardized criteria from the Diagnostic and Statistical Manual Fourth Edition (DSM-IV). Dementia diagnosis required impairment in memory defined as 1.5 SDs below the age-adjusted mean on Logical Memory [12] or a score of 24 or less on the Free and Cued Selective Reminding Test [9], impairment in one additional cognitive domain, and evidence of functional decline. AD was diagnosed in participants diagnosed with DSM- IV dementia meeting clinical criteria for probable or possible disease established by the National Institute of Neurological and Communication Disorders and Stroke and the Alzheimer Disease and Related Disorders Association (NINCDS-ADRDA). Probable or possible AD was determined at case conference based on information from the neurological exam, the EAS neuropsychological battery, self-reported personal and family health histories, the screen for depression symptoms (Geriatric Depression Scale), and responses to ADL questionnaires. [23].
2.2. Statistical software
For LCA modeling we used MPlus version 8 [24]. We used SPSS version 24 [25] for all other analyses; these include analyses of variance (ANOVAs) amongst the classes, cox proportional hazards models, and figure generation.
3. Results
Table 1 displays the demographic characteristics and cognitive performance of the sample (n = 1,345). The average age of the sample was 78 years (SD = 5.4 years), 61.6% were female, and 68% were non-Hispanic white. Average years of education was 13.6 years (SD = 3.5).
Table 1.
Demographic characteristics and cognitive test performance of the whole sample (n = 1,345).
| Characteristics | Whole sample | Did not develop dementia | Developed dementia | p |
|---|---|---|---|---|
| N (%) | 1,345 (100) | 1,119 (89.1) | 146 (10.9) | |
| Demographics | ||||
| Age, years (SD) | 78.0 (5.4) | 77.7 (5.3) | 80.5 (5.3) | <0.001 |
| Females (%) | 828 (61.6) | 0.194 | ||
| Education, years (SD) | 13.6 (3.5) | 13.7 (3.5) | 13.2 (3.6) | 0.105 |
| WRAT (SD) | 67.5 (15.2) | 83.8 (10.2) | 75.6 (10.7) | <0.001 |
| BIMC (SD) | 2.4 (2.3) | 2.2 (2.1) | 4.1 (2.9) | <0.001 |
| Non-Hispanic White | 914 (68) | 816 (68.2) | 98 (65.8) | 0.545 |
| African American | 359 (26.7) | 312 (26.1) | 47 (31.5) | |
| Other Race | 72 (7.3) | 32 (2.7) | 2 (1.3) | |
| Neuropsychological Performance | ||||
| Free Recall (SD) | 30.7 (6.2) | 31.3 (5.7) | 25.4 (7.3) | <0.001 |
| Boston Naming (SD) | 11.7 (2.6) | 11.9 (2.5) | 10.4 (3.0) | <0.001 |
| Digit Span (SD) | 13.8 (3.7) | 13.9 (0.4) | 12.7 (3.5) | <0.001 |
| Digit Symbol Coding (SD) | 40.0 (14.0) | 41.1 (13.9) | 31.5 (11.7) | <0.001 |
| Block Design (SD) | 21.4 (9.2) | 21.8 (9.2) | 17.4 (7.8) | <0.001 |
| Word Fluency (SD) | 34.8 (13.1) | 35.4 (13.0) | 30.1 (12.1) | <0.001 |
| Categories (SD) | 37.0 (9.2) | 37.7 (9.1) | 31.3 (8.6) | <0.001 |
| Logical Memory (SD) | 19.7 (7.0) | 20.2 (6.9) | 15.6 (6.5) | <0.001 |
| Trail Making Test A (SD) | 60.2 (26.5) | 58.4 (25.0) | 75.0 (34.0) | <0.001 |
| Trail Making Test B (SD) | 143.5 (7.04) | 139.5 (68.7) | 177.2 (76.3) | <0.001 |
Note. WRAT = Wide Range Achievement Test. BIMC = Blessed Information Memory Concentration Test.
