Abstract
Urologic prostate surgery has changed dramatically over the past decades. Following the introduction of the robot, the surgical approach has been modified and thanks to the magnification allowed by the robot a further level of precision can be achieved. Moreover, advances in the anatomical studies have provided new evidence regarding the periprostatic anatomy.
The aim of this review is to describe our approach to robot-assisted radical prostatectomy. Our holistic perspective towards patient selection, pre- and postoperative care is provided. In our center, robot-assisted radical prostatectomy is performed by means of an anterograde approach. A nonbladder-sparing dissection with a graded approach towards nerve preservation is carried out. The procedure is concluded with what we call ‘total anatomical reconstruction’.
Keywords: prostate cancer, prostatic anatomy, robot-assisted radical prostatectomy, total anatomical reconstruction
Introduction
The robotic approach towards radical prostatectomy started to be performed regularly in 2001.1 Since then, several modifications to the approach itself and to the technique have been described. Presently, the transperitoneal approach is the most widely adopted because of the quick and easy access to the peritoneal cavity.
Our previous studies have focused on the periprostatic anatomy, architecture of neural tissue as a ‘hammock’ and risk stratified gradation in nerve sparing (NS) to achieve better functional outcomes without compromising oncological control.2–4 We currently individualize the surgery to maximize cancer control, achieve urinary continence and maintain erectile ability.
The aim of this paper is to discuss our current approach towards robot-assisted radical prostatectomy (RARP).
Patient selection
Initially, RARP was reserved in cases of localized disease, however its safety and feasibility have extended the indication in the case of locally advanced disease and an oligometastatic setting (in collaboration with medical oncologists).5
Radical prostatectomy should be offered to patients with at least 10 years’ life expectancy as per guidelines. Proper patient counseling is pivotal in this regard. The relative contraindications to RARP include: morbid obesity, a history of extensive surgery involving the peritoneal or pelvic structures and glaucoma. We have however performed this operation even with ileal loop, functioning renal transplant and absence of the rectum and colon due to prior operations.
In our center, all patients are staged with preoperative multi-parametric magnetic resonance imaging (mpMRI). Currently, the vast majority of our patients undergo 3T mpMRI without endorectal coil, prior to surgery.6 All external biopsy slides are re-read by an expert uropathologist at our hospital. Whether a patient has undergone neither mpMRI nor biopsy prior to surgery, a mpMRI is generally obtained first, then a systematic 12-core prostate biopsy is obtained. When a lesion is identified on mpMRI, two to four target cores are obtained from the same lesion. According to the patient’s mpMRI, clinical data and probability of extracapsular extension (ECE) of prostate cancer, we counsel the patient and then plan the subsequent surgical approach as either complete NS, non-NS, or incremental NS.7 All patients are informed that the planned NS will be carried out, given no unexpected intraoperative findings.
All patients are asked about sexual health and continence status. Even in the case when a patient does not report adequate erections to sustain intercourse, given a low ECE probability, we try to spare the periprostatic nerves since this might be helpful in the continence recovery process.
Before surgery we highly encourage patients to exercise regularly according to their age, health status and comorbidities. Generally, we suggest walking at least 60 min per day at a steady peace, since this is helpful in the recovery process.
Presurgical steps
Once a patient has expressed his will to undergo RARP and has signed an informed consent for the procedure, he is admitted to the hospital on the very morning of the surgery. Patients are asked to fast from midnight before the operation.
After a brief doctor visit in the morning, the patient is taken to the operative room where the anesthesiologist and his team administer general anesthesia. The man is secured on the operating table, skin asepsis is achieved by means of iodopovidone and a 18-Ch Foley catheter is placed.
Port placement
The pneumoperitoneum is created according to the closed technique by means of the Veress needle. A transverse incision is made above the umbilicus for camera placement. Overall, four ports are then placed in a dome fashion: three for the robotic arms and one for laparoscopic assistance. The Air seal is activated, and the pressure is set to 12 mmHg. The patient is then placed in the Trendelenburg position and his legs are separated in order to dock the robot. The robotic arms are secured to the trocars. At this stage, we use monopolar curved scissors, Hot ShearsTM (right arm), the Maryland bipolar forceps (left arm) and the PrograspTM forceps (third arm).
