Abstract
As the worldwide population ages, the morbidity of neurodegenerative, cardiovascular, cerebrovascular, and endocrine diseases, such as diabetes and osteoporosis, continues to increase. The etiology of geriatric diseases is complex, involving the interaction of genes and the environment, which makes effective treatment challenging. Traditional Chinese medicine, unlike Western medicine, uses diverse bioactive ingredients to target multiple signaling pathways in geriatric diseases. Radix puerariae is one of the most widely used ancient traditional Chinese medicines and is also consumed as food. This review summarizes the evidence from in vivo and in vitro studies of the pharmacological effects of the main active components of the tuber of Radix puerariae on geriatric diseases.
Keywords: Aging, aging-related diseases, Radix pueraria
Introduction
Aging is the most important risk factor for most chronic diseases. The life expectancy worldwide has been accompanied by an ascending frequency in the prevalence of age-related diseases. Aging-associated diseases include atherosclerosis and cardiovascular disease, hypertension, cancer, arthritis, osteoporosis, Alzheimer's disease (AD), and Parkinson's disease (PD) due to the deterioration of the circulatory system, metabolic function, and immune system. The etiology of geriatric diseases is complicated, involving the interaction of genes and the environment, which makes effective treatment challenging. Traditional Chinese medicine has its own advantages, containing diverse bioactive ingredients to target multiple signaling pathways in geriatric diseases.[1,2,3,4,5,6,7,8,9,10]
Radix puerariae is one of the first medicinal herbs used in ancient China. The oldest Chinese written reference of the use of kudzu is described in classical poetry (Shih Ching), where it is described as a medicinal herb in the Divine Husbandman's Classic of Chinese Materia Medica (Shen Nong Ben Cao Jing). It was used for the relief of fever, diarrhea, and emesis. It is also mentioned in the Treatise on Fevers (Shang Han Lun) as a treatment of neck stiffness, lack of perspiration, and aversion to air drafts.[11] The Pharmacopoeia of the People's Republic of China describes R. pueraria as cool in nature, sweet and acrid in taste, and used to promote Spleen Yang to arrest diarrhea, encourage the production of body fluids, and indicated for the treatment of fever, acute dysentery, diarrhea, thirst, diabetes, and hypertension. In recent decades, the phytochemistry and pharmacological activities of constituents isolated from R. pueraria have been found to include effects on geriatric cerebrovascular, cardiovascular, and neurodegenerative diseases such as diabetes, hypertension, and hyperlipidemia. Its pharmacological effects include vascular dilation, heart and nerve protection, and antioxidant, anticancer, anti-inflammatory, and analgesic activity. It also promotes bone formation, suppresses alcohol intake, and decreases insulin resistance.[12,13,14,15,16,17] As the population ages, the morbidity of age-related diseases continues to increase. This review summarizes and discusses the pharmacology and mechanisms of the main active components of R. pueraria and its clinical applications in geriatric disease.
Constituents
Radix puerariae, also known as Gegen (Chinese name), kudzu root, Yegen or Kudzu vine root, is the dried root of Radix puerariae (Wild.) Ohwi and belongs to family Fabaceae. It grows in humid regions, monsoon forests, and coastal tracts. It is a twining perennial woody herb native to China, Korea, Japan, India, and the United States. In China, Radix puerariae is distributed throughout Henan, Hunan, Zhejiang, and Sichuan provinces.
Radix puerariae contains 20% to 25% starch, flavonoids, isoflavones, terpenoids, steroids the essential amino acids, lysine, phenylalanine, threonine, isoleucine, and leucine and other components. The steroid components include stigmasterol, β-sitosterol, and β-sitosterol-β-D-glc (daucosterol). The main active components are isoflavones including glycosides and aglycones [Figure 1]. High-performance liquid chromatography of R. pueraria tuber extract has identified flavonoids such as puerarin, daidzein, and genistein[18] as well as tuberosin, 4-methoxypuerarin, hydroxytuberosone, quercetin, biochanin A, biochanin B, irisolidon, tectoridin, robinin, and glycosides (C-glycoside 40, 6-diacetyl puerarin). Puerarin, an active isoflavone, is the most abundant secondary metabolite in R. pueraria, and is present at 1.88%–2.55% (w/w).[11] Such a high content of an active compound in a medicinal plant is rare.[19] Puerarin and its activities have been extensively studied because of its content in R. pueraria. Daidzein, and its 7-O-glycoside derivative daidzein were originally identified in soybeans and later in Yage and Fenge in w/w concentrations of 0.05%–0.47%.[11] Genistein and its 7-O-glycoside genistein are 5-hydroxylation products of daidzein and daidzin are additional isoflavones in Yege or Gegen, but are present in smaller amounts than daidzin and daidzein.[11] The effects of puerarin, genistein, and daidzein in geriatric diseases are shown in Table 1 and are reviewed below.
Figure 1.
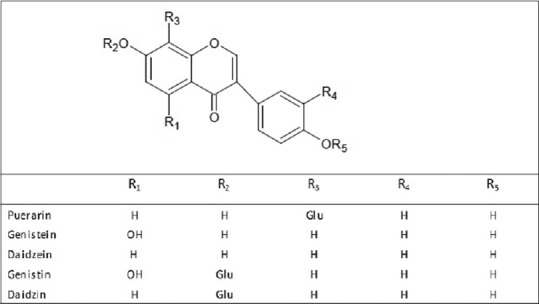
The structure of five main isoflavones in radix puerariae
Table 1.
Anti-aging effects and mechanisms of the components of Radix puerariae
| Component | Effects | Mechanisms |
|---|---|---|
| Puerarin | Alzheimer’s disease | Antioxidant |
| Parkinson disease | Increases the expression of TH and decreases the expression of GFAP; ameliorated MPTP-induced ROS formation | |
| Ischemic stroke | Decreases the level of serum vWF and sTM; increases the expression of BDNF and activates PI3K/AKT and MAPK/ERK signaling pathways | |
| Vascular dementia | Antioxidant | |
| Myocardial hypertrophy | Through (AMPK)/target of rapamycin (mTOR)-mediated signaling pathway; inhibits activation of the redox-sensitive p38 and the NF-κB pathway; blocks Rac1-dependent NADPH oxidase activation; blocks PI3K/Akt and JNK signaling pathways | |
| Hypertension | Improves EDR; increases the phosphorylation of eNOS and decreases the expression of gp91phox, p22phox, TGFβ1, and VCAM-1; reduces the expression of TGF-β and Smad3 mRNA and increases the expression of Smad7 mRNA | |
| Angina pectoris | Inhibits the expression of the protein and mRNA levels of CRP | |
| Acute myocardial ischemia | Decreases the upregulation of P2X3 mRNA and protein levels; opens the calcium-activated potassium channel and activates protein kinase C; increases NO concentration; antioxidant | |
| Diabetic retinopathy | Alleviates cell apoptosis; attenuates IL-1β-mediated leukostasis | |
| Diabetic nephropathy | Regulates the expression of glomerular extracellular matrix | |
| Osteoporosis | Promotes the serological level of osteocalcin, BMSC proliferation, the expression of ALP, and suppresses the serological level of adiponectin and adiposity | |
| Hyperlipidemia | Enhances the expression of 7alpha-hydroxylase (CYP7A1) mRNA and promotion of cholesterol and bile acids excretion in liver | |
| Digestive system cancer | Induces the loss of MMP and generation of ROS | |
| Breast cancer | Downregulates MDR1 expression | |
| Genistein | Ischemic stroke | Antioxidant; enhances eNOS phosphorylation/activation and NO-mediated thiol modification of Keap1; decreases thromboxane A2 concentration and leukocyte-platelet aggregates production |
| Myocardial hypertrophy | Regulates the MTA3/TAK1/MKK4/JNK signaling pathway | |
| Hypertension | Regulates the activity of eNOS and reverse endothelial dysfunction | |
| Diabetes | Improves islet cell survival and proliferation and facilitates insulin production; activates the cAMP/PKA-dependent extracelluar ERK1/2 signaling pathway; antioxidant; improves high glucose-impaired intracellular cAMP production and PKA activity | |
| Osteoporosis | Improves the balance between RANKL and its decoy receptor OPG | |
| Hyperlipidemia | Upregulates the expression of hepatic LDL receptor, estrogen receptor α(ERα), and ERβ mRNAs; lowers the levels of the plasma lipid and vWF | |
| Digestive system cancer | Inhibits phosphorylation of AKT and induces the mitochondrial pathway of apoptosis; inhibits β-catenin target genes | |
| Reproductive system cancer | Suppresses EMT and the migration capacities of BG-1 ovarian cancer cells via ER signaling and the downregulation of TGF-β signal | |
| Breast cancer | Activates ATR kinase and BRCA1 complex; downregulates microRNA-155 | |
| Daidzein | Hyperlipidemia | Enhances the cholesterol homeostasis genetic program; inhibits the activity of pancreatic lipase and lipoprotein lipase and the differentiation of rat preadipocytes |
| Diabetes | Activates AMP-activated protein kinase (AMPK) |
TH: Tyrosine hydroxylase, GFAP: Glial fibrillary acidic protein, MPTP: 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine, ROS: Reactive oxygen species, vWF: von Willebrand factor, sTM: Thrombomodulin, BDNF: Brain-derived neurotrophic factor, AMPK: 5’-adenosine monophosphate kinase, CRP: C-reactive protein, BMSC: Bone marrow stromal cell, ALP: Alkaline phosphatase, MMP: Matrix-metalloproteinases, OPG: Osteoprotegerin, EMT: Epithelial-mesenchymal transition, JNK: Jun N-terminal kinase, EDR: Endothelium-dependent relaxation, ATR: Ataxia telangiectasia-mutated and Rad3-related, NOS: Nitric oxide synthase, RANKL: Receptor activator of NF-κB ligand, TNF: Tumor necrosis factor, IL: Interleukin
Puerarin Effects on Aging-Related Diseases
Neurodegenerative diseases
Neurodegenerative diseases involve the continuing deterioration and loss of neurons or the myelin sheath, ultimately resulting in neurological dysfunction. Neuron loss progresses with aging. Consequently, advancing age in itself contributes to the increasing risk of disorders such as Alzheimer's and PDs.