3.1. Latent Class Analysis
3.1.1. Demographic Characteristics and Cognitive Profiles of the Classes.
Five classes were identified (Table 2): One class had poor scores on all cognitive measures when compared to the rest of the classes, which we labelled Mixed-Domain Impairment (n = 107). Two other classes showed dissociations in scores with one class displaying lower scores on episodic memory (Logical Memory) and verbal fluency (Categories), while the other had worse performance on tasks of attention and executive function (Digit Symbol Coding, Block Design, Trail Making Test A, Trail Making Test B). We labelled these classes as Memory-Specific (n = 457), and Frontal Impairment (n = 118) respectively. The other two classes scored relatively higher on all cognitive measures, with one class displaying superior performance to the rest of the classes, hence we refer to them as Average (n = 539), and Superior Cognition (n = 124). In sum, three classes demonstrated cognitive impairment in some area, while two other classes seemed to be cognitively intact. The cognitive impairment classes were the “Mixed-Domains Impairment”, “Memory-Specific Impairment”, and “Frontal Impairment” groups, while the cognitive intact classes were the “Average” and the “Superior Cognition” groups. Significant differences among the classes were present for age, education, premorbid IQ, and global cognition. Individuals in the Mixed-Domain Impairment, Memory-Specific Impairment, and Frontal Impairment classes were older and had lower levels of education compared to the Average and Superior Cognition classes; they also had poorer scores on the WRAT and the BIMC. Almost 50% of individuals making up the Mixed-Domain Impairment (49.5%) and Frontal Impairment (48.3%) classes were African American. Although the highest percentage of females was in the Superior Cognition class (69.4%), there were no significant differences for gender.
Table 2.
Demographic, cardiovascular risk variables, and cognitive test performance that were and were not included in the latent class model according to the five-class solution in (n=1,345).
| Cognition Impaired | Cognition Intact | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Mixed-Domain Impairment | Memory-Specific Impairment | Frontal Impairment | Average | Superior Cognition | F/X2 | p |
| N (%) | 107 (8.0) | 457 (34.0) | 118 (8.8) | 539 (40.1) | 124 (9.2) | ||
| Demographics | |||||||
| 1Age, years (SD) | 79.4 (6.2) | 79.0 (5.1) | 80.4 (6.1) | 77.1 (5.0) | 75.2 (3.9) | 26.5 (4, 1340) | <0.001 |
| 1Females (%) | 73 (68.2) | 268 (58.6) | 74 (62.7) | 327 (60.7) | 86 (69.4) | 7.1 | 0.132 |
| 1Education, years (SD) | 9.3 (3.1) | 12.8 (3.0) | 12.3 (3.6) | 14.8 (3.0) | 16.6 (2.6) | 117.5 (4, 1340) | <0.001 |
| WRAT (SD) | 49.7 (15.6) | 65.0 (13.7) | 63.1 (13.5) | 75.1 (10.8) | 83.8 (3.5) | 68.1 (4, 613) | <0.001 |
| BIMC (SD) | 4.7 (2.6) | 2.9 (2.3) | 3.7 (2.6) | 1.6 (1.7) | 0.7 (0.9) | 94.7 (4, 1339) | <0.001 |
| Non-Hispanic White (%) | 46 (43.0) | 298 (65.2) | 54 (45.8) | 406 (75.3) | 110 (88.7) | 96.9 | <0.001 |
| African American (%) | 53 (49.5) | 130 (28.4) | 57 (48.3) | 111 (20.6) | 8 (6.5) | ||
| Other Race (%) | 8 (7.5) | 29 (6.3) | 7 (5.9) | 22 (4.1) | 6 (4.8) | ||
| 1Neuropsychological Measures | |||||||
| Free Recall (SD) | 27.5 (6.7) | 28.5 (6.2) | 30.1 (6.1) | 32.1 (5.4) | 35.4 (4.1) | 54.1 (4, 1325) | <0.001 |
| Boston Naming (SD) | 7.5 (2.2) | 10.8 (2.4) | 11.1 (2.3) | 12.9 (1.7) | 14.2 (1.1) | 235.6 (4, 1320) | <0.001 |
