Abstract
Background
Down syndrome (DS) is the most common form of viable chromosomal abnormality. DS is associated with recurrent infections, auto-immunity and malignancies in children. Little is known about immunity and infections in DS at adulthood.
Methods
We studied two separate group of adults (> 18 years old) with DS in a single referral tertiary center (Strasbourg University Hospital). The first group included 37 ambulatory DS patients between November 2014 and May 2017. We analyzed exhaustive serological and immunobiological parameters, at one point, together with the prevalence of infections, autoimmune manifestations and malignancies. The second group included 64 hospitalized patients (138 stays) in the same center, between January 2005 and December 2016.
Results
One hundred and one adult patients with DS were included. Unlike children and despite a global lymphopenia, adults with DS underwent few infections in our ambulatory group. They did not experience any malignancy and, apart from hypothyroidism, they presented only occasional autoimmune manifestations. Hospitalized DS patients were older than ambulatory ones (median age 47 years (18–73) vs. 27 (18–52), p < 0.0001) and admitted mostly for infections (76.8%). Infections were associated with epilepsy and dementia (OR 6.5 (2.2–19), p = 0.001; p = 0.0006 in multivariate analysis) and higher mortality (OR 7.4 (1.4–37), p = 0.01).
Conclusion
Despite persistent immunobiological abnormalities at adulthood, young ambulatory adults with DS remain healthy with a low rate of infections. Infections are associated with neurological degeneration and increase the mortality arguing for a dedicated support of older DS patients.
Trial registration
ClinicalTrials.gov: NCT01663675 (August 13, 2012). Hospital Clinical Research Program (PHRC): number 2012-A00466–37 (Dr Y. Alembik).
Electronic supplementary material
The online version of this article (10.1186/s13023-018-0989-x) contains supplementary material, which is available to authorized users.
Keywords: Down syndrome, Immunodeficiency, Infectious risk, Neurological impairment, Adult
Introduction
Down syndrome (DS) caused by trisomy 21 is the most common form of viable chromosome abnormality in children and the prevalence of DS continues to increase with life expectancy [1]. Survival of patients with DS improved drastically in the past few decades, with the detection and the early surgical care of congenital heart malformations (atrioventricular septal, ventricular septal, and atrial septal defects or persistent patent ductus arteriosus) [2]. The median age at death is now mid-50’s compared to 10 years of age in the 1970’s [3, 4]. Children with DS have a high incidence of infections of the respiratory tracts [5]. Over the last 3 decades, these infections have been linked to both innate and adaptive immunological abnormalities. Studies describing the immune system of infants with DS report the reduction and an altered distribution of T and B cell populations [6, 7] coupled to a poor response to vaccines [8–11]. Thus, it has been suggested that children with DS share similarities with patients affected with primary immunodeficiency (PID) and some PIDs classifications include DS [12, 13]. In contrast with pediatric literature, there is a lack of information about infections and immune parameters in adults with DS. Herein we report the features of 101 adults with DS.
Methods
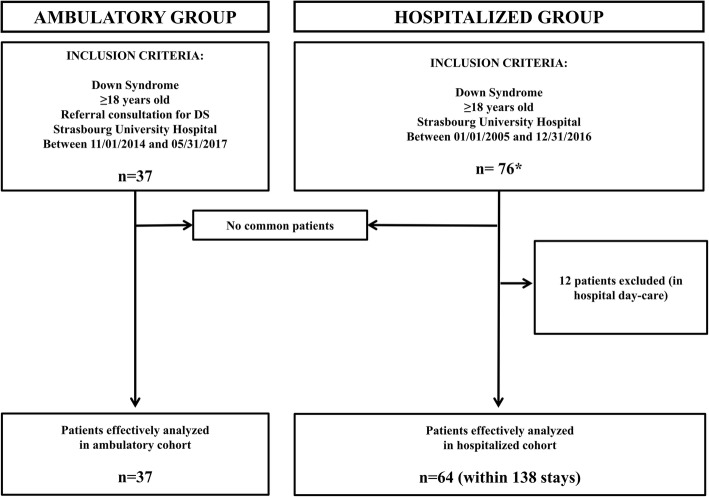
Following approvement of our Institutional Boards, we studied two separate groups of adults (> 18 years old) with DS in Strasbourg University Hospital (Fig. 1). The first group included ambulatory DS patients (Department of Medical Genetic) between 2014 and 2017. We analyzed at one point serological and immunobiological parameters, together with the retrospective prevalence of infections, autoimmune manifestations and malignancies. The second group included hospitalized patients between 2005 and 2016 with associated DS ICD codes or key words “Down syndrome” or “Trisomy 21” in medical letters with the mean of systematic research by the medical information department of the hospital. We excluded patients hospitalized for scheduled exams.
