Summary
Natural killer (NK) cells are highly specialized cytotoxic lymphocytes that provide protection against pathogens and malignant cells. They develop from common lymphoid progenitors via a multi‐stage lineage commitment and differentiation process that gives rise to mature NK cells with potent cytotoxic functionality. Although generally considered cells of the innate immune system, recent studies have demonstrated that NK cells have the capacity to mount immune responses with features of adaptive immunity, including robust antigen‐specific clonal‐like expansion and the generation of long‐lived memory cells that mediate enhanced recall responses. Here, we discuss specific transcription factors that have been shown to commonly and uniquely regulate NK cell development and effector and memory responses in experimental mouse models.
Keywords: development, epigenetics, memory, transcription factors
Abbreviations
- ATAC‐seq
Assay for Transposase‐Accessible Chromatin using sequencing
- BM
bone marrow
- CHILP
common helper innate lymphoid progenitor
- CHS
contact hypersensitivity
- CLP
common lymphoid progenitor
- cNK
classical NK
- EILP
early innate lymphoid progenitor
- ILC1p
ILC1 progenitor
- ILC1
type 1 ILC
- ILC
innate lymphoid cell
- iNK
immature NK cell
- Lin
lineage
- MCMV
murine cytomegalovirus
- mNK
mature NK cell
- mTOR
mechanistic target of rapamycin
- NK
natural killer
NKP
NK precursor
- PDK1
3′‐phosphoinositide‐dependent kinase 1
- pre‐pro NK
pre‐pro NK cells
- rNKP
refined NK precursor
- TF
transcription factor
Introduction
Natural killer (NK) cells are cytotoxic lymphocytes that provide defense against viral infection and malignancy. They have been classified as a subset of group 1 innate lymphoid cells (ILCs).1, 2 Activation of NK cells is regulated by germline‐encoded surface receptors and leads to the release of cytolytic molecules such as perforin and granzymes, and of cytokines such as interferon‐γ (IFN‐γ), which act to kill target cells and activate other cells of the immune system.
Development of NK cells occurs principally, although not exclusively, in the bone marrow (BM) during adulthood, and in the liver and thymus during fetal ontogeny.3, 4, 5 The liver and thymus also support the differentiation of unique tissue‐resident NK cell populations with developmental requirements that are distinct from those of ‘classical’ NK cells arising in the BM.6, 7 NK cells develop from common lymphoid progenitors (CLPs) through a lineage commitment process that is thought to involve transition through an early innate lymphoid progenitor (EILP) stage with the capacity to seed both the NK cell and ‘helper’ ILC lineages. Further differentiation leads to the generation of NK progenitor cells, which lack multi‐lineage potential.8, 9 Studies in mice have shown that these committed progenitors undergo a stepwise differentiation process from pre‐pro NK progenitor cells (pre‐pro NKPs), which are negative for major immune lineage markers (Lin–) but are Id2hiCD117+, to pre‐NKPs that up‐regulate expression of CD27, interleukin‐7 receptor‐α (IL‐7Rα; also known as CD127), and 2B4 (CD244), and finally to ‘refined’ NKPs (rNKPs) that acquire expression of CD122, the IL‐15 receptor β‐chain (Fig. 1). The latter marks the stage at which IL‐15 signaling becomes critical for NK cell development and drives further differentiation into immature NK (iNK) cells.10, 11, 12 Notably, IL‐15 regulates many aspects of NK cell biology after the rNKP stage, ranging from proliferation, survival, and metabolism during development to effector functions in the context of an active immune response.13 Immature NK cells first acquire expression of the classical NK activating receptors NK1.1 (in C57BL/6 and certain other strains of mice) and NKp46 at the NKP‐to‐iNK transition. Up‐regulation of additional receptors such as Ly49 family NK receptors and CD49b (also known as DX5), and acquisition of cytotoxic functionality marks the transition of iNK cells into naive mature NK (mNK) cells, which emigrate from the bone marrow and mediate surveillance of peripheral tissues.12, 14, 15 In the BM and periphery, NK cell maturation can be further tracked by down‐regulation of CD27 expression and up‐regulation of CD11b expression, with immature CD27+ NK cells exhibiting the greatest proliferative potential and more mature CD27− CD11b+ NK cells exhibiting greater effector potential.16, 17 Settings such as inflammation, malignancy, and/or cellular stress act on naive NK cells to drive their differentiation into effector cells and, in certain cases, into long‐lived memory cells.18, 19
Figure 1.
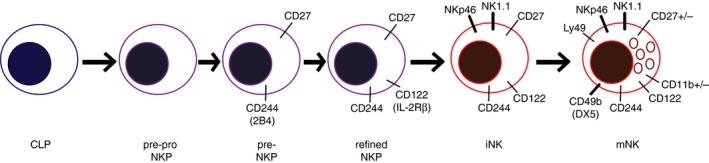
Schematic depicting the sequential stages of classical natural killer cell development in mice, including characteristic surface receptors that mark each stage.
In this review, we provide an overview of the specific transcription factors (TFs) that regulate NK cell development, maturation, and effector and memory cell generation, as defined by experimental studies in mice (Fig. 2).
Figure 2.
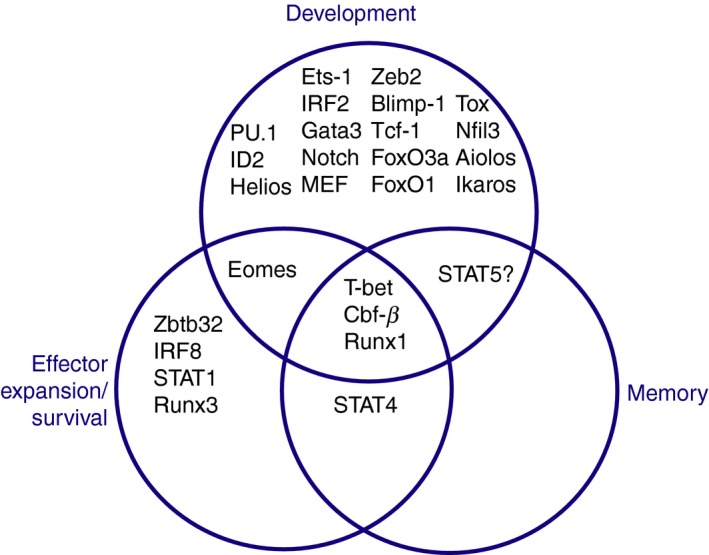
Venn diagram showing transcription factors that commonly and distinctly regulate natural killer cell development, effector cell expansion and survival, and memory cell formation following mouse cytomegalovirus infection.