Bladder drop
Following camera, trocar and instrument placement, the intraperitoneal space is under direct vision. If necessary, lysis of adhesions is carried out to release the bowel. The medial umbilical ligaments and median umbilical ligaments are then incised to access the space of Retzius. During this phase we rely on electrocoagulation for bleeding control. The prostate is then identified and the periprostatic fat is cleared in an effort to visualize the puboprostatic ligaments.
Incision of the endopelvic fascia
The endopelvic fascia is generally incised in case of a large prostate or aggressive cancer, in order to release the lateral prostatic fascia from the levator ani muscle fibers and mobilize the prostate on both sides. This is aimed to provide mobility (helpful when dissecting large prostates) and visual inspection of periprostatic tissue (in case of aggressive cases). The incision is realized by means of cold scissors medially to the white line sparing its distal part. This is in direct continuity with the puboprostatic ligaments. The archus tendinous is dissected and preserved. We call those structures the ‘prostatic collar’ which serves later as a scaffold for the anterior reconstruction of the bladder neck after specimen removal.8
In the case of a small prostate or nonaggressive disease, the endopelvic fascia is often not incised in order to maintain the anatomical integrity that may help with functional recovery.
Bladder neck transection
We always perform a nonbladder neck-sparing procedure. We incise the anterior bladder wall right above the prostatovesical junction, Figure 1. The bladder neck is subsequently identified and its anterior aspect is cut using cautery. The Foley catheter is then lifted and held ventrally using the fourth arm. The posterior bladder neck is dissected until we identify the retrotrigonal fibromuscular layer. This represents a musculofascial plane lying behind the posterior wall of the bladder neck.3
Figure 1.
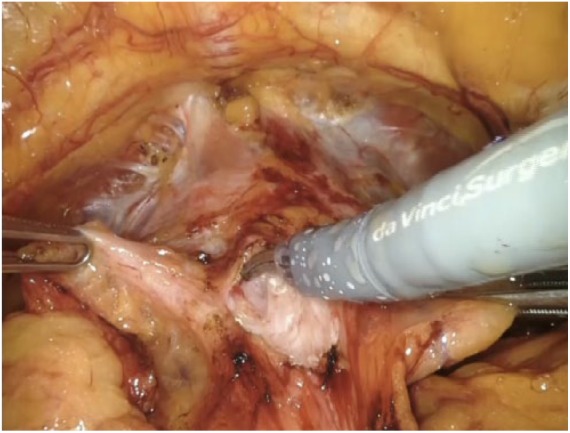
Incision of the anterior bladder wall. The assistant holds the right anterior portion of the bladder wall, the left one is held by the third arm. The Maryland forceps secures the bladder dome ventrally. The dissection is carried out by means of monopolar cautery.
Vasa deferentia and seminal vesicles dissection
The retrotrigonal layer is then sectioned to gain access to the vasa deferentia and seminal vesicles. First, the ipsilateral vas deferens is identified and sectioned. We strongly try to avoid complete electrocautery beyond this step. Successively, the seminal vesicles are carefully dissected medially first and then laterally. Clips are typically employed for vascular control to avoid pelvic plexus damage, especially lateral to the seminal vesicles. A careful dissection in close proximity to the tips of the seminal vesicles is of pivotal importance for the preservation of the nerves. In fact, the pelvic plexus continues in what we call the proximal neurovascular plate (PNP), and it is in direct contact with the most lateroventral aspects of the seminal vesicles. The PNP continues then in the predominant neurovascular bundle (PNB).2 Avoiding an injury to the PNP at this stage is extremely important. In fact, damages to the fibers at the lever of the PNP will prevent signal conduction to the most distal structures, namely the PNB and the accessory neurovascular pathways (ANPs).
After completion of vas sectioning and seminal vesicle dissection, the fourth arm is used to lift the prostate by the seminal vesicles. This sets up the surgical field for the start of nerve preservation, which begins with the posterior dissection of the prostate.