| Digit Span (SD) | 10.3 (2.9) | 12.5 (2.8) | 11.9 (2.9) | 14.9 (3.2) | 18.5 (3.6) | 156.0 (4, 1335) | <0.001 |
| Digit Symbol Coding (SD) | 19.5 (7.4) | 33.6 (8.1) | 28.7 (9.3) | 47.0 (9.5) | 60.4 (10.2) | 485.7 (4, 1327) | <0.001 |
| Block Design (SD) | 10.3 (6.1) | 17.8 (6.7) | 15.3 (7.1) | 24.3 (7.0) | 33.8 (8.1) | 213.9 (4, 1179) | <0.001 |
| Word Fluency (SD) | 19.0 (8.3) | 29.8 (9.7) | 27.9 (10.4) | 39.4 (10.8) | 50.4 (11.1) | 187.6 (4, 1234) | <0.001 |
| Categories (SD) | 27.1 (5.9) | 32.6 (6.6) | 33.6 (7.4) | 40.3 (7.1) | 50.7 (8.1) | 257.3 (4, 1330) | <0.001 |
| Logical Memory (SD) | 13.9 (6.1) | 16.5 (5.9) | 17.1 (5.9) | 22.2 (6.1) | 27.4 (5.7) | 136.5 (4, 1289) | <0.001 |
| Trail Making Test A (SD) | 121.2 (33.2) | 63.9 (17.8) | 75.8 (20.3) | 48.0 (13.4) | 39.0 (11.2) | 406.9 (4, 1248) | <0.001 |
| Trail Making Test B (SD) | 274.0 (46.0) | 149.5 (36.2) | 274.6 (30.5) | 102.7 (28.5) | 76.9 (22.2) | 1170.8 (4, 1248) | <0.001 |
| Vascular Risk and Diseases | |||||||
| Systolic blood pressure >140mmHg (%) | 72 (97.3) | 289 (91.5) | 76 (87.4) | 343 (86.0) | 73 (80.2) | 16.7 (4, 967) | <0.01 |
| Hypertension medication (%) | 72 (97.3) | 273 (60.8) | 65 (56.5) | 308 (59.1) | 60 (52.6) | 5.7 (4,1306) | 0.223 |
| HDL Cholesterol, mg/dl (SD) | 58.6 (17.4) | 58.9 (15.2) | 59.3 (16.7) | 56.7 (15.5) | 58.4 (16.5) | 0.6 (4, 492) | 0.682 |
| Total Cholesterol, mg/dl (SD) | 188.4 (33.1) | 185.9 (38.6) | 191.1 (42.8) | 185.7 (40.5) | 187.3 (40.4) | 0.5 (4, 492) | 0.682 |
| Current smoking (%) | 9 (8.7) | 26 (5.9) | 12 (10.7) | 41 (7.9) | 5 (4.4) | 5.3 (4, 1289) | 0.258 |
| Diabetes (%) | 18 (16.8) | 60 (13.7) | 12 (10.2) | 62 (11.5) | 10 (8.0) | 31.1 (4, 483) | <0.001 |
| 2Cumulative Vascular Disease (%) | 65 (60.7) | 225 (49.2) | 60 (50.8) | 247 (45.8) | 47 (37.9) | 9.8 (4, 1306) | 0.020 |
Note. Indicates variables that were used as covariates in the latent class model. WRAT = Wide Range Achievement Test.
Cumulative Vascular Disease = history of either claudication, stroke, myocardial infarction, angina, or heart failure.
Table 2 and Figure 1 summarize the cognitive measures for each class using summary domains of visual and spatial functions (Digit Symbol Coding, Block Design), executive function (Controlled Oral Word Fluency Test, TMTB), attention/working memory (Digit Span, TMTA), episodic memory (FCSRT, LM) and semantic/language fluency (BNT, Categories).
Figure 1.
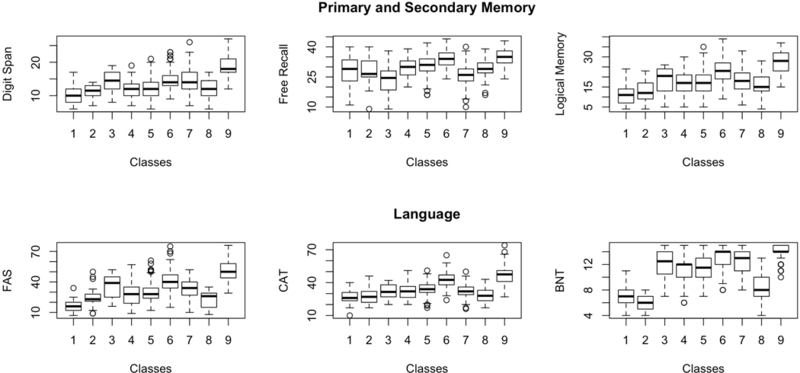
Figure illustrating how each of the classes performed on neuropsychological measures reflecting domains of episodic memory, semantic/ language fluency, visual and spatial function, attention/working memory, and executive function.
Vascular Risk factor profiles, not used in the LCA models are summarized in Table 2. In the Mixed-Domain Impairment class, 97.3% had systolic blood pressure over 140mmHg, and 97.3% were using anti-hypertensive medication; 60.7% of the individuals in this class also had history of vascular disease. The Mixed-Domains Impairment class had the highest proportion of individuals with diabetes (16.8%), followed by the Memory Impairment Class (13.7%), while the highest proportion of smokers belonged to the Frontal-Impairment class (10.7%). Up to 60.7% of Mixed-Domain Impairment and over 50% of Memory-Specific and Frontal Impairment had vascular disease.
3.2. Cox Proportional Hazards Models: Incidence of all-cause Dementia and Alzheimer’s Disease in each latent class.
Development of dementia across the latent classes.