Fig. 1.
Flow diagram of patients. *Within 190,740 stays - **Patients were excluded for insufficient data when the cause of hospitalization was not mentioned, or when clinical and biological data were unavailable
We used Fisher’s exact test to compare qualitative variables and Student test for quantitative variables in univariate analysis. Mann-Whitney test was used to compare non-normally distributed variables. Multivariate analyzes were done for a p-value < 0.10 in univariate analysis using ridge logistic regression. Statistical significance was defined by p < 0.05 in 2-tailed tests.
Results
Thirty-seven patients came to the medical genetic consultation for DS (Table 1). Median age was 27 years (18–52). None was in institutional care. Frequent infections were reported during childhood for 20 DS patients (54%), the leading manifestations involving respiratory tract (49%) and ENT (16%). Only one patient was identified with recurrent infectious events after the age of 18. Total lymphocyte counts of DS patients were low when compared to standards (1480 cells/μL vs. 2170 cells/μL, p = 0.004) concerning especially naïve and memory B cell subsets as described in DS children. 15 patients presented with hypergammaglobulinemia (IgG > 15 g/L) and increased IgG1 and IgG3 levels. Serological status following vaccination for DPT (Diphteria, Polio and Tetanus), Streptococcus pneumoniae and Haemophilus influenzae showed protective titers in most cases.
Table 1.
Ambulatory DS patients
| Demographics and clinical characteristics of ambulatory DS patients | n = 37 | |
| Age at inclusion (years) | 27 (18–52) | |
| Sex ratio (F/M) | 1.2 (20/17) | |
| Institutional living (n, %) | 0 | |
| Recurrent infections within childhood | ||
| Before 5 years old | 18 (49%) | |
| From 6 to 18 years old | 17 (46%) | |
| Recurrent respiratory tract infections at adulthooda | 1 (2.7%) | |
| Hypothyroidism | 20 (54%) | |
| Epilepsy | 2 (5.1%) | |
| Dementia | 0 | |
| Cardiac congenital disease | 13 (35%) | |
| Urogenital malformation | 0 | |
| Gastrointestinal malformation | 2 (5.1%) | |
| Type 1 diabetes | 0 | |
| Celiac disease | 1 (2.7%) | |
| Hematological malignancy | 0 | |
| Biological features of ambulatory DS patients | Normal values | |
| Neutrophils (cells/μL) | 1800-7900 | 2225 (1110-4970) |
| Lymphocytes (cells/μL) | 1943-2709 | 1480 (801–3132) |
| T cells (cells/μL) | 700–1900 | 1208 (514–2516) |
| CD4+ T cells (cells/μL) | 400–1300 | 612 (273–1307) |
| CD8+ T cells (cells/μL) | 200–700 | 474 (117–1365) |
| Naïve CD45RA+ (% of T-cells) | 34–60% | 31% (9–65) |
| Memory CD45RO+ (% of T-cells) | 33–65% | 65% (32–86) |
| Regulatory T cells CD3 + CD25 + FoxP3+ (% of CD4+) | 2.75–5.0% | 2.3% (0.3–5.3) |
| NK cells CD3-CD56+ (cells/μL) | 100–400 | 231 (53–633) |
