Abstract
Objective
To determine whether infection-prevention and control (IPC) interventions can reduce the colonisation and infection of intensive care unit (ICU)-acquired carbapenem-resistant Klebsiella pneumoniae (CRKP) in a general ICU ward in China.
Methods
We used a quasi-experimental before-and-after study design. The study was conducted in 4 stages: baseline period, January 2013–June 2013; IPC interventions period including de-escalation and targeted bundle interventions, July 2013–June 2014; modified IPC interventions period, July 2014–June 2015; and follow-up period, July 2015–June 2016. We used modified de-escalation interventions according to patient-risk assessments to prevent the transmission of CRKP.
Results
A total of 629 patients were enrolled in study. The incidence of ICU-acquired CRKP colonisation/infection was 10.08 (4.43–16.43) per 1000 ICU patient-days during the baseline period, and significantly decreased early during the IPC interventions, but the colonisation/infections reappeared in April 2014. During the modified IPC intervention and follow-up periods, the incidence of ICU-acquired CRKP colonisations/infections reduced to 5.62 (0.69–6.34) and 2.84 (2.80–2.89), respectively, with ongoing admission of cases with previously acquired CRKP. The incidence of ICU-acquired CRKP catheter-related bloodstream infections decreased from 2.54 during the baseline period to 0.41 during the follow-up period. The incidence of ventilator-associated pneumonia and skin and soft tissue infections showed a downward trend from 2.84 to 0.41 and from 3.4 to 0.47, respectively, with slight fluctuations.
Conclusions
Comprehensive IPC interventions including de-escalation and targeted bundle interventions showed a significant reduction in ICU-acquired CRKP colonisations/infections, despite ongoing admission of patients colonised/infected with CRKP.
Electronic supplementary material
The online version of this article (10.1186/s13756-018-0453-7) contains supplementary material, which is available to authorized users.
Keywords: Carbapenem-resistant Klebsiella pneumoniae; Infection-prevention and control intervention; De-escalation; Incidence; Catheter-related infection, intensive care unit
Introduction
Klebsiella pneumoniae infections are a serious contemporary problem in intensive care units (ICUs) worldwide and primarily affect critical and immunocompromised patients [1]. Carbapenems are the last class of β-lactam drugs that have retained their anti-Gram-negative activity; however, multidrug-resistant (MDR) bacteria, especially carbapenem-resistant Klebsiella pneumoniae (CRKP), have emerged and disseminated worldwide [2, 3]. In our hospital, the incidence of CRKP has increased remarkably from 7.3% in 2011 to 25% in 2015 (unpublished). Most of CRKP strains exhibit multidrug resistance and fail to respond to conventional therapy, which has resulted in a 28-day attributable mortality rate of 30–70% [4]. In 2013, global agencies such as the Centers for Disease Control and Prevention declared carbapenem-resistant Enterobacteriaceae spp. as an immediate public health threat that required urgent and aggressive action [5].
Promiscuous plasmids and clonal outbreaks exacerbate the worldwide spread of CRKP [6]. Risk factors associated with the acquisition of CRKP bacteria include poor functional status, comorbidities, ICU stay, use of invasive devices, immunosuppression, and exposure to multiple antibiotics before the initial culture [7]. Patient-to-patient cross-transmission and hand carriage by health care workers are the main modes of spread of CRKP [8, 9]. In addition to the antimicrobial stewardship programs, coordinated infection-prevention and control (IPC) interventions such as contact precautions, hand hygiene, and active surveillance of patients at risk for CRKP carriage and infection are advocated for effectively stopping the spread of CRKP outbreaks. Robust evidence for IPC interventions effectiveness in individual studies is limited in developed countries [10–12]. Additionally, the interventions vary widely by region and effect. Moreover, multifaceted implementation programs in low-income and middle-income countries, especially in hospital settings with restricted resources, are inconclusive.
This retrospective study aimed to evaluate the epidemiologic characteristics of CRKP in the general ICU during July 2013–June 2016 and assess the effect of collaborative IPC interventions to prevent the spread of CRKP in a teaching hospital in Shanghai.
Methods
Settings and ethics statement
The tertiary-level ICU of the Ruijin Hospital, Shanghai Jiao Tong University School of Medicine in Shanghai, consists of 12 beds including 8 private rooms and 2 double-occupancy rooms, and can hold approximately 180–250 critically ill patients annually. The study population consisted of all consecutive patients admitted to the ICU from 1 January 2013 through 30 June 2016. We employed a quasi-experimental before-and-after study design.
Bacterial isolation, antimicrobial susceptibility testing, and clinical data collection
When patients were admitted to the ICU ward, pathogens screening had been carried out immediately and the second ASCs had been done within a week. Then routine and active surveillance cultures (ASCs) from various samples (nasopharyngeal swabs, sputum, endotracheal aspirate, urinary tract, and other possible infection sites) had been collected to monitor the incidence of CRKP colonisation/infection twice a week on Monday and Thursday. All isolates were identified using the VITEK2 compact system (bioMérieux, France), and routine antibiotic susceptibility tests were performed by the disk-diffusion assay to identify carbapenem resistance; susceptibility breakpoints were interpreted as per the Clinical and Laboratory Standards Institute guidelines [13]. Two specialists in infection biology helped collect patients’ clinical and microbiological data on colonisation and/or CRKP infection, including demographic characteristics, comorbid conditions, severity of illness, type of specimen, invasive procedures, antibiotic therapy, and outcomes. Escherichia coli ATCC 25922 was used as the quality-control strain.