In total, there were 149 cases of incident all-cause dementia and 123 cases of incident AD. The highest proportion of incident cases was found in the Mixed-Domain Impairment class (29.9% all-cause dementia and 24.3% AD), followed by the Memory-Specific Impairment class (15.8% all-cause dementia and 13.3% AD).
Follow-up was also stratified into 4-year time-intervals. When stratified, the Mixed-Domain Impairment and Memory-Specific Impairment classes had more incident cases of both all-cause dementia and AD in the first 4 years of follow-up (84.4% and 86.6% in the Mixed-Domain Class and 61.1% and 62.3% in the Memory-Specific Class) while the Frontal Impairment class had more incident cases of both all-cause dementia and AD after 4 years of follow-up (53.9% and 60%). Table 2 shows the incidence rates of dementia and AD across the classes.
Since follow-up in our sample ranged from <1 to >19 years, we also ran the final models that were restricted to individuals with ≤ 8 years of follow-up (n = 655) as a sensitivity analysis to determine if loss-to-follow-up is associated with outcome. Our results did not differ from the analyses using the entire subsample cohort, thus we report here results for the entire sample.
Risk of incidence until end of follow-up.
Cox proportional hazards models showed that the Mixed-Domain Impairment, Memory-Specific Impairment, and Frontal Impairment - classes were associated with an elevated risk of incident all-cause dementia (HR = 9.2, 95% CI: 5.5 – 15.2; HR = 3.5, 95%CI: 2.3 – 5.4; and HR = 4.3, 95%CI: 2.2 – 8.2) and incident AD (HR = 9.0, 95%CI: 5.1 – 15.8; HR = 3.6, 95%CI: 2.2 – 5.8; and HR = 3.9, 95%CI: 1.9 – 8.3) when compared to the Average class (Table 4). Figures 2 and 3 show the cumulative incidence rates for all-cause dementia and AD for each of the classes.
Table 4.
Hazards ratios of incidence of all-cause dementia and Alzheimer’s Dementia until end of follow-up and stratified by time-to-event.
| Impaired Cognition | Intact Cognition | ||||
|---|---|---|---|---|---|
| Mixed-Domain Impairment | Memory-Specific Impairment | Frontal Impairment | Average | Superior Cognition | |
| HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | |
| All cause dementia | 9.2 (5.5 – 15.2)*** | 3.5 (2.3 – 5.4)*** | 4.3 (2.2 – 8.2) *** | Ref. | 0.4 (0.1 – 1.6) |
| <4 years | 13.2 (5.7– 30.3)*** | 5.6 (2.5 – 12.4)*** | 2.6 (0.9 – 7.9) | Ref. | NA |
| 4–8 years | 2.8 (0.9 – 8.6) | 1.7 (0.8 – 3.6) | 3.3 (1.3 – 8.7)* | Ref. | NA |
| ≥8 years | 2.5 (0.3 – 20.3) | 2.0 (0.8 – 4.7) | 6.6 (0.8 – 53.5) | Ref. | 1.8 (0.5 – 6.5) |
| Alzheimer’s disease | 9.00 (5.1 – 15.8)*** | 3.6 (2.2 – 5.8)*** | 3.9 (1.9 – 8.3)*** | Ref. | 0.4 (0.1 – 1.7)*** |
| <4 years | 10.7 (4.6 – 25.1)*** | 4.8 (2.1 – 10.8)*** | 1.7 (0.5 – 5.9) | Ref. | NA |
| 4–8 years | 3.1 (0.8 – 11.5) | 2.1 (0.9 – 4.7) | 4.8 (1.7 – 13.5)** | Ref. | NA |
| ≥8 years | 3.1 (3.8 – 25.2) | 1.8 (0.7 – 4.6) | NA | Ref. | 1.3 (0.3 – 6.3) |
Note. p<0.05
p<0.001
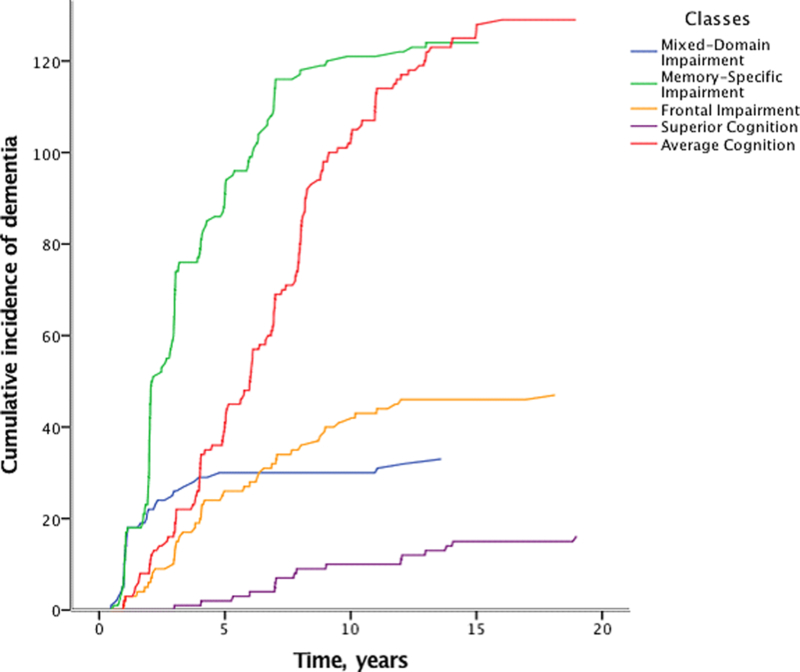
Figure 2.