| CD19+ B cells (cells/μL) | 169–271 | 71 (42–264) |
| Naive CD27- IgD+ (cells/μL) | 112–169 | 50 (4–246) |
| Transitional CD24 + CD38+ (cells/μL) | 2–6 | 2 (1–8) |
| Switched memory CD27 + IgD- (cells/μL) | 18–40 | 10 (2–22) |
| Marginal zone CD27 + IgD+ (cells/μL) | 22–54 | 3 (1–15) |
| Plasmablast CD27-CD38+ (cells/μL) | 1–3 | 3 (1–13) |
| CD21lowCD38low (cells/μL) | 4–11 | 2 (1–11) |
| IgG (g/L) | 7.2–14.7 | 14.2 (10.5–21.2) |
| IgG1 (g/L) | 3.41–10.3 | 10.1 (5.3–14) |
| IgG2 (g/L) | 2.07–6.59 | 3.5 (1.4–6.4) |
| IgG3 (g/L) IgG4 (g/L) IgM (g/L) |
0.21–1.45 0.013–1.03 0.48–3.10 |
1.4 (0.4–3.8) 0.1 (0.1–0.8) 0.7 (0.3–2.9) |
| IgA (g/L) | 1.1–3.6 | 3.1 (1.6–7.3) |
| Anti-nuclear antibodies (ANAs) (n, %) | ≤ 1/160 | 6 (16%) |
| Anti-ds DNA antibodies (anti-ds DNA Abs) (n, %) | < 50 U/ml | 0 |
| Anti-thyroperoxidase antibodiesb (anti-TPO Abs) (n, %) | <34kU/l | 2 (5.4%) |
| Serological status (protective titerc) | n = 37 | |
| Tetanus | 36 (97%) | |
| Polio | 33 (89%) | |
| Diphteria | 19 (51%) | |
| Haemophilus influenzae type b | 30 (81%) | |
| Streptococcus pneumoniae | 34 (92%) | |
a> 2 pneumoniae or bronchitis with need of antibiotherapy (after 18 years old) bAnti-TPO antibodies were searched for 26 patients c37patients were inoculated against diphteria tetanus and poliomyelitis (DTP vaccine), 35 (97%) against BCG (Bacillus of Calmette and Guerin), 27 (73%) against Bordetella pertussis, 8 (22%) against Haemophilus influenza type b and 2 (5%) against Streptococcus pneumoniae. Protective titers were defined using manufacturers’ values
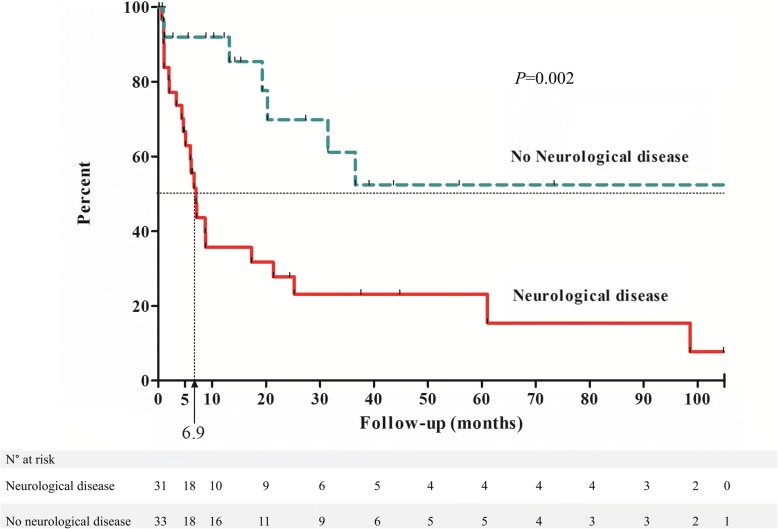
During an 11 year period (2005–2016), 64 DS patients were hospitalized at Strasbourg University Hospital, mainly in Internal Medicine, Infectious diseases, Pneumology and Intensive Care Units corresponding to 138 stays (a total of 190,740 stays were recorded in the same departments during this period), (Table 2). Median age was 47 years (18–73), older than ambulatory ones (p < 0.0001). Thirty-seven patients (58%) were in institutional care. The outstanding causes for hospitalization among DS patients were infections (n = 106/138), mostly aspiration pneumonia (n = 91/138). When available, lymphocyte counts and Ig levels were comparable to those of the ambulatory patients. Infections were associated with epilepsy and dementia (OR 6.5 (2.2–19), p = 0.001; p = 0.0006 in multivariate analysis) and higher mortality (OR 7.4 (1.4–37), p = 0.01). We found a median of second infectious event at 6.9 months in the group with neurological diseases vs. more than 120 months in the group without neurological diseases (p = 0.002, Fig. 2). Furthermore, the annual rate of infection dramatically increased with age in hospitalized group with a 5 fold increase of incidental infections after 50 years (Additional file 1: Figure S1).