We retrospectively analyzed the genetic relationships of the 18 collected CRKP isolates from October 2015 to June 2016 by carbapenemase genes (blaKPC, blaIMP, blaNDM, blaVIM, and blaOXA-48) sequencing, multilocus sequence typing (MLST) and pulsed-field gel electrophoresis (PFGE) methods to illustrate the main transmission clones in our ICU ward. Related methods were showed in Additional file 1 in details.
Infection-control interventions and data collection
The study was implemented in 4 stages (Table 1). The first stage was a 6-month baseline period included patients who were admitted to the ICU during January 2013–June 2013, during which no intervention was performed and regular culture surveys were conducted to measure the prevalence of CRKP colonisation/infection.
Table 1.
Interventions undertaken to curtail the epidemic spread of CRKP in 4 cumulative stages a
| Intervention | Description | Date begun |
|---|---|---|
| Baseline period | No intervention and regular surveillance cultures | Jan 2013- June 2013 |
| Period 1 | 1. Active surveillance cultures 2. De-escalation interventions |
July 2013–June 2014 |
| 2.1 First level interventions | ||
| • contact precautions • patient isolation: single room isolation or cohorting • cohorting of medical care • disinfection and sterilization | ||
| 2.2 Second level interventions | ||
| • contact precautions • disinfection and sterilization | ||
| IPC interventions | 2.3 Third level interventions | |
| • disinfection and sterilization | ||
| 3. Target bundles interventions | ||
| • intravascular catheter-related infection • ventilation associated pneumonia • catheter-associated urinary tract infection • skin and soft tissue infections. | ||
| Period 2 | 1. Active surveillance cultures 2. Modified de-escalation interventions |
July 2014–June 2015 |
| Modified IPC interventions |
+ Enhanced external medical staff education + Contact precautions of shared equipment + Enhanced terminal room disinfection |
|
| 2.1 First level interventions (as Period 1) 2.2 Second level interventions (as Period 1) 2.3 Third level interventions (as Period 1) |
||
| 3. Target bundles interventions (as Period 1) | ||
| Period 3 | Follow up period 2 | July 2015–June 2016 |
aThe procedures of infection control interventions are explicated in detail in the section of Materials and Methods
IPC Infection prevention and control, CRKP Carbapenem-resistant Klebsiella pneumoniae
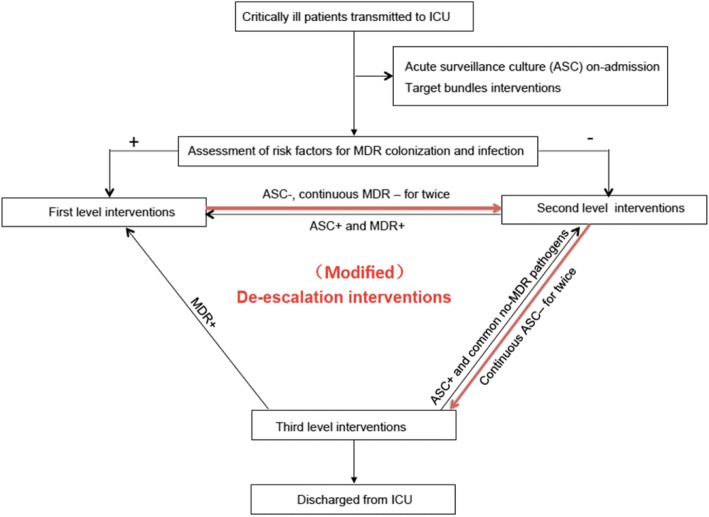
The second stage—standard IPC intervention period including contact precautions, patient isolation, cohorting of medical care and disinfection and sterilization with de-escalation strategy and targeted bundle interventions aiming at intravascular catheter-related infection, ventilator-associated pneumonia, catheter-associated urinary tract infection and skin and soft tissue infections were implemented (Fig. 1)—during July 2013–June 2014. ASCs for pathogen colonisation/infection were performed twice a week, and the risk factors for MDR bacteria colonisation/infection were assessed [14]. First-level interventions were performed for patients who were diagnosed with MDR pathogen colonisation/infection or transferred from a clinical department with a high prevalence of MDR bacteria to the ICU and had invasive devices and skin-barrier damage by endotracheal intubation, central venous catheter insertion, etc. The first-level interventions included the following: (i) Contact precautions: adherence to hand-hygiene protocols before and after patient care and wearing gowns and gloves before patient care. (ii) Patient isolation: single-room isolation or cohorting. Patients with same MDR pathogens were admitted in double-occupancy rooms or a concentrated area of the unit, managed by dedicated nursing staff, and supplied with separate disposable medical equipment. (iii) Disinfection and sterilisation: To decrease the risk of transmission and burden of organisms, terminal disinfection of rooms was performed after patients been transferred or discharged out of ICU, different from ultraviolet irradiation disinfection regardless of time and distance in the baseline period. Environmental cleaning in the intervention period was enhanced including cleaning of areas in close proximity to the patient and irradiation with ultraviolet light from a close distance for at least 24 h after patients release. Additionally, targeted bundle interventions including those for intravascular catheter-related infection, ventilator-associated pneumonia, catheter-associated urinary tract infection, and skin and soft tissue infections were performed as previously described [15]. For patients who were transferred from a clinical department with a lower prevalence of MDR bacteria than the ICU or were colonised/infected with common pathogens before ICU admittance, second-level interventions including contact precautions, disinfection, and sterilisation (irradiation with ultraviolet light from a close distance for at least 2 h) were implemented. For patients without bacterial colonisation/infection or high-risk factors, third-level interventions including hand hygiene, disinfection, and sterilisation measures were used. During the study, when patients receiving first-level interventions had 2 consecutive negative ASCs which should be carried out over at least 1 week, they were de-escalated to second-level interventions. When second-level patients showed 2 consecutive negative ASCs, they were de-escalated to third-level interventions. Patients in any level were upgraded to first-level interventions if MDR bacteria were detected during their ICU stay.
