Abstract
Cardiac fibroblasts deposit and maintain extracellular matrix during organogenesis and under physiological conditions. In the adult heart, activated cardiac fibroblasts also participate in the healing response after acute myocardial infarction and during chronic disease states characterized by augmented interstitial fibrosis and ventricular remodelling. However, delineation of the characteristics, plasticity, and origins of cardiac fibroblasts is an area of on-going investigation and controversy. A set of genetic mouse models has been developed that specifically addresses the nature of these cells, in terms of both their origins and their response during cardiac disease and ventricular remodelling. As our understanding of cardiac fibroblasts becomes more defined and refined, so does the potential to develop new therapeutic strategies to control fibrosis and adverse ventricular remodelling.
Heart disease is the leading cause of mortality in the developed world. Coronary artery disease and its sequelae, including myocardial infarction (MI) and heart failure, are underlying causes of this lethality1. MI injury causes acute necrosis of cardiomyocytes in the affected area of the heart, with concomitant generation of a reparative fibrotic scar that, in the short term, prevents ventricular wall rupture. However, in long-standing heart failure, interstitial fibrosis accumulates throughout the heart, leading to wall and septal stiffening and progressively worsening cardiac function2. Acute healing fibrosis after MI and longstanding progressive interstitial fibrosis during heart failure are primarily mediated by the activation and function of fibroblasts3−5. The cardiac fibroblast is a plentiful and definable cell type in the heart that expresses a defined array of extracellular matrix (ECM) proteins with type I collagen being the prototypical component. Disease-activated fibroblasts have traditionally been referred to as myofibroblasts, in part because they express contractile genes such as ACTA2, which encodes smooth muscle α-actin (α-SMA)2−5. These activated fibroblasts also become more highly specialized in the generation and secretion of fibrillar collagens and matricellular proteins2–5. However, the functional properties of these cells have been largely documented in culture6; consequently, the details of how activated fibroblasts perform in vivo are still a topic of investigation. Further insights are also needed to determine whether the term myofibroblasts, which designates a contractile cell, is appropriate for fibroblasts in the diseased heart. Indeed, in the injured lung, noncontractile but activated fibroblasts (therefore not myofibroblasts) seem to be even more adept at ECM production and driving the fibrotic response7.
Possibly owing to a lack of clear definitions for the cardiac fibroblast, many facets of fibroblast biology remain a topic of on-going debates4,5. The generation and characterization of genetic mouse models with fibroblast-specific or ‘activated fibroblast’-specific allele expression of Cre recombinase has allowed researchers to assess the role of cardiac fibroblasts in the mouse heart in more exact terms. In this Review, we discuss insights gained from the use of genetically engineered mice that allow a systematic evaluation of fibroblast identity8. The emerging picture suggests that, despite previous claims that activated fibroblasts transdifferentiate from a wide array of unrelated cell types, the majority of injury-activated, matrix-producing cells seem to expand from tissue-resident fibroblasts that are already present in the myocardium and poised to respond9−12. Given that the effectors involved in fibroblast activation have been reviewed previously13,14, this article focuses primarily on current efforts to define the fibroblast population in the adult myocardium.
Resident cardiac fibroblasts
Initial studies first using morphology and flow cytometry have estimated that fibroblasts constitute between 27% and 50% of the total cells in mouse and rat ventricles, respectively15−17. Subsequently, a combination of histological and flow cytometric measurements were used to quantify fibroblasts. Although similar approaches had been used previously, refinements such as optimized tissue digestion, multiparametric analysis, and use of several cardiac fibroblast-specific mouse lines allowed robust calculation of the relative amounts of noncardiomyocytes in the mouse and human hearts. In agreement with some previous findings, endothelial cells far outnumbered the fibroblasts, which were less abundant than previously thought. Approximately 10% of the total cell number in the adult mouse myocardium is comprised of resident adventitial and interstitial fibroblasts18.
These resident cardiac fibroblasts (FIG. 1) are critical in the structural and mechanical maintenance of the heart. They coordinate the production and remodelling of the collagen network that is critical in ensuring correct conductivity and rhythmicity throughout the heart2,19,20. However, the presumed basal functions of fibroblasts in regulating the ECM and structural support of the heart are somewhat implied. Although embryonic loss of fibroblasts results in hearts with substantial reduction in type I and type III collagens, perinatal lethality precludes further investigation into additional roles of fibroblasts in the adult heart21,22. Indeed, researchers have largely assumed that activated fibroblasts and/or myofibroblasts (FIG. 1) are the exclusive source of new collagen production (such as type I, II, III, V, and VI collagens). However, this concept needs further mechanistic evaluation in vivo because other cell types can make and secrete particular types of collagens, including epithelial and endothelial cells23−25, skeletal muscle myotubes and fibers26−28, and cardiac myocytes29,30. Therefore, to characterize all the roles of fibroblasts and activated fibroblasts in the heart, one would need to delete these cells or inactivate them at specific times. Researchers are beginning to undertake these mechanistic approaches in disease models or after acute injury, but little has been done to understand the long-term basal role of tissue-resident fibroblasts in the heart. Moreover, a thorough understanding of the regulation and function of activated fibroblasts or myofibroblasts after acute and chronic injury requires additional investigation (BOX 1).
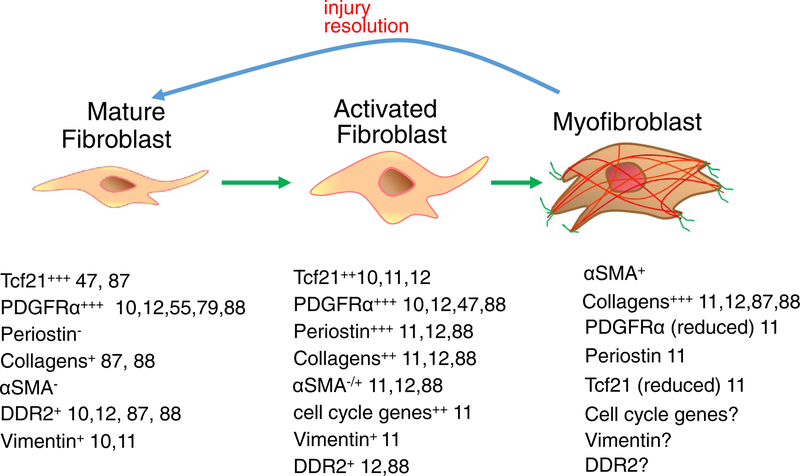
Figure 1 |. Current categories of resident cardiac fibroblasts.