Transcription factors that regulate NK cell development and maturation
The Ets family of winged helix‐turn‐helix TFs plays important roles in cellular differentiation and development. Ets TFs share a common Ets domain that binds purine‐rich DNA motifs with a conserved GGA core, which are enriched at many lymphocyte‐specific gene loci.20 Several Ets family members have been shown to regulate NK cell development. Mice lacking ETS proto‐oncogene 1 (Ets‐1) have a severely reduced NK cell compartment, corresponding with fewer rNKPs, iNK cells, and mNK cells in the BM, indicating that Ets‐1 acts at very early stages of NK cell development.12, 21, 22 Furthermore, Ets1 −/− NK cells have decreased expression of activating and elevated expression of inhibitory NK receptors, and fail to degranulate upon stimulation, underscoring maturation and functional defects in the few cells that do develop in Ets‐1‐deficient mice. Mechanistically, Ets‐1 was shown to directly bind the Id2 gene locus, and was required for normal expression of both ID2 and T‐bet, two TFs that also regulate NK cell development and are discussed below.22 Somewhat unexpectedly, Ets1 −/− NK cells were found to exhibit increased proliferation, size, granularity, and granzyme production following exposure to suboptimal doses of IL‐15, indicating that Ets‐1 may act to limit IL‐15 responsiveness.22
In addition to Ets‐1, two other Ets family members, Myeloid Elf‐1 Like Factor (MEF; encoded by Elf4) and Purine‐rich box 1 (PU.1; encoded by Spi1), contribute to NK cell development. Similar to animals lacking Ets‐1, MEF‐deficient mice harbor fewer and functionally impaired NK cells that produce less IFN‐γ and perforin, and are less cytotoxic against tumor cell lines.23 In contrast, PU.1‐deficient NK cells maintain cytotoxic functionality against tumor targets, but express reduced levels of CD117 and IL‐7Rα and proliferate less after exposure to IL‐2 and IL‐12.24
Notch family TFs act as early as the CLP stage and facilitate lymphocyte development, at least in part, by repressing a myeloid lineage fate. Specific Notch ligands, often expressed by stromal cells at the site of lymphocyte development, differentially influence commitment to the various innate or adaptive lymphocyte fates. For example, the Notch ligands Jagged1/2 and Delta‐like protein‐1 (DLL‐1) preferentially promote the in vitro development of NK cells and T cells, respectively.25, 26, 27 However, the requirement for Notch ligands in NK cell fate specification may not be specific or absolute, as NK cells can be generated in vitro, albeit in reduced numbers, even in the presence of DLL or in the absence of all Notch ligands.27, 28 Indeed, the NK lineage may represent the default fate for lymphoid progenitors; progenitors lacking TFs required for T cell or B cell commitment, e.g. Bcl11b or Pax5, respectively, develop into NK cells when cultured with Notch ligands in vitro.29, 30, 31
TCF‐1 (encoded by Tcf7) is another TF required for early NK cell development. Tcf7 −/− mice have severely reduced numbers of NK cells at all stages of development, from pre‐pro NKPs in the BM to mNK cells in the periphery.8 Although TCF‐1 is highly expressed in early developing and immature NK cells, its expression wanes in mNK cells and several lines of evidence suggest that TCF‐1 may actually antagonize later stages of mNK cell maturation. First, TCF‐1‐deficient mNK cells express lower levels of the inhibitory NK receptor Ly49A and higher levels of CD11b and KLRG1, a phenotype typically associated with terminal maturity.8, 32, 33 In addition, TCF‐1‐deficient NK cells express elevated levels of granzymes, consistent with the finding that TCF‐1 binds and promotes a closed chromatin configuration at regulatory elements near the Gzmb gene. Modulation of granzyme expression by TCF‐1 may protect developing NK cells from apoptosis, as both TCF‐1‐deficiency and granzyme overexpression impair the survival of developing NK cells.32
Signal transducer and activator of transcription 5 (STAT5; encoded by two adjacent genes, Stat5a and Stat5b) acts directly downstream of IL‐15 receptor (IL‐15R) signaling on NK cells. Like IL‐15 and IL‐15R, STAT5 is required for NK development and homeostasis, and NK cells are markedly reduced in settings of STAT5 deficiency, with the Stat5b paralog having a greater impact.34, 35, 36, 37 Early work showed that genetic deletion of Stat5a/b at the NKP‐iNK transition ablated NK cell development at and past the iNK stage. Subsequently, Villarino et al. used Stat5a +/– Stat5b –/– mice to show that STAT5 also contributes to the generation of NKPs and ILC1 progenitors (ILC1ps), indicating that STAT5 acts at the very earliest stages of NK cell development.34, 36 STAT5 directly regulates expression of many genes involved in NK cell differentiation, survival, and function, including the TF T‐bet (discussed below). Indeed, STAT5 and T‐bet appear to regulate expression of overlapping suites of genes in NK cells, underscoring the interrelated and cooperative relationship between these two important NK lineage‐defining TFs.36 More recently, it was shown that the oligomerization state of STAT5, plays an important role in the differential regulation of its target genes in NK cells. For example, STAT5 tetramers were shown to be dispensable for NK cell proliferation but necessary for survival through direct induction of Bcl2 expression downstream of IL‐2/IL‐15 signaling.38
Nfil3 (encoded by E4bp4) is a basic leucine zipper TF with diverse biological roles, ranging from circadian rhythms to lymphocyte differentiation and function.39, 40 Nfil3 is expressed by CLPs, before other NK lineage‐defining TFs such as ID2, T‐bet, and Eomes, and increases as NK cells undergo differentiation and maturation.41 Studies in E4bp4 −/− mice demonstrated that Nfil3 is essential for the generation of NK cells and other ILC subsets, although it is notably dispensable for the development of certain tissue‐resident NK cell populations in the liver and salivary gland.42, 43, 44, 45, 46, 47, 48, 49, 50 E4bp4 −/− mice harbor severely reduced numbers of ‘classical’ NK cell numbers in the BM, spleen, lung, and blood, and the few NK cells that do develop are poorly cytotoxic and produce less IFN‐γ.46 Early studies reported fewer iNK cells and mNK cells, but similar or increased numbers of NKPs in E4bp4 −/− compared with wild‐type (WT) mice;46, 48 however, later work refined these findings to show that pre‐NKPs and rNKPs were also reduced in the absence of Nfil3.41, 44 Notably, deletion of Nfil3 after the NKP stage had no discernible effect on NK development, homeostasis, or cytokine production.51, 52 Mechanistically, Nfil3 is thought to act at least partially downstream of IL‐15 signaling, as ectopic Nfil3 expression can rescue aspects of NK cell development in mice lacking components of the IL‐15 signaling pathway.41 IL‐15 promotes Nfil3 expression via the 3′‐phosphoinositide‐dependent kinase 1 and mechanistic target of rapamycin (PDK1‐mTOR) signaling axis, although Nfil3 itself reinforces IL‐15 signaling by maintaining CD122 expression in NK cells.46, 53, 54 Consistent with its key role in NK cell biology, Nfil3 expression is tightly regulated in NK cells, including through post‐transcriptional modifications such as SUMOylation and phosphorylation that influence its functions in development.55 Direct gene targets of Nfil3 include Eomes, ID2, and Notch, other TFs that critically shape NK cell development and function (discussed above and below), although one group reported normal ID2 expression in developing E4bp4 −/− NK cells.41, 44, 55 Ectopic overexpression of ID2, Eomes, or T‐bet, or short‐term exposure to Notch ligands was shown to restore development in E4bp4 −/− NK cells,41, 46, 55 further supporting the interpretation that Nfil3 acts upstream of and promotes the expression and/or function of these key NK lineage‐defining TFs. Together, these studies indicate that Nfil3 is critical for early NK cell development and function.