Denonvilliers’ fascia incision and nerve-sparing approach
With the prostate and seminal vesicles lifted, the Denonvilliers’ fascia (DF) is under direct vision, Figure 2. According to the patient’s risk of ECE, we dissect the DF: completely, leaving all of its layers on the specimen (extrafascial dissection); partly, in between the posterior and anterior layers, namely (interfascial dissection); or we do not dissect the DF at all. In the last case, the plane between the posterior prostatic capsule and the anterior layer of the DF fascia is gently developed.
Figure 2.
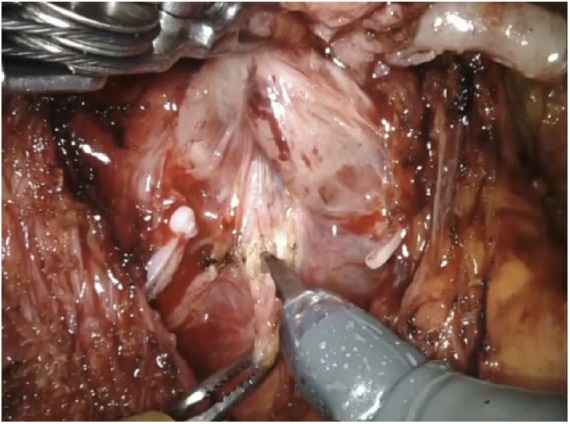
Dissection of the Denonvilliers’ fascia. The prostate and the dissected seminal vesicles are held by the third arm. Denonvilliers’ fascia is incised on the midline and the plane is developed laterally.
When moving laterally, the lateral prostatic fascia (LPF) is encountered. This structure encompasses three layers (Figure 3), thus four levels of dissection are possible laterally to the prostate. These levels of dissection correspond to what we call the NS grades. Specifically, the grade 1 NS corresponds to a plane of dissection developed completely between the prostatic pseudocapsule and the LPF. None of the LPF layers are sacrificed in the grade 1 NS, thus an intrafascial dissection is carried out.
Figure 3.
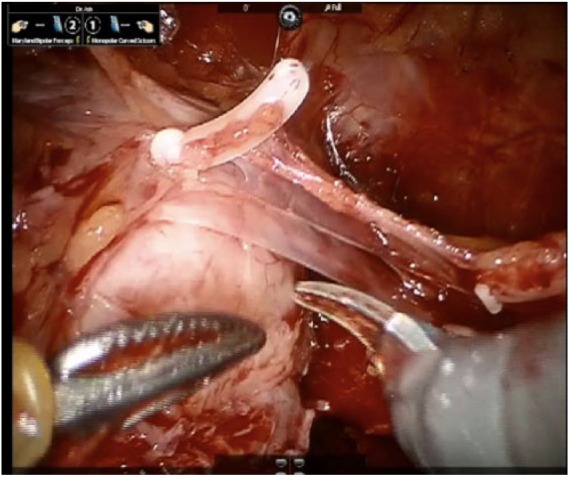
The three-layer structure of the lateral prostatic fascia is demonstrated.
When an interfascial dissection is performed, a grade 2 NS is achievable by sectioning the periprostatic venous layer of the LPF. This layer is in direct contact with the prostatic pseudocapsule. A grade 3 NS is performed when all but the outermost layer of the LPF are sacrificed.
The grade 4, or non-NS, corresponds to the extrafascial dissection where the LPF is dissected in toto and left on the specimen. In this case the dissection plane is developed between the LPF and the levator ani muscle.
During the dissection of the LPF and DF, our efforts are aimed to achieve a traction-free dissection in an effort to avoid postoperative neurapraxia. To control the vessels of the prostatic pedicles we use Hem-o-lok® clips. During the lateral dissection, the fibers from the ANPs directed to the prostate are sectioned.