Table 2.
Hospitalized DS patients (n = 64): comparison between DS patients with or without recurrent infections
| Infectionsa (n = 31) |
No Infections (n = 33) |
OR /HR |
Univariate
p* |
Multivariate
p** |
|
|---|---|---|---|---|---|
| Age (years) | 51 (19–73) | 45 (18–65) | / | 0.01 | 0.12 |
| Sex ratio (F/M) | 0.35 (8/23) | 0.8 (15/18) | 0.41 (0.14–1.2) | 0.12 | 0.16 |
| Institutional living (n, %) | 21 (68) | 16 (48) | 2.2 (0.8–6.1) | 0.12 | 0.89 |
| Hypothyroidism (n, %) | 14 (45) | 13 (40) | 1.2 (0.46–3.4) | 0.8 | / |
| Cardiac congenital disease (n, %) | 5 (16) | 5 (15) | 1.1 (0.27–4.1) | 1 | 0.06 |
| Gastrointestinal malformation (n, %) | 2 (6.4) | 2 (6) | 1.1 (0.14–8.0) | 1 | / |
| Neurological diseaseb(n, %) | 22 (71) | 9 (27) | 6.5 (2.2–19) | 0.001 | 0.0006 |
| Epilepsy (n, %) | 16 (52) | 7 (21) | 3.9 (1.3–12) | 0.02 | / |
| Dementia (n, %) | 13 (42) | 3 (9) | 7.2 (1.8–29) | 0.003 | / |
| Hematological malignancy (n, %) | 0 | 1 (3) | 0.34 (0.1–9) | 1 | / |
| Lymphocytes rate (/μL) | 1340 (650–2580) | 1575 (558–3710) | / | 0.09 | 0.13 |
| Lymphocytes < 1000/mm3 (n, %) | 9 (29) | 5 (15) | 2.3 (0.67–7.8) | 0.23 | / |
| Immunoglobulin rate (g/L) | 12 (7–23) | 16 (9–18) | / | 0.22 | / |
| Mortality (n, %) | 10 (32) | 2 (6) | 7.4 (1.4–37) | 0.01 | / |
aTotal number of infectious events: 106 (/138 stays); intensive care unit admission 11/106; Need of pressor amines 7/106, Acute lung injury syndrome 3/18; Pneumonia 96/106 (91%). The other reasons were seizures (n = 8), heart failure (n = 5), occlusive syndrome (n = 5), arterial cardiovascular event (n = 5), syncope (n = 4), acute renal disease (n = 2), pancreatitis, venous thrombosis, deep weight loss (n = 1 each)
b Neurological disease was defined as epilepsy or dementia
*Difference between infections and no infections groups using Fischer’s test for categorical variables and Mann-Whitney test for quantitative variables
**Using multiple logistic regression. OR: Odd Ratio – HR: Hazard Ratio
Statistically significant results are marked in boldface
Fig. 2.