At least three gene-expression profiles can be used to describe fibroblasts in the adult heart10−12,87,88. Mature, resident fibroblasts are interspersed in the myomesial space throughout the heart and are proposed to maintain the extracellular matrix (ECM). They have a low level of proliferation and do not express smooth muscle α-actin (α-SMA) or periostin. After injury, a population of fibroblasts, often associated with inflammatory cell accumulation and cardiomyocyte death, rapidly proliferate and become activated to express many of the gene products shown. Later in the response, a smaller population of these activated fibroblasts further differentiate into presumed myofibroblasts, which express α-SMA and other genetic signatures shown. In the past, these myofibroblasts were reported to have increased ECM deposition and contractile capacities89, although in vivo contractile activity has been predominantly documented in skin myofibroblasts90. Myofibroblasts have also been shown to regress their activated gene-expression profile and morphology back to the more ‘quiescent’ and resident fibroblast state11. Ddr2, discoidin domain-containing receptor 2; NA, not available; Pdgfr-α, platelet-derived growth factor receptor-α; Tcf21; transcription factor 21.
Box 1 | How are fibroblasts activated?
In response to cardiac injury, both cytokine and neurohumoral factors are released and profound changes occur in the mechanical strain relationships within the ventricular wall (or septum), which together are thought to underlie fibroblast activation. A central cytokine involved in fibroblast activation, at least as defined in cultured fibroblasts, is transforming growth factor-β (TGF-β)82. TGF-β binds to and causes heterodimerization of TGF-β receptor 1 and 2 that in turn causes direct phosphorylation of the cytoplasmically-localized transcription factors SMAD2/3, which then translocate to the nucleus in complex with SMAD4 to promote fibroblast differentiation-specific gene expression83. TGF-β receptor signalling as well as signalling from other neurohumoral factor receptors, such as angiotensin II receptor, also initiate fibroblast activation through engagement of mitogen-activated protein kinase (MAPK) effectors, such p38 (REFS 82,83). For example, activation of p38 in fibroblasts and other mesenchymal cell types induces collagen and smooth muscle a-actin (a-SMA) transcriptional activity as well as the appearance of α-SMA stress fibres84–86. A priority is to elucidate the entire molecular circuitry that regulates fibroblast activation, with the aim of identifying novel therapeutic approaches that might limit progressive cardiac fibrosis, as well as different stages of fibroblast differentiation in longstanding disease.
Another issue in attempting to elucidate the fundamental roles of cardiac fibroblasts is the somewhat nonspecific genetic tools that have previously been used to manipulate the activity or viability of these cells. For example, as discussed below, we have generated mice expressing a tamoxifen-inducible Cre protein from the periostin (Postn gene) locus, which was used to induce a diphtheria toxin expression cassette to eliminate newly activated fibroblasts in the heart after MI injury11. This approach increased lethality in mice owing to inefficient collagen production and ventricular rupture11, emphasizing a critical role for activated fibroblasts in wound healing and scar formation.
Previous studies aimed at elucidating the baseline and disease effects of cardiac fibroblasts have been somewhat equivocal, because they relied on markers that were only partially specific to this presumed cell type8. For example, discoidin domain-containing receptor 2 (DDR2), fibroblast-specific protein 1 (FSP1, also called protein S100-A4), Thy-1 membrane glycoprotein (CD90), and vimentin, which have all been used in past studies putatively to identify cardiac fibroblasts, are expressed by additional cell types in the heart. FSP1 or its promoter are expressed in many other cardiac cell types, with fibroblasts being in the minority34. Thy-1 is expressed on most immune and endothelial cells35, and DDR2 and vimentin are expressed in endothelial cells36−38. Herein lies the difficulty, as researchers had not reached a consensus definition of the fibroblast and its markers. Although still nascent, a consistent group of fibroblast markers is now emerging (FIG. 1) that has led to more consistency in defining this cell type and its function in the heart.
The latest data support the hypothesis that resident fibroblasts derived from the epicardium during embryonic development are the source of disease-relevant activated fibroblasts or myofibroblasts in the adult heart. However, past studies suggested that cardiac fibroblasts originated from heterogeneous pools of cells that required transdifferentiation to achieve a fibroblast phenotype39. This latter concept disregards the presence of fibroblasts that already reside in the myocardium to mediate tissue remodelling and ECM production. Moreover, tissue-resident fibroblasts are geometrically interspersed between cardiomyocytes and are of the appropriate molecular programme to allow rapid responsiveness after injury. The hypothesis that activated fibroblasts in the heart emerge from endothelial cells, pericytes, or immune cells would require that they first undergo molecular transdifferentiation, a process that would lack rapid responsiveness and homogenous spatial coverage throughout the tissue.