ANPs represent putative accessory neural pathways that are found around the prostate. ANPs can be observed as a triangular extension of the PNP and converge near the apex. They have been demonstrated to carry neural impulses to the penile tissue outside the classical PNB.9 The unpredictability of the return to sexual function after RARP and the lack of correlation between the surgeon’s perspective regarding the quality of NS and functional results, could be partly attributed to those pathways.2
In an effort to preserve the nerves running in close proximity to the apical prostatic region, we perform the high anterior release of the LPF. This entails the dissection and release of the LPF more medially and anteriorly with respect to standard techniques and has been demonstrated to determine better functional outcomes after prostatectomy.10
Circumferential apical dissection
This step represents a crucial one in RARP. In fact, its correct execution has implications in oncological and functional outcomes. Positive surgical margins (PSMs) are more frequently reported at the prostatic apex11 and the occurrence of a PSM at the apex has been associated with a higher risk of recurrence.12 At the apical level, the prostate subtly merges with the urethra and it is very difficult to discriminate between those two structures. To avoid apical PSMs, the urethra could be transected a little farther from the prostatic apex but this could affect urinary continence after RARP.13 To overcome those issues, we have implemented what we called the ‘retroapical approach’ for the urethral sectioning.14 The rationale for this approach relies on the posterior urethral anatomy: once approached posteriorly, the urethra is only ‘covered’ by the DF; whereas when approaching the urethra anteriorly, the Santorini complex prevents direct urethra visualization.
During the apical dissection, the prostate is completely freed posteriorly and posterolaterally. At this point, the prostate is lifted anteriorly towards the pubic symphysis. This movement opens the space behind the urethra and compresses the Santorini plexus against the pubic bones, occluding its flow temporarily. We change the camera lens to 30° optic to approach the prostate urethral junction. We believe that in this position one can accomplish a clear and separate view of the prostatic apex (white) and the urethral sphincter complex (Figure 4). When this distinction is not completely evident, the assistant, by moving the Foley catheter, can help in discriminating the two structures.
Figure 4.
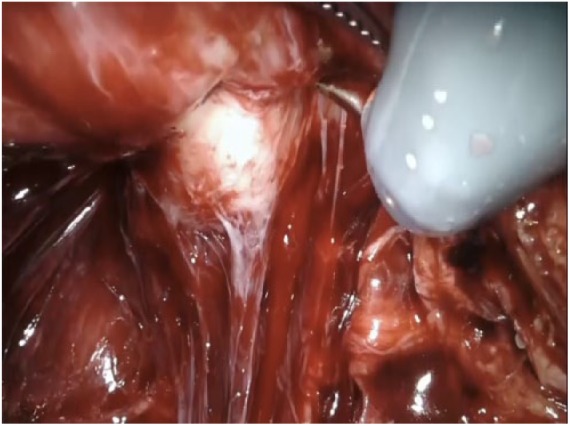
The urethra can be easily identified thanks to its white color. This help distinguishing the urethra from the prostatic apex.
At this point, we incise the posterior hemi-circumference of the urethra with cold curved scissors approximately 1 mm distal from the prostatic apex. The Foley catheter is then exposed and its tip is retracted till the distal urethral opening. This allows for the direct visualization of the anterior hemi-circumference of the urethra. Again, the anterior portion of the urethra is dissected sharply. Now the urethral sphincteric complex is fully transected.
Control of the Santorini plexus
The lens is now changed to 0° optic and the dissection is continued anteriorly. The prostate is retracted and the dissection is carried out in a craniocaudal fashion. When the preoperative mpMRI had not identified any suspicious lesion in the anterior prostate, the Santorini plexus along with the puboprostatic ligaments is sectioned 1 mm away from the anterior margin of the prostate. We try to preserve as much as possible the puboprostatic ligaments and the archus tendineous that will serve for the reconstructive phase. The Santorini plexus is finally ligated to avoid postoperative bleeding.
Specimen removal
At this point the balloon of the Foley catheter is inflated with 20–30 ml of sterile saline solution. The convex part of the Foley catheter’s balloon faces the pubic symphysis in an effort to determine a gentle compression on the deep venous complex to aid in the hemostatic process.
The specimen is now removed and lymph node dissection is carried out according to the D’Amico risk group categories.
In the meantime, the specimen is sent to the pathology department for frozen section examination. Following the experience by Schlomm and colleagues we have implemented the neurovascular adjacent frozen section examination (NeuroSAFE) technique in our department since 2016.15 This allows for a direct assessment of a PSM at the time of surgery. When a PSM is detected on frozen section a further resection is carried out according to the PSM location.
