Abstract
Background:
The aim of this work was to assess the use of prostate-specific membrane antigen (PSMA)-labelled radiotracers in detecting the recurrence of prostate cancer. PSMA is thought to have higher detection rates when utilized in positron emission tomography (PET)/computed tomography (CT) scans, particularly at lower prostate-specific antigen (PSA) levels, compared with choline-based scans.
Methods:
A systematic review was conducted comparing choline and PSMA PET/CT scans in patients with recurrent prostate cancer following an initial curative attempt. The primary outcomes were overall detection rates, detection rates at low PSA thresholds, difference in detection rates and exclusive detection rates on a per-person analysis. Secondary outcome measures were total number of lesions, exclusive detection by each scan on a per-lesion basis and adverse side effects.
Results:
Overall detection rates were 79.8% for PSMA and 66.7% for choline. There was a statistically significant difference in detection rates favouring PSMA [OR (M–H, random, 95% confidence interval (CI)) 2.27 (1.06, 4.85), p = 0.04]. Direct comparison was limited to PSA < 2 ng/ml in two studies, with no statistically significant difference in detection rates between the scans [OR (M–H, random, 95% CI) 2.37 (0.61, 9.17) p = 0.21]. The difference in detection on the per-patient analysis was significantly higher in the PSMA scans (p < 0.00001). All three studies reported higher lymph node, bone metastasis and locoregional recurrence rates in PSMA.
Conclusions:
PSMA PET/CT has a better performance compared with choline PET/CT in detecting recurrent disease both on per-patient and per-lesion analysis and should be the imaging modality of choice while deciding on salvage and nonsystematic metastasis-directed therapy strategies.
Keywords: choline, PET/CT, positron emission tomography, prostate cancer, PSMA
Introduction
The role of contemporary morphological and functional imaging in the setting of recurrent prostate cancer following primary curative treatment is limited due to poor detection rates. Nuclear imaging with positron emission tomography (PET) has mitigated a number of limitations of traditional morphological and functional imaging modalities, making PET scans of pivotal diagnostic value in this cohort of patients. Furthermore, improved detection rates with PET scans at low prostate-specific antigen (PSA) thresholds have driven recent advancements in metastasis-directed treatment strategies of oligometastatic prostate cancer.
Choline PET scans have been the traditional imaging modality of choice in restaging patients following biochemical relapse.1 However, multiple studies have shown low sensitivity and specificity, particularly at low PSA levels,2–7 which can result in delays in salvage therapy. Although known since the 1980s, prostate-specific membrane antigen (PSMA) has recently come to the fore in the imaging of prostate cancer due to promising preliminary data.8–11 PSMA is a cell surface protein expressed in normal prostatic tissue, hyperplastic prostate tissue, prostatic intraepithelial neoplasia, as well as extraprostatic locations (kidney, small bowel, salivary glands), but is known to be expressed most in prostate cancer,12–15 including in metastatic disease.16,17 Radio-labelling of PSMA with 68Ga (amongst other tracers) has enabled its detection with PET scanning, opening a new chapter in prostate cancer imaging.8,11
PSMA labelled radiotracers are thought to have higher detection rates than choline-labelled tracers in the biochemical recurrence (BCR) setting, particularly at lower PSA levels.18
In this paper we have systematically reviewed the world literature comparing the performance of PSMA and choline-based PET/computed tomography (CT) scans in patients with biochemical recurrence following initial treatment with curative intent.
Methods
Evidence acquisition
Criteria for considering studies for this review
The inclusion criteria were all randomized trials and observational studies comparing choline and PSMA PET/CT scans in patients with suspected recurrence following initial primary curative treatment for prostate cancer.
Search strategy and study selection
The systematic review was performed in accordance with the Cochrane guidelines and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.19 Bibliographic databases searched were MEDLINE (2000 to March 2017), EMBASE (2000 to March 2017), Cochrane Central Register of Controlled Trials (CENTRAL; in the Cochrane Library, Issue 1, 2017), CINAHL (2000 to March 2017). As well as hand-searching individual urological journals, citation and reference lists were also evaluated. The search was conducted on 28 March 2017.