Time to second infectious event within the DS hospitalised group. The median duration of second infectious event-free survival was 6.9 months within the group with neurological disease, as compared with a median over the last follow up (105 months) in the group with no neurological disease. Hazard ratio 0.05 (p = 0.002)
Discussion
In DS children, epidemiological [1, 14–18] and pathophysiological [6, 19–22] evidences argue for a higher risk of infectious events, hematological malignancies and autoimmunity. Our work correlates for the first time detailed immunological findings and infectious events in adult patients with DS. Despite persistent T and B cell alterations, young ambulatory adults with DS have a low risk of infections, suggesting offsetting mechanisms in adulthood. However, infections, mostly bacterial aspiration pneumonia, remain the first cause of hospitalization. The major factor associated with infectious complications and premature death is the occurrence of neurological diseases such as seizures and dementia [23]. Seizures are frequent in adults with Down’s syndrome with about 10 times increased incidence as compared to general population. Seizures are associated with aging and cognitive impairment in DS [24]. Development of dementia in DS syndrome dramatically increases after age of 40 and is one of the main cause of institutionalization and hospitalization [4, 24]. Considering pediatric studies and our work, infections in DS occur early in life up and in the second adulthood period -after 50 years-old- especially when neurological comorbidities are associated. Indeed, neurological impairment marks a turning point in the infectious complications of adults patients with DS and this should be kept in mind by physicians.
Additional file
Figure S1. Annual rate of infection by 5-years range within the hospitalized DS patients. (TIF 4139 kb)
Acknowledgments
Additional contributions:
PHRC T21 study group: Emmanuel Andres, MD, PhD, Xavier Argemi, MD, PhD, Frédéric Blanc, MD, PhD, Isabelle Brun, MD, Salima El Chehadeh, MD, Jean-Luc Davideau, MD, Jean-Louis Dietemann, MD, PhD, Hélène Dollfus, MD, PhD, Elise Gazzano, MD, Bernard Goichot, MD, PhD, Julie Helms, MD, Jean-Michel Hiebel, MD, Georges Kaltenbach, MD, PhD, Anne Koenig, MD, PhD, Marie-Cécile Manière, PhD, Yaumara Perdomo, MD, Hélène Petit-Eisenmann, MD, PhD, Alain Pradignac, MD, PhD, Anne-Elisabeth Quoix, MD, PhD, Philippe Sauder MD PhD, Elise Schaefer, MD, Francis Schneider, MD, PhD, Dana Timbolschi, MD, Jean-Christophe Weber, MD (Strasbourg University Hospital) contributed clinical and administrative collaborative efforts. Insightful comments on the manuscript: Laurent Arnaud MD, PhD (Strasbourg University Hospital) and Hans Hartmut Peter MD, PhD (Universitätsklinikum Freiburg). No compensation was received.
We thank the patients, families and staff for their time and dedication.
Funding
Fundings/grants:
Dr. Yves Alembik (grant from the French Ministry of Health PHRC 2012-A00466–37),
Pr. Anne-Sophie Korganow (grant from EU-funded (ERDF) project INTERREG V “RARENET”).
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its additional files].
Abbreviations
- DPT
Diphtheria, Polio and Tetanus
- DS
Down Syndrome
- ENT
Ear, Nose and Throat
- ICD-10
International Classification of Diseases (version 10)
- mAb
monoclonal Antibody
- PID
Primary Immune Deficiency
Authors’ contributions
AG (corresponding author) had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: AG, YD, YA, ASK, Acquisition, analysis, or interpretation of data: all authors, Drafting of the manuscript: AG, YD, ASK, Critical revision of the manuscript for important intellectual contents: all authors, Statistical analysis: AG, YD, ASK, Administrative, technical, or material supports: all authors, Study supervision: AG, YA, ASK. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Ambulatory patients involved in PHRC T21 “Trisomy 21 in Adulthood”: (Dr Alembik Yves): All subjects were recruited under a protocol approved by ethical board CPP-Est IV N°12/47 (research ethical board of Strasbourg University Hospital) including a written informed consent.