Fig. 1.
The procedures of (Modified) de-escalation interventions in period 2 and 3. Patients were active surveillance culture (ASC) for pathogens colonization and infection twice a week when admitted to the ICU, and assesse the risk factors of MDR bacteria colonization and infection immediately. For patients were defined infection/colonization with MDR pathogens, or came from clinical department with high prevalence of MDR bacteria before admitted to ICU, with invasive devices and damaged skin barrier such as endotracheal intubation, central venous catheter and urinary catheter etc., first level interventions measures were taken. For patients came from clinical department with low detection rate of MDR bacteria and just infected/colonized with common pathogens before admitted to ICU, second level interventions were taken. However, patients without bacteria infection/colonization or high risk factors, simple third level interventions were implemented. When patients in first level interventions have 2 consecutive negative tests which should be carried out over at least 1 week, descend to second level interventions. When continuous twice ASC negative results for second level patients, descend to third level interventions, and upgrade to first level interventions once MDR bacteria was detected during their ICU stay
The third stage—modified IPC intervention period—during July 2014–June 2015. Modified IPC interventions, in addition to the standard IPC interventions, which included extensive external medical staff education (all consulting staff, rehabilitative physicians, and external nurses should receive lecture and practices on infection prevention education once a month, including the importance and procedures of infection control measures); extensive contact precautions and cleaning of shared equipment (radiography and ultrasound machines); and extensive terminal room disinfection, especially for areas in close proximity to CRKP carriers such as surfaces around sinks. Once the CRKP patients were discharged, extensive terminal cleaning of CRKP patient rooms and monitoring of the cleaning process were performed to ensure that all surfaces were adequately cleaned and disinfected.
The fourth stage—follow-up period—involved a follow-up in addition to the modified IPC interventions during July 2015–June 2016.
Definitions
ICU-acquired infection was confirmed when pathogens were not present at the time of admission, but detected by ASC after ICU admission for > 48 h [16]. Isolates not susceptible to imipenem, meropenem, or ertapenem were considered carbapenem-resistant. Central line-associated bloodstream infection, ventilator-associated pneumonia, and catheter-associated urinary tract infection were defined as per the Centers for Disease Control guidelines [15, 17–19].
The incidence of CRKP colonisations/infections detected by cultures was measured and standardised to the number of cases per 1000 ICU patient-days according to the Centers for Disease Control criteria [14]. The monthly incidence of CRKP positivity was calculated. CRKP colonisation/infection was measured and compared with the overall periodic incidence. The primary endpoint was the monthly incidence of ICU-acquired CRKP patients (no. of cases/1000 ICU patient-days) during the baseline and different intervention periods. Subsequently, the incidence of infections form different sites (no. of cases per 1000 catheter-days/ventilator-days /ICU patient-days) was calculated.
Statistical analysis
All statistical tests were performed using SPSS software, version 17.0 (Chicago, IL, USA). Discrete variables were summarised as frequency (%) and continuous variables, as mean and standard deviation or median and interquartile range. Student’s t-test and the Mann-Whitney U-test were used to compare continuous variables. Continuous variables were compared among multiple groups using variance analysis and Students-Newman-Keuls test (normal distribution and equal variances assumed) or rank sum test (non-normal distribution or equal variances not assumed). Categorical variables were compared using the χ2 test or Fisher’s test, as appropriate. All tests were 2-tailed, and values of P < 0.05 were considered statistically significant. Segmented (interrupted) linear regression (Additional file 1: Table S2) was performed to examine whether the use of predetermined IPC interventions affected incidence.
Results
A total of 629 patients were enrolled during the entire study: 74 cases in the baseline period, 187 cases in the standard IPC intervention period, 222 cases in the modified IPC intervention period, and 146 cases in the follow-up period. Additionally, 87 patients had CRKP, of which 69 (79.3%) patients acquired the infection in the ICU and 18 (20.7%) acquired the infection prior to ICU admission. Patient data such as general characteristics, presence of chronic disease, organ failure, risk factors of CRKP colonisation/infection were described in Table 2. The Acute Physiology and Chronic Health Evaluation II score on ICU admission, proportion of patients with organ failure such as congestive heart failure and acute respiratory failure, and exposure to carbapenems before CRKP colonisation/infection were higher during the intervention periods as compared to the baseline. However, the number of invasive operations such as tracheal intubation, surgeries, central line catheter insertions, and indwelling urinary catheter insertions before CRKP colonisation/infection were lower in the follow-up period than in the intervention periods. All other patient characteristics during the ICU stay were similar among the 4 study periods.
Table 2.