All studies comparing choline PET/CT scans with PSMA PET/CT scans in prostate cancer diagnostics were evaluated. No language restrictions were applied. Animal studies were excluded. Search terms included (not limited to): ‘prostate cancer’, ‘PSMA PET’, ‘choline PET’, ‘prostatectomy’, ‘radiotherapy’, ‘lymphadenectomy’, ‘biochemical recurrence’ and ‘metastasis’. Boolean operators (AND, OR) were employed to augment the search process. Medical subjecting heading phrases included: (PSMA), (choline PET), (prostatectomy), (radiotherapy), (biochemical recurrence) and (lymphadenectomy).
Primary outcomes measures
Per-patient analysis
(1) Overall detection rates following biochemical recurrence (defined as at least one pathological lesion)
(2) Detection rates following biochemical recurrence at low PSA thresholds (defined as at least one pathological lesion)
(3) Difference in detection rates and exclusive detection (number of patients with at least one pathological lesion captured only by one of the PET scans and missed by the other PET scan)
Secondary outcome measures
Per-lesion analysis
(1) Total number of lesions in PSMA and choline PET CT/CT scans.
(2) Exclusive detection by each scan (number of lesions captured only by one of the PET Scans and missed by the other PET scan)
(3) Adverse side effects
Quality assessment of evidence
Study quality was assessed according to QUADAS-2 analysis, as detailed in the Cochrane Handbook for Systematic Review of Diagnostic Accuracy Tests.20 The grading of recommendations assessment, development and evaluation (GRADE) approach was used to rate the quality of evidence.20
Data extraction and analysis
Two reviewers (MM, BR) independently identified all studies that appeared to fit the inclusion criteria for full review. Disagreement was resolved by consensus between the authors. Comparable data from each study were combined in a meta-analysis where possible. A Mantel–Haenszel Chi-square test was used for continuous data and expressed as the mean difference with 95% confidence interval (CI) and for dichotomous data an inverse variance was used and expressed as an odds ratio (OR) or risk ratio with a 95% CI. The p value was considered significant if it was <0.05. Heterogeneity was analysed using a Chi-square test on N-1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test.19 I² values of 25%, 50% and 75% corresponded to low, medium and high levels of heterogeneity respectively. A fixed-effect model was used unless statistically significant high heterogeneity (I² >75% was considered as significantly high heterogeneity) existed between studies. A random effects model was employed if heterogeneity existed. If the data available were deemed not suitable for a meta-analysis, they have been described in a narrative fashion. Differences in the detection rate were tested using McNemar’s test. A p value <0.05 was deemed as significant.
Results
Literature search
A total of 474 papers were identified in the initial search from which 4 were evaluated for a comprehensive evaluation (supplemental Figure 1). One study was excluded as PSMA PET/CT scans were only performed on individuals who had negative choline PET/CTs.21 Overall, three studies were included in the final review.22–24 All three studies were observational comparative studies. Morigi and colleagues22 was a prospective study. The other two studies were retrospective studies.23,24 Overall 178 men with suspected recurrent prostate cancer had 68GA-PSMA PET/CT and choline PET/CT scans. Choline tracers used were 11C-labelled choline by Schwenck and colleagues23 and 18F-labelled choline used by Afshar-Oromieh and colleagues24 and Morigi and colleagues22 The demographics of individual studies are shown in Table 1.
Table 1.
Individual study demographics.
| Schwenck and colleagues25 | Morigi and colleagues4 | Afshar-Oromieh and colleagues26 | |
|---|---|---|---|
| Type of study | Retrospective | Prospective | Retrospective |
| Total number of patients | 103 | 38 | 37 |
| Age (years) | • n/a | • Mean (range) = 68 (54–81) |
• Mean ± SD = 69.3 (±7.1) • Median (range) = 70 (57–85) |
| PSA (ng/ml) at the time of scan | • Median = 2.7 | • Mean ± SD = 1.72 ± 2.54 • Range 0.04–12.0 |
• Mean ± SD = 11.1 ± 24.1 • Median (range) = 4.0 (0.01–116) |
| Gleason score | • n/a | • G6–7 = 23/38 (61%) • G8–9 = 15/38 (39%) |
• Mean ± SD = 7.4 ± 1.1 • Median (range) = 7 (5–9) |
| Initial treatment | • Radical prostatectomy or radiotherapy (no further data available) |
• Radical prostatectomy = 22 • Radical prostatectomy + salvage radiotherapy = 12 • Radical radiotherapy = 4 |
• Radical prostatectomy = 28 • Radical radiotherapy +ADT = 9 |
ADT, androgen deprivation therapy; PSA, prostate-specific antigen; SD, standard deviation.
