Abstract
The treatment of acute ischemic stroke patients with a proximal large vessel occlusion (LVO) in the anterior circulation has seen tremendous advances initially with the demonstration of the substantial benefit of thrombectomy within 6-h of stroke onset and then with the demonstration of thrombectomy in carefully selected patients up to 24-h from onset. In both the early and late time windows, imaging played an important role in patient selection, especially in the later time window trials where very strict imaging inclusion criteria were employed to identify patients with a small/moderate sized ischemic core on computed tomography perfusion scanning and diffusion-weighted magnetic resonance imaging. In clinical practice, it is important to identify LVO patients quickly so several scoring scales have been developed to help route appropriate patients to a thrombectomy capable center. The recently reported thrombectomy trials left many unanswered questions such as do patients with more distal vessel occlusions benefit, do patients with LVO and mild clinical deficits benefit from thrombectomy, what is the largest extent of baseline ischemic core that still benefits from thrombectomy and what is the best approach to anesthesia with thrombectomy. These questions and other are being addressed in ongoing and future clinical trials that will likely expand the indications and safety for this powerfully effective therapy and also determine if neuroprotection is synergistic with thrombectomy.
Keywords: Imaging, prehospital, stroke, thrombectomy
Introduction
The treatment of acute ischemic stroke (AIS) in patients with large vessel occlusion (LVO) has entered a golden age with the demonstration of the highly beneficial effects of thrombectomy both in the early time window up to 6 h from stroke onset and a much later time window up to 24 h in highly selected patients. In 2015, the results of five clinical trials comparing thrombectomy to standard treatment, tissue plasminogen activator (tPA) in most control patients revealed the substantial benefits of endovascular therapy in patients who were primarily treated within 6 h of stroke onset.[1,2,3,4,5] A sixth trial, THRACE, published in 2016 also demonstrated the benefits of thrombectomy in patients largely selected with magnetic resonance imaging (MRI), but the magnitude of the treatment effect was less robust than in the prior five trials.[6] In 2018, two clinical trials, DAWN and DEFUSE-3 demonstrated that thrombectomy improved outcomes of AIS patients up to 24 h from stroke onset.[7,8] In these two late time window trials, AIS patients were carefully selected based on clinical and stringent imaging criteria using MRI and computed tomography perfusion (CTP). These imaging criteria led to the inclusion of AIS patients with very small ischemic cores pretreatment, a median of 8 ml in DAWN and 10 ml in DEFUSE-3. The results of the early and late time window thrombectomy trials has led to a paradigm shift in the treatment of LVO patients, and the intriguing question is how to apply the inclusion criteria to daily clinical practice. This review will focus on the identification of LVO patients prehospital, the best approaches to the routing of these patients how to approach imaging of suspected LVO patients and paradigms for patients being transferred from a primary to a tertiary care hospital. Finally, the review will discuss future directions for LVO clinical trials and patient care.
Prehospital Assessment
Ambulance evaluation of patients’ symptoms and physical signs are essential for triaging patients to primary or comprehensive stroke centers. Some assessment scales have been developed and will be reviewed.
The field assessment stroke triage for emergency destination (FAST-ED) scale consists of five items which were derived from the National Institutes of Health Stroke Scale (NIHSS) scale, facial palsy (scored 0–1), arm weakness (0–2), speech changes (0–2), time (documentation for decision-making but no points), eye deviation (0–2), and denial/neglect (0–2), with a total score of 0–9.[1] The FAST-ED scale provides three distinct groups for determining the likelihood of LVOS: 0–1: <15%, 2–3: ~30%, and ≥4: ~60% or higher.[9] For patients with a FAST-ED score ≥4, a comprehensive stroke center may be the first choice for routing of a patient, if the travel time is not too long. Based on the FAST-ED scale, an app FAST-ED is being utilized in Atlanta, Georgia, USA.[10] After entering the information of the scoring in the application (APP), the APP will automatically provide a list of primary or comprehensive hospitals nearby based on the FAST-ED scale and travel time. A decision can then be made as to what is the most expeditious way to route an individual patient.