Clinical characteristics of the ICU patients, according to study period
| Variable | Baseline period | Period 1 | Period 2 | Period 3 | P value |
|---|---|---|---|---|---|
| General characteristics | |||||
| Patients (no.) | 74 | 187 | 222 | 146 | |
| Age (yr), mean ± SD | 63 ± 17 | 62 ± 18 | 61 ± 18 | 63 ± 19 | 0.869 |
| Male sex (%) | 52(70.3%) | 114(61%) | 134(60.4%) | 90(61.6%) | 0.476 |
| APACHEII score, mean ± SD | 15 ± 7 | 15 ± 7 | 16 ± 8 | 19 ± 9 | 0.001 |
| Chronic disease | |||||
| Malignancy(%) | 29(39.2%) | 90(48.1%) | 108(48.6%) | 59(40.4%) | 0.249 |
| Chronic obstructive pulmonary disease(%) | 3(4.1%) | 6(3.2%) | 13(5.9%) | 8(5.5%) | 0.591 |
| Diabetes(%) | 12(16.2%) | 24(12.8%) | 35(15.8%) | 20(13.7%) | 0.811 |
| Hypertension(%) | 14(18.9%) | 55(29.4%) | 60(27%) | 54(37%) | 0.035 |
| Coronary heart disease(%) | 5(6.8%) | 10(5.3%) | 19(8.6%) | 7(4.8%) | 0.454 |
| Organ failure | |||||
| Congestive heart failure(%) | 4(5.4%) | 8(4.3%) | 26(11. 7%) | 19(13%) | 0.011 |
| Acute respiratory failure(%) | 12(16.2%) | 51(27.3%) | 72(32.4%) | 66(45.2%) | P < 0.001 |
| Acute renal injury(%) | 16(21.6%) | 41(21.9%) | 50(22.5%) | 46(31.5%) | 0.147 |
| Acute gastrointestinal injury (%) | 5(6.80%) | 25(13.4%) | 25(11.3%) | 33(22.6%) | 0.003 |
| Hepatic insufficiency(%) | 6(8.1%) | 20(10.7%) | 26(11.7%) | 26(17.8%) | 0.123 |
| Exposure to antimicrobial therapy before CRKP colonization/infection | |||||
| Carbapenem | 44(59.5%) | 99(52.9%) | 115(51.8%) | 94(64.4%) | 0.078 |
| Cephalosporin antibiotics | 56(75.7%) | 135(72.7%) | 177(79.7%) | 94(64.4%) | 0.012 |
| Sulbactam/ cefoperazone | 12(16.2%) | 29(15.5%) | 43(19.4%) | 27(18.5%) | 0.749 |
| Mechanical ventilation before CRKP colonization/infection | |||||
| Tracheal intubation | 63(85.1%) | 162(86.6%) | 197(88.7) | 110(75.3%) | 0.004 |
| Tracheotomy | 11(4.9%) | 22(11.8%) | 23(10.8%) | 25(17.1%) | 0.318 |
| Days of mechanical ventilation, days, mean ± SD | 7 ± 14 | 7 ± 20 | 5 ± 14 | 10 ± 23 | 0.104 |
| Surgery or invasive devices before CRKP colonization/infection | |||||
| Surgery | 61(82.4%) | 160(85.6%) | 179(80.6%) | 103(70.5%) | 0.007 |
| Central line catheter | 71(95.9%) | 165(88.2%) | 180(81.1%) | 113(72.4%) | P < 0.001 |
| Thoracentesis | 9(12.2%) | 17(9.1%) | 23(10.4%) | 24(16.4%) | 0.184 |
| Abdominal paracentesis | 5(6.8%) | 25(13.4%) | 21(9.5%) | 18(12.3%) | 0.356 |
| Continuous renal replacement therapy | 1(1.4%) | 26(13.9%) | 22(9.9%) | 19(13%) | 0.022 |
| Indwelling urinary catheter | 70(94.6%) | 182(97.3%) | 218(98.2%) | 135(92.5%) | 0.027 |
The microbiological characteristics and genetic relatedships of the 18 collected CRKP isolates from October 2015 to June 2016 were retrospectively analyzed. 13 blaKPC positive, 3 blaVIM positive and one blaOXA-48 positive CRKP isolates were detected. MLST data showed that these 13 KPC-2 carbapenemases producing CRKP isolates belonged to the ST11 group and that their PFGE patterns were highly similar between on-admission and ICU-acquired CRKP isolates. (Additional file 1: Table S1 and Figure S1). However, genome of detected K. pneumonia should be sequenced and compared to clarify the main transmission clones of ICU-acquired CRKP in our future infection control measures and studies.
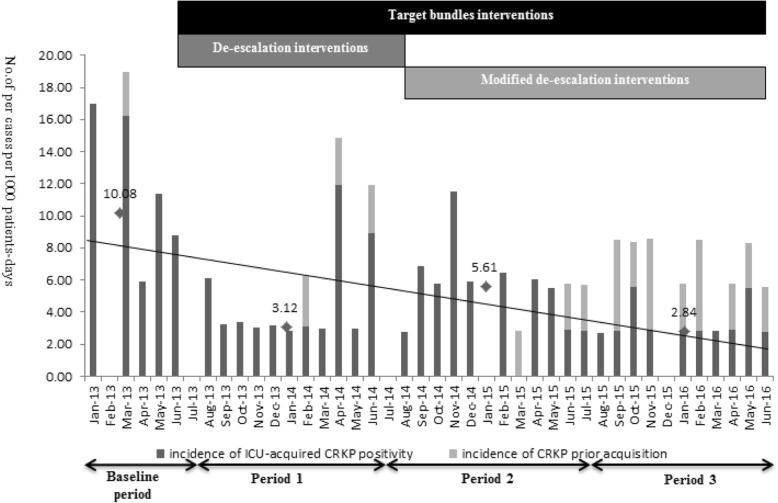
The monthly incidence of totally CRKP colonisation/infections including ICU-acquired CRKP and CRKP acquisition prior ICU admission were showed in Fig. 2 and Table 3. After the modified IPC intervention, a significant reduction in the overall incidence was observed (Table 3). The monthly incidence of ICU-acquired CRKP was 10.08 (4.43–16.43) cases per 1000 ICU patient-days at the baseline, and IPC interventions were associated with a clear significant decrease to 3.12 (2.98–5.40), but was followed by several increases in the incidence in April 2014(Fig. 2). The prevalence rates stabilised and decreased with implementation of the modified IPC interventions in the modified IPC intervention (5.62 (0.69–6.34)) and follow-up periods (2.84 (2.80–2.89)) as compared to the baseline period (P = 0.032 and 0.021). There was no significant difference in the monthly incidence rates between the standard and modified IPC intervention periods. Although patients who acquired infections prior to ICU admission showed an increasing distribution during the follow-up period, there was a continuous declination in the incidence of ICU-acquired CRKP infections with respect to compliance with modified IPC interventions, (P = 0.032). Segmented (interrupted) linear regression was added to examine whether the use of predetermined IPC interventions affected incidence. IPC interventions period did not showed a significant change in incidence due to short time of baseline period. However, compared with IPC interventions period, the success of modified IPC interventions was shown by the negative slope value which was associated with continuous declination of monthly incidence of ICU-acquired CRKP colonization/ infection (p = 0.036). Results were shown in Additional file 1: Table S2.