Members of the Runx family of TFs play prominent roles in hematopoiesis.56, 57 Runx TFs share a conserved Runt domain that mediates heterodimerization with the non‐DNA binding cofactor, core‐binding factor‐β (CBF‐β). Heterodimerization with CBF‐β is essential for the function of Runx TFs, as it increases the affinity of Runx TFs for DNA and facilitates the recruitment of coactivators and corepressors that regulate transcription by remodeling the chromatin landscape at target loci. The Runx TF Runx3 is expressed in developing NK cells and increases with NK maturation. Defects in Runx3 or CBF‐β expression or activity have been shown to impair iNK and mNK cell development in vitro and in vivo.58, 59, 60, 61 Regulatory targets of Runx3 in NK cells include the gene encoding CD122, and Runx3‐deficiency reduces CD122 expression and, as a result, responsiveness to IL‐15.59, 60 Additionally, Runx3‐deficiency alters expression of genes involved in proliferation, survival, migration, and NK cell function, including many that are up‐regulated in response to IL‐2 or IL‐15 signaling. Notably, a subset of these genes also contains Ets‐binding motifs, suggestive of co‐regulation by Runx3 and Ets1 at these loci.60, 62
Inhibitor of DNA binding (ID) TFs function by inhibiting E protein TFs, many of which promote B cell and T cell differentiation.63 Inhibition is achieved by heterodimeric interactions between ID and E proteins through shared helix‐loop‐helix (HLH) domains, which prevents E protein binding at target loci. The ID family TF ID2 (encoded by Id2) contributes to the development of NK cells, as well as the helper ILC lineages. ID2 is expressed throughout early NK cell development and peaks in mNK cells.10, 64, 65 While Id2 −/− mice have normal numbers of NKPs and iNK cells, the most mature CD27− CD11b+ subset of mNK cells is significantly reduced.64, 66, 67 Consistent with a role for ID2 in inhibiting E protein activity, mNK cell development in the BM (although not spleen) was normalized in mice lacking both ID2 and the E protein, E2A.64 At a transcriptional level, Id2 −/− NK cells ectopically express many T cell‐related genes and have aberrantly open chromatin at T cell‐related gene loci, but express lower levels of NK cell‐related genes. These findings suggest that ID2 acts to suppress a T cell and promote an NK cell gene signature.65, 67 In addition, ID2 may also act to tune IL‐15 responsiveness, as treatment of Id2 −/− NK cells with high doses of IL‐15, or deletion of the negative regulator of IL‐15 signaling, suppressor of cytokine signaling 3 (SOCS3), was sufficient to overcome the requirement for ID2 in NK cell development.65 ID2 expression and activity are subject to multiple levels of regulation. For example, the histone H2A deubiquitinase MYSM1 promotes NK cell development by maintaining an open chromatin configuration at the Id2 locus and by facilitating the recruitment of Nfil3 to the Id2 promoter.68 More recently, it was shown that a cis‐regulatory element in the long non‐coding RNA (lncRNA), RNA demarcated regulatory region of Id2 (Rroid), that is located upstream of Id2 critically regulates NK cell identity and homeostasis.69 Acting through long‐range interactions, Rroid promotes chromatin accessibility and STAT5 recruitment to the Id2 locus, thereby enhancing ID2 expression. The pattern of Rroid expression mirrors that of ID2, and Rroid is likewise necessary for late‐stage NK cell development but dispensable for early NK lineage specification. Altogether, these studies highlight key roles for ID2 in titrating E protein activity to support NK cell maturation and repress T cell fates.
The thymocyte‐selection associated high mobility group box (TOX) TF is up‐regulated early in NK cell differentiation shortly after the CLP stage, peaks at the iNK and mNK stages in the BM, and then is down‐regulated in peripheral mNK cells.70 Tox −/− mice harbor normal numbers of CLPs, NKPs, and iNK cells, but reduced numbers of mNK cells in both the BM and spleen, pointing to a role for TOX in later stages of NK cell development and maturation. Consistent with this, Tox −/− NK cells express normal levels of CD122 and expand appropriately in response to IL‐15 in vitro, but are less cytotoxic against target cells in vivo.70 TOX expression is likely regulated at least in part by Nfil3, based on findings that Nfil3‐deficiency reduces TOX expression in CLPs, Nfil3 can bind the Tox promoter in mouse lymphoma cell lines, and TOX overexpression is sufficient to rescue NK cell development in Nfil3‐deficient BM progenitors.50 Future studies are needed to define the mechanisms, including specific gene targets, by which TOX controls NK cell development.70
The Forkhead box class O TFs FoxO1 and FoxO3a typically act as negative regulators of lymphocyte development and function downstream of nutrient and growth factor signaling pathways such as Akt and mTOR. Under normal growth conditions, post‐translational modifications such as phosphorylation restrict nuclear entry of FoxO TFs, but nutrient or growth factor deprivation can overcome this restriction, facilitating FoxO‐mediated induction of target genes such as pro‐apoptotic Bim and Puma. Interleukin‐15 signaling normally acts to promote the phosphorylation, and hence inactivation, of FoxO3a and FoxO1 in developing NK cells.71, 72 Consistent with a negative regulatory role for FoxO3a in NK development, NK cells lacking FoxO3a are resistant to IL‐15‐deprivation‐induced apoptosis and exhibit a hypermaturation phenotype characterized by elevated KLRG1 and CD11b expression.71 Similarly, FoxO1‐deficient NK cells have features of hypermaturation, due in part to their elevated and early expression of T‐bet.72 However, while Deng et al. reported that deletion of FoxO1 in iNK cells led to an accumulation of mNK cells, Wang et al. reported a loss of mNK cells due to impaired autophagy.72, 73 Hence, the precise role of Foxo1 in NK cell development and maturation remains to be clarified.