Oxidative stress is involved in the pathogenesis of AD. Intracerebroventricular administration of puerarin in a mouse model of sporadic AD protected against learning and memory disability. Puerarin increased the activities of glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) and decreased that of malondialdehyde (MDA).[20] It also acted as an intracellular reactive oxidation species (ROS) scavenger. In amyloid precursor protein/presenilin-1 (APP/PS1) mice, an established AD model, oral puerarin resulted in a significant decrease in lipid peroxidation (LPO) and reduction in cognitive impairment. Puerarin has been associated with induction of heme oxygenase 1 (HO-1) in the hippocampus.[21] In a cytoplasmic cell hybrid (cybrid) cell AD model, it protected neurons against oxidative stress-induced apoptosis by downregulating the Bax/Bcl-2 expression ratio, which blocked the activation of the c-Jun N-terminal kinase (JNK), p38, and caspase-3.[22]
PD is a degenerative disorder characterized by apoptosis or necrosis of dopaminergic motor neurons in the substantia nigra. In a mouse PD model, puerarin increased spontaneous activity and travel distance in an open field assessment associated with increased tyrosine hydroxylase expression and decreased expression of the glial fibrillary acidic protein.[23] In other mouse PD models, puerarin inhibited 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neuronal degeneration by modulating the expression of glial cell line-derived neurotrophic factor expression, the PI3K/Akt pathway, and glutathione (GSH) activation. The reduction in MPTP-induced ROS formation and lysosome-associated membrane protein (Lamp) 2A expression protected against cell loss.[24,25] In SH-SY5Y human neuroblastoma cells, puerarin protected against MPP (+)-induced apoptosis by regulating the activity of the ubiquitin-proteasome system.[26] The available evidence from animal models shows that antioxidant and antiapoptosis activities of puerarin protect neurons against damage in dementia and PD.
Cerebrovascular disease
Puerarin has been shown to decrease the morbidity of ischemic stroke. A clinical study in 45 patients with acute cerebral infarction found that puerarin plus aspirin decreased serum von Willebrand factor (vWF) and thrombomodulin (sTM), and was protective against damage to the vascular endothelium.[12] It improved neurological function, decreased infarct volume, and increased peri-infarct collateral vessel density in an experimental rat model.[27] Puerarin accelerated the recovery of brain function in a rat cerebral ischemia/reperfusion model. It inhibited astrocyte apoptosis, possibly by modulating the expression of apoptosis-related factors, brain-derived neurotrophic factor, and activating phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt), and mitogen-activated protein kinase/extracellular regulated protein kinase (MAPK/ERK) signaling.[28]
Puerarin has also been evaluated preclinically in an animal ischemia/reperfusion model where it decreased MDA, nitric oxide (NO), nuclear factor kappa B (NF-κB), tumor necrosis factor-α (TNF-α), interleukin (IL)-1 β, and IL-6 produced in response to oxidative stress and inflammation. It also increased the expression of growth factors including vascular endothelial growth factor, erythropoietin, erythropoietin receptor, and growth-associated protein-43. Physiological effects included improved recovery of neurological functions and neurovascular protection resulting from the anti-oxidative and anti-inflammatory activity.[29] Ischemic stroke is complicated by vascular dementia, which has a significant impact on the quality of life of the elderly. Puerarin improved impaired learning and memory functions in a rat model of vascular dementia induced by chronic ischemia. It was associated with decreased MDA content, restoration of GSH-Px activity and total thiol concentration, increased cell viability, reduced apoptosis, and ROS generation. Puerarin also upregulated nuclear respiratory factor 2 (Nrf2), and forkhead box protein (Fox) O1, FoxO3, and FoxO4.[30] The combination of salvianolic acid-B and puerarin was more neuroprotective than either compound alone, which may prove significant in the treatment of ischemic stroke.[31] Puerarin is often used to treat headache, dizziness, stiff neck, tinnitus, limb paralysis, and other symptoms in patients with hypertensive encephalopathy. It is often used in conjunction with other antihypertensive drugs.
Cardiovascular disease
Aging is accompanied by many changes in the cardiovascular system, including systolic hypertension, increased central vascular stiffness, and increased pulse pressure. Recently, puerarin injection has become widely used to treat coronary heart disease and angina pectoris. The effect of puerarin on paroxysmal chest pain or chest discomfort, myocardial ischemia associated with angina pectoris has been clinically evaluated. In a series of 388 patients, puerarin injection was more effective than danshen, a widely used antiangina prescription in China, for control of angina symptoms, without clinically significant adverse reactions.[32] Two meta-analyses and a systematic review found that combining puerarin with conventional Western medicines improved the treatment of unstable angina pectoris.[33,34] However, the finding should be interpreted with caution because of the very low methodological quality of studies and potential publication bias. A more rigorously designed, the randomized double-blind placebo-controlled trial is needed to confirm the findings.
Puerarin was also reported to inhibit the expression of C-reactive protein and mRNA in lipopolysaccharide-induced peripheral blood mononuclear cells isolated from patients with unstable angina pectoris. Puerarin inhibited the NF-κB signaling pathway.[35] Puerarin activity has also been extensively investigated in animal models of acute myocardial ischemia (AMI). Pegylated puerarin was found to target myocardial tissue affected by ischemia in an isoprenaline-induced rat AMI model.[36] P2 ×3 receptors are trimeric adenosine triphosphate (ATP)-activated ion channels permeable to Na+, K + and Ca2+ present in ganglia neurons responsible for excitatory sympathetic reflexes induced by ischemia-related myocardial cell injury. Puerarin reduced systolic blood pressure (BP) and heart rate and relieved pain after AMI. It also decreased upregulated P2X3 mRNA and protein expression in stellate, superior cervical, and cervical dorsal root ganglia inhibited upregulated ATP-activated currents in dorsal root ganglion neurons and depressed upregulated excitatory sympathetic responses.[14] The available evidence indicates that puerarin may relieve myocardial ischemic damage by blocking P2X₃ signaling and depressing excitatory sympathetic reflexes. Other results obtained in isolated rat hearts is consistent with the association of the therapeutic effect of puerarin on AMI, the opening of calcium-activated potassium channels, and protein kinase C (PKC) activity.[37]
Puerarin was also found to promote vasodilation after myocardial infarction in a rat model, with increased serum NO concentration, NO synthase (eNOS) expression, and protein kinase B (Akt/PKB) phosphorylation in myocardial endothelial cells.[38] Compound medicines including puerarin can also protect against AMI. Shenge, which contains puerarin and danshensu isolated from the Chinese herb, Salvia miltiorrhiza, decreased ST elevation, reduced the size of the ischemic area, serum creatine kinase isoenzyme-MB, lactate (LDH), and MDA, and increased serum SOD in a dose-dependent manner.[39] The evidence indicates that the cardioprotective effects of Shenge depend on its antioxidant and anti-LPO activities.