In addition to the FAST-ED scale, the rapid arterial occlusion evaluation (RACE) scale and the Cincinnati prehospital stroke severity (CPSS) scale[11,12] were found to be useful for identifying LVO. Table 1 shows comparisons of the scales to FAST-ED. FAST-ED had a higher accuracy for identifying LVO than RACE and CPSS (area under the receiver operating characteristic curve: FAST-ED = 0.81 as reference; RACE = 0.77, P = 0.02; and CPSS = 0.75, P = 0.002). FAST-ED ≥4 had sensitivity of 0.60, specificity of 0.89 versus RACE ≥5 of 0.55 and 0.87, and CPSS ≥2 of 0.56 and 0.85, respectively.[9]
Table 1.
Comparisons of large vessel occlusion scales
| FAST-ED[1] | RACE[2] | CPSSS | ||||
|---|---|---|---|---|---|---|
| Facial palsy | Normal or minor paralysis | 0 | Absent | 0 | ||
| Partial or complete paralysis | 1 | Mild | 1 | |||
| Moderate to severe | 2 | |||||
| Arm weakness | No drift | 0 | Normal to mild | 0 | Normal to mild | 0 |
| Drift or some effort against gravity | 1 | Moderate | 1 | Cannot hold arm (either left, right or both) up for 10 seconds before arm (s) falls to bed | 1 | |
| No effort against gravity or no movement | 2 | Severe | 2 | |||
| Speech changes | Absent | 0 | Performs both tasks correctly | 0 | ||
| Mild to moderate | 1 | Performs 1 task correctly | 1 | |||
| Severe, global aphasia or mute | 2 | Performs neither tasks | 2 | |||
| Eye deviation | Absent | 0 | Absent | 0 | Absent | 0 |
| Partial | 1 | Present | 1 | Present | 2 | |
| Forced deviation | 2 | |||||
| Absent | 0 | |||||
| Extinction to bilateral simultaneous | ||||||
| Stimulation in only one sensory modality | 1 | |||||
| Does not recognize own hand or orients only to one side of the body | 2 | |||||
| Agnosia (if left hemiparesis) | Patient recognizes his/her arm and the impairment | 0 | ||||
| Does not recognized his/her arm or the impairment | 1 | |||||
| Does not recognize his/her arm nor the impairment | 2 | |||||
| Level of consciousness | Alert | 0 | ||||
| Incorrectly answers at least one of two level of consciousness questions on NIHSS (age or current month) and does not follow at least one of two commands (close eyes, open and close hand) | 1 | |||||
| Total score | 0-9 | 0-9 | 0-4 | |||
| Cut-off | 4 | 5 | 2 | |||
| LVO sensitivity and specificity | 60% and 89% | 85% and 68% | 83% and 40% | |||
LVO: Large vessel occlusion, FAST-ED: Field assessment stroke triage for emergency destination, RACE: Rapid arterial occlusion evaluation, CPSSS: Cincinnati Prehospital Stroke Severity, NIHSS: National Institutes of Health Stroke Scale
Ambulance assessment by telemedicine and onboard CT is very helpful for reducing prehospital delays. A telemedicine-enabled mobile stroke treatment unit (MSTU) equipped with a CT was proposed by the Cleveland pre-hospital acute stroke treatment Group. A vascular neurologist evaluated each patient through telemedicine and a neuroradiologist and vascular neurologist remotely assessed images obtained by the MSTU CT. They found that MSTU significantly decreased time to imaging and treatment with intravenous (IV) tPA compared with a traditional ambulance, with a median alarm-to-CT scan completion times of 33 min with the MSTU versus 56 min controls (P < 0.0001) and median alarm-to-thrombolysis initiation time of 55.5 min with the MSTU versus94 min for controls, (P < 0.0001).[13]
In Beijing China, the government authorized Xuanwu hospital to develop a stroke emergency map to reduce prehospital delays. There are 66 centers in the region that can provide IV t-PA. An APP connected the emergency medical system with the hospitals. The EMS staff can quickly find out the nearest hospital for the patient, and notify the hospital by the APP. This will presumably alter prehospital and hospital delays in the future in Beijing.