Cumulative incidence of dementia for each of the classes.
Figure 3.
Cumulative incidence of Alzheimer’s dementia for each of the classes.
Risk of incidence stratified into 4-year time bins.
When stratified into time-bins (Table 4), the Mixed-Domain Impairment and Memory-Specific Impairment classes were associated with a higher risk of incident dementia (HR = 13.6, 95%CI = 5.9 −31.2 and HR = 5.8, 95%CI = 2.6 – 12.8) and incident AD (HR = 11.1, 95%CI = 4.7 – 25.9, and HR = 5.0, 95%CI = 2.2 – 11.2) in the first four years after baseline assessment, while the Frontal Impairment class was associated with a higher risk of incident all-cause dementia and incident AD between four and eight years of follow-up (HR = 6.0, 95%CI = 2.5 – 14.3, and HR = 7.1, 95%CI = 2.7 – 18.4).
4. Discussion
This study investigated the five-class solution based on cognitive function in older adults in the EAS, and estimated rates of onset for all-cause dementia and for AD. The classes we identified based on cognitive profiles were shown to differ in pre-morbid IQ and vascular risk factors at baseline, variables not used to define the classes. We show herein that the groups defined by LCA varied in risk of incident dementia and AD from negligible risk to high risk. Classes with worse cognitive performance also had a higher vascular risk profile.
A novel finding from our study is that membership in specific latent classes based on cognitive performance at baseline were differentially associated with the time-frame for the onset of all-cause dementia and AD. Specifically, the Mixed-Domain Impairment and Memory-Specific Impairment classes were associated with higher risks for incident AD and all-cause dementia within the first 4 years of follow-up, while the Frontal Impairment class was associated with higher risks of dementia and AD between 4 and 8 years of follow-up. The presence of subgroups of individuals with specific cognitive profiles that are linked to future onset of AD and all-cause dementia has at least three important clinical implications.
First, these findings indicate that cross-sectional cognitive measures can be used to flag individuals at high risk for adverse cognitive outcomes for further evaluation. Members of the subgroups at highest risk of AD may be candidates for possible enrollment into clinical trials. Randomized control trials are not generally designed to distinguish amongst individuals using sophisticated methods. Cognitive profiling could be used to identify subgroups for enrollment or exclusion; it could also be used as a basis for stratified randomization or as a basis for pragmatic clinical trials. In the health care setting, this type of approach may eventually lead to a simplified risk-assessment sheet to help clinicians distinguish amongst individuals requiring further diagnostic testing, those eligible for prevention strategies, and others who may benefit more from tailored interventions. Identifying classes, and characterizing them in terms of their impairments, will additionally help us identify residual cognitive assets i.e. cognitive systems that are still intact and which may be used as compensatory mechanisms in intervention programs with aims of compressing dementia morbidity.
Second, group-based approaches that classify individuals based on latent class models might offer a more individualized approach to treatment. Potentially, patients from memory and referral clinics could be classified into phenotypic groups based on cognitive performance. Previous studies show that when compared to other biomarkers, baseline cognitive function has at least comparable and to superior prediction for progression to dementia [26–28]. Additionally, biomarker data is still often expensive, invasive, and not part of clinical routine; the availability of a classification system that identifies individuals based on their cognitive profile may offer insight into underlying pathological processes [29–31].