Aging is associated with arterial stiffening and results in hypertension and cardiac hypertrophy. Endothelium-dependent relaxation (EDR) refers to a vasodilation response to a variety of endothelial factors. It can occur with the conversion of L-Arg to NO by NO synthase promoted by drugs or physiological stimuli. EDR is impaired in rats by infusion angiotensin II along with reduction of phospho-eNOS (Ser 1177) expression and increases in the expression of gp91phox, p22phox, TGFβ1, and VCAM-1. Puerarin has been shown to improve EDR and reverse the changes in angiotensin II (Ang II)-induced protein expression of the above molecules.[40] Ang II induces macrophage aggregation in inflammatory responses, and the macrophages secrete cytokines like TGF-β1 that act through Smad proteins to promote myocardial collagen synthesis, deposition, and fibrosis.[41] Puerarin was found to protect against myocardial injury in spontaneously hypertensive rats and was associated with reduced TGF-β and Smad3 expression and increased Smad7 mRNA expression.[42]
Thickening of the myocardium can be caused by diseases, but it can also result from pathophysiological change related to aging. Puerarin has been shown to protect against cardiomyocyte hypertrophy and apoptosis via 5’-adenosine monophosphate kinase (AMPK)/target of rapamycin-mediated signaling[43] and can attenuate Ang II-induced cardiac hypertrophy by inhibiting activation of the redox-sensitive p38 and the NF-κB pathway.[44] Puerarin also has antihypertrophic effects by blocking Rac1-dependent NADPH oxidase activation and downstream redox-sensitive AP-1 signaling.[45] In another mouse model, aortic banding induced cardiac hypertrophy. The available evidence indicates that puerarin slows the progression of cardiac hypertrophy and apoptosis probably by the blockade of PI3K/Akt and JNK signaling pathways.[46] Evidence from modern medicine indicates that puerarin can dilate coronary arteries, increase coronary blood flow, reduce myocardial oxygen consumption and increase oxygen supply, and reduce peripheral resistance. It also has an antihypertensive effect and can alleviate the “stiff neck” symptoms of hypertension patients.
Endocrine diseases
Puerarin has been investigated in diabetes mellitus and its complications, hyperlipidemia, and osteoporosis. Diabetes mellitus is an aging-related disease characterized by high blood glucose and impaired glucose tolerance resulting from insulin secretion deficiency, insulin resistance, or both. In addition to insulin resistance, aging may increase the susceptibility of diabetes mellitus because of increased inducible NOS (iNOS) expression, and S-nitrosation of the insulin receptor substrate-1, or the activation of AKT/PKB in skeletal muscle.[15,47] Both diabetes itself and its related complications are responsible for increased morbidity and mortality. The antidiabetes activity of puerarin includes reduced body weight gain, improved blood glucose control, and improved glucose tolerance. R. pueraria has been used to treat diabetes for thousands of years, and Puerarin can reduce blood sugar and increase insulin receptor sensitivity in patients with type 2 diabetes. Puerarin may increase β-cell proliferation in high-fat-diet-induced db/db diabetic mice by activation of glucagon-like peptide-1 receptor signaling and its downstream targets[48] and by inhibition of β-cell apoptosis in streptozotocin (STZ)-induced diabetic mice by the mediation of PI3K/Akt signaling.[49]
Chronic hyperglycemia results in retinal microvascular abnormalities, neovascularization, macular edema, and retinal detachment that ultimately lead to blindness. Puerarin has been shown to attenuate inflammation-induced retinal injury by inhibiting apoptosis caused by proinflammatory factors and by interfering with inflammatory signaling. IL-β can be responsible for retinal cell inflammation and apoptosis following breakdown of the blood-retina barrier. Puerarin attenuated leukostasis and cell apoptosis and prevented molecular events induced by upstream and downstream signaling in TR-iBRB2 retinal capillary endothelial cells pretreated with IL-1 β for 24 h.[50] Puerarin alleviated neuropathic pain by inhibiting inflammation mediated by suppression of NF-κB activation and cytokine upregulation in the spinal cord of rats with rats with STZ-induced diabetes.[51]
Diabetic nephropathy is characterized by the accumulation of the glomerular extracellular matrix, proteinuria, and progressive renal failure. Puerarin regulated the production of glomerular extracellular matrix and protected against the progression of other events in rats with diabetic nephropathy induced by STZ.[52] Puerarin was found to protect against renal injury caused by hyperglycemia and advanced glycation end products (AGEs). It reduced the AGE content and decreased the expression of AGE-specific cellular receptor (RAGE) mRNA in the kidneys of rats with STZ-induced diabetes.[53]
Puerarin has beneficial effects on hyperlipidemia. Large multicenter epidemiology studies conducted in China confirmed that total cholesterol (TC), triglycerides (TGs), and low-density lipoprotein cholesterol (LDL-C) all increase with age.[54] Hepatic cholesterol 7 alpha-hydroxylase (CYP7A1) is a membrane-bound cytochrome P450 enzyme that converts cholesterol to 7-alpha-hydroxycholesterol, which is the first and rate-limiting step in the synthesis of bile acid. Puerarin inhibited the increase in serum and liver TC concentration caused by a cholesterol-rich diet in Sprague-Dawley rats in part by enhancing CYP7A1 mRNA expression and promoting excretion of cholesterol and bile acids by the liver.[16]
Reduction of bone mass without alteration of bone composition leads to fractures in postmenopausal and age-related primary osteoporosis. Puerarin is an isoflavone with a chemical structure like that of estrogen. As it has estrogen activity but is less toxic. Its effects on bone metabolism have been investigated. Puerarin and/or zinc gavage decreased bone loss, increased serum osteocalcin, bone marrow stromal cell proliferation, and alkaline phosphatase expression, and reduced serum adiponectin and bone marrow adiposity in ovariectomized rats. Coadministration had a stronger effect on bone loss and biomechanical strength than puerarin or zinc monotherapy.[55]
Cancer
The incidence of cancer increases with age and is related to changes in molecular physiology. The inhibition of the proliferation of BGC-823 gastric cancer cells was stronger in response to combination treatment with puerarin and 5-fluorouracil than to monotherapy, with no increase in side effects.[56] Similar effects were observed in Eca109 esophageal cancer cells in vitro and in nude mice.[57] High concentrations of puerarin have also been reported to inhibit cell proliferation, increase apoptosis, and change the morphology of SMMC-7721 human hepatocellular carcinoma cells in a time-dependent and dose-dependent manner. Puerarin-induced apoptosis was associated with the loss of matrix-metalloproteinases and generation of ROS.[58] The anticancer activity of a puerarin nanosuspension was demonstrated by changes in tumor volume, body weight, and survival of mice bearing HT-29 human colon cancer cell tumors.[59] Multidrug resistance (MDR) is a major obstacle to effective cancer chemotherapy and may be mediated by the expression of the MDR1 gene, which was purified in 1979. Puerarin was shown to downregulate MDR1 expression and improve drug toxicity via NF-κB and cAMP-responsive element and transcription-dependent upregulation of AMPK in MCF-7/Adriamycin human breast MDR cancer cells.[60]
Puerarin summary
Puerarin is the bioactive ingredient of Radix puerariae that improves blood circulation, has antiarrhythmic effects, lowers elevated BP, blood lipids and blood sugar, and has neuroprotective and antitumor activity. Currently, injection, tablet, and capsule formulations of puerarin are available for clinical use.[61] The injection formulation has been approved by the State Food and Drug Administration in China[62] and is used to treat coronary heart disease, angina pectoris, myocardial infarction, and viral myocarditis in children. It is also used to treat ischemic cerebrovascular disease, diabetes mellitus, retinal arteriole and vein occlusion, and sudden deafness. Its effects on the reversal of multidrug tumor resistance need further confirmation in clinical trials and practice.