Fig. 2.
Epidemic of observed monthly incidence rate of patients (No. of cases per 1000 ICU patient-days) colonized or infected with CRKP in the intensive care unit during the baseline and different intervention periods. Black bars show the incidence rate of ICU-acquired CRKP positivity, and gray bars represent the incidence rate of CRKP patients at ICU admission surveillance. Trend lines of ICU-acquired CRKP (No. of cases per 1000 ICU patient-days) for each period are shown in black solid bold type, showed a downward incidence rate trend from 10.08 to 2.84 after the implement of modified IPC interventions (P<0.05)
Table 3.
CRKP distribution and the incidence of ICU-acquired infections from different infection sites
| Variable | Baseline period | Period 1 | Period 2 | Period 3 | p |
|---|---|---|---|---|---|
| No. cases of total CRKP | 22 | 20 | 20 | 25 | |
| No. cases of ICU-on-admission CRKP | 1 | 3 | 2 | 12 | |
| No. cases of ICU-acquired CRKP | 21 | 17 | 18 | 13 | |
| Probable source of colonization/ infection of ICU-acquired CRKP | |||||
| Bloodstream n(%) | 3/21(14.3) | 6/17(35.3) | 1/18(5.6) | 1/13(7.7) | 0.11 |
| Respiratory tract n(%) | 4/21(19) | 3/17(17.6) | 5/18(27.8) | 5/13(38.5) | 0.555 |
| Skin and soft tissue n(%) | 13/21(61.9) | 7/17(41.2) | 12/18(66.7) | 3/13(23.1) | 0.058 |
| Urine tract n(%) | 1/21(4.8) | 1/17(5.9) | 0/18(0) | 4/13(25) | 0.073 |
| Incidence-ICU acquired | |||||
| Incidence of CRBSI (No. of cases per 1000 catheter days) | 2.54 | 2.44 | 0.43 | 0.41 | 0.352 |
| Incidence of VAP (No. of cases per 1000 ventilator-days) | 2.84 | 0.48 | 0.49 | 0.81 | 0.41 |
| Incidence of skin and soft tissue infections (No. of cases per 1000 ICU patient-days) | 3.40 | 0.99 | 1.46 | 0.47 | 0.531 |
| Incidence of catheter-associated urinary tract infection (No. of cases per 1000 catheter days) | 0 | 0 | 0 | 0.34 | 0.63 |
During the entire study, the probable source of colonisation/infection of ICU-acquired CRKP was the bloodstream, respiratory tract, skin and soft tissues, and urinary tract (Table 4). The incidence of ICU-acquired CRKP catheter-related bloodstream infections per 1000 central line catheter-days decreased from 2.54 during the baseline period to 0.41 during the follow-up period, although the difference was not statistically significant. Additionally, there was a downward trend in the incidence of ICU-acquired CRKP ventilator-associated pneumonia and skin and soft tissue infections from 2.84 to 0.41 infections per 1000 ventilator-days and from 3.4 to 0.47 infections per 1000 ICU patient-days, with slight fluctuations in the trend. In contrast, there was no difference in the incidence of urinary tract infections.
Table 4.
Monthly incidence rates of totally CRKP colonization/ infection and ICU-acquired CRKP (No. of cases per 1000 ICU patient-days)
| Intervention (period) | Incidence | Incidence | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of cases per 1000 ICU patient-days (totally CRKP) | No. of cases per 1000 ICU patient-days (ICU-acquired CRKP) | |||||||
| Mean | Median | Pa | Pb | Mean | Median | Pc | Pd | |
| Baseline period | 10.33 ± 7.05 | 10.08 (4.43–17.46) | / | / | 9.88 ± 6.44 | 10.08 (4.43–16.43) | / | / |
| Period 1 | 5.05 ± 4.25 | 3.18 (2.98–6.18) | 0.014 | / | 4.30 ± 3.2 | 3.12 (2.98–5.40) | 0.024 | / |
| Period 2 | 4.94 ± 3.18 | 5.76 (2.77–6.34 | 0.013 | 0.945 | 4.47 ± 3.46 | 5.62 (0.69–6.34) | 0.032 | 0.9 |
| Period 3 | 5.88 ± 2.81 | 5.78 (3.52–8.47) | 0.037 | 0.577 | 3.05 ± 1.42 | 2.84 (2.80–2.89) | 0.021 | 0.032 |
aCompared with baseline period
bCompared with period 1
cCompared with baseline period
dCompared with period 1
Discussion
This report describes the management of an outbreak of CRKP colonisation/infection until final control of the epidemic and includes clinical data of > 600 ICU patients over a period of almost 4 years. Implementation of an infection-prevention bundle and control interventions led to clinically important and statistically significant decreases in ICU-acquired CRKP colonisations/infections at a tertiary-level ICU in a developing country. To our knowledge, this is one of the first few comprehensive IPC studies including de-escalation and targeted bundle interventions that aimed at reducing the incidence of ICU-acquired CRKP in China.