Third, the use of actuarial procedures based on multiple neuropsychological measures results in greater diagnostic stability [4, 32]. Our results showed that 3 different classes constituting different proportions of cognitive impairment predicted incident dementia within specific time-frames. One of these classes was dominated by non-amnestic impairment related to executive function, attention, and visual and spatial skills; however, these individuals still were at elevated risk of developing all-cause dementia and AD. Previous studies indicating mixed- and executive-specific domain impairment in MCI as a measure for preclinical AD and in vascular dementia also showed similar results [4, 5, 33]. Possibly, a subgroup with frontal impairment is undergoing the aging process with specific underlying biological processes making it qualitatively different than other better-known aging processes (e.g. amnestic).
Strengths, limitations, and Future Directions.
Strengths of this study include the large well-characterized and diverse sample in terms of demographics, race/ethnicity, and cognitive status, which enables identification of specific and meaningful groups; the extensive cognitive battery representing five major cognitive domains; and the relatively long clinical follow-up. Previous research studies have performed similar analysis using smaller samples [34, 35], fewer cognitive measures [36], and shorter follow-up [37, 38].Our community based sample is more representative of older adults in the Bronx than a sample seeking medical care for cognitive difficulties.
Our study has limitations. The generalizability of our findings to clinic-based samples or community-based samples with different demographic characteristics is unknown. Nor is it clear, if these results depend upon the specific neurocognitive battery used in the EAS; the use of different tests, different domains, and number of tests per domain may affect the number of classes generated. For example, we may have missed a considerable number of individuals with visual memory impairment [39]. The follow-up time differed significantly amongst the classes, with the Frontal impairment and Mixed-Domains classes having the shortest follow-up (3.0 and 3.5 years) and the Superior Cognition having the longest follow-up (5.1 years), thus the results need to be interpreted with caution, especially with regards to time-intervals. Lastly, these classes may represent different stages of a single illness rather than biologically distinct subtypes of dementia. For example, the Mixed-Domain class may have started off as a Memory-Specific Impairment class, and the Memory-Specific Impairment Class may progress to the Mixed-Domain Impairment class in a few years. Since the Mixed-Impairment class is a more advanced stage, different paths may result in similar class assignment e.g. Memory-Specific, Frontal-Impairment, or an alternate path which we may not have captured. Alternatively, individuals in the Frontal Impairment Class may be undergoing a different biological process and have a distinct type or distinct types of dementia. In future research we will follow individuals over time to see if class membership changes as disease progresses. In future analyses we are also planning to find out if our latent classes correspond to biological subtypes. The use of imaging and pathology data to supplement our results with biomarkers would be insightful, revelatory, and possibly confirmatory of our classes.
Our results are presented within a research framework – they need to be modified, replicated, and validated. We realize that there are gaps in our study, and that the use of biomarkers (e.g. Aβ and pathologic tau for AD specific profiles), imaging markers, and genetic and clinical data would help refine and define the classes better. The application of a precision medicine approach will allow various fields to come together to materialize the breath of information and translate it into clinical applicability. Until more refined methods are concretely developed we suggest cognition to be assessed via a thorough neuropsychological evaluation to acquire all information necessary to classify (and treat) patients accordingly. The use of coordinated approaches [40, 41] on aging cohorts to replicate and validate findings would make use of a better platform to harmonize studies and compare results by capitalizing on data from various sources.
5. Conclusion
The current study revealed that during the course of late-life aging, improved parsing of cognitive heterogeneity and early diagnosis are necessary tools. Results revealed that the majority of older adults maintain good cognitive function, with smaller subgroups exhibiting uneven patterns of cognitive impairment and signs of imminent risk. A novel and important finding was that some subgroups were associated with increased risk of incident dementia within 4 years of follow-up, while other subgroups had a delayed risk, implying room for intervention. Pragmatically, these results illustrate a need to develop various intervention and treatment programs to address group-based and individual-level needs. We are fitting latent class models in other longitudinal aging studies to determine if similar results are obtained in different samples using independent cognitive tests.
Supplementary Material
Table 3.
Incidence of dementia across the five classes (% in parentheses).
| N (%) | Person-years | Dementia cases (%) | Incidence-rate per 100 person-years | AD cases (%) | Incidence-rate per 100 person-years | |
|---|---|---|---|---|---|---|
| Mixed-Domain Impairment | 107 (8) | 370.8 | 32 (21.5) | 8.6 | 26 (21.1) | 7.0 |
| Memory-Specific Impairment | 457 (34) | 1933.0 | 72 (48.3) | 3.7 | 61 (49.6) | 3.2 |
| Frontal Impairment | 118 (8.8) | 2683.8 | 29 (19.5) | 1.1 | 10 (8.1) | 0.4 |
| Average | 539 (40.1) | 350.9 | 13 (8.7) | 3.7 | 24 (19.5) | 6.8 |
| Superior Cognition | 124 (9.2) | 589.6 | 3 (2.0) | 0.5 | 2 (1.6) | 0.3 |
| Total | 1,345 (100) | 5928.1 | 149 (100) | 2.5 | 123 (100) | 2.2 |
Note. Percentages are column percentages. AD = Alzheimer’s disease.