Transfer Patients
Neurologists and neurointerventionist are striving to organize systems of care to maximize the delivery of acute stroke therapy. Regionalized care systems are needed. Primary stroke centers to triage patients and give IV tPA therapy or direct referral to tertiary centers for some LVO patients in some locales.[14] Many hospitals have helicopter transfer capability in the United State, which dramatically shortens the transfer time for acute stroke patient. Imaging at primary centers will need to include vessel imaging (CT angiography [CTA]) to allow for the identification of LVO and penumbral imaging (CTP) would be ideal for referral of appropriate patients to tertiary centers for endovascular therapy, especially in the late time window. Time matters regarding what imaging to repeat or to do de novo at the tertiary center. Table 2 shows our imaging recommendations. In patients with LVO seen within 6 h at the primary hospital, a repeat head CT and CTA to detect hemorrhage and recanalization or persistent occlusion, respectively, will be needed at the tertiary center, if the patient had t-PA at the primary center or the transfer time was prolonged. In patients with acute LVO within the 7–16 h time window, a repeat CTA may be needed to confirm persistent LVO when a patient's symptoms (NIHSS score) improve quickly. Diffusion-weighted MRI and perfusion MR or perfusion CTP will be needed in most late time window patients to evaluate the extent of the ischemic core and penumbra if not performed at the primary hospital A repeat CTA and CTP or MRI may be needed to confirm a persistent LVO if the patient's symptoms (NIHSS score) significantly improve or transport to the tertiary center was prolonged. In patients with acute LVO within the 17–24 h time window, diffusion-weighted MRI or CTP in the tertiary center are needed to evaluate clinical-core mismatch based on the DAWN trial criteria for thrombectomy patient selection.[8]
Table 2.
Repeated imaging in the tertiary center for patients transfer from primary center
| ≤6 h | 7-16 h | 17-24 h | |
|---|---|---|---|
| CT | ✓* | ||
| CTA | ✓* | ||
| CTP | ✓ | ✓ | |
| DWI | ✓ | ✓ | |
| MRP | ✓ |
*In patients with t-PA given. CTA: CT angiography, CTP: CT perfusion, MRI: Magnetic resonance imaging, DWI: Diffusion-weighted MRI, MRP: MR perfusion, CT: Computed tomography
Imaging Selection in the Thrombectomy Clinical Trials
In the six earlier time window thrombectomy clinical trials imaging with both CT and MRI were utilized, although most patients were imaged with CT pretreatment, aside from in the THRACE trial. The minimum requirement for baseline imaging was to exclude intracranial hemorrhage with a standard head CT or MRI and to identify an LVO amenable to thrombectomy on CTA or magnetic resonance angiography (MRA). In addition, the extent of early infarction was evaluated by the Alberta stroke program early CT score (ASPECTS) rating or its MRI equivalent in all trials, and the score was used to exclude patients in 4/6 of the trials if it was <5 or 6, Figure 1.[15] The median ASPECTS score in the trials was highly favorable in all of the trials, indicating that the patients included on an average had small- to modest-sized ischemic cores despite the presence of proximal LVO s on CTA or MRA. The inclusion of such patients was an important factor in the success of these trials. A relatively small number of patients with low ASPECTS scores were included in these trials, and a meta-analysis suggested that an ASPECTS score of 3–5 was associated with a favorable 90-day clinical outcome, but a score of 0–2 was not.[16] The results of this meta-analysis should be viewed with caution because it pooled a similar number of CT and MRI based ASPECTS scores which are not equivalent and had a much larger treatment benefit in the MRI ASPECTS patients. The CT ASPECTS score is problematic for several reasons. The score is not well correlated with ischemic core volume on diffusion-weighted MRI when the two scans are obtained in close temporal proximity.[17] This discrepancy may occur because a favorable CT ASPECTS score may miss extensive regions of cortical infarction, if it is primarily derived from abnormalities in subcortical regions. In addition, the ASPECTS score may be influenced by other factors besides ischemic injury such as leukoaraiosis, edema, brain atrophy, and motion artifacts that can cause an artificially reduced score. The CT ASPECTS score also has a large degree of interobserver variability that leads to concerns about its reliability.[18] Automated approaches to CT ASPECTS are now available, and they appear to reduce the problem of reading variability.