Thus far, there are no universally useful and successful infection-control measures in hospital settings in low- and middle-income countries. The current medical literature does not provide sufficient data to determine which model of infection control is the most appropriate and effective in controlling the spread of CRKP in the ICU [20, 21]. Our proposed intervention comprises 3 main components: ASC; de-escalation interventions; and targeted bundle interventions. Although the efficacy of ASC has been described previously [22, 23], our study showed that ASC allowed early identification of CRKP carriers and guided de-escalation interventions to stop contact precautions and initiate patient isolation in a timely manner. Through this approach, patients with different risk factors were provided hierarchical prevention and control measures and were upgraded or de-escalated according to the ASC results. A 4-year perspective study in Israel [10] showed that isolation precaution alone was ineffective. Our bundled IPC interventions, including isolation precaution, showed a rapid decrease in the incidence of ICU-acquired CRKP colonisations/infections. In addition to de-escalation interventions, targeted bundles led to a downward trend in the incidence of ICU-acquired CRKP infections including central line-associated bloodstream infection, ventilator-associated pneumonia, and skin and soft tissue infections.
When carbapenem-resistant pathogen outbreaks occurred with multimodal interventions, it was difficult to determine which of the interventions impacted the transmission of CRKP. A study on carbapenem-resistant Acinetobacter baumannii outbreak in an Italian ICU [24] showed that medical staff should focus on standard contact precautions, especially hand-washing with alcohol-based solutions and personal protective equipment. In contrast, a 5-year Greek study [25] suggested that the most-influential factors responsible for a decrease in the incidence of carbapenem-resistant pathogen in the bloodstream infections were increased participation in educational courses. Feedback from screening services, rapid turnaround time and efficient communication were correlated with overall institutional success in outbreak control [26, 27]. In our study, although de-escalation and targeted bundle interventions helped to reduce the incidence of CRKP during IPC interventions, the reappearance of ICU-acquired CRKP in April 2014 suggested that additional factors were involved. Therefore, the modified IPC interventions were implemented according to the monitoring and analysis of screening results and proved successful in preventing the transmission of CRKP with prompt implementation of organizational measures such as non-ICU medical staff education, contact precautions for shared equipment, and multiple rigorous cleaning and disinfection interventions. The incidence of ICU-acquired CRKP colonisations and infections continued to decrease despite the extremely high number of cases of previous CRKP acquisition at ICU admission in period 2 and 3, suggesting that these measures may contributed to decrease the incidence of ICU-acquired infections. The integration of epidemiological and microbiological data and the strict application of infection-control measures played a decisive role in preventing against the spread of CRKP in our hospital.
In developing countries, shortage of medical supplies is known to contribute to a low adherence to adequate IPC practices and increase the risk of nosocomial infections [25]. The follow-up period is more important than the other periods, as it involves evaluation of the effectiveness and practicality of IPC interventions during a prolonged national outbreak [28, 29]. In a 1-year follow-up period, the incidence of ICU-acquired CRKP colonisations and infections continued to decrease despite the extremely high number of cases of previous CRKP acquisition at ICU admission.
During the entire study period, we implemented a de-escalation antimicrobial therapy based on culture results and the clinical status of patients. But the impact of de-escalation antimicrobial therapy on antimicrobial resistance was not analyzed. While other studies have shown a relationship between antimicrobial use and resistance. Antimicrobial stewardship programs especially de-escalation antimicrobial therapy has been shown to improve patient outcomes, reduce antimicrobial adverse events, and decrease antimicrobial resistance [30, 31].
Our study had a few limitations. Firstly, this was a single-centre study; therefore, the number of included patients was relatively small and the results may not be generalizable to other institutions. Further multicentre, prospective studies are needed to confirm our findings. Secondly, the interventions described in this study were multimodal due to clinical necessity, which precludes determination of the effectiveness of any single measure. Thirdly, compliance with various interventions during the study period was not assessed.
Conclusion
In summary, comprehensive IPC interventions including modified de-escalation and targeted bundle interventions played a pivotal role in controlling the epidemic spread of ICU-acquired CRKP colonisations/infections in our hospital in China.
Additional file
Table S1. The microbiological characteristics and genetic relatedness of the 18 collected CRKP isolates. Table S2. Central distribution values and regression slopes for the monthly incidence of colonization/Infection with ICU-acquired CRKP (No. of cases per 1000 ICU patient-days). Figure S1. Pulsed-field gel electrophoresis (PFGE) of XbaI-digested DNA of 13 KPC-2 producing CRKP isolates. PFGE marker, Salmonella enterica H9812. (DOCX 651 kb)
Acknowledgements
The authors are grateful to Chenrong Mi (Department of nosocomial infection management, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine) for their assistance in analysing the data needed for the study.
Funding
This study was supported by National Key R&D Program of China (2017YFC1309700, 2017YFC1309705).
Availability of data and materials
The raw data can be made available to the interested researchers by the authors of this article if requested.
Abbreviations
- ASC
Active surveillance cultures
- CRKP
Carbapenem-resistant Klebsiella pneumonia
- ICU
Intensive care unit
- IPC
Infection-prevention and control
- MDR
Multidrug-resistant
Authors’ contributions
HQ and JL designed the study. ML, XW, JW and RT analysed the data. JS, LL, JH, JW, QG and YZ performed the surveillance. ML and XW wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study protocol was approved by the Institutional Ethic Committees of the Shanghai Jiao Tong University School of Medicine and Ruijin Hospital (2013 NO. 29) and written consent was obtained from all participating patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Meiling Li, Email: merlin-163@163.com.