Acknowledgments:
We thank the EAS research participants. We thank Charlotte Magnotta, Diane Sparracio and April Russo for assistance in participant recruitment; Betty Forro, Wendy Ramratan, and Mary Joan Sebastian for assistance in clinical and neuropsychological assessments; Michael Potenza for assistance in data management.
Funding sources: Research reported in this publication was supported by the National Institute On Aging of the National Institutes of Health under Award Number K01AG054700; by the Einstein Aging Study (PO1 AG03949) from the National Institutes on Aging program; the National Institutes of Health CTSA (1UL1TR001073) from the National Center for Advancing Translational Sciences (NCATS), the Sylvia and Leonard Marx Foundation, and the Czap Foundation. The Memory and Aging Project was supported by grant R01AG17917 from the National Institute on Aging. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflicts of Interest: All authors declare that there are no financial, personal, or other potential conflicts of interest.
References
- 1.Hampel H, O’Bryant SE, Durrleman S, Younesi E, Rojkova K, Escott-Price V, et al. A Precision Medicine Initiative for Alzheimer’s disease: the road ahead to biomarker-guided integrative disease modeling. Climacteric : the journal of the International Menopause Society 2017;20(2):107–18. [DOI] [PubMed] [Google Scholar]
- 2.Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimer’s Research & Therapy 2014;6(4):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Godyn J, Jonczyk J, Panek D, Malawska B. Therapeutic strategies for Alzheimer’s disease in clinical trials. Pharmacol Rep 2016;68(1):127–38. [DOI] [PubMed] [Google Scholar]
- 4.Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis 2014;42(1):275–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark LR, Delano-Wood L, Libon DJ, McDonald CR, Nation DA, Bangen KJ, et al. Are empirically-derived subtypes of mild cognitive impairment consistent with conventional subtypes? J Int Neuropsychol Soc 2013;19(6):635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edmonds EC, Delano-Wood L, Clark LR, Jak AJ, Nation DA, McDonald CR, et al. Susceptibility of the conventional criteria for mild cognitive impairment to false-positive diagnostic errors. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 2015;11(4):415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zammit AR, Hall CB, Lipton RB, Katz MJ, Muniz-Terrera G. Identification of Heterogeneous Cognitive Subgroups in Community-Dwelling Older Adults: A Latent Class Analysis of the Einstein Aging Study. J Int Neuropsychol Soc 2018:1–13. [DOI] [PMC free article] [PubMed]
- 8.Katz MJ, Lipton RB, Hall CB, Zimmerman ME, Sanders AE, Verghese J, et al. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer disease and associated disorders 2012;26(4):335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buschke H Selective reminding for analysis of memory and learning. Journal of Verbal Learning and Verbal Behavior 1973;12:543–50. [Google Scholar]
- 10.Buschke H Cued recall in amnesia. J Clin Neuropsychol 1984;6(4):433–40. [DOI] [PubMed] [Google Scholar]
- 11.Grober E, Dickson D, Sliwinski MJ, Buschke H, Katz M, Crystal H, et al. Memory and mental status correlates of modified Braak staging. Neurobiol Aging 1999;20(6):573–9. [DOI] [PubMed] [Google Scholar]
- 12.Wechsler D Wechsler Memory Scale - Revised San Antonio: The Psychological Corporation; 1987. [Google Scholar]
- 13.Benton AL, Hamsher K. Multilingual Aphasia Examination Iowa City AJA Assoc; 1989. [Google Scholar]
- 14.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test Second Edition ed. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 15.Wechsler D Adult Intelligence Scale-III 3rd ed. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 16.Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol 1992;49(12):1253–8. [DOI] [PubMed] [Google Scholar]
- 17.Battery AIT. Manual of Directions and Scoring Washington DC: War Department, Adjutant General’s Office; 1944. [Google Scholar]
- 18.Wilson RS, Boyle PA, Yu L, Barnes LL, Sytsma J, Buchman AS, et al. Temporal course and pathologic basis of unawareness of memory loss in dementia. Neurology 2015;85(11):984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson RS, Aggarwal NT, Barnes LL, Bienias JL, Mendes de Leon CF, Evans DA. Biracial population study of mortality in mild cognitive impairment and Alzheimer disease. Archives of neurology 2009;66(6):767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franzen MD, Burgess EJ, Smith-Seemiller L. Methods of Estimating Premorbid Functioning. Archives of Clinical Neuropsychology 1997;12(8):711–38. [PubMed] [Google Scholar]