The TF GATA binding protein 3 (Gata3) is essential for the generation of thymic NK (tNK) cells, which are distinct from classical NK cells in their reliance on IL‐7 signaling, but not T‐bet or ID2, for development.7, 44, 45, 74, 75, 76 Compared with tNK cells, the role of Gata3 in ‘classical’ NK cell development is more nuanced. Although early studies reported normal numbers of Gata3‐deficient NK cells in fetal liver chimeric mice, later work showed that genetic deletion of Gata3 at the iNK stage led to reduced numbers of NK cells in the BM and spleen.75, 77 Furthermore, Gata‐3‐deficient NK cells maintain cytotoxicity against tumor target cells, but exhibit an immature phenotype characterized by higher CD27 expression, lower expression of T‐bet, CD11b, CD43, and several Ly49 receptors, as well as diminished IFN‐γ production in vitro.75, 77
The TF interferon regulatory factor 2 (IRF2), part of a larger family of IRF TFs that bind interferon‐stimulated response elements (ISREs) in target genes, also contributes to NK cell development and maturation. Global IRF2‐deficiency, or IRF2‐deficiency in the hematopoietic compartment, reduces mNK cells in the spleen and liver, respectively. In the BM, IRF2‐deficiency does not alter the number of immature CD11blo NK cells, but reduces the number of mature NK cells expressing CD11b and CD43. Additionally, IRF2‐deficient NK cells are more apoptotic and produce less IFN‐γ, yet maintain cytotoxic functionality against tumor target cells.78, 79 These studies indicate that IRF2 regulates later stages of NK cell maturation, function, and peripheral homeostasis.
The T‐box family TFs T‐bet (encoded by Tbx21) and Eomes regulate late NK cell development at the iNK and mNK stages, respectively.80 NK cells lacking T‐bet or Eomes are reduced in the periphery, but not BM, whereas those lacking both TFs are severely reduced in all organs, indicating a degree of functional compensation between T‐bet and Eomes TFs during NK development.80, 81 Phenotypically, Tbx21 –/– NK cells are both more proliferative and more apoptotic and fail to progress past the CD27+ CD11b+ stage of maturation.82 Tbx21 –/– NK cells also express higher levels of Eomes, and it has been proposed that T‐bet stabilizes the iNK‐to‐mNK cell transition by transiently suppressing Eomes expression.80, 83 Eomes is thought to act after T‐bet in NK cell development, but Eomes‐deficient NK cells also exhibit features of impaired maturation, including failure to up‐regulate CD11b, lower levels of CD49b (DX5), and expression of a limited Ly49 receptor repertoire.80 Like many other NK‐defining TFs, T‐bet and Eomes are regulated by IL‐15 and mTOR signaling.53 In addition, Eomes has been shown to directly bind the Il2rb promoter and enhance CD122 expression, and hence IL‐15 responsiveness, in both NK cells and CD8+ T cells.81
Zinc Finger E‐box Binding Homeobox 2 (Zeb2) is another TF that regulates NK cell maturation. Similar to T‐bet‐deficient NK cells, NK cells lacking Zeb2 arrest at the CD27+ CD11b+ stage, and exhibit survival and BM egress defects.84 T‐bet is both necessary and sufficient for expression of Zeb2, and NK cells lacking either T‐bet or Zeb2, or both, exhibit strikingly similar phenotypes, suggesting that Zeb2 and T‐bet act cooperatively to promote NK cell maturation.
B lymphocyte‐induced maturation protein 1 (Blimp‐1; encoded by Prdm1) is another TF involved in NK cell maturation. Blimp‐1 is up‐regulated in response to IL‐15 at the NKP‐to‐iNK transition in a T‐bet‐dependent manner and remains constitutively expressed by resting naive NK cells in the BM and periphery. Experiments involving fetal liver chimeras demonstrated that Blimp‐1‐deficiency impairs the generation of mature CD27− CD11bhi CD43+ KLRG1+ NK cells in the BM and periphery, but enhances NK cell cytotoxicity and proliferation.85
Members of the Ikaros family of zinc finger TFs, including Ikaros, Helios, and Aiolos, also contribute to NK cell development and maturation. Early studies revealed that Ikaros (encoded by Ikzf1) is necessary for the generation of NK cells from CLPs, acting in part through the regulation of c‐Kit and Flt3 expression by early hematopoietic progenitors.86 Similar deficits in NK cell generation were demonstrated in mice harboring a C‐terminal truncation in Ikaros, and the few NK cells that developed in these mice were functionally impaired and unable to kill target cells even at high effector‐to‐target ratios.87 Helios (encoded by Ikzf2) is expressed in iNK cells, although it is down‐regulated at later stages of maturation. Notably, Helios‐deficient NK cells exhibit increased IFN‐γ production and degranulation and can clear viral infections more effectively, suggesting a negative regulatory role for this TF in NK cell effector responses.88 In contrast, Aiolos (encoded by Ikzf3) is expressed constitutionally and is necessary for peripheral NK cells to differentiate into the most mature CD27− CD11bhi subset.89 Furthermore, while Aiolos‐deficient NK cells are less able to control mouse cytomegalovirus (MCMV) infection, they exhibit superior tumor killing, suggesting that Aiolos, like Helios, may also negatively regulate NK cell effector responses in some settings.
Transcription factors that regulate effector and memory NK cells
Although traditionally classified as innate immune cells, a growing number of studies indicate that NK cells are capable of immune responses with features of adaptive immunity, including antigen‐specific clonal‐like expansion and differentiation into long‐lived memory cells with enhanced functionality during recall responses.19 Early studies demonstrated NK cell memory responses in mice in the context of contact hypersensitivity (CHS) reactions against chemical haptens and in settings of MCMV infection.19, 90 Although the latter model has been used more extensively to define the TFs that regulate NK memory responses, and is the focus of studies discussed below, the aryl hydrocarbon receptor (AhR) TF was recently shown to contribute to the generation of the hapten‐specific memory NK cells that mediate CHS reactions.91
Infection with MCMV in mice drives the robust clonal‐like expansion of NK cells expressing the activating NK receptor, Ly49H. This expansion is antigen‐specific and is driven by engagement of Ly49H with its viral ligand, the MCMV‐encoded glycoprotein m157.92 Effector NK cells reach peak numbers at day 7, but most eventually die off in the following weeks, leaving behind a small pool of long‐lived memory cells.19 The expansion, contraction, and memory phases of the anti‐MCMV NK cell response have distinct transcriptional requirements and current understanding of the specific TFs that regulate these phases is described below.93, 94
Signal transducer and activator of transcription 4 (STAT4) is arguably a master regulator of the overall anti‐MCMV response by Ly49H+ NK cells. STAT4 acts downstream of IL‐12 receptor (IL‐12R) signaling and is essential for NK cell activation, expansion, and memory cell persistence following MCMV infection.18 STAT4 directly promotes the expression of Zbtb32, T‐bet, interferon regulatory factor 8 (IRF8), Runx1, and Runx3,93, 94, 95, 96, 97 TFs that also contribute to the MCMV‐driven Ly49H+ NK cell response (see below). Functionally, STAT4 acts in part by directing a ‘permissive’ chromatin state, likely through the recruitment of histone remodeling enzymes, around target gene loci. Indeed, H3K4 trimethylation at the Runx, Cbfb, and Irf8 promoters was recently shown to occur in a STAT4‐dependent manner during NK cell activation in vitro.94, 95 The related TF, STAT1 also contributes to the expansion of MCMV‐specific Ly49H+ NK cells, acting downstream of type 1 interferon (IFN‐I) signals that serve to protect NK cells from NKG2D‐dependent fratricide.98 Recent Assay for Transposase‐Accessible Chromatin using sequencing (ATAC‐seq) studies underscore the distinct contributions of STAT4 and STAT1 to the chromatin landscape of NK cells during early MCMV infection.99 STAT4 was shown to transiently bind and facilitate open chromatin at enhancer regions for genes that are dynamically expressed during early MCMV infection. In contrast, STAT1 was shown to occupy and promote closed chromatin at promoter regions, but these decreases in chromatin accessibility were less dynamic and modest. Furthermore, STAT4 and STAT1 appear to partially cross‐regulate each other during early MCMV infection, with STAT4 actively repressing expression of STAT1 and STAT1 target genes, and STAT1 limiting STAT4‐induced IFN‐γ production.99
The Broad‐complex Tramtrack Bric‐a‐brac Zinc Finger (BTB‐ZF) TF, Zbtb32, is highly and transiently up‐regulated during the early stages of MCMV infection in response to signals from the pro‐inflammatory cytokines IL‐12, IL‐18, and IFN‐I.96 Zbtb32 was shown to be essential for the proliferation of MCMV‐specific NK cells and, mechanistically, acted by antagonizing Blimp‐1.96 More recently, the TF IRF8 was shown to bind within the Zbtb32 locus and to regulate the proliferative expansion of MCMV‐specific NK cells in a manner that was strikingly similar to Zbtb32, suggesting that IRF8 acts upstream of Zbtb32 in this key signaling pathway.95 Notably, although Zbtb32 and IRF8 critically regulate MCMV‐induced NK cell expansion, they do not appear to be required for generation or long‐term persistence of MCMV‐specific memory NK cells.