Reference standard for individual studies
No reference standard was reported by the included studies. Histological confirmation was selectively used. In the study by Schwenck and colleagues23 only two patients with lung metastases had histological confirmation. Both metastases were shown on the PSMA scan, and only in one patient was choline uptake shown. In the study by Morigi and colleagues22 histopathologic confirmation was performed on 9 out of 38 patients. All nine lesions positive with PSMA were confirmed to be true-positive. Of the two lesions that were positive on the choline scan, one was true-positive the other was false-positive (but true-negative with PSMA). In the study by Afshar-Oromieh and colleagues24 PSMA-positive lesions were confirmed with histology in seven cases.
Primary outcomes measures
Per-patient analysis
(1) Overall detection rates following biochemical recurrence
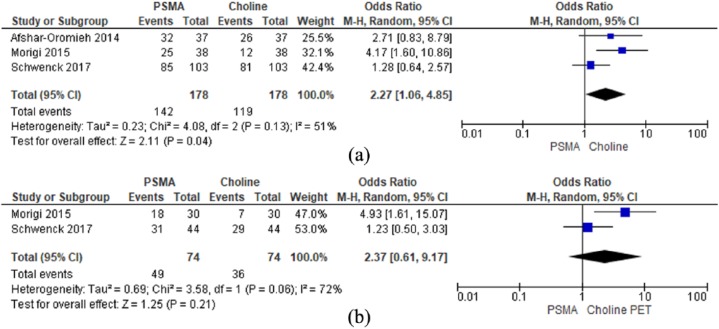
Overall detection rates for biochemical recurrence were 79.8% for PSMA and 66.9% for choline PET/CT (Table 2). All three studies reported on overall detection rates following biochemical recurrence and were suitable for meta-analysis. A random model was used for analysis as there was a moderate degree of heterogeneity (I2 = 51%). There was a statistically significant difference in overall detection rates between the choline and PSMA scans, favouring the PSMA scans. OR (M–H, random, 95% CI) 2.27 (1.06, 4.85), p = 0.04 [Figure 1(a)].
Table 2.
The differences between choline PET and PSMA PET scans.
| Study | n | Choline PET/CT | PSMA PET/CT |
|---|---|---|---|
| Overall detection rates for individual studies | |||
| Schwenck and colleagues25 | 103 | 81 (78.6%) | 85 (82.5%) |
| Morigi and colleagues4 | 38 | 12 (31.6%) | 25 (65.8%) |
| Afshar-Oromieh and colleagues26 | 37 | 26 (70.3%) | 32 (86.5%) |
| Total | 178 | 119 (66.9%) | 142 (79.8%) |
| Total number of lesions (per-lesion analysis) | |||
| Schwenck and colleagues25 | 554 | 839 | |
| Morigi and colleagues4 | 29 | 59 | |
| Afshar-Oromieh and colleagues26 | 56 | 78 | |
| Total | 639 | 976 | |
| Exclusive detection | |||
| Schwenck and colleagues25 | 38 | 323 | |
| Afshar-Oromieh and colleagues26 | 0 | 22 | |
| Total | 38 | 345 | |
CT, computed tomography; PET, positron emission tomography; PSMA, prostate-specific membrane antigen.
Figure 1.
(a): Overall detection rates; (b): detection rates at PSA thresholds less than 2 ng/ml.
PSA, prostate-specific antigen.