Embryonic origins of cardiac fibroblasts
Progenitors of resident ventricular fibroblasts invade the mouse myocardium from the epicardium at embryonic (E) day 13.5, with molecularly distinct fibroblasts forming and interspersing throughout the ventricle by E17.5-E18.521,22,40–43 (FIG. 2). This embryonic primary source of early epicardial-derived fibroblasts is defined by expression of the marker genes Tcf21 (encoding transcription factor 21)22, Wt1 (Wilms tumor protein)44,45, and Tbx18 (T-box transcription factor TBX18)46. A second, minor developmental source of fibroblasts from the embryonic endothelium, identified by two groups using lineage tracing, was shown to constitute ~10–20% of the tissue-resident fibroblasts in particular regions of the ventricular septum and right ventricle10,12 (FIG. 2). Of note, these cells arise early during embryonic development and are not generated by transdifferentiation of mature endothelial cells. In addition to Tcf21, Wt1, and Tbx18 being markers of early embryonic fibroblasts of epicardial origin, platelet-derived growth factor receptor-α (Pdgfr-α)21 is also used to identify these cells, although only Tcf21 and Pdgfr-α are expressed by resident mature fibroblasts of the adult heart11,21,22,47.
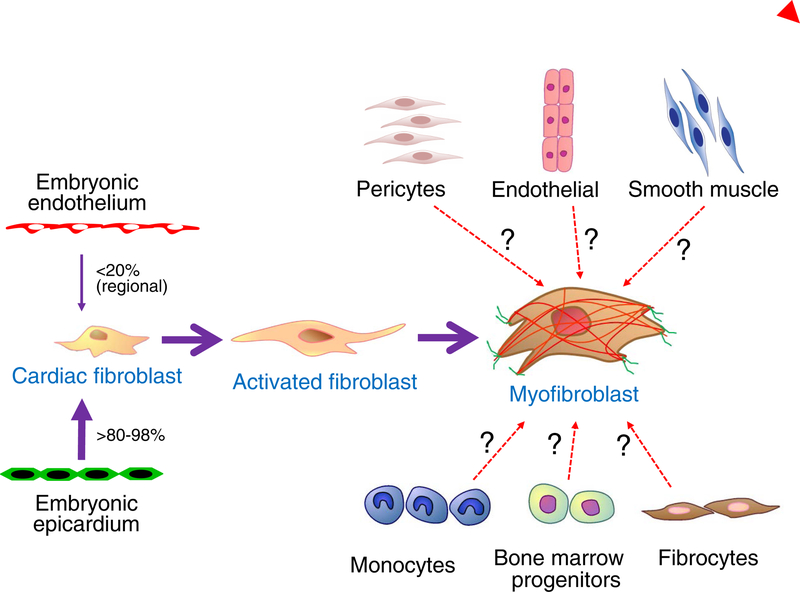
Figure 2 |. Developmental and alternative sources of fibroblasts.
There are two documented sources of tissue resident cardiac fibroblasts, the developmental epicardium and developmental endothelial cells. The epicardial-derived fibroblasts emerge by the process of epithelial-to-mesenchymal transition of the epicardium at embryonic day E13.5 in the mouse, and constitute a majority of the ventricular and atrial cardiac fibroblasts. How the endothelial-derived cardiac fibroblasts arise during development is uncertain, but they contribute tissue-resident fibroblasts to regions of the right ventricle and of the ventricular septum. Studies have suggested a number of nonfibroblast cellular sources, such as endothelial cells, smooth muscle cells, monocytes, fibrocytes, and bone-marrow progenitors (designated with question marks), as the primary origin for newly generated activated fibroblasts and myofibroblasts in the heart after injury. However, recent studies with more highly refined genetic markers have not confirmed these results, and instead have shown that tissue-resident cardiac fibroblasts of developmental origin generate all the activated fibroblasts and myofibroblasts after injury. Pericytes have been reported to have fibroblast-like qualities in the heart when activated, but further work is needed to understand the potential role of these cells54.
Origins of activated cardiac fibroblasts
The common method of classifying activated fibroblasts is by detection of gene expression associated with matrix remodelling (encoding selected collagen types, collagen-assembly proteins, and proteases) and/or those expressed by mesenchymal cells (encoding α-SMA, fibronectin, and vimentin). However, the successful detection of these proteins and use to infer fibroblast identity or activation has had technical problems, leading to conclusions that were generally inconsistent between laboratories. The diverse cell types that reportedly give rise to activated fibroblasts include endothelial cells48,49, bone-marrow-derived fibrocytes or immune cells50–52, mesenchymal stem/progenitor perivascular cells53,54, and adult epicardium55,56 (FIG. 2). Some of these reports examined gene expression of cultured fibroblasts, in which the fibroblast was identified by adhesion to plastic or flow cytometry. Others have relied predominantly on immunohistochemistry with some form of lineage tracing. Surprisingly, few studies implicated the resident fibroblast as a contributor to cardiac remodelling despite their fairly high abundance and obvious capacity to differentiate quickly into myofibroblasts without the need for transdifferentiation16,18.
Recent studies have demonstrated that the past conclusions regarding extracardiac sources and transdifferentiation of endothelial cells as major contributors to fibrotic activities might not be quantitatively accurate10–12 (FIG. 2). Multiple research groups, using bone-marrow chimeras, parabiosis, and bone-marrow-specific Cre lines, have reported either no or only modest contribution of bone-marrow-derived cells to the injury-responsive fibroblast population10−12. For example, lineage tracing of bone-marrow-derived cells with LysM-Cre57 or Kit-Cre58 showed no significant contribution of these lineages to the periostin-expressing activated fibroblasts after MI injury11. Similar results were obtained using Vav-Cre lineage tracing that demonstrated little contribution of the haematopoietic lineages to activated fibroblasts in the hearts of mice subjected to pressure-overload stress10. Further experiments with bone-marrow chimerism and parabiosis also showed minimal contribution of bone-marrow-derived cells to the proliferating fibroblast population after pressure overload12.