Xiaoli Wang, Email: xiaoliwang0714@163.com.
Jiahui Wang, Email: otakubecoming@163.com.
Ruoming Tan, Email: sandratan37@hotmail.com.
Jingyong Sun, Email: 13671578899@126.com.
Lei Li, Email: Lileiys1023@yeah.net.
Jie Huang, Email: seaky_huang@yaoo.com.
Jun Wu, Email: tonywujun@126.com.
Qiuying Gu, Email: gqy20769@rjh.com.cn.
Yujin Zhao, Email: 2631219516@qq.coom.
Jialin Liu, Email: ljl11243@rjh.com.cn.
Hongping Qu, Phone: +8602153305091, Email: hongpingqu0412@hotmail.com.
References
- 1.Bassetti M, Poulakou G, Ruppe E, Bouza E, Van Hal SJ, Brink A. Antimicrobial resistance in the next 30 years, humankind, bugs and drugs: a visionary approach. Intensive Care Med. 2017;43:1464–1475. doi: 10.1007/s00134-017-4878-x. [DOI] [PubMed] [Google Scholar]
- 2.Guan X, He L, Hu B, Hu J, Huang X, Lai G, et al. Laboratory diagnosis, clinical management and infection control of the infections caused by extensively drug-resistant gram-negative bacilli: a Chinese consensus statement. Clin Microbiol Infect. 2016;22(Suppl 1):S15–S25. doi: 10.1016/j.cmi.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Tian L, Tan R, Chen Y, Sun J, Liu J, Qu H, et al. Epidemiology of Klebsiella pneumoniae bloodstream infections in a teaching hospital: factors related to the carbapenem resistance and patient mortality. Antimicrob Resist Infect Control. 2016;5:48. doi: 10.1186/s13756-016-0145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman ND, Carmeli Y, Walton AL, Schwaber MJ. Carbapenem-resistant Enterobacteriaceae: a strategic roadmap for infection control. Infect Control Hosp Epidemiol. 2017;38:580–594. doi: 10.1017/ice.2017.42. [DOI] [PubMed] [Google Scholar]
- 5.Solomon SL, Oliver KB. Antibiotic resistance threats in the United States: stepping back from the brink. Am Fam Physician. 2014;89(12):938–941. [PubMed] [Google Scholar]
- 6.Xu Y, Gu B, Huang M, Liu H, Xu T, Xia W, et al. Epidemiology of carbapenem resistant Enterobacteriaceae (CRE) during 2000-2012 in Asia. J Thorac Dis. 2015;7(3):376–385. doi: 10.3978/j.issn.2072-1439.2014.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Duijn PJ, Verbrugghe W, Jorens PG, Spohr F, Schedler D, Deja M, et al. The effects of antibiotic cycling and mixing on antibiotic resistance in intensive care units: a cluster-randomised crossover trial. Lancet Infect Dis. 2018;18:401–409. doi: 10.1016/S1473-3099(18)30056-2. [DOI] [PubMed] [Google Scholar]
- 8.Hayden MK, Lin MY, Lolans K, Weiner S, Blom D, Moore NM, et al. Prevention of colonization and infection by Klebsiella pneumoniae carbapenemase-producing enterobacteriaceae in long-term acute-care hospitals. Clin Infect Dis. 2015;60:1153–1161. doi: 10.1093/cid/ciu1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Loon K, Voor In’t Holt AF, Vos MC. A Systematic Review and Meta-analyses of the Clinical Epidemiology of Carbapenem-Resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2017;62(1). 10.1128/AAC.01730-17. [DOI] [PMC free article] [PubMed]
- 10.Cohen MJ, Block C, Levin PD, Schwartz C, Gross I, Weiss Y, et al. Institutional control measures to curtail the epidemic spread of carbapenem-resistant Klebsiella pneumoniae: a 4-year perspective. Infect Control Hosp Epidemiol. 2011;32:673–678. doi: 10.1086/660358. [DOI] [PubMed] [Google Scholar]
- 11.Laurent C, Rodriguez-Villalobos H, Rost F, Strale H, Vincent JL, Deplano A, et al. Intensive care unit outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae controlled by cohorting patients and reinforcing infection control measures. Infect Control Hosp Epidemiol. 2008;29:517–524. doi: 10.1086/588004. [DOI] [PubMed] [Google Scholar]
- 12.Borer A, Eskira S, Nativ R, Saidel-Odes L, Riesenberg K, Livshiz-Riven I, et al. A multifaceted intervention strategy for eradication of a hospital-wide outbreak caused by carbapenem-resistant Klebsiella pneumoniae in southern Israel. Infect Control Hosp Epidemiol. 2011;32:1158–1165. doi: 10.1086/662620. [DOI] [PubMed] [Google Scholar]
- 13.Zhang F, Wang X, Xie L, Zheng Q, Guo X, Han L, et al. A novel transposon, Tn6306, mediates the spread of blaIMI in Enterobacteriaceae in hospitals. Int J Infect Dis. 2017;65:22–26. doi: 10.1016/j.ijid.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Magiorakos AP, Burns K, Rodriguez Bano J, Borg M, Daikos G, Dumpis U, et al. Infection prevention and control measures and tools for the prevention of entry of carbapenem-resistant Enterobacteriaceae into healthcare settings: guidance from the European Centre for Disease Prevention and Control. Antimicrob Resist Infect Control. 2017;6:113. doi: 10.1186/s13756-017-0259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krein SL, Greene MT, Apisarnthanarak A, Sakamoto F, Tokuda Y, Sakihama T, et al. Infection prevention practices in Japan, Thailand, and the United States: results from National Surveys. Clin Infect Dis. 2017;64:S105–s111. doi: 10.1093/cid/cix073. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Pueyo MJ, Olaechea-Astigarraga P, Palomar-Martinez M, Insausti-Ordenana J, Alvarez-Lerma F. Quality control of the surveillance programme of ICU-acquired infection (ENVIN-HELICS registry) in Spain. J Hosp Infect. 2013;84:126–131. doi: 10.1016/j.jhin.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Lo E, Nicolle LE, Coffin SE, Gould C, Maragakis LL, Meddings J, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:464–479. doi: 10.1086/675718. [DOI] [PubMed] [Google Scholar]