(2) Detection rates after BCR at lower PSA values
Morigi and colleagues reported detection rates 50% and 12.5% respectively in PSA thresholds of <0.5 ng/ml for PSMA and choline scans respectively. Schwenk and colleagues reported detection rates 61% and 45% respectively at PSA thresholds of <1 ng/ml for PSMA and choline PET/CT scans respectively. Due to differing stratification methods used in each of the studies direct comparison was limited to PSA < 2 ng/ml in two studies. A random model was used for analysis as there was a high degree of heterogeneity (I2 = 72%). There was no statistically significant difference in detection rates between the choline and PSMA PET/CT scans at PSA thresholds <2 ng/ml. OR (M–H, random, 95% CI) 2.37 (0.61, 9.17) p = 0.21 [Figure 1(b)].
(3) Difference in detection rates:
The difference in detection in the per-patients analysis was significantly higher in the PSMA PET/CT scans (p < 0.00001). On a per-patient analysis 27 patients had lesions detected exclusively with PSMA and 4 patients had lesions detected exclusively detected with choline PET/CT scans [Table 3(a)].
Table 3.
(a). Difference in detection rates (per-patient analysis), (p < 0.00001).
| Choline –ve | Choline +ve | |
|---|---|---|
| PSMA –ve | 31 | 4 |
| PSMA +ve | 27 | 116 |
Secondary outcomes measures
Per-lesion analysis
(1) Total number of lesions in PSMA and choline PET/CT scans
On cumulative analysis, the total number of lesions detected by PSMA and choline PET/CT Scans was 976 versus 639 respectively (Table 2). All three studies reported higher lymph node, bone metastasis and locoregional recurrence in PSMA in comparison with choline PET/CT [Table 3(b)].
(b). Total number of lesions at specific sites in PSMA and choline PET-scans (per-lesion analysis).
| Bone metastasis |
Lymph nodes metastasis |
Local recurrence |
Other sites |
|||||
|---|---|---|---|---|---|---|---|---|
| PSMA | Choline | PSMA | Choline | PSMA | Choline | PSMA | Choline | |
| Afshar-Oromieh and colleagues 26 | 23 | Unclear | 40 | Unclear | 10 | Unclear | 5 | Unclear |
| Morigi and colleagues 4 | 16 | 9 | 36 | 18 | 7 | 2 | 0 | 0 |
| Schwenck and colleagues 25 | 372 | 242 | 439 | 287 | 26 | 24 | 2 | 1 |
PET, positron emission tomography; PSMA, prostate-specific membrane antigen.
(2) Exclusive detection by each scan
All three studies individually reported higher exclusive lesions detected with PSMA as compared with choline PET/CT Scans. Cumulative analysis was possible only on data from two studies. The number of pathological lesions exclusively detected by PSMA and choline PET/CT Scans was 345 and 38 respectively (Table 2).
(3) Adverse effects with radiotracers
There were no reports of any adverse effects in any of the publications.
Quality assessment of studies
The patients in all the studies have received differing treatments and hence represent a heterogeneous cohort. All the studies were hence judged to have a high degree of risk of bias for patient selection. There was no consistent reference standard with selective use of histological confirmation to compare the outcomes of the two types of scan. There is therefore a concern with false positivity. The authors have hence judged the risk of bias for index test as unclear. The risk of bias for reference standard is judged as high. It is unclear if the timing between choline and PSMA PET/CT scans would have influenced the detection rates. The authors have therefore judged the risk of bias for flow and timing as unclear (supplemental Figure 2). There was low concern for applicability domains (supplemental Figure 2). Adopting the GRADE approach, the quality of evidence for ‘overall detection rates’ and ‘detection rates after at PSA thresholds less than 2 ng/ml’ was rated as ‘low’ and ‘very low’ respectively (supplementary Table 1).
Discussion
Principal findings
The review highlights the superior performance of PSMA PET/CT scans when compared with choline PET/CT Scans in detecting recurrent prostate cancer. Overall detection rates of PSMA and choline PET/CT scans were 80% and 67% respectively. At PSA thresholds of <2 ng/ml the detection rates of PSMA and choline PET/CT scans were 66% versus 49% respectively. The overall detection rates on a per-patient analysis of PSMA scans was significantly better than choline scans in identifying recurrent disease in patients who have had an initial attempt at curative therapy. On a per-lesion analysis, a higher number of patients with local recurrence were detected in the PSMA cohort.