Endothelial-to-mesenchymal transition has been proposed as a mechanism for fibroblast generation after MI and experimental diabetes mellitus on the basis of endothelial lineage tracing with Tie1-Cre and Tie2-Cre lines, but both of these studies also relied on FSP1 to identify fibroblasts (FIG. 2)10,34. FSP1 is expressed in multiple cell types and is not a specific indicator of the fibroblast lineage in vivo, and the Tie1-Cre and Tie2-Cre lines are also expressed in immune cell lineages10,34. With the use of a range of distinct endothelial Cre lines, several reports now show that endothelial cell conversion to fibroblasts after injury is very low. When Cdh5-Cre, VE-cadherin-CreERT2 or Tie2-Cre lineage tagging was used to label pre-existing vascular endothelial cells, no fibroblasts were found to arise from this population after pressure overload or MI injury10–12. Finally, using single-cell RNAseq, endothelial, smooth muscle, and haematopoietic lineage cells were found not to have a marker profile consistent with an activated fibroblast (defined by a-SMA, periostin, and vimentin expression)11.
Interestingly, Ubil and colleagues reported that fibroblasts in the mouse heart, as lineage traced using a Col1a2-CreERT2 transgene, generated new endothelial cells in the infarcted myocardium, indicating mesenchymal-to-endothelial transition59. However, Kanisicak and colleagues did not observe re-expression of the endothelial cell marker CD31 after MI in two distinct cardiac fibroblast lineages, suggesting that tissue-resident and activated fibroblasts do not generate CD31-expressing endothelial cells11. After MI, smooth muscle cells and fibroblasts emerged from adult epicardium56,60, but after pressure overload by thoracic aortic constriction, few fibroblasts were found to originate from adult epicardium10,12. Data generated by ndependent laboratories using lineage tracing and chimeric mouse models, therefore, suggests hat injury-activated, matrix-producing cells from sources such as endothelium, smooth muscle, bone marrow, and blood might be substantially less abundant than previously thought (FIG. 2).
Injury-induced fibroblasts in the heart
More recent studies have made a convincing case that tissue-resident fibroblasts are the primary source of activated fibroblasts underlying tissue fibrosis and disease remodelling in the heart10–12,60. First, Kanisicak and colleagues used a newly generated genetic tool in which a tamoxifen-regulated mutated oestrogen receptor-Cre (mCrem) was inserted into the Postn locus. This process generated a highly specific means of lineage tracing essentially all activated fibroblasts in the heart subjected to pressure overload, MI injury, or neuroendocrine stimulation, without labelling of other cell types11. Using the PostnmCrem allele together with a ROSA26DTA, cellular killing allele61, activated fibroblasts were shown to be required for healing and scar formation after MI injury in vivo11. The same study also showed that the tissue-resident fibroblasts already in the heart were the overwhelming source of activated fibroblasts after MI injury, pressure overload, or infusion of a fibrosis-promoting neuroendocrine agonist cocktail11 (FIG. 2). Of note, during development and in other tissues, periostin is not exclusive to activated fibroblasts31−33. However, in adult, stressed cardiac ventricles, periostin is uniquely expressed by activated fibroblasts11.
The Tcf21 genetic locus was also used to express a tamoxifen-regulated Cre protein for lineage tracing, but this time to show that the overwhelming majority of tissue-resident fibroblasts in the heart were of developmental epicardial origin, and that this Cre-expressing knock-in allele was highly specific in the heart11,22 (FIG. 3). In support of this conclusion, Wt1Cre-EGFP lineage-traced epicardial derivatives44 also generated the vast majority of tissue-resident fibroblasts in the heart after pressure overload10. Although Wt1 and Tbx18 are expressed in embryonic fibroblast progenitors, these genes are not expressed by most mature fibroblasts in the adult heart62,63. However, Tcf21 continues to be expressed by adult cardiac fibroblasts47 (FIG. 3).
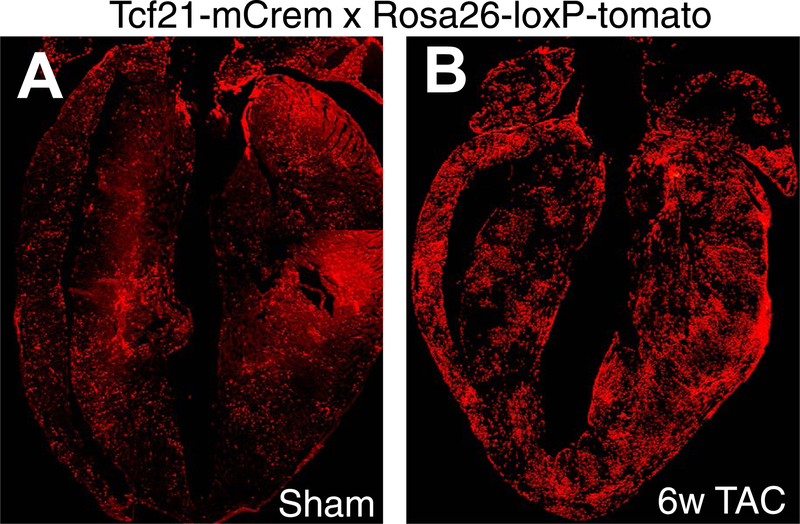
Figure 3 |. Lineage tracing of resident cardiac fibroblasts.
Hearts from adult Tcf21mCrem mice containing a Cre-dependent ROSA26TdT indicator that was induced with tamoxifen perinatally. Tissue-resident fibroblasts are labelled red. Mice were subjected to either a | a sham surgical procedure (no injury) or b | 6 weeks of a thoracic aortic constriction surgical procedure before harvesting. The images show that Tcf21-lineage tissue-resident fibroblasts are uniformly distributed throughout the heart and that they expand with pressure-overload stimulation.
When investigating the endothelial origin of the ventricular septal and right ventricular fibroblasts, two groups have demonstrated that these fibroblast populations can produce ECM in response to pressure overload10,12. Compared with the epicardial-derived fibroblasts, gene expression and proliferation were similar in the two cell populations after injury. In conclusion, the tissue-resident fibroblasts generated during development are the primary source of the majority of activated fibroblasts after cardiac injury. Activated tissue-resident fibroblasts also secrete factors that affect cardiac hypertrophy, but that are also likely to enhance the acute injury response and scar formation (reviewed previously14,64).