Several TFs are notable for being required for both NK cell development and effector/memory cell generation during viral infection. For example, the TFs Runx1 and Runx3 are up‐regulated in NK cells during early MCMV infection, and along with their binding partner CBF‐β, are required for optimal NK cell expansion and antiviral defense during MCMV infection.94 RNA‐seq studies revealed the substantial down‐regulation of cell cycle and proliferation genes in Runx1‐deficient and IRF8‐deficient NK cells from MCMV‐infected animals, demonstrating that Runx1 and IRF8 drive transcriptional programs that promote NK cell proliferation during MCMV infection.94, 95 In addition, CBF‐β and Runx1 are distinctly required for memory NK cell generation, as inducible deletion of either TF after the expansion phase greatly reduced memory cell numbers in the liver and spleen at later time points.93, 94 Like the Runx TFs, T‐bet and Eomes are also essential for MCMV‐driven expansion of Ly49H+ NK cells, although only T‐bet is required for memory cell formation.93
Recent studies have harnessed the power of high‐throughput sequencing platforms to define the global changes in transcription and chromatin accessibility that occur in Ly49H+ NK cells during the course of MCMV infection. A key finding of these studies was that memory NK cells and memory CD8+ T cells share a common transcriptional and epigenetic signature, and many of the regulated genes such as Tcf7, Zeb2, and Tox have known roles in NK cell development.8, 32, 70, 84, 99, 100 Moreover, open chromatin regions in memory cells were enriched for binding sites for AP‐1 and underrepresented for TCF‐LEF binding sites, presenting novel pathways that may be involved in memory NK cell formation and/or maintenance.99
Concluding remarks
Collectively, these studies demonstrate that developing, effector, and memory NK cells have unique and shared TF requirements, mirroring their distinct and overlapping biological activities (Fig. 2). Moreover, recent studies using whole genome next‐generation sequencing approaches have highlighted the dynamic global transcriptional and epigenetic changes that accompany NK cell differentiation at every stage. With the advent of sophisticated molecular technologies that enable investigation of chromatin accessibility and transcriptomes at the single‐cell level, and transcription factor binding in rare cell types,101, 102, 103, 104 it will be possible to obtain a more thorough and refined understanding of how specific TFs and epigenomic remodeling enzymes regulate NK cell differentiation and function. Ultimately, these studies have potential to inform clinical applications that target NK cells, including vaccines and cellular immunotherapies to treat cancer and infection.
Disclosures
The author has no conflict of interests to disclose.
Authorship
MB and AMB wrote the manuscript and created the figures.
Acknowledgements
We thank members of the Beaulieu laboratory for helpful discussions and review of the manuscript. This work was supported by funding from the NIH‐NIAID (K22 AI116802 to AMB and T32 AI1125185‐02 to MB).
References
- 1. Artis D, Spits H. The biology of innate lymphoid cells. Nature 2015; 517:293–301. [DOI] [PubMed] [Google Scholar]
- 2. Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G et al Innate lymphoid cells – a proposal for uniform nomenclature. Nat Rev Immunol 2013; 13:145–9. [DOI] [PubMed] [Google Scholar]
- 3. Di Santo JP. Natural killer cell developmental pathways: a question of balance. Annu Rev Immunol 2006; 24:257–86. [DOI] [PubMed] [Google Scholar]
- 4. Sitnicka E. Early cellular pathways of mouse natural killer cell development. J Innate Immun 2011; 3:329–36. [DOI] [PubMed] [Google Scholar]
- 5. Tang Y, Peitzsch C, Charoudeh HN, Cheng M, Chaves P, Jacobsen SE et al Emergence of NK‐cell progenitors and functionally competent NK‐cell lineage subsets in the early mouse embryo. Blood 2012; 120:63–75. [DOI] [PubMed] [Google Scholar]
- 6. Peng H, Jiang X, Chen Y, Sojka DK, Wei H, Gao X et al Liver‐resident NK cells confer adaptive immunity in skin‐contact inflammation. J Clin Invest 2013; 123:1444–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vosshenrich CA, Garcia‐Ojeda ME, Samson‐Villeger SI, Pasqualetto V, Enault L, Richard‐Le Goff O et al A thymic pathway of mouse natural killer cell development characterized by expression of GATA‐3 and CD127. Nat Immunol 2006; 7:1217–24. [DOI] [PubMed] [Google Scholar]
- 8. Yang Q, Li F, Harly C, Xing S, Ye L, Xia X et al TCF‐1 upregulation identifies early innate lymphoid progenitors in the bone marrow. Nat Immunol 2015; 16:1044–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature 2014; 508:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carotta S, Pang SH, Nutt SL, Belz GT. Identification of the earliest NK‐cell precursor in the mouse BM. Blood 2011; 117:5449–52. [DOI] [PubMed] [Google Scholar]
- 11. Fathman JW, Bhattacharya D, Inlay MA, Seita J, Karsunky H, Weissman IL. Identification of the earliest natural killer cell‐committed progenitor in murine bone marrow. Blood 2011; 118:5439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosmaraki EE, Douagi I, Roth C, Colucci F, Cumano A, Di Santo JP. Identification of committed NK cell progenitors in adult murine bone marrow. Eur J Immunol 2001; 31:1900–9. [DOI] [PubMed] [Google Scholar]
- 13. Wu Y, Tian Z, Wei H. Developmental and functional control of natural killer cells by cytokines. Front Immunol 2017; 8:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huntington ND, Tabarias H, Fairfax K, Brady J, Hayakawa Y, Degli‐Esposti MA et al NK cell maturation and peripheral homeostasis is associated with KLRG1 up‐regulation. J Immunol 2007; 178:4764–70. [DOI] [PubMed] [Google Scholar]
- 15. Kim S, Iizuka K, Kang HS, Dokun A, French AR, Greco S et al In vivo developmental stages in murine natural killer cell maturation. Nat Immunol 2002; 3:523–8. [DOI] [PubMed] [Google Scholar]
- 16. Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol 2006; 176:1517–24. [DOI] [PubMed] [Google Scholar]
- 17. Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4‐stage developmental program. Blood 2009; 113:5488–96. [DOI] [PubMed] [Google Scholar]