Overall, two studies in the review individually reported better detection rates of PSMA when compared with choline PET/CT scans at PSA thresholds of <1 ng/ml. The meta-analysis in the review could only analyze data at PSA thresholds <2 ng/ml due to differing PSA stratifications by individual studies. The analysis suggested a trend towards better detection rates with PSMA at this threshold however; this did not achieve statistical significance. Additionally, the number of lesions detected by PSMA scans was significantly higher than choline scans. The exclusive detection on both per-patient and per-lesion analysis was consistently higher with PSMA. No adverse effects were reported with either scans.
Morigi and colleagues22 reported that 54% of patients had a major to moderate change on their management in recurrent prostate cancer purely based on the findings of PSMA PET/CT, while choline PET/CT scans did not exclusively change management in any case. Bluemel and colleagues21 reported a 43.8% increased detection rate from PSMA PET/CT in patients with choline negative scans. The exclusive detection rates both on a patient basis and lesion basis in this review were higher in PSMA PET/CT compared with choline PET/CT. These findings would affirm the suggestion that PSMA PET/CT scanning is more likely to influence management in patients with recurrent prostate cancer.
Implications in clinical practice
Salvage treatment
The timing and indications of local salvage treatment options after initial prostatectomy or radiotherapy is an area of much contention and debate. In contemporary practice, salvage therapy is administered mostly based on PSA kinetics without an overt reliance on morphological imaging. Pfister and colleagues27 in a pooled analysis of seven retrospective studies of early radiotherapy after radical prostatectomy reported a 5-year biochemical-free survival of 71.1% at PSA < 0.5 ng/ml and concluded biochemical-free survival rates were significantly better when salvage radiotherapy was offered at low PSA thresholds. While salvage therapy has the potential to cure if offered early, it will plausibly result in overtreatment in a cohort of patients.
Our findings strengthen the arguments made by current evidence. Castellucci and colleagues28 reported a detection rate of 28.4% in 605 patients with BCR and PSA values <2 ng/ml. Von Eyben and colleagues29 in a meta-analysis reported overall detection rates and detection rates at PSA thresholds < 0.5 ng/ml of 81% and 50% respectively on a per-patient analysis corroborating the findings of this review. Furthermore a meta-analysis by Perera and colleagues30 with a total of 1309 patients showed a detection rate of 76% for BCR (PSA < 2 ng/ml).
These findings would suggest that PSMA scans, when integrated with other factors such as PSA kinetics, initial stage, grade and surgical margin state, are more likely to offer appropriate guidance on the timing of salvage treatment options than choline PET/CT Scans. In addition, if salvage radiotherapy is contemplated, accurate information on the sites of recurrence will enable clinicians to decide on the appropriate type, dose and field of salvage radiotherapy.
Nonsystemic metastatic directed therapy
Nonsystemic metastatic directed therapy (MDT) with options such as stereotactic radiotherapy or salvage lymphadenectomy in low volume oligometastatic disease is a promising and evolving treatment option for patients with recurrent prostate cancer. The potential benefits of an MDT strategy include the possibility of curing cancers previously thought to be incurable, delay hormonal manipulations and reduce treatment related toxicity. Steuber and colleagues25 in a retrospective multi-centre study of 2079 men compared the standard of care (early versus delayed androgen deprivation therapy (ADT)) versus MDT (stereotactic radiotherapy/salvage lymphadenectomy) in men with PET-detected nodal recurrence at PSA progression. At a median follow up of 70 months, MDT had a better cancer specific survival (CSS) in comparison with the standard of care (5-year CSS for MDT and standard of care (SOC) was 98.6% and 95.7% respectively). Ost and colleagues26 in a phase II multi-centre randomized trial, compared surveillance and MDT in patients with three or fewer extracranial metastatic lesions on choline PET/CT with biochemical recurrence following initial prostate cancer treatment with curative intent. The trial reported significantly longer ADT-free survival in the MDT cohort in comparison with surveillance at a median follow-up time of 3 years (21 months versus 13 months). Despite the potential benefits of an MDT approach its success hinges on the accurate detection of all oligorecurrent lesions on imaging. In this review the overall number of metastatic lesions detected by PSMA PET/CT and choline PET/CT was 976 and 639 respectively. Also, the number of bone and lymph nodes metastasis was consistently higher in the PSMA cohort. This trend emphasizes that PSMA PET/CT scans are better equipped compared with choline-based scans to direct a MDT strategy.