Human cardiac fibroblasts and fibrosis
Much of our knowledge about cardiac fibroblasts has been derived from studies using experimental animal models. Indeed, we know considerably less about the biology of human cardiac fibroblasts. Many studies on human cardiac fibroblasts have relied on primary in vitro culture techniques for their expansion and subsequent manipulation6. Other studies have examined fibroblasts using histological analysis of post-mortem hearts and the use of α-SMA to identify myofibroblasts65. One study using morphometric analysis demonstrated that mesenchymal cells, which include fibroblasts and pericytes, comprise roughly 50% of the cells in the healthy human heart. This study used 14C dating of cells from pathology-free, post-mortem hearts to calculate fibroblast longevity. The entire population of mesenchymal cells was estimated to be renewed about twice during a normal human lifespan and that numbers of these cells peak at age 30 years and then decline66.
Although post-mortem studies can provide useful information about fibroblast location and numbers, lack of definitive methods to identify cardiac fibroblasts in the human heart is still problematic. The alternative is to measure the products of fibroblast activity by either imaging or biochemical assays. The noninvasive visualization of fibrotic scarring in the heart using MRI has become widespread, and has suggested that the pattern of scarring is dependent on the severity and type of insult67. A second method for monitoring emergent fibrosis is measurement of circulating factors68. Indeed, some biomarkers have been identified that can be used to indicate the extent of matrix deposition, although how this parameter might correlate with total fibroblast content in the heart is uncertain14.
Given that ECM production or remodelling is a hallmark of fibroblast activation, a number of individual ECM, remodelling, and matricellular proteins have been proposed as markers for fibrosis, although some studies have not identified a reliable correlation between the amount of myocardial fibrosis and circulating collagen components69–71. A combination of biomarkers predicting type I collagen crosslinking (ratio of circulating type I collagen to matrix metalloproteinase 1 [MMP1; also known as interstitial collagenase]) and deposition (procollagen type I peptide) has been examined. In this study, poor outcomes were associated with low type I collagen crosslinking and high type I collagen deposition in patients with hypertension and heart failure72. Another complication is that some presumed fibrosis-indicating proteins can be secreted by inflammatory cells or possibly even other cell types present in the myocardium during injury or longstanding fibrotic disease. Galectin 3, a proposed mediator of vascular fibrosis and inflammation, might be a more reliable biomarker for heart failure with associated fibrosis73,74. The fibrosis-associated microRNAs miR-21 and miR-19b have been suggested as circulating measures of cardiac fibrosis and heart failure75,76. However, the current measures of fibroblast number and activity in the human heart, as well as total fibrotic content, remain unreliable and often indeterminate. Further molecular dissection of cardiac fibroblasts in animal models is likely to suggest alternative markers for application to human hearts to improve evaluation and tracking of ongoing disease, which is important for advances in treatment of fibrotic disease states with emerging therapeutic agents or strategies.
Unanswered questions
Using the newly generated and verified genetic lineage-tracing mouse models discussed throughout this Review, researchers can now more reliably address fundamental concepts in fibroblast biology that remain unexplored. One very simple question is what happens to activated fibroblasts in a heart with injury resolution after MI, such as those cells within a stable scar after the acute healing process? The same question holds for the fate of activated fibroblasts in hearts when a pressure-overload stimulus is removed. The two possibilities are cellular apoptosis or reversion of the cell to its previous basal state. Studies have shown a dramatic expansion of activated fibroblast numbers in the heart from tissue-resident fibroblasts after MI injury or pressure-overload10,11. Kanisicak and colleagues also conducted a specific experiment in which fibroblasts were activated and lineage traced in hearts with the PostnmCrem mouse line during angiotensin II and phenylephrine infusion, followed by histological analysis 2 weeks after the infusion was stopped so that the injury response regressed. The data showed that lineage-traced fibroblasts from the activation period were still present in the heart, and that they had a molecular programme more similar to a resident fibroblast than an activated fibroblast11. However, total PostnmCrem lineage-traced fibroblast numbers were also substantially reduced, suggestive of cellular loss11. Many activated fibroblasts are, therefore, likely to undergo apoptosis after injury resolution in vivo, but some revert back to a baseline, ‘resting’ state. These concepts need additional in-depth analysis to understand the dynamics of fibroblast differentiation plasticity in vivo. Related to this concept, it is intriguing why fully differentiated, a-SMA-expressing fibroblasts lack cellular stability and longevity, suggesting that they are not a truly differentiated cell type as classically defined, but are instead a ‘supraphysiological’ state that requires continuous input for their maintenance. However, myofibroblasts resident in the scar of an MI-injured heart might also remain indefinitely, given the ongoing stress and strain environment of the scar. If the contractile activity of myofibroblasts is indeed important, their presence might help to maintain scar tension and stability over time (BOX 1).
Another question is whether stable, intermediate states of fibroblast activation exist, or instead whether a scalable continuum of differentiation states exist that interpret the degree of cytokine and mechanical stimulation. For example, one of the first events in fibroblast activation seems to be periostin expression, followed by α-SMA expression11. One possible means of examining the question of lineage substates is single-cell RNA sequencing of activated fibroblasts isolated from the hearts of PostnmCrem lineage-traced mice11. These cells could be removed for analysis from the same region at different time points after injury, or from different regions of the heart so that cells in the scar that experience greater mechanical load could be compared with cells in the border zone with increased compliance. These gene-expression profiles could be clustered in an attempt to identify stable, intermediate differentiated states based on marker genes, or to build a model of a more fluid continuum of differentiated gene expression.