[19] However, even with automated CT ASPECTS assessment, its ability to predict a favorable clinical outcome remains problematic.[20] CTP imaging, Figure 2, was also acquired in some of the patients in the six thrombectomy trials, aside from THRACE in which CT was only performed in approximately 25% of the patients. Only the EXTEND-IA used the CTP results as an inclusion/exclusion criteria, requiring that the ischemic core be <70 ml.[2] Interestingly, the absolute benefit of thrombectomy on the 90-day outcome as assessed by the modified Rankin score (mRS) was the greatest in this trial, 31%. In the SWIFT-PRIME trial, CTP was obtained in 85% of the enrolled patients and was initially used for inclusion/exclusion.[4] Small ischemic cores were seen on CTP when it was obtained at baseline. When CTP was obtained an automated assessment of core volume as determined by ischemic core and ischemic penumbra or hypoperfused tissue at risk of infarction was determined by using predefined thresholds of cerebral blood flow, 30% of the contralateral homologous brain region for ischemic core and a Tmax delay >6 s for penumbral tissue. Recent studies suggest that the standard singular value (singular-value decomposition) method for analyzing CTP data is prone to systematic errors because of its high sensitivity to noise and problems related to delayed arrival of the bolus contrast employed with CTP in ischemic patients.[21] Correction for these factors by including delay time for bolus arrival or a Bayesian method of data analysis have a much more robust correlation with core volume as determined by DWI.[22,23] Post hoc analyses of the MR CLEAN and THRACE trials did demonstrate some benefit of patients with large ischemic cores on CTP or DWI but to a lesser extent than patients with smaller ischemic cores, but the treatment benefit was not seen in older patients.[24,25] It remains unresolved what the lowest ASPECTS score and the largest ischemic core volume on CTP is associated with a favorable outcome. In addition to the scores on these CT parameters, other factors such as patient age, lesion location, metabolic status, and collateral blood vessel status will also likely be important in predicting outcome and will have to be factored into future clinical trials addressing this issue.
Figure 1.
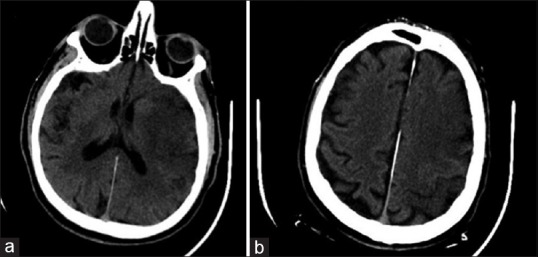
(a and b) Acute ischemic stroke with an APSECTS score of 2
Figure 2.
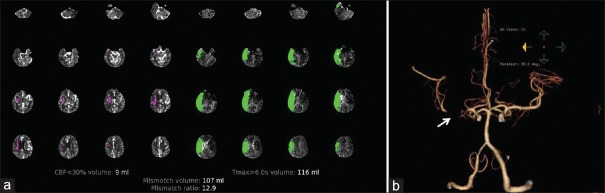
Computed tomography perfusion and computed tomography angiography images. (a) A computed tomography perfusion showing a small ischemic core with large penumbra (RAPID iSchemaView), and (b) the corresponding right middle cerebral artery M1 occlusion on computed tomography angiography
The two late window thrombectomy trials, DAWN and DEFUSE-3, had very strict imaging enrollment criteria utilizing both CTP and MRI with the former used in the majority of patients [Table 3].[7,8] The baseline ischemic core volumes were quite small, with a median of 8 ml in DAWN and 10 ml in DEFUSE-3. In DEFUSE-3, the volume of the ischemic penumbra was much larger than the ischemic core identified by CTP and DWI and this was also likely in DAWN, although not specified. The absolute percentage of patients with a favorable benefit of thrombectomy at 90-days as measured by the mRS outcome was 33% in DAWN and 28% in DEFUSE-3 and when pooled together it was better than that observed in pooled results from the early time window trials.[26] This enhanced benefit observed in the late time window trials is counterintuitive but likely occurred because of more stringent patient selection as required on the baseline advance imaging and also because very few patients received intravenous tPA prior to inclusion in the late window trials, while in the earlier window thrombectomy trials most of the control patients did receive tPA. In the late time window, current recommendations suggest that advanced imaging with CTP or MRI be employed and that patients be treated who fulfill the imaging criteria that were used in DAWN and DEFUSE-3.[27] It should be noted that the criteria used in DEFUSE-3 were somewhat more liberal than in DAWN and would allow for more patients to be treated with thrombectomy up to 16 h from stroke onset. Further studies are needed to identify what the upper limit is of ischemic core volume that still benefits from thrombectomy, but is highly likely that much more substantial ischemic core volumes will benefit from thrombectomy than were seen in the two late window trials which primarily enrolled patients with relatively small ischemic cores. As in the early time window, other factors such as age, infarct location, and metabolic status will influence treatment response in addition to ischemic lesion volume.