Strengths and limitations
The current study has several important limitations. Due to the limited number of studies that have directly compared PSMA and choline PET/CT scans, the low number of patients included in this review impacted on some of the results. Despite this the overall statistical significance shown illustrates why there is a rapidly increasing volume of research utilizing PSMA PET/CT scans, implying there is sufficient evidence is available to convince clinicians of its merits over conventional imaging.
Each study analyzed had a heterogeneous cohort of patients that received differing treatment strategies. In the setting of recurrent prostate cancer where the evidence is often inconclusive and a variety of treatment options available, this is inevitable. The authors were also unable to perform a meta-analysis at a PSA threshold of <1 ng/ml due to differing PSA stratification used by individual studies. In the setting of recurrent prostate cancer, the diagnostic accuracy of PET/CT scans cannot be evaluated with absolute confidence due to the inability to choose a reference standard. Also, the studies have only selectively used histological evaluation to confirm whether detectable disease was true-positive. In reality achieving histological confirmation may be impractical and potentially unethical. The quality of the evidence based on the GRADE classification was low, primarily due to the bias of individual results and the inconsistency of results. While randomized trials comparing the two radiotracers in the setting of recurrent prostate cancer may confirm the superiority of PSMA PET/CT scans with confidence; it is the author’s view that the current review, despite the poor quality, provides adequate data to confirm the superiority of PSMA PET/CT scans over choline PET/CT scans.
Areas of future interest and impact on ongoing research
The authors do believe that future research in a randomized setting must concentrate on evaluating the role of PSMA PET/CT scans in defining appropriate salvage strategies in the event of recurrence following initial curative treatment. The proPSMA31 study seeks to answer some of these questions. A possible criticism of ongoing research trials such as the Surveillance or metastasis-directed Therapy for OligoMetastatic Prostate cancer recurrence (STOMP) trial32 and the Conventional care or Radioablation in the treatment of Extracranial metastases (CORE) trial33 is the use of inferior imaging modalities to define oligometastatic disease. The outcomes of this review would suggest that establishing whether an individual truly has oligometastatic disease based on contemporary definitions is dictated by the imaging modality employed. In the recurrent prostate cancer setting it is therefore vital that PSMA PET/CT scans, with their superior detection rates, be used as the standard imaging modality of choice in trials evaluating the efficacy of MDT strategies.
Conclusion
PSMA PET/CT scans have a better performance compared with choline PET/CT scans in detecting recurrent disease following initial curative treatment for prostate cancer, both on a per-patient and per-lesion analysis. PSMA PET/CT scans should be the imaging modality of choice while deciding on salvage and nonsystematic metastasis-directed therapy strategies. Research trials evaluating treatment outcomes in the oligometastatic setting should use PSMA PET/CT scans as the imaging modality of choice to evaluate outcomes.
Supplemental Material
Supplemental material, Supplemental for Detection rates of recurrent prostate cancer: 68Gallium (Ga)-labelled prostate-specific membrane antigen versus choline PET/CT scans. A systematic review by Masood Moghul, Bhaskar Somani, Tim Lane, Nikhil Vasdev, Brian Chaplin, Clive Peedell, Gokul Vignesh KandaSwamy and Bhavan Prasad Rai in Therapeutic Advances in Urology
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Supplemental material: Supplemental material is available for this article online.
ORCID iDs: Masood Moghul  https://orcid.org/0000-0001-7957-5031
https://orcid.org/0000-0001-7957-5031
Bhaskar Somani  https://orcid.org/0000-0002-6248-6478
https://orcid.org/0000-0002-6248-6478
Contributor Information
Masood Moghul, Barts Health NHS Trust, Department of Urology, The Royal London Hospital, London, UK.
Bhaskar Somani, University Hospital Southampton NHS Trust, Southampton, UK.
Tim Lane, Hertfordshire and South Bedfordshire Urological Cancer Centre, Department of Urology, Lister Hospital, Stevenage, UK.