Retrospective hospitalized patients: An approval statement from the local ethical committee has been obtained (ethical committee of the Faculties of Medicine and Dentistry of Strasbourg N°2018–8).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aurélien Guffroy, Phone: (0033) 369 550 521, Email: aurelien.guffroy@chru-strasbourg.fr, Email: aurelienguffroy@hotmail.com.
Yannick Dieudonné, Email: yannick.dieudonne@chru-strasbourg.fr.
Beatrice Uring-Lambert, Email: beatrice.lambert@chru-strasbourg.fr.
Joelle Goetz, Email: joelle.goetz@chru-strasbourg.fr.
Yves Alembik, Email: yves.alembik@chru-strasbourg.fr.
Anne-Sophie Korganow, Email: anne-sophie.korganow@chru-strasbourg.fr.
References
- 1.Irving C, Basu A, Richmond S, Burn J, Wren C. Twenty-year trends in prevalence and survival of Down syndrome. Eur J Hum Genet. 2008;16:1336–1340. doi: 10.1038/ejhg.2008.122. [DOI] [PubMed] [Google Scholar]
- 2.Jensen KM, Bulova PD. Managing the care of adults with Down’s syndrome. BMJ. 2014;349:g5596. doi: 10.1136/bmj.g5596. [DOI] [PubMed] [Google Scholar]
- 3.Genes and human disease. In: Who World Health Organization. Available from: http://www.who.int/genomics/public/geneticdiseases/en Accessed 18 Dec 2018.
- 4.Englund A, Jonsson B, Zander CS, Gustafsson J, Annerén G. Changes in mortality and causes of death in the Swedish Down syndrome population. Am J Med Genet A. 2013;161:642–649. doi: 10.1002/ajmg.a.35706. [DOI] [PubMed] [Google Scholar]
- 5.Ram G, Chinen J. Infections and immunodeficiency in Down syndrome: immunodeficiency in Down syndrome. Clin Exp Immunol. 2011;164:9–16. doi: 10.1111/j.1365-2249.2011.04335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verstegen RHJ, Driessen GJ, Bartol SJW, van Noesel CJM, Boon L, van der Burg M, et al. Defective B-cell memory in patients with Down syndrome. J Allergy Clin Immunol. 2014;134:1346–1353. doi: 10.1016/j.jaci.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Schoch J, Rohrer TR, Kaestner M, Abdul-Khaliq H, Gortner L, Sester U, et al. Quantitative, phenotypical, and functional characterization of cellular immunity in children and adolescents with Down syndrome. J Infect Dis. 2017;215:1619–1628. doi: 10.1093/infdis/jix168. [DOI] [PubMed] [Google Scholar]
- 8.Kusters M a A, Manders NCC, de Jong B a W, van Hout RWNM, Rijkers GT, de Vries E. Functionality of the pneumococcal antibody response in Down syndrome subjects. Vaccine. 2013;31:6261–6265. doi: 10.1016/j.vaccine.2013.09.070. [DOI] [PubMed] [Google Scholar]
- 9.Kusters MA, Bok VLA, Bolz WEA, Huijskens EGW, Peeters MF, de Vries E. Influenza a/H1N1 vaccination response is inadequate in Down syndrome children when the latest cut-off values are used. Pediatr Infect Dis J. 2012;31:1284–1285. doi: 10.1097/INF.0b013e3182737410. [DOI] [PubMed] [Google Scholar]
- 10.Kusters MA. Jol-Van Der Zijde ECM, Gijsbers RHJM, de Vries E. Decreased response after conjugated meningococcal serogroup C vaccination in children with Down syndrome Pediatr Infect Dis J. 2011;30:818–819. doi: 10.1097/INF.0b013e31822233f9. [DOI] [PubMed] [Google Scholar]
- 11.Kusters MA, Jol-van der Zijde CM, van Tol MJ, Bolz WE, Bok LA, Visser M, et al. Impaired avidity maturation after tetanus toxoid booster in children with Down syndrome. Pediatr Infect Dis J. 2011;30:357–359. doi: 10.1097/INF.0b013e3181ff85a8. [DOI] [PubMed] [Google Scholar]