Genistein Effects on Aging-Related Disease
Cardiovascular and cerebrovascular disease
Genistein can attenuate neuronal degeneration caused by cerebral ischemia by reducing oxidative damage. Genistein inhibited decreases in SOD activity and Nrf1 expression that resulted from ischemia and decreased MDA, apoptosis-related cysteine peptidase caspase-3, and caspase-9 expression.[63] Genistein also protected ischemia/reperfusion damage to hippocampal CA1 neurons by reducing oxidative stress. The mechanism may have involved enhancement of eNOS phosphorylation/activation, NO-mediated thiol modification of Keap1, and subsequent upregulation of Nrf2/HO-1 antioxidative signaling.[64] Genistein may also protect against ischemia/reperfusion injury by decreasing thromboxane A2, formation of leukocyte-platelet aggregates, and increasing U-46619-and decreasing platelet releasate-elicited contractile responses.[65] Genistein attenuated pressure overload-induced cardiac dysfunction, hypertrophy, in an experimental mouse model. The mechanism may have involved regulation of metastasis-associated gene 3 (MTA3)/TGFβ and activated kinase 1 (TAK1)/MKK4/JNK signaling.[66]
In animal hypertension studies, genistein decreased BP by regulating eNOS activity and reversing endothelial dysfunction. Angiotensin-converting enzyme inhibition, PKC-βII activation, and increased kinin and NO activity may have contributed to the BP-lowering activity genistein.[17] Genistein also reduced endothelial dysfunction in spontaneously hypertensive rats, possibly by increasing eNOS activity and calmodulin-1 expression and decreasing superoxide generation;[67] the antihypertensive effects and reversal of endothelial expression were increased by combined treatment with Mg.[68]
Endocrine diseases
Genistein has been evaluated in diabetes mellitus and its complications, hyperlipidemia, and osteoporosis. The anti-diabetes effects of genistein have been demonstrated in various animal models. Genistein improved islet-cell survival and proliferation and facilitated insulin production in pancreatic β-cell cultures after alloxan injury[69] and improved glucose tolerance and blood insulin level in mice with STZ-induced diabetes via activation of cAMP/PKA-dependent extracellular ERK1/2 signaling.[70] It was also reported to inhibit STZ diabetes-induced retinal inflammation by interfering with inflammatory signaling by ERK and P38 MAPKs in activated microglia.[71] Oxidative damage is involved in the development of diabetes-induced cardiovascular diseases, and genistein was found to increase the antioxidant reserve of the hearts of rats with STZ-induced diabetes. The anti-inflammatory and antioxidant effects of genistein were shown by NF-κB immunohistochemical staining and amelioration of ultrastructural degenerative changes in myocardial tissue.[72] Continuing daidzein treatment of rats with diabetes prevented abnormal changes of vascular reactivity in aortic tissue through mediation of prostaglandin-related pathways and attenuation of oxidative stress.[73]
Monocytes that adhere to arterial endothelial cells and enter the intima participate in the development of atherosclerosis. Genistein has been shown to inhibit hyperglycemia-induced adhesion of monocytes to cultured human aortic endothelial cells, suppress monocyte chemotactic protein-1 (MCP-1), IL-8, impaired intracellular cAMP production, and PKA activity.[74] Genistein also suppressed diabetes-induced adhesion of monocytes to endothelial cell and endothelial secretion of adhesion molecules in mice with diabetes.[74] It also improved wound healing and angiogenesis in mice with STZ-induced type 1 diabetes by suppression of FoxO1, iNOS activity, and oxidative stress.[75]
The estrogen activity of genistein on lipid metabolism has been associated with reduced atherosclerosis in animal models. Dietary genistein improved hyperlipidemia in hamsters by upregulating the expression of hepatic LDL receptor, estrogen receptor alpha (ERα), and ERβ mRNAs[76] and 7-difluoromethyl-5,4′-dimethoxygenistein (DFMG), a genistein derivative, increased plasma nitrite, decreased plasma lipids and vWF, decreased fatty infiltration of aortic lesions, and improved EDR in DFMG-treated mice.[77] DFMG also slowed the development of atherosclerosis, reduced TC, LDL cholesterol, and LPO, increased smooth muscle cell and collagen content of thoracic aorta atheromas in a rabbit model.[78]
Receptor activator of NF-κB ligand (RANKL) and its decoy receptor osteoprotegerin (OPG) regulate bone resorption and homeostasis in normal bone remodeling. In a group of osteopenic, postmenopausal women, 24 months of dietary supplementation with genistein, calcium, and Vitamin D3 improved bone turnover and sRANKL-OPG balance in a cohort of osteopenic, postmenopausal women. Coadministration had a stronger effect than the calcium and Vitamin D3 control.[79]
Cancer
Genistein was shown to inhibit the proliferation of SNU-449 human hepatocellular carcinoma cells in a concentration-dependent manner by downregulation of thioredoxin-1.[80] It also inhibited proliferation and induced Akt phosphorylation and mitochondrial apoptosis of HCT-116 and LoVo cells colorectal cancer cells.[81] Other studies have associated the antiproliferative effect of genistein-27 in colitis-associated colorectal cancer with the p65-CDX2-β-catenin axis and inhibition of β-catenin target genes.[82] About one-sixth of breast cancers are triple-negative cancers, named after the absence of the expression of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2. An analysis of 5,445 phosphorylation sites on 2,008 phosphoproteins in genistein-treated triple-negative MDA-MB-231 breast cancer cells revealed that genistein inhibited proliferation by regulating the cell cycle and DNA damage by activation of ataxia telangiectasia-mutated and Rad3-related (ATR) kinases and the BRCA1 complex.[83]
Genistein-enhanced trichostatin A-induced p53-dependent apoptosis of lung cancer cells that was associated with histone/nonhistone protein acetylation.[84] It also inhibited the proliferation of human multiple myeloma cells by upregulating miR-29b and inhibiting NF-κB.[85] Genistein promoted programmed or nonprogrammed cell death of MES-SA, MES-SA-Dx5, and SK-UT-1 uterine sarcoma cells,[86] and inhibited proliferation in LNCaP, LAPC-4, and PC-3 prostate cancer cells by increasing expression. It acted by reducing methylation of the ER-β promoter.[87] Methylation of 58 genes in DU-145 and LNCaP prostate cancer cells was altered by genistein and daidzein, which led to inhibition of cell proliferation and promotion of apoptosis.[88] The anticancer effects of genistein in metastatic breast cancer have been attributed to downregulation of miRNA-155.[89]
Epithelial-mesenchymal transition (EMT) is important for tumor cell migration, and it was found that 7-difluoromethoxyl-5,4′-di-n-octyl genistein reversed the EMT phenotype of gastric cancer stem-like cells by modulation of forkhead box M1 (FoxM1) and decrease of Twist1 expression.[90] Genistein also inhibited colon cancer progression and suppressed the expression of colon stem-cell markers, including CD133, CD44, and β-catenin in 1,2-dimethylhydrazine-induced colon cancer in rats.[91] Bisphenol A and nonylphenol are endocrine disrupting chemicals that promote EMT and migration of estrogen-responsive cancers. Genistein suppressed EMT and the migration of BG-1 ovarian cancer cells enhanced by 17 β-estradiol, bisphenol A and nonylphenol by downregulating ER and TGF-β signaling.[92]
The association of the anticarcinogenic and antiproliferative effects of soybean isoflavonoids and signal transduction pathways has been investigated. Genistein and genistin have weak estrogen activity. However, in the presence of estradiol, they downregulate estradiol-induced alkaline phosphatase activity and reduce cell proliferation much like antiestrogens. This effect warrants further study to evaluate potential clinical benefits.
Daidzein
Cerebrovascular disease
Daidzein enhanced the cholesterol homeostasis genetic program, including expression of the Lxr and the downstream transporter ApoE, Abca1, and Abcg1 genes in vitro. It also improved motor/gait function in chronic stroke and increased synaptophysin expression. The effects were abolished in ApoE-knockout mice, indicating the role of daidzein-induced ApoE upregulation in stroke recovery.[93]
Endocrine diseases
Daidzein has been evaluated in diabetes mellitus and hyperlipidemia. Daidzein may decrease glucose absorption. It is an α-glucosidase inhibitor and can suppress postprandial hyperglycemia caused by starch,[94] and was found to promote glucose uptake by activation of AMP-activated protein kinase (AMPK) and GLUT4 translocation to the plasma membrane of muscle cells.[95] A 6-month dietary supplementation with daidzein decreased TGs and uric acid in a randomized, double-blind, placebo-controlled trial including 210 adults with hypercholesterolemia. ESRβ activity and the Rsal genotype were both involved.[96] In obese mice fed a high-fat diet, daiazein improved hyperlipoidemia by inhibiting the activities of pancreatic lipase and lipoprotein lipase, the differentiation of rat preadipocytes, and by stimulating lipolysis by activating hormone-sensitive lipase.[97]
Cancer
Ovarian cancer is the deadliest of all gynecologic cancers. The growth of ovarian cancer cells is inhibited by the anti-inflammatory and proapoptotic activity of natural ERβ agonists.[98] Daidzein is an ER modulator that inhibits cell migration, invasion, proliferation, and sphere formation by regulating focal adhesion kinase and PI3K/AKT signaling in ovarian cancer cells.[99] It has been shown to induce apoptosis and cell cycle arrest in SKOV3 human ovarian cancer cells by inhibiting the Raf/MEK/ERK cascade.[100] Despite an initial response to chemotherapy, most ovarian cancer patients relapse with an incurable disease attributable to development chemotherapy resistance.