Table 3.
Imaging criteria for inclusion in the DAWN and DEFUSE-3 trials
| DAWN-included patients 6-24 h from stroke onset |
| Patients uded patients 6-24 h from stroke onsetP or DWI |
| Patients ≤80, NIHSS 10-19, ischemic core volume ≤30 ml on CTP or DWI |
| Patients ≤80, NIHSS 20 or higher, ischemic core volume ≤20 ml on CTP or DWI |
| Occlusion of the intracranial internal carotid artery or middle cerebral artery on CTA or MRA |
| DEFUSE-3-included patients from 6-16 h from stroke onset |
| Ischemic core volume <70 ml on CTP or DWI |
| Ischemic penumbra at least 180% larger than the ischemic core volume |
| Ischemic penumbra at least 15 ml or larger |
| Occlusion of the internal carotid artery or middle cerebral artery on CTA or MRA |
NIHSS: National Institutes of Health Stroke Scale, DAWN, DEFUSE, CT: Computed tomography, CTP: CT perfusion, MRI: Magnetic resonance imaging, DWI: Diffusion-weighted MRI
Improving the Timeliness of Thrombectomy
In the earlier time window, it is especially important to perform thrombectomy as quickly as possible because the sooner the LVO is opened the more likely a patient will have a favorable outcome.[28] As discussed routing LVO patients to a thrombectomy capable center should be done when appropriate. For LVO patients initially evaluated at a primary stroke center where IV tPA can be initiated, the time from admission there to transfer to a thrombectomy center should be done in an efficient and timely manner. When patients arrive at a thrombectomy center either as their initial admission site or as a hospital to hospital transfer, their evaluation and imaging should be performed as quickly as possible. For suspected LVO patients who arrive directly at a thrombectomy center, a quick initial clinical screen can be done in the ED by personnel alerted to their impending arriving by emergency medical services in the field or ambulance. If their screening LVO scale suggests a reasonable likelihood of an LVO, they can bypass the radiology department and have their screening head CT and CTA in the angiography suite, if this capability is available there. Bypassing radiology has been shown to reduce the time from ED arrival to groin puncture.[29] For patients being transferred from another hospital, a head CT will have been obtained in most cases and in an increasing number of patients, a CTA documenting an LVO will also have to be obtained. These patients can go directly to the angiography suite in most cases because the thrombectomy team will already know that the patient is a candidate for thrombectomy. If the transport time is short, i.e., <1 h and tPA was not given, the procedure can begin without additional imaging. If IV tPa was given a repeat head CT and CTA in the angiography suite is reasonable to exclude an intracranial hemorrhage related to tPA and to determine if vessel recanalization has occurred, obviating the need for the recanalization procedure. For transfer LVO patients with long transit time, i.e., over 1 h, CTP in addition to a head CT and CTA in the radiology department should be considered to determine if the ischemic core has progressed to a volume where thrombectomy is likely to have little chance of benefit. For later time window patients, more than 6–8 h from stroke onset, who either present to the thrombectomy center directly or who are transferred from another hospital, the complete CT evaluation with a head CT, CTA, and CTP or an MRI/MRA should be done in most cases. This will be necessary to determine if they fulfill the imaging criteria used in the DAWN and DEFUSE-3 trials for late thrombectomy. Since late time window patients who are eligible for thrombectomy are by definition slow evolvers of their ischemic core, time to groin puncture is less critical than in the earlier time window patients in whom the speed of core evolution is more heterogeneous.[30]
Future Considerations