- 12.Ming JE, Stiehm ER, Graham JM. Syndromic immunodeficiencies: genetic syndromes associated with immune abnormalities. Crit Rev Clin Lab Sci. 2003;40:587–642. doi: 10.1080/714037692. [DOI] [PubMed] [Google Scholar]
- 13.Cuadrado E, Barrena MJ. Immune dysfunction in Down’s syndrome: primary immune deficiency or early senescence of the immune system? Clin Immunol Immunopathol. 1996;78:209–214. doi: 10.1006/clin.1996.0031. [DOI] [PubMed] [Google Scholar]
- 14.Bloemers BLP, Broers CJM, Bont L, Weijerman ME, Gemke RJBJ, van Furth AM. Increased risk of respiratory tract infections in children with Down syndrome: the consequence of an altered immune system. Microbes Infect Inst Pasteur. 2010;12:799–808. doi: 10.1016/j.micinf.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Hasle H, Clemmensen IH, Mikkelsen M. Risks of leukaemia and solid tumours in individuals with Down’s syndrome. Lancet Lond Engl. 2000;355:165–169. doi: 10.1016/S0140-6736(99)05264-2. [DOI] [PubMed] [Google Scholar]
- 16.Roizen NJ, Patterson D. Down’s syndrome. Lancet. 2003;361:1281–1289. doi: 10.1016/S0140-6736(03)12987-X. [DOI] [PubMed] [Google Scholar]
- 17.Yang Q, Rasmussen SA, Friedman JM. Mortality associated with Down’s syndrome in the USA from 1983 to 1997: a population-based study. Lancet Lond Engl. 2002;359:1019–1025. doi: 10.1016/S0140-6736(02)08092-3. [DOI] [PubMed] [Google Scholar]
- 18.Linabery AM, Li W, Roesler MA, Spector LG, Gamis AS, Olshan AF, et al. Immune-related conditions and acute leukemia in children with Down syndrome: a Children’s oncology group report. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2015;24:454–458. doi: 10.1158/1055-9965.EPI-14-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bloemers BLP, van Bleek GM, Kimpen JLL, Bont L. Distinct abnormalities in the innate immune system of children with Down syndrome. J Pediatr. 2010:804–9. [DOI] [PubMed]
- 20.Bloemers BLP, Bont L, de Weger RA, Otto SA, Borghans JA, Tesselaar K. Decreased Thymic output accounts for decreased naive T cell numbers in children with Down syndrome. J Immunol. 2011;186:4500–4507. doi: 10.4049/jimmunol.1001700. [DOI] [PubMed] [Google Scholar]
- 21.Carsetti R, Valentini D, Marcellini V, Scarsella M, Marasco E, Giustini F, et al. Reduced numbers of switched memory B cells with high terminal differentiation potential in Down syndrome: clinical immunology. Eur J Immunol. 2015;45:903–914. doi: 10.1002/eji.201445049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verstegen RHJ, Kusters MAA, Gemen EFA, DE Vries E. Down syndrome B-lymphocyte subpopulations, intrinsic defect or decreased T-lymphocyte help. Pediatr Res. 2010;67:563–569. doi: 10.1203/PDR.0b013e3181d4ecc1. [DOI] [PubMed] [Google Scholar]
- 23.Lott IT, Dierssen M. Cognitive deficits and associated neurological complications in individuals with Down’s syndrome. Lancet Neurol. 2010;9:623–633. doi: 10.1016/S1474-4422(10)70112-5. [DOI] [PubMed] [Google Scholar]
- 24.Menéndez M. Down syndrome, Alzheimer’s disease and seizures. Brain and Development. 2005;27:246–252. doi: 10.1016/j.braindev.2004.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Annual rate of infection by 5-years range within the hospitalized DS patients. (TIF 4139 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its additional files].