- 18.Marschall J, Mermel LA, Fakih M, Hadaway L, Kallen A, O'Grady NP, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:753–771. doi: 10.1086/676533. [DOI] [PubMed] [Google Scholar]
- 19.Klompas M, Branson R, Eichenwald EC, Greene LR, Howell MD, Lee G, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:915–936. doi: 10.1086/677144. [DOI] [PubMed] [Google Scholar]
- 20.Bassetti M, Giacobbe DR, Giamarellou H, Viscoli C, Daikos GL, Dimopoulos G, et al. Management of KPC-producing Klebsiella pneumoniae infections. Clin Microbiol Infect. 2018;24:133–144. doi: 10.1016/j.cmi.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 21.Tacconelli E, Cataldo MA, Dancer SJ, De Angelis G, Falcone M, Frank U, et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. 2014;20(Suppl 1):1–55. doi: 10.1111/1469-0691.12427. [DOI] [PubMed] [Google Scholar]
- 22.Gagliotti C, Cappelli V, Carretto E, Marchi M, Pan A, Ragni P, et al. Control of carbapenemase-producing Klebsiella pneumoniae: a region-wide intervention. Euro Surveill. 2014;19(43). [DOI] [PubMed]
- 23.Langer AJ, Lafaro P, Genese CA, McDonough P, Nahass R, Robertson C. Using active microbiologic surveillance and enhanced infection control measures to control an outbreak of health care-associated extended-spectrum beta-lactamase-producing Klebsiella pneumoniae infections--New Jersey, 2007. Am J Infect Control. 2009;37:73–75. doi: 10.1016/j.ajic.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Bianco A, Quirino A, Giordano M, Marano V, Rizzo C, Liberto MC, et al. Control of carbapenem-resistant Acinetobacter baumannii outbreak in an intensive care unit of a teaching hospital in southern Italy. BMC Infect Dis. 2016;16:747. doi: 10.1186/s12879-016-2036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kousouli E, Zarkotou O, Politi L, Polimeri K, Vrioni G, Themeli-Digalaki K, et al. Infection control interventions affected by resource shortages: impact on the incidence of bacteremias caused by carbapenem-resistant pathogens. Eur J Clin Microbiol Infect Dis. 2018;37(1):43–50. doi: 10.1007/s10096-017-3098-1. [DOI] [PubMed] [Google Scholar]
- 26.Lyles RD, Moore NM, Weiner SB, Sikka M, Lin MY, Weinstein RA, et al. Understanding staff perceptions about Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae control efforts in Chicago long-term acute care hospitals. Infect Control Hosp Epidemiol. 2014;35:367–374. doi: 10.1086/675596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Apisarnthanarak A, Ratz D, Khawcharoenporn T, Patel PK, Weber DJ, Saint S, et al. National Survey of practices to prevent methicillin-resistant Staphylococcus aureus and multidrug-resistant Acinetobacter baumannii in Thailand. Clin Infect Dis. 2017;64:S161–s166. doi: 10.1093/cid/cix045. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Wang Q, Yin Y, Chen H, Jin L, Gu B, et al. Epidemiology of Carbapenem-Resistant Enterobacteriaceae Infections: Report from the China CRE Network. Antimicrob Agents Chemother. 2018;62(2). 10.1128/AAC.01882-17. [DOI] [PMC free article] [PubMed]
- 29.Gurieva T, Dautzenberg MJD, Gniadkowski M, Derde LPG, Bonten MJM, Bootsma MCJ. The transmissibility of antibiotic-resistant Enterobacteriaceae in intensive care units. Clin Infect Dis. 2018;66:489–493. doi: 10.1093/cid/cix825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly AA, Jones MM, Echevarria KL, Kralovic SM, Samore MH, Goetz MB, et al. A report of the efforts of the veterans health administration national antimicrobial stewardship initiative. Infect Control Hosp Epidemiol. 2017;38:513–520. doi: 10.1017/ice.2016.328. [DOI] [PubMed] [Google Scholar]
- 31.Dodds Ashley ES, Kaye KS, DePestel DD, Hermsen ED. Antimicrobial stewardship: philosophy versus practice. Clin Infect Dis. 2014;59(Suppl 3):S112–S121. doi: 10.1093/cid/ciu546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The microbiological characteristics and genetic relatedness of the 18 collected CRKP isolates. Table S2. Central distribution values and regression slopes for the monthly incidence of colonization/Infection with ICU-acquired CRKP (No. of cases per 1000 ICU patient-days). Figure S1. Pulsed-field gel electrophoresis (PFGE) of XbaI-digested DNA of 13 KPC-2 producing CRKP isolates. PFGE marker, Salmonella enterica H9812. (DOCX 651 kb)
Data Availability Statement
The raw data can be made available to the interested researchers by the authors of this article if requested.