The recent advances provided by the early and late time window thrombectomy trials for patients with proximal LVO have energized the field of AIS therapy. These trials, however, left many unanswered questions that will need to be addressed by future clinical trials. As already discussed, the lowest ASPECTS score and largest ischemic core volume where thrombectomy is no longer beneficial needs to be determined. LVO patients with mild neurological deficits were not included in the prior trials and additional trials are underway to determine if thrombectomy is beneficial in this clinical setting. Not many patients with more distal middle cerebral artery occlusions, i.e., M2, were studied and this is another focus of ongoing trials. Occlusions of other intracerebral arteries such as the anterior cerebral and basilar artery also need to be studied regarding the efficacy of thrombectomy. It remains unclear if general anesthesia or conscious sedation is equally appropriate for thrombectomy and trials are underway addressing this question. Pediatric stroke patients were not included in the prior trials, and this group needs to be studied. Finally, trials combining neuroprotection either before thrombectomy should be considered to determine if pretreatment can increase the number of LVO patients who benefit from thrombectomy when transport time and distances are long.[31] Neuroprotection targeted at reperfusion injury should be assessed after successful reperfusion to determine if this combined treatment is associated with a better 90-day outcome than reperfusion alone.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A xrandomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 2.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–30. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–95. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 5.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 6.Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): A randomised controlled trial. Lancet Neurol. 2016;15:1138–47. doi: 10.1016/S1474-4422(16)30177-6. [DOI] [PubMed] [Google Scholar]
- 7.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–18. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 9.Lima FO, Silva GS, Furie KL, Frankel MR, Lev MH, Camargo ÉC, et al. Field assessment stroke triage for emergency destination: A simple and accurate prehospital scale to detect large vessel occlusion strokes. Stroke. 2016;47:1997–2002. doi: 10.1161/STROKEAHA.116.013301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nogueira RG, Silva GS, Lima FO, Yeh YC, Fleming C, Branco D, et al. The FAST-ED app: A smartphone platform for the field triage of patients with stroke. Stroke. 2017;48:1278–84. doi: 10.1161/STROKEAHA.116.016026. [DOI] [PubMed] [Google Scholar]
- 11.Pérez de la Ossa N, Carrera D, Gorchs M, Querol M, Millán M, Gomis M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: The rapid arterial occlusion evaluation scale. Stroke. 2014;45:87–91. doi: 10.1161/STROKEAHA.113.003071. [DOI] [PubMed] [Google Scholar]
- 12.Katz BS, McMullan JT, Sucharew H, Adeoye O, Broderick JP. Design and validation of a prehospital scale to predict stroke severity: Cincinnati prehospital stroke severity scale. Stroke. 2015;46:1508–12. doi: 10.1161/STROKEAHA.115.008804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taqui A, Cerejo R, Itrat A, Briggs FB, Reimer AP, Winners S, et al. Reduction in time to treatment in prehospital telemedicine evaluation and thrombolysis. Neurology. 2017;88:1305–12. doi: 10.1212/WNL.0000000000003786. [DOI] [PubMed] [Google Scholar]
- 14.Gerschenfeld G, Muresan IP, Blanc R, Obadia M, Abrivard M, Piotin M, et al. Two paradigms for endovascular thrombectomy after intravenous thrombolysis for acute ischemic stroke. JAMA Neurol. 2017;74:549–56. doi: 10.1001/jamaneurol.2016.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muir KW, Buchan A, von Kummer R, Rother J, Baron JC. Imaging of acute stroke. Lancet Neurol. 2006;5:755–68. doi: 10.1016/S1474-4422(06)70545-2. [DOI] [PubMed] [Google Scholar]