Nikhil Vasdev, Hertfordshire and South Bedfordshire Urological Cancer Centre, Department of Urology, Lister Hospital, Stevenage, UK.
Brian Chaplin, NHS Foundation Trust, Consultant Urological Surgeon, South Tees Hospitals NHS Foundation Trust, UK.
Clive Peedell, James Cook University Hospital, Middlesbrough, UK.
Gokul Vignesh KandaSwamy, Morriston Hospital, Abertawe Bro Morgannwg University Health Board, Swansea.
Bhavan Prasad Rai, Department of Urology, Freeman Hospital, Newcastle upon Tyne, UK.
References
- 1. Rai BP, Baum RP, Patel A, et al. The role of positron emission tomography with (68)Gallium (Ga)-labeled prostate-specific membrane antigen (PSMA) in the management of patients with organ-confined and locally advanced prostate cancer prior to radical treatment and after radical prostatect. Urology 2016; 95: 11–15. [DOI] [PubMed] [Google Scholar]
- 2. Pelosi E, Arena V, Skanjeti A, et al. Role of whole-body 18F-choline PET/CT in disease detection in patients with biochemical relapse after radical treatment for prostate cancer. Radiol Med 2008; 113: 895–904. [DOI] [PubMed] [Google Scholar]
- 3. Heinisch M, Dirisamer A, Loidl W, et al. Positron emission tomography/computed tomography with F-18-fluorocholine for restaging of prostate cancer patients: meaningful at PSA <5 ng/ml? Mol Imaging Biol 2006; 8: 43–48. [DOI] [PubMed] [Google Scholar]
- 4. Krause BJ, Souvatzoglou M, Tuncel M, et al. The detection rate of [11C]choline-PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer. Eur J Nucl Med Mol Imaging 2008; 35: 18–23. [DOI] [PubMed] [Google Scholar]
- 5. Kwee SA, DeGrado T. Prostate biopsy guided by 18F-fluorocholine PET in men with persistently elevated PSA levels. Eur J Nucl Med Mol Imaging 2008; 35: 1570. [DOI] [PubMed] [Google Scholar]
- 6. Husarik DB, Miralbell R, Dubs M, et al. Evaluation of [18 F]-choline PET/CT for staging and restaging of prostate cancer. Eur J Nucl Med Mol Imaging 2008; 253–263. [DOI] [PubMed] [Google Scholar]
- 7. Schmid DT, John H, Zweifel R, et al. Fluorocholine PET/CT in patients with prostate cancer: initial experience. Radiology 2005; 235: 623–628. [DOI] [PubMed] [Google Scholar]
- 8. Hillier SM, Maresca KP, Femia FJ, et al. Preclinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Cancer Res 2009; 69: 6932–6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu H, Moy P, Kim S, et al. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res 1997; 57: 3629. [PubMed] [Google Scholar]
- 10. Bander NH. Technology insight: monoclonal antibody imaging of prostate cancer. Nat Clin Pract Urol 2006; 3: 216–225. [DOI] [PubMed] [Google Scholar]
- 11. Eder M, Schafer M, Bauder-Wust U, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem 2012; 23: 688–697. [DOI] [PubMed] [Google Scholar]
- 12. Silver DA, Pellicer I, Fair WR, et al. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res 1997; 3: 81–85. [PubMed] [Google Scholar]
- 13. Mannweiler S, Amersdorfer P. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol Oncol Res 2009; 15: 167–172. [DOI] [PubMed] [Google Scholar]
- 14. Murphy GP, Elgamal AA, Su SL, et al. Current evaluation of the tissue localization and diagnostic utility of prostate specific membrane antigen. Cancer 1998; 83: 2259–2269. [PubMed] [Google Scholar]
- 15. Sweat SD, Pacelli A, Murphy GP, et al. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology 1998; 52: 637–640. [DOI] [PubMed] [Google Scholar]
- 16. Ross JS, Sheehan CE, Fisher HAG, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res 2003; 9: 6357–6362. [PubMed] [Google Scholar]
- 17. Rajasekaran AK, Anilkumar G, Christiansen JJ. Is prostate-specific membrane antigen a multifunctional protein? Am J Physiol Cell Physiol 2005; 288: C975–C981. [DOI] [PubMed] [Google Scholar]