As discussed previously, fibroblasts are likely to be the primary mediators of the production of selected ECM proteins in the heart, as well as in most other tissues in vertebrate organisms. However, cardiomyocytes contain a basal lamina of selected ECM proteins (especially fibronectin, but also α1 type I collagen and type VI collagen) that form early in cardiac development before fibroblasts enter the heart. Moreover, lower organisms such Drosophila lack the fibroblast cell type despite having full capacity to mount a fibrotic response with collagen secretion by tissue parenchymal cells77−81. Therefore, whether fibroblasts are the only relevant source of collagen and ECM production in the heart, either at baseline or with acute and chronic injury, remains to be tested. Indeed, mammalian cardiac myocytes can express and secrete collagens that could easily contribute to healing in the border areas of an MI29,30.
The capacity of activated fibroblasts to contract and remodel the heart in vivo through expression of smooth muscle-related contractile genes is also of unproven mechanistic importance. This unanswered question has direct implications in naming activated fibroblasts as myofibroblasts or possibly something else. Indeed, mitogen-activated protein kinase-activated protein kinase 2 (Mapkapk2)-deficient mice lack expression of important contractile genes in their activated fibroblasts during lung injury, even though these activated fibroblasts produce ECM even more abundantly than control fibroblasts replete in Mapkapk27. In the future, it will be interesting to distinguish the ECM-producing functions of activated fibroblasts from their presumed contractile activity in the heart.
Conclusions
Many cellular functions have been attributed to cardiac fibroblasts during homeostasis and cardiovascular remodelling, but the lack of a clear definition of a fibroblast might have led to inaccurate assumptions about this population. Our improved capacity to track fibroblasts in vivo is now providing exciting new avenues to understand and target maladaptive fibroblast activities in the heart, as well as potentially important adaptive or physiological functions. A stronger grasp of the identity and behaviour of cardiac fibroblasts and their different functional stages with defined molecular signatures will be critical in generating and evaluating new antifibrotic therapies for cardiovascular disease in humans, as well as designing new biomarkers to track fibrotic remodelling and evaluate treatment efficacy.
Acknowledgements
We thank Jill T. Kuwabara from the Tallquist laboratory at the University of Hawaii for original images in Figure 3.
Author biographies
Jeffery D. Molkentin, PhD. Is a Professor in the Department of pediatrics, University of Cincinnati and Cincinnati Children’s Hospital (USA). He received his PhD from the Medical College of Wisconsin in 1994, after which he performed postdoctoral training with Dr. Eric Olson in Texas from 1994–1997, followed by his first faculty appointment in 1997 at the Cincinnati Children’s Hospital Medical Center where he remains today. Dr. Molkentin is a full investigator of the Howard Hughes Medical Institute since 2008. Dr Molkentin has placed more than 20 of his past trainees into academics as laboratory principle investigators. Dr. Molkentin’s research program continues to focus on the identification of candidate genes and signaling pathways involved in cardiac hypertrophy, contractility, cell death, heart failure, fibrosis and muscular dystrophy, as well as mitochondria-dependent necrosis.
Michelle D. Tallquist Ph.D is an Associate Professor in the Department of Medicine, in the Center for Cardiovascular Research at the University of Hawaii. She received a BA in Chemistry from Kalamazoo College in 1989. After obtaining her degree, she worked several years as an analytical chemist at The Upjohn Company. In 1992 she entered graduate school at the Mayo Clinic, where she studied immunology. For her thesis work she investigated the molecular basis of graft rejection. Her post-doctoral work was carried out in Seattle at the Fred Hutchinson Cancer Research Center where she used the power of mouse molecular genetics to investigate Platelet Derived Growth Factor signaling in vivo. For 10 years, she was faculty at the University of Texas Southwestern Medical Center in Dallas. Her research focuses on understanding the signals that direct fibroblast differentiation and activation during organogenesis and disease processes. Using the mouse, she has defined signaling pathways that are necessary for cardiac fibroblast formation and is generating models to manipulate fibroblast numbers and activation during tissue homeostasis and disease. The long term goal of her research is to understand the beneficial and detrimental actions of the extracellular matrix and growth factors produced by fibroblasts and to identify signaling pathways unique to the fibroblast
Footnotes
Competing interests statement
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mozaffarian D et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 131, e29–322 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Gourdie RG, Dimmeler S & Kohl P Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease. Nat. Rev. DrugDiscov 15, 620–638 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shinde AV & Frangogiannis NG Fibroblasts in myocardial infarction: a role in inflammation and repair. J. Mol. Cell. Cardiol 70, 74–82 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis J & Molkentin JD Myofibroblasts: trust your heart and let fate decide. J. Mol. Cell. Cardiol 70, 9–18 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borthwick LA, Wynn TA & Fisher AJ Cytokine mediated tissue fibrosis. Biochim. Biophys. Acta 1832, 1049–1060 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter KE & Turner NA Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol. Ther 123, 255–278 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Liu T et al. Lack of MK2 inhibits myofibroblast formation and exacerbates pulmonary fibrosis. Am. J. Respir. Cell. Mol. Biol 37, 507–517 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swonger JM, Liu JS, Ivey MJ & Tallquist MD Genetic tools for identifying and manipulating fibroblasts in the mouse. Differentiation 92, 66–83 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore-Morris T, Guimaraes-Camboa N, Yutzey KE, Puceat M & Evans SM Cardiac fibroblasts: from development to heart failure. J. Mol. Med. (Berl.) 93, 823–830 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore-Morris T et al. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J. Clin. Invest 124, 2921–2934 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanisicak O et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun 7, 12260 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali SR et al. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ. Res 115, 625–635 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Weber KT, Sun Y, Bhattacharya SK, Ahokas RA & Gerling IC Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat. Rev. Cardiol 10, 15–26 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Weber KT & Diez J Targeting the cardiac myofibroblast secretome to treat myocardial fibrosis in heart failure. Circ. Heart Fail 9, e003315 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Nag AC Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios 28, 41–61 (1980). [PubMed] [Google Scholar]