Total anatomical reconstruction
The posterior bladder neck and the Denonvilliers’ musculofascial plate reconstruction represent the first step of our total anatomical technique. The rationale behind this step is in the omega-shaped rhabdosphincter of the urethra. By reconstructing the posterior plate first, we secure enough support to the posterior urethra, where the ‘opening’ of the omega lies. For this surgical step we use two needle holders. Initially, we create a muscular flap behind the bladder neck, according to the Pagano principle.16 The classical Pagano suture consists in a midline stitch of a right and left detrusor flap behind the bladder neck. We perform the same steps, but we also incorporate in the Pagano suture the retrotrigonal flap in an effort to provide more support to the posterior bladder neck.3 We subsequently suture the retrotrigonal layer to the DF that has been dissected to access the posterior urethra, in keeping with the Rocco principle.17 At this point, the vesicourethral anastomosis can be carried out virtually in a tension-free fashion by using a continuous suture.
We conclude the total anatomical reconstruction by suturing the new bladder neck to the archus tendineous by means of a running suture.4 The operation is now complete, Figure 5. Generally, we do not sew the peritoneum.
Figure 5.
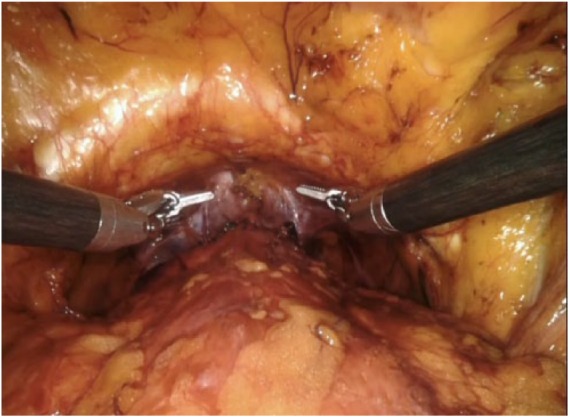
Total anatomical reconstruction.
Postoperative care
The vast majority of the patients are discharged home on the first postoperative day. We emphasize the importance of moderate physical activity. We advise the patient to walk every day for at least 30–60 min according to his health status. In general, the catheter is removed 7 days after surgery.
Overview
Surgery, like all branches of medicine is constantly updated. Several modifications to the traditional aspects of it have occurred over the past decades. One of the greatest changes in the field was provided by the introduction of the NS concept. More recently, thanks to the introduction of the robotic approach and to focused anatomical studies, a better understanding of the periprostatic anatomy has been achieved. The magnification allowed by the robot has changed the way of preserving the periprostatic tissues and the ways of performing the reconstructive phase after the removal of the prostate.
This review represents a ‘picture’ of the current evidences and the preferences of the Authors in performing RARP are described. It is not unlikely that this review in 10 (or less) years would be going ‘out of fashion’ given the speed at which our field is progressing.
Acknowledgments
We thank Ms Mary Marcelle Niles for the linguistic revision.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Alberto Martini, Department of Urology, Icahn School of Medicine at Mount Sinai Hospital, New York, NY, USA.
Ashutosh Kumar Tewari, Department of Urology, Icahn School of Medicine at Mount Sinai, 1425 Madison Avenue, New York, NY 10029, USA.