- 16.Román LS, Menon BK, Blasco J, Hernández-Pérez M, Dávalos A, Majoie CB, et al. Imaging features and safety and efficacy of endovascular stroke treatment: A meta-analysis of individual patient-level data. Lancet Neurol. 2018;17:895–904. doi: 10.1016/S1474-4422(18)30242-4. [DOI] [PubMed] [Google Scholar]
- 17.Schröder J, Thomalla G. A critical review of alberta stroke program early CT score for evaluation of acute stroke imaging. Front Neurol. 2016;7:245. doi: 10.3389/fneur.2016.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McTaggart RA, Jovin TG, Lansberg MG, Mlynash M, Jayaraman MV, Choudhri OA, et al. Alberta stroke program early computed tomographic scoring performance in a series of patients undergoing computed tomography and MRI: Reader agreement, modality agreement, and outcome prediction. Stroke. 2015;46:407–12. doi: 10.1161/STROKEAHA.114.006564. [DOI] [PubMed] [Google Scholar]
- 19.Nagel S, Sinha D, Day D, Reith W, Chapot R, Papanagiotou P, et al. E-ASPECTS software is non-inferior to neuroradiologists in applying the ASPECT score to computed tomography scans of acute ischemic stroke patients. Int J Stroke. 2017;12:615–22. doi: 10.1177/1747493016681020. [DOI] [PubMed] [Google Scholar]
- 20.Demeestere J, Scheldeman L, Cornelissen SA, Heye S, Wouters A, Dupont P, et al. Alberta stroke program early CT score versus computed tomographic perfusion to predict functional outcome after successful reperfusion in acute ischemic stroke. Stroke. 2018;49:2361–7. doi: 10.1161/STROKEAHA.118.021961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almeida SR, Vicentini J, Bonilha L, De Campos BM, Casseb RF, Min LL, et al. Brain connectivity and functional recovery in patients with ischemic stroke. J Neuroimaging. 2017;27:65–70. doi: 10.1111/jon.12362. [DOI] [PubMed] [Google Scholar]
- 22.Lin L, Bivard A, Kleinig T, Spratt NJ, Levi CR, Yang Q, et al. Correction for delay and dispersion results in more accurate cerebral blood flow ischemic core measurement in acute stroke. Stroke. 2018;49:924–30. doi: 10.1161/STROKEAHA.117.019562. [DOI] [PubMed] [Google Scholar]
- 23.Sakai Y, Delman BN, Fifi JT, Tuhrim S, Wheelwright D, Doshi AH, et al. Estimation of ischemic core volume using computed tomographic perfusion. Stroke. 2018;49:2345–52. doi: 10.1161/STROKEAHA.118.021952. [DOI] [PubMed] [Google Scholar]
- 24.Yoo AJ, Berkhemer OA, Fransen PS, van den Berg LA, Beumer D, Lingsma HF, et al. Effect of baseline alberta stroke program early CT score on safety and efficacy of intra-arterial treatment: A subgroup analysis of a randomised phase 3 trial (MR CLEAN) Lancet Neurol. 2016;15:685–94. doi: 10.1016/S1474-4422(16)00124-1. [DOI] [PubMed] [Google Scholar]
- 25.Gautheron V, Xie Y, Tisserand M, Raoult H, Soize S, Naggara O, et al. Outcome after reperfusion therapies in patients with large baseline diffusion-weighted imaging stroke lesions: A THRACE trial (Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke) subgroup analysis. Stroke. 2018;49:750–3. doi: 10.1161/STROKEAHA.117.020244. [DOI] [PubMed] [Google Scholar]
- 26.Albers GW. Late time window paradox. Stroke. 2018;49:768–71. doi: 10.1161/STROKEAHA.117.020200. [DOI] [PubMed] [Google Scholar]
- 27.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A Guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 28.Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: A meta-analysis. JAMA. 2016;316:1279–88. doi: 10.1001/jama.2016.13647. [DOI] [PubMed] [Google Scholar]
- 29.Mendez B, Requena M, Aires A, Martins N, Boned S, Rubiera M, et al. Direct transfer to angio-suite to reduce workflow times and increase favorable clinical outcome. Stroke. 2018;49:2723–7. doi: 10.1161/STROKEAHA.118.021989. [DOI] [PubMed] [Google Scholar]
- 30.Rocha M, Jovin TG. Fast versus slow progressors of infarct growth in large vessel occlusion stroke: Clinical and research implications. Stroke. 2017;48:2621–7. doi: 10.1161/STROKEAHA.117.017673. [DOI] [PubMed] [Google Scholar]
- 31.Savitz SI, Baron JC, Yenari MA, Sanossian N, Fisher M. Reconsidering neuroprotection in the reperfusion era. Stroke. 2017;48:3413–9. doi: 10.1161/STROKEAHA.117.017283. [DOI] [PubMed] [Google Scholar]