ER-positive breast cancer is the most frequent cause of cancer death in women worldwide, making development of novel treatments a priority. An oral form of isoflavone daidzein was synergistic with centchroman, an oral contraceptive with anticancer activity, to induce apoptosis and inhibit PI3K/Akt pathway signaling in MCF-7/MDA MB-231 human breast cancer cells.[101] The phytoestrogen activity of daidzein was found to inhibit the drug transporter BCRP/ABCG2 in breast cancer cells.[102] Long-term human studies monitoring free estrogens and their conjugates are highly warranted to evaluate the potential side effects of high-dose genistein and daidzein, especially in patients diagnosed with ERα+ breast cancer.[103] Daidzein was also reported to suppress TNF-α-induced migration and invasion of human breast cancer cells by inhibiting hedgehog/Gli1 signaling[104] and to cause cytochrome c-mediated apoptosis via Bcl-2 in human hepatic cancer cells,[105] The evidence supports daidzein is a potent inducer of mitochondrial activation of apoptosis in hepatic cancer cells via mitochondrial pathway.
Conclusions
With a rapidly aging population, the morbidity of age-related neurodegenerative, cardio- and cerebrovascular, and endocrine diseases including diabetes and osteoporosis is increasing. The etiology of these diseases is complicated and involves the interaction of gene and environment, and the availability of effective therapies is limited. Traditional Chinese medicines differs from Western medicines by containing more than one active ingredient and can target multiple signal pathways.[1,2,3,4,5,6,7,8,9,10] R. puerariae has an important place in Chinese traditional medicine. It has been shown effective in the treatment and management of cardiovascular diseases, diabetes, hypertension, and cerebrovascular diseases. The application of Radix puerariae in human diseases is based on the theory of Chinese medicine and clinical research. Pharmacological activities of the tuber extract and its constituents have been identified. The mechanisms of action of the extract have not been fully described and warrant continuing study. Its active ingredients are thought to include puerarin, genistein, and daidzein. The available evidence supports the demonstrated or potential benefits of puerarin in diverse clinical applications. The evidence warrants continuing study of its molecular mechanisms and targets and for improving its oral bioavailability and reducing its side effects. Puerarin injection is currently approved in coronary heart and ischemic cerebrovascular diseases because of its fibrinolytic and vascular activity, which may be shared with daidzein and genistein. Study of the fibrinolytic mechanisms of daidzein and genistein independently, combined with each other and with puerarin would help to discover synergy in their fibrinolytic activity. Interdisciplinary studies are required for a better understanding of the pharmacological mechanisms of Radix puerariae extract and its individual constituents. Comparative studies with other medicines are needed to confirm its safety.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This project was supported by the Natural Science Foundation of China (81471340) and the National Key Clinical Specialty (Traditional Chinese Medicine, no. 122).
References
- 1.Yang Y, Ren C, Zhang Y, Wu X. Ginseng: An nonnegligible natural remedy for healthy aging. Aging Dis. 2017;8:708–20. doi: 10.14336/AD.2017.0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu P, Zhao H, Luo Y. Anti-aging implications of Astragalus membranaceus (Huangqi): A well-known Chinese tonic. Aging Dis. 2017;8:868–86. doi: 10.14336/AD.2017.0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J, Cao B, Zhao H, Feng J. Emerging roles of Ganoderma lucidum in anti-aging. Aging Dis. 2017;8:691–707. doi: 10.14336/AD.2017.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang N, Ji S, Zhang H, Mei S, Qiao L, Jin X, et al. Herba cistanches: Anti-aging. Aging Dis. 2017;8:740–59. doi: 10.14336/AD.2017.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao Y, Wei Y, Wang Y, Gao F, Chen Z. Lycium barbarum: A traditional Chinese herb and A promising anti-aging agent. Aging Dis. 2017;8:778–91. doi: 10.14336/AD.2017.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cakova V, Bonte F, Lobstein A. Dendrobium: sources of active ingredients to treat age-related pathologies. Aging Dis. 2017;8:827–49. doi: 10.14336/AD.2017.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Z, Feng W, Shen Q, Yu N, Yu K, Wang S, et al. Rhizoma coptidis and berberine as a natural drug to combat aging and aging-related diseases via anti-oxidation and AMPK activation. Aging Dis. 2017;8:760–77. doi: 10.14336/AD.2016.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao H, Han Z, Li G, Zhang S, Luo Y. Therapeutic potential and cellular mechanisms of panax notoginseng on prevention of aging and cell senescence-associated diseases. Aging Dis. 2017;8:721–39. doi: 10.14336/AD.2017.0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuo W, Yan F, Zhang B, Li J, Mei D. Advances in the studies of Ginkgo biloba leaves extract on aging-related diseases. Aging Dis. 2017;8:812–26. doi: 10.14336/AD.2017.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang W, Huang X, Chen W. The effects of baicalin and baicalein on cerebral ischemia: A review. Aging Dis. 2017;8:850–67. doi: 10.14336/AD.2017.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z, Lam TN, Zuo Z. Radix puerariae: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2013;53:787–811. doi: 10.1002/jcph.96. [DOI] [PubMed] [Google Scholar]
- 12.Hu HT, Fen F, Ding MP. Effects of puerarin with aspirin on the markers of damaged vascular endothelial cells in patients with acute cerebral infarction. Zhongguo Zhong Yao Za Zhi. 2008;33:2827–9. [PubMed] [Google Scholar]
- 13.Bagheri M, Joghataei MT, Mohseni S, Roghani M. Genistein ameliorates learning and memory deficits in amyloid β(1-40) rat model of Alzheimer's disease. Neurobiol Learn Mem. 2011;95:270–6. doi: 10.1016/j.nlm.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Liu S, Yu S, Xu C, Peng L, Xu H, Zhang C, et al. Puerarin alleviates aggravated sympathoexcitatory response induced by myocardial ischemia via regulating P2X3 receptor in rat superior cervical ganglia. Neurochem Int. 2014;70:39–49. doi: 10.1016/j.neuint.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Ropelle ER, Pauli JR, Cintra DE, da Silva AS, De Souza CT, Guadagnini D, et al. Targeted disruption of inducible nitric oxide synthase protects against aging, S-nitrosation, and insulin resistance in muscle of male mice. Diabetes. 2013;62:466–70. doi: 10.2337/db12-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan LP, Chan SW, Chan AS, Chen SL, Ma XJ, Xu HX, et al. Puerarin decreases serum total cholesterol and enhances thoracic aorta endothelial nitric oxide synthase expression in diet-induced hypercholesterolemic rats. Life Sci. 2006;79:324–30. doi: 10.1016/j.lfs.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Palanisamy N, Venkataraman AC. Beneficial effect of genistein on lowering blood pressure and kidney toxicity in fructose-fed hypertensive rats. Br J Nutr. 2013;109:1806–12. doi: 10.1017/S0007114512003819. [DOI] [PubMed] [Google Scholar]