- 18. Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med 2012; 209:947–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature 2009; 457:557–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bassuk AG, Leiden JM. The role of Ets transcription factors in the development and function of the mammalian immune system. Adv Immunol 1997; 64:65–104. [DOI] [PubMed] [Google Scholar]
- 21. Barton K, Muthusamy N, Fischer C, Ting CN, Walunas TL, Lanier LL et al The Ets‐1 transcription factor is required for the development of natural killer cells in mice. Immunity 1998; 9:555–63. [DOI] [PubMed] [Google Scholar]
- 22. Ramirez K, Chandler KJ, Spaulding C, Zandi S, Sigvardsson M, Graves BJ et al Gene deregulation and chronic activation in natural killer cells deficient in the transcription factor ETS1. Immunity 2012; 36:921–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lacorazza HD, Miyazaki Y, Di Cristofano A, Deblasio A, Hedvat C, Zhang J et al The ETS protein MEF plays a critical role in perforin gene expression and the development of natural killer and NK‐T cells. Immunity 2002; 17:437–49. [DOI] [PubMed] [Google Scholar]
- 24. Colucci F, Samson SI, DeKoter RP, Lantz O, Singh H, Di Santo JP. Differential requirement for the transcription factor PU.1 in the generation of natural killer cells versus B and T cells. Blood 2001; 97:2625–32. [DOI] [PubMed] [Google Scholar]
- 25. DeHart SL, Heikens MJ, Tsai S. Jagged2 promotes the development of natural killer cells and the establishment of functional natural killer cell lines. Blood 2005; 105:3521–7. [DOI] [PubMed] [Google Scholar]
- 26. Jaleco AC, Neves H, Hooijberg E, Gameiro P, Clode N, Haury M et al Differential effects of Notch ligands Delta‐1 and Jagged‐1 in human lymphoid differentiation. J Exp Med 2001; 194:991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lehar SM, Dooley J, Farr AG, Bevan MJ. Notch ligands Delta 1 and Jagged1 transmit distinct signals to T‐cell precursors. Blood 2005; 105:1440–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmitt TM, Ciofani M, Petrie HT, Zuniga‐Pflucker JC. Maintenance of T cell specification and differentiation requires recurrent notch receptor‐ligand interactions. J Exp Med 2004; 200:469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carotta S, Brady J, Wu L, Nutt SL. Transient Notch signaling induces NK cell potential in Pax5‐deficient pro‐B cells. Eur J Immunol 2006; 36:3294–304. [DOI] [PubMed] [Google Scholar]
- 30. Li L, Leid M, Rothenberg EV. An early T cell lineage commitment checkpoint dependent on the transcription factor Bcl11b. Science 2010; 329:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li P, Burke S, Wang J, Chen X, Ortiz M, Lee SC et al Reprogramming of T cells to natural killer‐like cells upon Bcl11b deletion. Science 2010; 329:85–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jeevan‐Raj B, Gehrig J, Charmoy M, Chennupati V, Grandclement C, Angelino P et al The transcription factor Tcf1 contributes to normal NK cell development and function by limiting the expression of granzymes. Cell Rep 2017; 20:613–26. [DOI] [PubMed] [Google Scholar]
- 33. Held W, Kunz B, Lowin‐Kropf B, van de Wetering M, Clevers H. Clonal acquisition of the Ly49A NK cell receptor is dependent on the trans‐acting factor TCF‐1. Immunity 1999; 11:433–42. [DOI] [PubMed] [Google Scholar]
- 34. Eckelhart E, Warsch W, Zebedin E, Simma O, Stoiber D, Kolbe T et al A novel Ncr1‐Cre mouse reveals the essential role of STAT5 for NK‐cell survival and development. Blood 2011; 117:1565–73. [DOI] [PubMed] [Google Scholar]
- 35. Imada K, Bloom ET, Nakajima H, Horvath‐Arcidiacono JA, Udy GB, Davey HW et al Stat5b is essential for natural killer cell‐mediated proliferation and cytolytic activity. J Exp Med 1998; 188:2067–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Villarino AV, Sciume G, Davis FP, Iwata S, Zitti B, Robinson GW et al Subset‐ and tissue‐defined STAT5 thresholds control homeostasis and function of innate lymphoid cells. J Exp Med 2017; 214:2999–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yao Z, Cui Y, Watford WT, Bream JH, Yamaoka K, Hissong BD et al Stat5a/b are essential for normal lymphoid development and differentiation. Proc Natl Acad Sci USA 2006; 103:1000–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lin JX, Du N, Li P, Kazemian M, Gebregiorgis T, Spolski R et al Critical functions for STAT5 tetramers in the maturation and survival of natural killer cells. Nat Commun 2017; 8:1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cowell IG. E4BP4/NFIL3, a PAR‐related bZIP factor with many roles. BioEssays 2002; 24:1023–9. [DOI] [PubMed] [Google Scholar]
- 40. Yin J, Zhang J, Lu Q. The role of basic leucine zipper transcription factor E4BP4 in the immune system and immune‐mediated diseases. Clin Immunol (Orlando, Fla) 2017; 180:5–10. [DOI] [PubMed] [Google Scholar]
- 41. Male V, Nisoli I, Kostrzewski T, Allan DS, Carlyle JR, Lord GM et al The transcription factor E4bp4/Nfil3 controls commitment to the NK lineage and directly regulates Eomes and Id2 expression. J Exp Med 2014; 211:635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cortez VS, Fuchs A, Cella M, Gilfillan S, Colonna M. Cutting edge: salivary gland NK cells develop independently of Nfil3 in steady‐state. J Immunol 2014; 192:4487–91. [DOI] [PubMed] [Google Scholar]
- 43. Crotta S, Gkioka A, Male V, Duarte JH, Davidson S, Nisoli I et al The transcription factor E4BP4 is not required for extramedullary pathways of NK cell development. J Immunol 2014; 192:2677–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seillet C, Huntington ND, Gangatirkar P, Axelsson E, Minnich M, Brady HJM et al Differential requirement for Nfil3 during NK cell development. J Immunol 2014; 192:2667–76. [DOI] [PubMed] [Google Scholar]
- 45. Sojka DK, Plougastel‐Douglas B, Yang L, Pak‐Wittel MA, Artyomov MN, Ivanova Y et al Tissue‐resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife 2014; 3:24714492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gascoyne DM, Long E, Veiga‐Fernandes H, de Boer J, Williams O, Seddon B et al The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat Immunol 2009; 10:1118–24. [DOI] [PubMed] [Google Scholar]