- 18. Afshar-Oromieh A, Haberkorn U, Eder M, et al. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18F-FECH. Eur J Nucl Med Mol Imaging 2012; 39: 1085–1086. [DOI] [PubMed] [Google Scholar]
- 19. Moher D Liberati A Tetzlaff J et al.;. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–269, W64. [DOI] [PubMed] [Google Scholar]
- 20. Higgins JPT, Green S. (eds). Cochrane handbook for systematic reviews of interventions. Version 5.1.0. 2011], The Cochrane Collaboration, http://handbook.cochrane.org [Google Scholar]
- 21. Bluemel C, Krebs M, Polat B, et al. 68Ga-PSMA-PET/CT in patients with biochemical prostate cancer recurrence and negative18F-Choline-PET/CT. Clin Nucl Med 2016; 41: 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morigi JJ, Stricker PD, van Leeuwen PJ, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med 2015; 56: 1185–1190. [DOI] [PubMed] [Google Scholar]
- 23. Schwenck J, Rempp H, Reischl G, et al. Comparison of 68Ga-labelled PSMA-11 and 11C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging 2017; 44: 92–101. [DOI] [PubMed] [Google Scholar]
- 24. Afshar-Oromieh A, Zechmann CM, Malcher A, et al. Comparison of PET imaging with a 68Ga-labelled PSMA ligand and 18F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2014; 41: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steuber T, Jilg C, Tennstedt P, et al. Standard of care versus metastases-directed therapy for pet-detected nodal oligorecurrent prostate cancer following multimodality treatment: a multi-institutional case-control study. Eur Urol Focus 2018; pii: S2405-S4569(18)30077-4. [DOI] [PubMed] [Google Scholar]
- 26. Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase ii trial. J Clin Oncol 2018; 36: 446–453. [DOI] [PubMed] [Google Scholar]
- 27. Pfister D, Bolla M, Briganti A, et al. Early salvage radiotherapy following radical prostatectomy. Eur Urol 2014; 65: 1034–1043. [DOI] [PubMed] [Google Scholar]
- 28. Castellucci P, Ceci F, Graziani T, et al. Early biochemical relapse after radical prostatectomy: which prostate cancer patients may benefit from a restaging 11C-Choline PET/CT scan before salvage radiation therapy? J Nucl Med 2014; 55: 1424–1429. [DOI] [PubMed] [Google Scholar]
- 29. von Eyben FE, Picchio M, von Eyben R, et al. 68Ga-labeled prostate-specific membrane antigen ligand positron emission tomography/computed tomography for prostate cancer: a systematic review and meta-analysis. Eur Urol Focus 2016; pii: S2405-S4569(16)30160-2. [DOI] [PubMed] [Google Scholar]
- 30. Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive (68)Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol 2016; 70: 926–937. [DOI] [PubMed] [Google Scholar]
- 31. Hofman MS, Murphy DG, Williams SG, et al. A prospective randomized multicentre study of the impact of gallium-68 prostate-specific membrane antigen (PSMA) PET/CT imaging for staging high-risk prostate cancer prior to curative-intent surgery or radiotherapy (proPSMA study): clinical trial protocol. BJU Int 2018; 122: 783–793. [DOI] [PubMed] [Google Scholar]
- 32. ClinicalTrials.gov. Non-systemic treatment for patients with low-volume metastatic prostate cancer [Internet]. Report no.: NCT01558427. Available from: https://clinicaltrials.gov/show/NCT01558427.
- 33. ClinicalTrials.gov. Conventional care versus radioablation (stereotactic body radiotherapy) for extracranial oligometastases [Internet]. Report no.: NCT02759783. Available from: https://clinicaltrials.gov/ct2/show/NCT02759783.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental for Detection rates of recurrent prostate cancer: 68Gallium (Ga)-labelled prostate-specific membrane antigen versus choline PET/CT scans. A systematic review by Masood Moghul, Bhaskar Somani, Tim Lane, Nikhil Vasdev, Brian Chaplin, Clive Peedell, Gokul Vignesh KandaSwamy and Bhavan Prasad Rai in Therapeutic Advances in Urology