- 16.Banerjee I, Fuseler JW, Price RL, Borg TK & Baudino TA Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am. J. Physiol. Heart Circ. Physiol 293, H1883–H1891 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Zak R Development and proliferative capacity of cardiac muscle cells. Circ. Res 35 (Suppl. II), 17–26 (1974). [PubMed] [Google Scholar]
- 18.Pinto AR et al. Revisiting cardiac cellular composition. Circ. Res 118, 400–409 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caulfield JB & Borg TK The collagen network of the heart. Lab. Invest 40, 364–372 (1979). [PubMed] [Google Scholar]
- 20.Camelliti P, Borg TK & Kohl P Structural and functional characterisation of cardiac fibroblasts. Cardiovasc. Res 65, 40–51 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Smith CL, Baek ST, Sung CY & Tallquist MD Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circ. Res 108, e15–e26 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acharya A et al. The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development 139, 2139–2149 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaage J & Lindblad WJ Production of collagen type I by mouse peritoneal macrophages. J. Leukoc. Biol 48, 274–280 (1990). [DOI] [PubMed] [Google Scholar]
- 24.Vaage J & Harlos JP Collagen production by macrophages in tumour encapsulation and dormancy. Br. J. Cancer 63, 758–762 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi M et al. Secretion of collagen types I and II by epithelial and endothelial cells in the developing chick cornea demonstrated by in situ hybridization and immunohistochemistry. Development 103, 27–36 (1988). [DOI] [PubMed] [Google Scholar]
- 26.Alexakis C, Partridge T & Bou-Gharios G Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am. J. Physiol. Cell. Physiol 293, C661–C669 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Liu E et al. Secreted collagen induced by ascorbic acid in L5 cloned muscle cultures does not affect acetylcholine receptor expression. Exp. Cell Res 209, 76–81 (1993). [DOI] [PubMed] [Google Scholar]
- 28.Zanotti S et al. Altered extracellular matrix transcript expression and protein modulation in primary Duchenne muscular dystrophy myotubes. Matrix Biol 26, 615–624 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Borg TK, Johnson LD & Lill PH Specific attachment of collagen to cardiac myocytes: in vivo and in vitro. Dev. Biol 97, 417–423 (1983). [DOI] [PubMed] [Google Scholar]
- 30.Fisher SA & Periasamy M Collagen synthesis inhibitors disrupt embryonic cardiocyte myofibrillogenesis and alter the expression of cardiac specific genes in vitro. J. Mol. Cell. Cardiol 26, 721–731 (1994). [DOI] [PubMed] [Google Scholar]
- 31.Snider P et al. Origin of cardiac fibroblasts and the role of periostin. Circ. Res 105, 934–947 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snider P et al. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ. Res 102, 752–760 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian L et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 485, 593–598 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong P, Christia P, Saxena A, Su Y & Frangogiannis NG Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. Am. J. Physiol. Heart Circ. Physiol 305, H1363–H1372 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hudon-David F, Bouzeghrane F, Couture P & Thibault G Thy-1 expression by cardiac fibroblasts: lack of association with myofibroblast contractile markers. J. Mol. Cell. Cardiol 42, 991–1000 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Perez-Pomares JM, Macias D, Garcia-Garrido L & Munoz-Chapuli R Contribution of the primitive epicardium to the subepicardial mesenchyme in hamster and chick embryos. Dev. Dyn 210, 96–105 (1997). [DOI] [PubMed] [Google Scholar]
- 37.Zhang S et al. A host deficiency of discoidin domain receptor 2 (DDR2) inhibits both tumour angiogenesis and metastasis. J. Pathol 232, 436–448 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Goldsmith EC, Zhang X, Watson J, Hastings J & Potts JD The collagen receptor DDR2 is expressed during early cardiac development. Anat. Rec. (Hoboken) 293, 762–769 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeisberg EM & Kalluri R Origins of cardiac fibroblasts. Circ. Res 107, 1304–1312 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asli N, Xaymardan M & Harvey R Epicardial origin of resident mesenchymal stem cells in the adult mammalian heart. J. Dev. Biol 2, 117–137 (2014). [Google Scholar]
- 41.Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, Gourdie RG & Poelmann RE Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ. Res 82, 1043–1052 (1998). [DOI] [PubMed] [Google Scholar]
- 42.Mikawa T & Gourdie RG Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev. Biol 174, 221–232 (1996). [DOI] [PubMed] [Google Scholar]
- 43.Dettman RW, Denetclaw W Jr., Ordahl CP & Bristow J Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev. Biol 193, 169–181 (1998). [DOI] [PubMed] [Google Scholar]
- 44.Zhou B et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454, 109–113 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wessels A et al. Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Dev. Biol 366, 111–124 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai CL et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature 454, 104–108 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braitsch CM, Kanisicak O, van Berlo JH, Molkentin JD & Yutzey KE Differential expression of embryonic epicardial progenitor markers and localization of cardiac fibrosis in adult ischemic injury and hypertensive heart disease. J. Mol. Cell. Cardiol 65, 108–119 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeisberg EM et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med 13, 952–961 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Widyantoro B et al. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation 121, 2407–2418 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Haudek SB et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc. Natl Acad. Sci. USA 103, 18284–18289 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mollmann H et al. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc. Res 71, 661–671 (2006). [DOI] [PubMed] [Google Scholar]
- 52.van Amerongen MJ et al. Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. J. Pathol 214, 377–386 (2008). [DOI] [PubMed] [Google Scholar]