- 21.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry 1983;140(6):734–9. [DOI] [PubMed] [Google Scholar]
- 22.D’Agostino RB Sr., Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117(6):743–53. [DOI] [PubMed] [Google Scholar]
- 23.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34(7):939–44. [DOI] [PubMed] [Google Scholar]
- 24.Muthén LK, Muthén BO. MPlus User’s Guide Seventh Edition ed. Los Angeles CA: Muthén & Muthén; 1998–2016. [Google Scholar]
- 25.SPSS Inc. IBM SPSS Statistics for Windows Armonk, NY: IBM Corp.; Released 2016. [Google Scholar]
- 26.Devanand DP, Liu X, Tabert MH, Pradhaban G, Cuasay K, Bell K, et al. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer’s disease. Biological psychiatry 2008;64(10):871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomar JJ, Bobes-Bascaran MT, Conejero-Goldberg C, Davies P, Goldberg TE. Utility of combinations of biomarkers, cognitive markers, and risk factors to predict conversion from mild cognitive impairment to Alzheimer disease in patients in the Alzheimer’s disease neuroimaging initiative. Archives of general psychiatry 2011;68(9):961–9. [DOI] [PubMed] [Google Scholar]
- 28.Landau SM, Harvey D, Madison CM, Reiman EM, Foster NL, Aisen PS, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology 2010;75(3):230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang YL, Bondi MW, Fennema-Notestine C, McEvoy LK, Hagler DJ Jr., Jacobson MW, et al. Brain substrates of learning and retention in mild cognitive impairment diagnosis and progression to Alzheimer’s disease. Neuropsychologia 2010;48(5):1237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salmon DP, Bondi MW. Neuropsychological Assessment of Dementia. Annual review of psychology 2009;60:257–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jak AJ, Bangen KJ, Wierenga CE, Delano-Wood L, Corey-Bloom J, Bondi MW. Contributions of neuropsychology and neuroimaging to understanding clinical subtypes of mild cognitive impairment. International review of neurobiology 2009;84:81–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 2009;17(5):368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eppig JS, Edmonds EC, Campbell L, Sanderson M, Delano-Wood L, Bondi MW, et al. Statistically-Derived Subtypes and Associations with Cerebrospinal Fluid and Genetic Biomarkers in Mild Cognitive Impairment: A Latent Profile Analysis. Journal of the International Neuropsychological Society : JINS 2017;23(7):564–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ylikoski R, Ylikoski A, Keskivaara P, Tilvis R, Sulkava R, Erkinjuntti T. Heterogeneity of cognitive profiles in aging: successful aging, normal aging, and individuals at risk for cognitive decline. Eur J Neurol 1999;6(6):645–52. [DOI] [PubMed] [Google Scholar]
- 35.Libon DJ, Xie SX, Eppig J, Wicas G, Lamar M, Lippa C, et al. The heterogeneity of mild cognitive impairment: a neuropsychological analysis. Journal of the International Neuropsychological Society : JINS 2010;16(1):84–93. [DOI] [PubMed] [Google Scholar]
- 36.Zahodne LB, Schupf N, Brickman AM, Mayeux R, Wall MM, Stern Y, et al. Dementia Risk and Protective Factors Differ in the Context of Memory Trajectory Groups. Journal of Alzheimer’s disease : JAD 2016;52(3):1013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peter J, Abdulkadir A, Kaller C, Kummerer D, Hull M, Vach W, et al. Subgroups of Alzheimer’s disease: stability of empirical clusters over time. Journal of Alzheimer’s disease : JAD 2014;42(2):651–61. [DOI] [PubMed] [Google Scholar]
- 38.Lam LC, Leung T, Lui VW, Leung VP, Chiu HF. Association between cognitive function, behavioral syndromes and two-year clinical outcome in Chinese subjects with late-onset Alzheimer’s disease. Int Psychogeriatr 2006;18(3):517–26. [DOI] [PubMed] [Google Scholar]
- 39.Alladi S, Arnold R, Mitchell J, Nestor PJ, Hodges JR. Mild cognitive impairment: applicability of research criteria in a memory clinic and characterization of cognitive profile. Psychological medicine 2006;36(4):507–15. [DOI] [PubMed] [Google Scholar]
- 40.Hofer SM, Piccinin AM. Integrative data analysis through coordination of measurement and analysis protocol across independent longitudinal studies. Psychological methods 2009;14(2):150–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofer SM, Piccinin AM. Toward an integrative science of life-span development and aging. The Journals of Gerontology: Series B 2010;65B(3):269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