- 47. Geiger TL, Abt MC, Gasteiger G, Firth MA, O'Connor MH, Geary CD et al Nfil3 is crucial for development of innate lymphoid cells and host protection against intestinal pathogens. J Exp Med 2014; 211:1723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kamizono S, Duncan GS, Seidel MG, Morimoto A, Hamada K, Grosveld G et al Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo . J Exp Med 2009; 206:2977–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Seillet C, Rankin LC, Groom JR, Mielke LA, Tellier J, Chopin M et al Nfil3 is required for the development of all innate lymphoid cell subsets. J Exp Med 2014; 211:1733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yu X, Wang Y, Deng M, Li Y, Ruhn KA, Zhang CC et al The basic leucine zipper transcription factor NFIL3 directs the development of a common innate lymphoid cell precursor. eLife 2014; 3:25310240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Firth MA, Madera S, Beaulieu AM, Gasteiger G, Castillo EF, Schluns KS et al Nfil3‐independent lineage maintenance and antiviral response of natural killer cells. J Exp Med 2013; 210:2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Seillet C, Mielke LA, Amann‐Zalcenstein DB, Su S, Gao J, Almeida FF et al Deciphering the innate lymphoid cell transcriptional program. Cell Rep 2016; 17:436–47. [DOI] [PubMed] [Google Scholar]
- 53. Yang M, Chen S, Du J, He J, Wang Y, Li Z et al NK cell development requires Tsc1‐dependent negative regulation of IL‐15‐triggered mTORC1 activation. Nat Commun 2016; 7:12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang M, Li D, Chang Z, Yang Z, Tian Z, Dong Z. PDK1 orchestrates early NK cell development through induction of E4BP4 expression and maintenance of IL‐15 responsiveness. J Exp Med 2015; 212:253–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kostrzewski T, Borg AJ, Meng Y, Filipovic I, Male V, Wack A et al Multiple levels of control determine how E4bp4/Nfil3 regulates NK cell development. J Immunol 2018; 200:1370–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Voon DC, Hor YT, Ito Y. The RUNX complex: reaching beyond haematopoiesis into immunity. Immunology 2015; 146:523–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wong WF, Kohu K, Chiba T, Sato T, Satake M. Interplay of transcription factors in T‐cell differentiation and function: the role of Runx. Immunology 2011; 132:157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ebihara T, Song C, Ryu SH, Plougastel‐Douglas B, Yang L, Levanon D et al Runx3 specifies lineage commitment of innate lymphoid cells. Nat Immunol 2015; 16:1124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guo Y, Maillard I, Chakraborti S, Rothenberg EV, Speck NA. Core binding factors are necessary for natural killer cell development and cooperate with Notch signaling during T‐cell specification. Blood 2008; 112:480–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Levanon D, Negreanu V, Lotem J, Bone KR, Brenner O, Leshkowitz D et al Transcription factor Runx3 regulates interleukin‐15‐dependent natural killer cell activation. Mol Cell Biol 2014; 34:1158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ohno S, Sato T, Kohu K, Takeda K, Okumura K, Satake M et al Runx proteins are involved in regulation of CD122, Ly49 family and IFN‐gamma expression during NK cell differentiation. Int Immunol 2008; 20:71–9. [DOI] [PubMed] [Google Scholar]
- 62. Lotem J, Levanon D, Negreanu V, Leshkowitz D, Friedlander G, Groner Y. Runx3‐mediated transcriptional program in cytotoxic lymphocytes. PLoS ONE 2013; 8:e80467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kee BL. E and ID proteins branch out. Nat Rev Immunol 2009; 9:175–84. [DOI] [PubMed] [Google Scholar]
- 64. Boos MD, Yokota Y, Eberl G, Kee BL. Mature natural killer cell and lymphoid tissue‐inducing cell development requires Id2‐mediated suppression of E protein activity. J Exp Med 2007; 204:1119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Delconte RB, Shi W, Sathe P, Ushiki T, Seillet C, Minnich M et al The helix‐loop‐helix protein ID2 governs NK cell fate by tuning their sensitivity to interleukin‐15. Immunity 2016; 44:103–15. [DOI] [PubMed] [Google Scholar]
- 66. Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S et al Development of peripheral lymphoid organs and natural killer cells depends on the helix‐loop‐helix inhibitor ID2. Nature 1999; 397:702–6. [DOI] [PubMed] [Google Scholar]
- 67. Zook EC, Li ZY, Xu Y, de Pooter RF, Verykokakis M, Beaulieu A et al Transcription factor ID2 prevents E proteins from enforcing a naive T lymphocyte gene program during NK cell development. Sci Immunol 2018; 3:eaao2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nandakumar V, Chou Y, Zang L, Huang XF, Chen SY. Epigenetic control of natural killer cell maturation by histone H2A deubiquitinase, MYSM1. Proc Natl Acad Sci USA 2013; 110:E3927–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mowel WK, McCright SJ, Kotzin JJ, Collet MA, Uyar A, Chen X et al Group 1 innate lymphoid cell lineage identity is determined by a cis‐regulatory element marked by a long non‐coding RNA. Immunity 2017; 47:435–49 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Aliahmad P, de la Torre B, Kaye J. Shared dependence on the DNA‐binding factor TOX for the development of lymphoid tissue‐inducer cell and NK cell lineages. Nat Immunol 2010; 11:945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Huntington ND, Puthalakath H, Gunn P, Naik E, Michalak EM, Smyth MJ et al Interleukin 15‐mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl‐1. Nat Immunol 2007; 8:856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Deng Y, Kerdiles Y, Chu J, Yuan S, Wang Y, Chen X et al Transcription factor Foxo1 is a negative regulator of natural killer cell maturation and function. Immunity 2015; 42:457–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang S, Xia P, Huang G, Zhu P, Liu J, Ye B et al FoxO1‐mediated autophagy is required for NK cell development and innate immunity. Nat Commun 2016; 7:11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vosshenrich CA, Ranson T, Samson SI, Corcuff E, Colucci F, Rosmaraki EE et al Roles for common cytokine receptor γ‐chain‐dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo . J Immunol 2005; 174:1213–21. [DOI] [PubMed] [Google Scholar]
- 75. Ali AK, Oh JS, Vivier E, Busslinger M, Lee SH. NK cell‐specific Gata3 ablation identifies the maturation program required for bone marrow exit and control of proliferation. J Immunol 2016; 196:1753–67. [DOI] [PubMed] [Google Scholar]