References
- 1. Tewari A, Menon M. Vattikuti Institute prostatectomy: surgical technique and current results. Curr Urol Rep 2003; 4: 119–123. [DOI] [PubMed] [Google Scholar]
- 2. Tewari A, Takenaka A, Mtui E, et al. The proximal neurovascular plate and the tri-zonal neural architecture around the prostate gland: importance in the athermal robotic technique of nerve-sparing prostatectomy. BJU Int 2006; 98: 314–323. [DOI] [PubMed] [Google Scholar]
- 3. Tewari A, El-Hakim A, Rao S, et al. Identification of the retrotrigonal layer as a key anatomical landmark during robotically assisted radical prostatectomy. BJU Int 2006; 98: 829–832. [DOI] [PubMed] [Google Scholar]
- 4. Tewari A, Jhaveri J, Rao S, et al. Total reconstruction of the vesico-urethral junction. BJU Int 2008; 101: 871–877. [DOI] [PubMed] [Google Scholar]
- 5. Gandaglia G, Abdollah F, Hu J, et al. Is robot-assisted radical prostatectomy safe in men with high-risk prostate cancer? Assessment of perioperative outcomes, positive surgical margins, and use of additional cancer treatments. J Endourol 2014; 28: 784–791. [DOI] [PubMed] [Google Scholar]
- 6. Martini A, Gupta A, Cumarasamy S, et al. Re: Rita Faria, Marta O. Soares, Eldon Spackman, et al. Optimising the Diagnosis of Prostate Cancer in the Era of Multiparametric Magnetic Resonance Imaging: A Cost-effectiveness Analysis Based on the Prostate MR Imaging Study (PROMIS). Eur Urol 2018; 73:23–30. Eur Urol 2018; 73: e108–e109. [DOI] [PubMed] [Google Scholar]
- 7. Martini A, Gupta A, Lewis SC, et al. Development and internal validation of a side-specific, multiparametric magnetic resonance imaging-based nomogram for the prediction of extracapsular extension of prostate cancer. BJU Int. Epub ahead of print April 19 2018. DOI: 10.1111/bju.14353. [DOI] [PubMed] [Google Scholar]
- 8. Takenaka A, Tewari AK, Leung RA, et al. Preservation of the puboprostatic collar and puboperineoplasty for early recovery of urinary incontinence after robotic prostatectomy: anatomic basis and preliminary outcomes. Eur Urol 2007; 51: 433–440. [DOI] [PubMed] [Google Scholar]
- 9. Terada N, Arai Y, Kurokawa K, et al. Intraoperative electrical stimulation of cavernous nerves with monitoring of intracorporeal pressure to confirm nerve sparing during radical prostatectomy: early clinical results. Int J Urol 2003; 10: 251–256 [DOI] [PubMed] [Google Scholar]
- 10. Kaul S, Savera A, Badani K, et al. Functional outcomes and oncological efficacy of Vattikuti Institute prostatectomy with Veil of Aphrodite nerve sparing: an analysis of 154 consecutive patients. BJU Int 2006; 97: 467–472. [DOI] [PubMed] [Google Scholar]
- 11. Novara G, Ficarra V, Mocellin S, et al. Systematic review and meta-analysis of studies reporting oncologic outcome after robot-assisted radical prostatectomy. Eur Urol 2012; 62: 382–404. [DOI] [PubMed] [Google Scholar]
- 12. Dev HS, Wiklund P, Patel V, et al. Surgical margin length and location affect recurrence rates after robotic prostatectomy. Urol Oncol 2015; 33: 109.e7–109.e13. [DOI] [PubMed] [Google Scholar]
- 13. Hinata N, Sejima T, Takenaka A. Progress in pelvic anatomy from the viewpoint of radical prostatectomy. Int J Urol 2013; 20: 260–270. [DOI] [PubMed] [Google Scholar]
- 14. Tewari AK, Srivastava A, Mudaliar K, et al. Anatomical retro-apical technique of synchronous (posterior and anterior) urethral transection: a novel approach for ameliorating apical margin positivity during robotic radical prostatectomy. BJU Int 2010; 106: 1364–1373. [DOI] [PubMed] [Google Scholar]
- 15. Schlomm T, Tennstedt P, Huxhold C, et al. Neurovascular structure-adjacent frozen-section examination (NeuroSAFE) increases nerve-sparing frequency and reduces positive surgical margins in open and robot-assisted laparoscopic radical prostatectomy: experience after 11,069 consecutive patients. Eur Urol 2012; 62: 333–340. [DOI] [PubMed] [Google Scholar]
- 16. Moinzadeh A, Shunaigat AN, Libertino JA. Urinary incontinence after radical retropubic prostatectomy: the outcome of a surgical technique. BJU Int 2003; 92: 355–359. [DOI] [PubMed] [Google Scholar]
- 17. Rocco B, Gregori A, Stener S, et al. Posterior reconstruction of the rhabdosphincter allows a rapid recovery of continence after transperitoneal video laparoscopic radical prostatectomy. Eur Urol 2007; 51: 996–1003. [DOI] [PubMed] [Google Scholar]