- 18.Jiang RW, Lau KM, Lam HM, Yam WS, Leung LK, Choi KL, et al. A comparative study on aqueous root extracts of Pueraria thomsonii and Pueraria lobata by antioxidant assay and HPLC fingerprint analysis. J Ethnopharmacol. 2005;96:133–8. doi: 10.1016/j.jep.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 19.Wong KH, Li GQ, Li KM, Razmovski-Naumovski V, Chan K. Kudzu root: Traditional uses and potential medicinal benefits in diabetes and cardiovascular diseases. J Ethnopharmacol. 2011;134:584–607. doi: 10.1016/j.jep.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Zhao SS, Yang WN, Jin H, Ma KG, Feng GF. Puerarin attenuates learning and memory impairments and inhibits oxidative stress in STZ-induced SAD mice. Neurotoxicology. 2015;51:166–71. doi: 10.1016/j.neuro.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Xie N, Li L, Zou Y, Zhang X, Dong M, et al. Puerarin alleviates cognitive impairment and oxidative stress in APP/PS1 transgenic mice. Int J Neuropsychopharmacol. 2014;17:635–44. doi: 10.1017/S146114571300148X. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Liu Y, Lao M, Ma Z, Yi X. Puerarin protects Alzheimer's disease neuronal cybrids from oxidant-stress induced apoptosis by inhibiting pro-death signaling pathways. Exp Gerontol. 2011;46:30–7. doi: 10.1016/j.exger.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Jiang M, Yun Q, Niu G, Gao Y, Shi F, Yu S, et al. Puerarin prevents inflammation and apoptosis in the neurocytes of a murine Parkinson's disease model. Genet Mol Res. 2016;15 doi: 10.4238/gmr.15047501. gmr15047501. [DOI] [PubMed] [Google Scholar]
- 24.Zhu G, Wang X, Wu S, Li X, Li Q. Neuroprotective effects of puerarin on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson's disease model in mice. Phytother Res. 2014;28:179–86. doi: 10.1002/ptr.4975. [DOI] [PubMed] [Google Scholar]
- 25.Cai P, Ye J, Zhu J, Liu D, Chen D, Wei X, et al. Inhibition of endoplasmic reticulum stress is involved in the neuroprotective effect of bFGF in the 6-OHDA-induced Parkinson's disease model. Aging Dis. 2016;7:336–449. doi: 10.14336/AD.2016.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng YF, Zhu GQ, Wang M, Cheng H, Zhou A, Wang N, et al. Involvement of ubiquitin proteasome system in protective mechanisms of puerarin to MPP(+)-elicited apoptosis. Neurosci Res. 2009;63:52–8. doi: 10.1016/j.neures.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Wu M, Liang S, Ma L, Han Y, Zhang X, Xu C, et al. Effects of delayed puerarin treatment in long-term neurological outcomes of focal ischemic stroke in rats. Indian J Pharmacol. 2014;46:157–60. doi: 10.4103/0253-7613.129305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang N, Zhang Y, Wu L, Wang Y, Cao Y, He L, et al. Puerarin protected the brain from cerebral ischemia injury via astrocyte apoptosis inhibition. Neuropharmacology. 2014;79:282–9. doi: 10.1016/j.neuropharm.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Xue Q, Liu Y, He R, Yang S, Tong J, Li X, et al. Lyophilized powder of catalpol and puerarin protects neurovascular unit from stroke. Int J Biol Sci. 2016;12:367–80. doi: 10.7150/ijbs.14059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Guo W, Tian B, Sun M, Li H, Zhou L, et al. Puerarin attenuates cognitive dysfunction and oxidative stress in vascular dementia rats induced by chronic ischemia. Int J Clin Exp Pathol. 2015;8:4695–704. [PMC free article] [PubMed] [Google Scholar]
- 31.Ling C, Liang J, Zhang C, Li R, Mou Q, Qin J, et al. Synergistic effects of salvianolic acid B and puerarin on cerebral ischemia reperfusion injury. Molecules. 2018;23 doi: 10.3390/molecules23030564. pii: E564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo ZK, Liu Y, Li HM. A clinical efficacy and safety study on coronary heart disease and angina treatment with puerarin injection. Zhonghua Liu Xing Bing Xue Za Zhi. 2012;33:614–6. [PubMed] [Google Scholar]
- 33.Gao Z, Wei B, Qian C. Puerarin injection for treatment of unstable angina pectoris: A meta-analysis and systematic review. Int J Clin Exp Med. 2015;8:14577–94. [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, Wu T, Chen X, Ni J, Duan X, Zheng J, et al. Puerarin injection for unstable angina pectoris. Cochrane Database Syst Rev. 2006;5:CD004196. doi: 10.1002/14651858.CD004196.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Yang X, Hu W, Zhang Q, Wang Y, Sun L. Puerarin inhibits C-reactive protein expression via suppression of nuclear factor kappaB activation in lipopolysaccharide-induced peripheral blood mononuclear cells of patients with stable angina pectoris. Basic Clin Pharmacol Toxicol. 2010;107:637–42. doi: 10.1111/j.1742-7843.2010.00548.x. [DOI] [PubMed] [Google Scholar]
- 36.Xia CF, Ye ZG, Zhou XN, Tang TT, Wen LJ, Liu XY, et al. Tissue distribution of PEGylated puerarin in acute myocardial ischemia mode rats. Yao Xue Xue Bao. 2014;49:1413–7. [PubMed] [Google Scholar]
- 37.Gao Q, Yang B, Ye ZG, Wang J, Bruce IC, Xia Q, et al. Opening the calcium-activated potassium channel participates in the cardioprotective effect of puerarin. Eur J Pharmacol. 2007;574:179–84. doi: 10.1016/j.ejphar.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 38.Zhang SY, Chen G, Wei PF, Huang XS, Dai Y, Shen YJ, et al. The effect of puerarin on serum nitric oxide concentration and myocardial eNOS expression in rats with myocardial infarction. J Asian Nat Prod Res. 2008;10:373–81. doi: 10.1080/10286020801892250. [DOI] [PubMed] [Google Scholar]
- 39.Wu L, Qiao H, Li Y, Li L. Protective roles of puerarin and danshensu on acute ischemic myocardial injury in rats. Phytomedicine. 2007;14:652–8. doi: 10.1016/j.phymed.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 40.Li X, Lin Y, Zhou H, Li Y, Wang A, Wang H, et al. Puerarin protects against endothelial dysfunction and end-organ damage in ang II-induced hypertension. Clin Exp Hypertens. 2017;39:58–64. doi: 10.1080/10641963.2016.1200603. [DOI] [PubMed] [Google Scholar]
- 41.Kohno T, Anzai T, Naito K, Sugano Y, Maekawa Y, Takahashi T, et al. Angiotensin-receptor blockade reduces border zone myocardial monocyte chemoattractant protein-1 expression and macrophage infiltration in post-infarction ventricular remodeling. Circ J. 2008;72:1685–92. doi: 10.1253/circj.cj-08-0115. [DOI] [PubMed] [Google Scholar]
- 42.Zhang NB, Huang ZG, Cui WD, Ding BP. Effects of puerarin on expression of cardiac smad3 and smad7 mRNA in spontaneously hypertensive rat. J Ethnopharmacol. 2011;138:737–40. doi: 10.1016/j.jep.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Liu B, Wu Z, Li Y, Ou C, Huang Z, Zhang J, et al. Puerarin prevents cardiac hypertrophy induced by pressure overload through activation of autophagy. Biochem Biophys Res Commun. 2015;464:908–15. doi: 10.1016/j.bbrc.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 44.Chen G, Pan SQ, Shen C, Pan SF, Zhang XM, He QY, et al. Puerarin inhibits angiotensin II-induced cardiac hypertrophy via the redox-sensitive ERK1/2, p38 and NF-κB pathways. Acta Pharmacol Sin. 2014;35:463–75. doi: 10.1038/aps.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gang C, Qiang C, Xiangli C, Shifen P, Chong S, Lihong L, et al. Puerarin suppresses angiotensin II-induced cardiac hypertrophy by inhibiting NADPH oxidase activation and oxidative stress-triggered AP-1 signaling pathways. J Pharm Pharm Sci. 2015;18:235–48. doi: 10.18433/j3n318. [DOI] [PubMed] [Google Scholar]
- 46.Yuan Y, Zong J, Zhou H, Bian ZY, Deng W, Dai J, et al. Puerarin attenuates pressure overload-induced cardiac hypertrophy. J Cardiol. 2014;63:73–81. doi: 10.1016/j.jjcc.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 47.Evans JL, Goldfine ID. Aging and insulin resistance: Just say iNOS. Diabetes. 2013;62:346–8. doi: 10.2337/db12-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang L, Yao D, Yang H, Wei Y, Peng Y, Ding Y, et al. Puerarin protects pancreatic β-cells in obese diabetic mice via activation of GLP-1R signaling. Mol Endocrinol. 2016;30:361–71. doi: 10.1210/me.2015-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Shangguan Z, Liu Y, Wang J, Li X, Yang S, et al. Puerarin protects pancreatic β-cell survival via PI3K/Akt signaling pathway. J Mol Endocrinol. 2014;53:71–9. doi: 10.1530/JME-13-0302. [DOI] [PubMed] [Google Scholar]
- 50.Zhu X, Xie M, Wang K, Zhang K, Gao Y, Zhu L, et al. The effect of puerarin against IL-1β-mediated leukostasis and apoptosis in retinal capillary endothelial cells (TR-iBRB2) Mol Vis. 2014;20:1815–23. [PMC free article] [PubMed] [Google Scholar]