- 76. Gabrielli S, Sun M, Bell A, Zook EC, de Pooter RF, Zamai L et al Murine thymic NK cells are distinct from ILC1s and have unique transcription factor requirements. Eur J Immunol 2017; 47:800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Samson SI, Richard O, Tavian M, Ranson T, Vosshenrich CA, Colucci F et al GATA‐3 promotes maturation, IFN‐γ production, and liver‐specific homing of NK cells. Immunity 2003; 19:701–11. [DOI] [PubMed] [Google Scholar]
- 78. Lohoff M, Duncan GS, Ferrick D, Mittrucker HW, Bischof S, Prechtl S et al Deficiency in the transcription factor interferon regulatory factor (IRF)‐2 leads to severely compromised development of natural killer and T helper type 1 cells. J Exp Med 2000; 192:325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Taki S, Nakajima S, Ichikawa E, Saito T, Hida S. IFN regulatory factor‐2 deficiency revealed a novel checkpoint critical for the generation of peripheral NK cells. J Immunol 2005; 174:6005–12. [DOI] [PubMed] [Google Scholar]
- 80. Gordon SM, Chaix J, Rupp LJ, Wu J, Madera S, Sun JC et al The transcription factors T‐bet and Eomes control key checkpoints of natural killer cell maturation. Immunity 2012; 36:55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR et al Effector and memory CD8+ T cell fate coupled by T‐bet and eomesodermin. Nat Immunol 2005; 6:1236–44. [DOI] [PubMed] [Google Scholar]
- 82. Soderquest K, Powell N, Luci C, van Rooijen N, Hidalgo A, Geissmann F et al Monocytes control natural killer cell differentiation to effector phenotypes. Blood 2011; 117:4511–8. [DOI] [PubMed] [Google Scholar]
- 83. Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA et al T‐bet regulates the terminal maturation and homeostasis of NK and Vα14i NKT cells. Immunity 2004; 20:477–94. [DOI] [PubMed] [Google Scholar]
- 84. van Helden MJ, Goossens S, Daussy C, Mathieu AL, Faure F, Marcais A et al Terminal NK cell maturation is controlled by concerted actions of T‐bet and Zeb2 and is essential for melanoma rejection. J Exp Med 2015; 212:2015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kallies A, Carotta S, Huntington ND, Bernard NJ, Tarlinton DM, Smyth MJ et al A role for Blimp1 in the transcriptional network controlling natural killer cell maturation. Blood 2011; 117:1869–79. [DOI] [PubMed] [Google Scholar]
- 86. Boggs SS, Trevisan M, Patrene K, Geogopoulos K. Lack of natural killer cell precursors in fetal liver of Ikaros knockout mutant mice. Nat Immun 1998; 16:137–45. [DOI] [PubMed] [Google Scholar]
- 87. Wang JH, Nichogiannopoulou A, Wu L, Sun L, Sharpe AH, Bigby M et al Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity 1996; 5:537–49. [DOI] [PubMed] [Google Scholar]
- 88. Narni‐Mancinelli E, Jaeger BN, Bernat C, Fenis A, Kung S, De Gassart A et al Tuning of natural killer cell reactivity by NKp46 and Helios calibrates T cell responses. Science 2012; 335:344–8. [DOI] [PubMed] [Google Scholar]
- 89. Holmes ML, Huntington ND, Thong RP, Brady J, Hayakawa Y, Andoniou CE et al Peripheral natural killer cell maturation depends on the transcription factor Aiolos. EMBO J 2014; 33:2721–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell‐ and B cell‐independent adaptive immunity mediated by natural killer cells. Nat Immunol 2006; 7:507–16. [DOI] [PubMed] [Google Scholar]
- 91. Zhang LH, Shin JH, Haggadone MD, Sunwoo JB. The aryl hydrocarbon receptor is required for the maintenance of liver‐resident natural killer cells. J Exp Med 2016; 213:2249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, Yokoyama WM. Specific and nonspecific NK cell activation during virus infection. Nat Immunol 2001; 2:951–6. [DOI] [PubMed] [Google Scholar]
- 93. Madera S, Geary CD, Lau CM, Pikovskaya O, Reiner SL, Sun JC. Cutting edge: divergent requirement of T‐Box transcription factors in effector and memory NK cells. J Immunol 2018; 200:1977–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rapp M, Lau CM, Adams NM, Weizman OE, O'Sullivan TE, Geary CD et al Core‐binding factor β and Runx transcription factors promote adaptive natural killer cell responses. Sci Immunol 2017; 2:eaan3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Adams NM, Lau CM, Fan X, Rapp M, Geary CD, Weizman OE et al Transcription factor IRF8 orchestrates the adaptive natural killer cell response. Immunity 2018; 48:1172–82.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Beaulieu AM, Zawislak CL, Nakayama T, Sun JC. The transcription factor Zbtb32 controls the proliferative burst of virus‐specific natural killer cells responding to infection. Nat Immunol 2014; 15:546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Madera S, Sun JC. Cutting edge: stage‐specific requirement of IL‐18 for antiviral NK cell expansion. J Immunol 2015; 194:1408–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Madera S, Rapp M, Firth MA, Beilke JN, Lanier LL, Sun JC. Type I IFN promotes NK cell expansion during viral infection by protecting NK cells against fratricide. J Exp Med 2016; 213:225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lau CM, Adams NM, Geary CD, Weizman OE, Rapp M, Pritykin Y et al Epigenetic control of innate and adaptive immune memory. Nat Immunol 2018; 19:963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Seehus CR, Aliahmad P, de la Torre B, Iliev ID, Spurka L, Funari VA et al The development of innate lymphoid cells requires TOX‐dependent generation of a common innate lymphoid cell progenitor. Nat Immunol 2015; 16:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA‐binding proteins and nucleosome position. Nat Methods 2013; 10:1213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Papalexi E, Satija R. Single‐cell RNA sequencing to explore immune cell heterogeneity. Nat Rev Immunol 2018; 18:35–45. [DOI] [PubMed] [Google Scholar]
- 103. Skene PJ, Henikoff S. An efficient targeted nuclease strategy for high‐resolution mapping of DNA binding sites. eLife 2017; 6:e21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zheng GX, Terry JM, Belgrader P, Ryvkin P, Bent ZW, Wilson R et al Massively parallel digital transcriptional profiling of single cells. Nat Commun 2017; 8:14049. [DOI] [PMC free article] [PubMed] [Google Scholar]