- 53.Ieronimakis N et al. Coronary adventitial cells are linked to perivascular cardiac fibrosis via TGFbeta1 signaling in the mdx mouse model of Duchenne muscular dystrophy. J. Mol. Cell. Cardiol 63, 122–134 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kramann R et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16, 51–66 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Wijk B, Gunst QD, Moorman AF & van den Hoff MJ Cardiac regeneration from activated epicardium. PLoS ONE 7, e44692 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou B et al. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J. Clin. Invest 121, 1894–1904 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clausen BE, Burkhardt C, Reith W, Renkawitz R & Forster I Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 8, 265–277 (1999). [DOI] [PubMed] [Google Scholar]
- 58.van Berlo JH et al. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 509, 337–341 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ubil E et al. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature 514, 585–590 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruiz-Villalba A et al. Interacting resident epicardium-derived fibroblasts and recruited bone marrow cells form myocardial infarction scar. J. Am. Coll. Cardiol 65, 2057–2066 (2015). [DOI] [PubMed] [Google Scholar]
- 61.Ivanova A et al. In vivo genetic ablation by Cre-mediated expression of diphtheria toxin fragment A. Genesis 43, 129–135 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duim SN, Kurakula K, Goumans MJ & Kruithof BP Cardiac endothelial cells express Wilms’ tumor-1: Wt1 expression in the developing, adult and infarcted heart. J. Mol. Cell. Cardiol 81, 127–135 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Guimaraes-Camboa N et al. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell 20, 345–359.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fujiu K & Nagai R Fibroblast-mediated pathways in cardiac hypertrophy. J. Mol. Cell. Cardiol 70, 64–73 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Willems IE, Havenith MG, De Mey JG & Daemen MJ The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am. J. Pathol 145, 868–875 (1994). [PMC free article] [PubMed] [Google Scholar]
- 66.Bergmann O et al. Dynamics of cell generation and turnover in the human heart. Cell 161, 1566–1575 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Mewton N, Liu CY, Croisille P, Bluemke D & Lima JA Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J. Am. Coll. Cardiol 57, 891–903 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tziakas DN et al. Independent and additive prognostic ability of serum carboxy-terminal telopeptide of collagen type-I in heart failure patients: a multi-marker approach with high-negative predictive value to rule out long-term adverse events. Eur. J. Prev. Cardiol 19, 62–71 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Ellims AH et al. Evaluating the utility of circulating biomarkers of collagen synthesis in hypertrophic cardiomyopathy. Circ. Heart Fail 7, 271–278 (2014). [DOI] [PubMed] [Google Scholar]
- 70.Kupari M, Laine M, Turto H, Lommi J & Werkkala K Circulating collagen metabolites, myocardial fibrosis and heart failure in aortic valve stenosis. J. Heart Valve Dis 22, 166–176 (2013). [PubMed] [Google Scholar]
- 71.Gyongyosi M et al. Myocardial fibrosis: biomedical research from bench to bedside. Eur. J. Heart Fail 19, 177–191 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ravassa S et al. Phenotyping of myocardial fibrosis in hypertensive patients with heart failure. Influence on clinical outcome. J. Hypertens 35, 853–861 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Ho JE et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J. Am. Coll. Cardiol 60, 1249–1256 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lopez-Andres N et al. Association of galectin-3 and fibrosis markers with long-term cardiovascular outcomes in patients with heart failure, left ventricular dysfunction, and dyssynchrony: insights from the CARE-HF (Cardiac Resynchronization in Heart Failure) trial. Eur. J. Heart Fail 14, 74–81 (2012). [DOI] [PubMed] [Google Scholar]
- 75.Villar AV et al. Myocardial and circulating levels of microRNA-21 reflect left ventricular fibrosis in aortic stenosis patients. Int. J. Cardiol 167, 2875–2881 (2013). [DOI] [PubMed] [Google Scholar]
- 76.Beaumont J et al. MicroRNA-19b is a potential biomarker of increased myocardial collagen cross-linking in patients with aortic stenosis and heart failure. Sci. Rep 7, 40696 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bunt S et al. Hemocyte-secreted type IV collagen enhances BMP signaling to guide renal tubule morphogenesis in Drosophila. Dev. Cell 19, 296–306 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bunt S, Denholm B & Skaer H Characterisation of the Drosophila procollagen lysyl hydroxylase, dPlod. Gene Expr. Patterns 11, 72–78 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shahab J et al. Loss of SPARC dysregulates basal lamina assembly to disrupt larval fat body homeostasis in Drosophila melanogaster. Dev. Dyn 244, 540–552 (2015). [DOI] [PubMed] [Google Scholar]
- 80.Zang Y et al. Plasma membrane overgrowth causes fibrotic collagen accumulation and immune activation in Drosophila adipocytes. Elife 4, e07187 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pastor-Pareja JC & Xu T Shaping cells and organs in Drosophila by opposing roles of fat body-secreted Collagen IV and perlecan. Dev. Cell 21, 245–256 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stempien-Otero A, Kim DH & Davis J Molecular networks underlying myofibroblast fate and fibrosis. J. Mol. Cell. Cardiol 97, 153–161 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Derynck R & Zhang YE Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425, 577–584 (2003). [DOI] [PubMed] [Google Scholar]
- 84.Stambe C et al. The role of p38alpha mitogen-activated protein kinase activation in renal fibrosis. J. Am. Soc. Nephrol 15, 370–379 (2004). [DOI] [PubMed] [Google Scholar]
- 85.Wang L, Ma R, Flavell RA & Choi ME Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for activation of p38alpha and p38delta MAPK isoforms by TGF-beta 1 in murine mesangial cells. J. Biol. Chem 277, 47257–47262 (2002). [DOI] [PubMed] [Google Scholar]
- 86.Davis J, Burr AR, Davis GF, Birnbaumer L & Molkentin JD A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev. Cell 23, 705–715 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Furtado MB et al. Cardiogenic genes expressed in cardiac fibroblasts contribute to heart development and repair. Circ. Res 114, 1422–1434 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaur H et al. Targeted ablation of periostin-expressing activated fibroblasts prevents adverse cardiac remodeling in mice. Circ. Res (2016). [DOI] [PubMed] [Google Scholar]
- 89.van Putten S, Shafieyan Y & Hinz B Mechanical control of cardiac myofibroblasts. J. Mol. Cell. Cardiol 93, 133–142 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR & Majno G Granulation tissue as a contractile organ. A study of structure and function. J. Exp. Med 135, 719–734 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]