- 51.Liu M, Liao K, Yu C, Li X, Liu S, Yang S, et al. Puerarin alleviates neuropathic pain by inhibiting neuroinflammation in spinal cord. Mediators Inflamm 2014. 2014:485927. doi: 10.1155/2014/485927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Q, Xiao Y, Gong H, Shen D, Zhu F, Wu Q, et al. Effect of puerarin on the expression of extracellular matrix in rats with streptozotocin-induced diabetic nephropathy. Natl Med J India. 2009;22:9–12. [PubMed] [Google Scholar]
- 53.Shen JG, Yao MF, Chen XC, Feng YF, Ye YH, Tong ZH, et al. Effects of puerarin on receptor for advanced glycation end products in nephridial tissue of streptozotocin-induced diabetic rats. Mol Biol Rep. 2009;36:2229–33. doi: 10.1007/s11033-008-9438-6. [DOI] [PubMed] [Google Scholar]
- 54.Li JH, Wang LM, Li YC, Bi YF, Jiang Y, Mi SQ, et al. Epidemiologic characteristics of dyslipidemia in Chinese adults 2010. Zhonghua Yu Fang Yi Xue Za Zhi. 2012;46:414–8. [PubMed] [Google Scholar]
- 55.Liu H, Li W, Ge X, Jia S, Li B. Coadministration of puerarin (low dose) and zinc attenuates bone loss and suppresses bone marrow adiposity in ovariectomized rats. Life Sci. 2016;166:20–6. doi: 10.1016/j.lfs.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 56.Guo XF, Yang ZR, Wang J, Lei XF, Lv XG, Dong WG, et al. Synergistic antitumor effect of puerarin combined with 5-fluorouracil on gastric carcinoma. Mol Med Rep. 2015;11:2562–8. doi: 10.3892/mmr.2014.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J, Yang ZR, Guo XF, Song J, Zhang JX, Wang J, et al. Synergistic effects of puerarin combined with 5-fluorouracil on esophageal cancer. Mol Med Rep. 2014;10:2535–41. doi: 10.3892/mmr.2014.2539. [DOI] [PubMed] [Google Scholar]
- 58.Zhang WG, Liu XF, Meng KW, Hu SY. Puerarin inhibits growth and induces apoptosis in SMMC-7721 hepatocellular carcinoma cells. Mol Med Rep. 2014;10:2752–8. doi: 10.3892/mmr.2014.2512. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Ma Y, Zheng Y, Song J, Yang X, Bi C, et al. In vitro and in vivo anticancer activity of a novel puerarin nanosuspension against colon cancer, with high efficacy and low toxicity. Int J Pharm. 2013;441:728–35. doi: 10.1016/j.ijpharm.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 60.Hien TT, Kim HG, Han EH, Kang KW, Jeong HG. Molecular mechanism of suppression of MDR1 by puerarin from pueraria lobata via NF-kappaB pathway and cAMP-responsive element transcriptional activity-dependent up-regulation of AMP-activated protein kinase in breast cancer MCF-7/adr cells. Mol Nutr Food Res. 2010;54:918–28. doi: 10.1002/mnfr.200900146. [DOI] [PubMed] [Google Scholar]
- 61.Zhou YX, Zhang H, Peng C. Puerarin: A review of pharmacological effects. Phytother Res. 2014;28:961–75. doi: 10.1002/ptr.5083. [DOI] [PubMed] [Google Scholar]
- 62.Hou SZ, Su ZR, Chen SX, Ye MR, Huang S, Liu L, et al. Role of the interaction between puerarin and the erythrocyte membrane in puerarin-induced hemolysis. Chem Biol Interact. 2011;192:184–92. doi: 10.1016/j.cbi.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 63.Aras AB, Guven M, Akman T, Alacam H, Kalkan Y, Silan C, et al. Genistein exerts neuroprotective effect on focal cerebral ischemia injury in rats. Inflammation. 2015;38:1311–21. doi: 10.1007/s10753-014-0102-0. [DOI] [PubMed] [Google Scholar]
- 64.Wang R, Tu J, Zhang Q, Zhang X, Zhu Y, Ma W, et al. Genistein attenuates ischemic oxidative damage and behavioral deficits via eNOS/Nrf2/HO-1 signaling. Hippocampus. 2013;23:634–47. doi: 10.1002/hipo.22126. [DOI] [PubMed] [Google Scholar]
- 65.Cortina B, Torregrosa G, Castelló-Ruiz M, Burguete MC, Moscardó A, Latorre A, et al. Improvement of the circulatory function partially accounts for the neuroprotective action of the phytoestrogen genistein in experimental ischemic stroke. Eur J Pharmacol. 2013;708:88–94. doi: 10.1016/j.ejphar.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 66.Qin W, Du N, Zhang L, Wu X, Hu Y, Li X, et al. Genistein alleviates pressure overload-induced cardiac dysfunction and interstitial fibrosis in mice. Br J Pharmacol. 2015;172:5559–72. doi: 10.1111/bph.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vera R, Sánchez M, Galisteo M, Villar IC, Jimenez R, Zarzuelo A, et al. Chronic administration of genistein improves endothelial dysfunction in spontaneously hypertensive rats: Involvement of eNOS, caveolin and calmodulin expression and NADPH oxidase activity. Clin Sci (Lond) 2007;112:183–91. doi: 10.1042/CS20060185. [DOI] [PubMed] [Google Scholar]
- 68.Sun L, Zhao T, Ju T, Wang X, Li X, Wang L, et al. A combination of intravenous genistein plus mg2+enhances antihypertensive effects in SHR by endothelial protection and BKCa channel inhibition. Am J Hypertens. 2015;28:1114–20. doi: 10.1093/ajh/hpv005. [DOI] [PubMed] [Google Scholar]
- 69.Yang W, Wang S, Li L, Liang Z, Wang L. Genistein reduces hyperglycemia and islet cell loss in a high-dosage manner in rats with alloxan-induced pancreatic damage. Pancreas. 2011;40:396–402. doi: 10.1097/MPA.0b013e318204e74d. [DOI] [PubMed] [Google Scholar]
- 70.Fu Z, Zhang W, Zhen W, Lum H, Nadler J, Bassaganya-Riera J, et al. Genistein induces pancreatic beta-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology. 2010;151:3026–37. doi: 10.1210/en.2009-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ibrahim AS, El-Shishtawy MM, Peña A, Jr, Liou GI. Genistein attenuates retinal inflammation associated with diabetes by targeting of microglial activation. Mol Vis. 2010;16:2033–42. [PMC free article] [PubMed] [Google Scholar]
- 72.Gupta SK, Dongare S, Mathur R, Mohanty IR, Srivastava S, Mathur S, et al. Genistein ameliorates cardiac inflammation and oxidative stress in streptozotocin-induced diabetic cardiomyopathy in rats. Mol Cell Biochem. 2015;408:63–72. doi: 10.1007/s11010-015-2483-2. [DOI] [PubMed] [Google Scholar]
- 73.Roghani M, Vaez Mahdavi MR, Jalali-Nadoushan MR, Baluchnejadmojarad T, Naderi G, Roghani-Dehkordi F, et al. Chronic administration of daidzein, a soybean isoflavone, improves endothelial dysfunction and attenuates oxidative stress in streptozotocin-induced diabetic rats. Phytother Res. 2013;27:112–7. doi: 10.1002/ptr.4699. [DOI] [PubMed] [Google Scholar]
- 74.Babu PV, Si H, Fu Z, Zhen W, Liu D. Genistein prevents hyperglycemia-induced monocyte adhesion to human aortic endothelial cells through preservation of the cAMP signaling pathway and ameliorates vascular inflammation in obese diabetic mice. J Nutr. 2012;142:724–30. doi: 10.3945/jn.111.152322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tie L, An Y, Han J, Xiao Y, Xiaokaiti Y, Fan S, et al. Genistein accelerates refractory wound healing by suppressing superoxide and FoxO1/iNOS pathway in type 1 diabetes. J Nutr Biochem. 2013;24:88–96. doi: 10.1016/j.jnutbio.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 76.Tang C, Zhang K, Zhao Q, Zhang J. Effects of dietary genistein on plasma and liver lipids, hepatic gene expression, and plasma metabolic profiles of hamsters with diet-induced hyperlipidemia. J Agric Food Chem. 2015;63:7929–36. doi: 10.1021/acs.jafc.5b01590. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y, Li L, You J, Cao J, Fu X. Effect of 7-difluoromethyl-5, 4’-dimethoxygenistein on aorta atherosclerosis in hyperlipidemia apoE(-/-) mice induced by a cholesterol-rich diet. Drug Des Devel Ther. 2013;7:233–42. doi: 10.2147/DDDT.S37512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao H, Li C, Cao JG, Xiang HL, Yang HZ, You JL, et al. 7-difluoromethyl-5,4’-dimethoxygenistein, a novel genistein derivative, has therapeutic effects on atherosclerosis in a rabbit model. J Cardiovasc Pharmacol. 2009;54:412–20. doi: 10.1097/FJC.0b013e3181bad280. [DOI] [PubMed] [Google Scholar]
- 79.Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, et al. OPG and sRANKL serum concentrations in osteopenic, postmenopausal women after 2-year genistein administration. J Bone Miner Res. 2008;23:715–20. doi: 10.1359/jbmr.080201. [DOI] [PubMed] [Google Scholar]
- 80.Roh T, Kim SW, Moon SH, Nam MJ. Genistein induces apoptosis by down-regulating thioredoxin-1 in human hepatocellular carcinoma SNU-449 cells. Food Chem Toxicol. 2016;97:127–34. doi: 10.1016/j.fct.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 81.Qin J, Teng J, Zhu Z, Chen J, Huang WJ. Genistein induces activation of the mitochondrial apoptosis pathway by inhibiting phosphorylation of akt in colorectal cancer cells. Pharm Biol. 2016;54:74–9. doi: 10.3109/13880209.2015.1014921. [DOI] [PubMed] [Google Scholar]
- 82.Du Q, Wang Y, Liu C, Wang H, Fan H, Li Y, et al. Chemopreventive activity of GEN-27, a genistein derivative, in colitis-associated cancer is mediated by p65-CDX2-β-catenin axis. Oncotarget. 2016;7:17870–84. doi: 10.18632/oncotarget.7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fang Y, Zhang Q, Wang X, Yang X, Wang X, Huang Z, et al. Quantitative phosphoproteomics reveals genistein as a modulator of cell cycle and DNA damage response pathways in triple-negative breast cancer cells. Int J Oncol. 2016;48:1016–28. doi: 10.3892/ijo.2016.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu TC, Lin YC, Chen HL, Huang PR, Liu SY, Yeh SL, et al. The enhancing effect of genistein on apoptosis induced by trichostatin A in lung cancer cells with wild type p53 genes is associated with upregulation of histone acetyltransferase. Toxicol Appl Pharmacol. 2016;292:94–102. doi: 10.1016/j.taap.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 85.Xie J, Wang J, Zhu B. Genistein inhibits the proliferation of human multiple myeloma cells through suppression of nuclear factor-κB and upregulation of microRNA-29b. Mol Med Rep. 2016;13:1627–32. doi: 10.3892/mmr.2015.4740. [DOI] [PubMed] [Google Scholar]
- 86.Yeh CC, Fan Y, Jiang L, Yang YL, He B, You L, et al. Genistein suppresses growth of human uterine sarcoma cell lines via multiple mechanisms. Anticancer Res. 2015;35:3167–73. [PubMed] [Google Scholar]
- 87.Mahmoud AM, Al-Alem U, Ali MM, Bosland MC. Genistein increases estrogen receptor beta expression in prostate cancer via reducing its promoter methylation. J Steroid Biochem Mol Biol. 2015;152:62–75. doi: 10.1016/j.jsbmb.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karsli-Ceppioglu S, Ngollo M, Adjakly M, Dagdemir A, Judes G, Lebert A, et al. Genome-wide DNA methylation modified by soy phytoestrogens: Role for epigenetic therapeutics in prostate cancer? OMICS. 2015;19:209–19. doi: 10.1089/omi.2014.0142. [DOI] [PubMed] [Google Scholar]
- 89.de la Parra C, Castillo-Pichardo L, Cruz-Collazo A, Cubano L, Redis R, Calin GA, et al. Soy isoflavone genistein-mediated downregulation of miR-155 contributes to the anticancer effects of genistein. Nutr Cancer. 2016;68:154–64. doi: 10.1080/01635581.2016.1115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cao X, Ren K, Song Z, Li D, Quan M, Zheng Y, et al. 7-difluoromethoxyl-5,4’-di-n-octyl genistein inhibits the stem-like characteristics of gastric cancer stem-like cells and reverses the phenotype of epithelial-mesenchymal transition in gastric cancer cells. Oncol Rep. 2016;36:1157–65. doi: 10.3892/or.2016.4848. [DOI] [PubMed] [Google Scholar]
- 91.Sekar V, Anandasadagopan SK, Ganapasam S. Genistein regulates tumor microenvironment and exhibits anticancer effect in dimethyl hydrazine-induced experimental colon carcinogenesis. Biofactors. 2016;42:623–37. doi: 10.1002/biof.1298. [DOI] [PubMed] [Google Scholar]
- 92.Kim YS, Choi KC, Hwang KA. Genistein suppressed epithelial-mesenchymal transition and migration efficacies of BG-1 ovarian cancer cells activated by estrogenic chemicals via estrogen receptor pathway and downregulation of TGF-β signaling pathway. Phytomedicine. 2015;22:993–9. doi: 10.1016/j.phymed.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 93.Kim E, Woo MS, Qin L, Ma T, Beltran CD, Bao Y, et al. Daidzein augments cholesterol homeostasis via apoE to promote functional recovery in chronic stroke. J Neurosci. 2015;35:15113–26. doi: 10.1523/JNEUROSCI.2890-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park MH, Ju JW, Park MJ, Han JS. Daidzein inhibits carbohydrate digestive enzymes in vitro and alleviates postprandial hyperglycemia in diabetic mice. Eur J Pharmacol. 2013;712:48–52. doi: 10.1016/j.ejphar.2013.04.047. [DOI] [PubMed] [Google Scholar]
- 95.Cheong SH, Furuhashi K, Ito K, Nagaoka M, Yonezawa T, Miura Y, et al. Daidzein promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and improves glucose homeostasis in type 2 diabetic model mice. J Nutr Biochem. 2014;25:136–43. doi: 10.1016/j.jnutbio.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 96.Qin Y, Shu F, Zeng Y, Meng X, Wang B, Diao L, et al. Daidzein supplementation decreases serum triglyceride and uric acid concentrations in hypercholesterolemic adults with the effect on triglycerides being greater in those with the GA compared with the GG genotype of ESR-β rsaI. J Nutr. 2014;144:49–54. doi: 10.3945/jn.113.182725. [DOI] [PubMed] [Google Scholar]
- 97.Guo Y, Wu G, Su X, Yang H, Zhang J. Antiobesity action of a daidzein derivative on male obese mice induced by a high-fat diet. Nutr Res. 2009;29:656–63. doi: 10.1016/j.nutres.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 98.Liu J, Viswanadhapalli S, Garcia L, Zhou M, Nair BC, Kost E, et al. Therapeutic utility of natural estrogen receptor beta agonists on ovarian cancer. Oncotarget. 2017;8:50002–14. doi: 10.18632/oncotarget.18442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chan KK, Siu MK, Jiang YX, Wang JJ, Leung TH, Ngan HY, et al. Estrogen receptor modulators genistein, daidzein and ERB-041 inhibit cell migration, invasion, proliferation and sphere formation via modulation of FAK and PI3K/AKT signaling in ovarian cancer. Cancer Cell Int. 2018;18:65. doi: 10.1186/s12935-018-0559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hua F, Li CH, Chen XG, Liu XP. Daidzein exerts anticancer activity towards SKOV3 human ovarian cancer cells by inducing apoptosis and cell cycle arrest, and inhibiting the raf/MEK/ERK cascade. Int J Mol Med. 2018;41:3485–92. doi: 10.3892/ijmm.2018.3531. [DOI] [PubMed] [Google Scholar]
- 101.Kaushik S, Shyam H, Sharma R, Balapure AK. Dietary isoflavone daidzein synergizes centchroman action via induction of apoptosis and inhibition of PI3K/Akt pathway in MCF-7/MDA MB-231 human breast cancer cells. Phytomedicine. 2018;40:116–24. doi: 10.1016/j.phymed.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 102.Rigalli JP, Scholz PN, Tocchetti GN, Ruiz ML, Weiss J. The phytoestrogens daidzein and equol inhibit the drug transporter BCRP/ABCG2 in breast cancer cells: Potential chemosensitizing effect. Eur J Nutr. 2017 doi: 10.1007/s00394-017-1578-9. doi: 10.1007/s00394-017-1578-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 103.Poschner S, Maier-Salamon A, Zehl M, Wackerlig J, Dobusch D, Pachmann B, et al. The impacts of genistein and daidzein on estrogen conjugations in human breast cancer cells: A targeted metabolomics approach. Front Pharmacol. 2017;8:699. doi: 10.3389/fphar.2017.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bao C, Namgung H, Lee J, Park HC, Ko J, Moon H, et al. Daidzein suppresses tumor necrosis factor-α induced migration and invasion by inhibiting hedgehog/Gli1 signaling in human breast cancer cells. J Agric Food Chem. 2014;62:3759–67. doi: 10.1021/jf500231t. [DOI] [PubMed] [Google Scholar]
- 105.Park HJ, Jeon YK, You DH, Nam MJ. Daidzein causes cytochrome c-mediated apoptosis via the bcl-2 family in human hepatic cancer cells. Food Chem Toxicol. 2013;60:542–9. doi: 10.1016/j.fct.2013.08.022. [DOI] [PubMed] [Google Scholar]


