Abstract
Background:
Whether intraoperative use of hydroxyethyl starch (HES) solutions is associated with postoperative acute kidney injury (AKI) continues to be researched. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is validated for early detection of AKI. Previous studies are limited and use empirically predefined volumes of HES solutions with serum creatinine as marker for AKI.
Materials and Methods:
Adults scheduled for orthopedic surgery under general anesthesia with >200–300 mL blood loss expected were included; 40 were randomized to receive 6% HES 130/0.4 (tetrastarch) (group HES) or Ringer's lactate (group RL) boluses when stroke volume variation (SVV) >10% in supine or lateral position, or >14% in prone position. Incidence of early postoperative AKI using urinary NGAL (>100 ng/mL) was the primary outcome, and using derangement of serum creatinine was the secondary measure.
Results:
In 38 patients, intervention was completed, and incidence of AKI (postoperative urinary NGAL >100 ng/mL) among them was 0% in both groups. Patients with urinary NGAL >50 ng/mL were insignificantly higher for group RL versus group HES (6/19 vs. 4/19) (P = 0.461), as were those with incidence of AKI as per creatinine values (5/19 vs. 4/19) (P = 1.000). Group RL had significantly higher requirement of fluid (1211 ± 758 mL vs. 689 ± 394 mL) (P = 0.013) and lower cardiac index (P < 0.05) versus group HES.
Conclusion:
SVV-guided tetrastarch and Ringer's lactate do not result in postoperative AKI diagnosed by urinary NGAL >100 ng/mL; however, an insignificant trend for better renal functions as well as significantly more efficacious volume expansion and hemodynamic stability were seen with tetrastarch instead.
Keywords: Acute kidney injury, hydroxyethyl starch, Ringer's lactate, tetrastarch
Introduction
Hydroxyethyl starch (HES) solutions are effective for management of intravascular volume in surgical and critical care settings. Their use in critically ill patients is no longer recommended due to increased incidences of adverse renal effects such as acute kidney injury (AKI) and need of renal replacement therapy, as well as raised mortality.[1,2,3,4] Safety of HES solutions in surgical settings may differ because of lack of capillary leakage and organ dysfunctions that are frequently seen in critically ill patients. This leads to differing pharmacokinetics in surgical patients when compared with those who are critically ill.[5,6] The debate regarding safety of HES solutions in surgical patients nevertheless exists.
To enhance safety of HES solutions in surgical patients, it is recommended to use newer generation products in least volumes. One of the recent newer generation HES solutions is tetrastarch (6% HES 130/0.4). Goal-directed therapy using various predictors of fluid responsiveness is a modality that helps use lowered effective volumes of intravenous fluids. Of the several predictors used for goal-directed therapy, stroke volume variation (SVV) is a common and successful one.[7,8,9]
The existing literature presents only limited data evaluating renal safety of HES solutions in surgical settings and is heterogeneous with respect to the fluid regimens used. These trials have mostly used predetermined fixed volumes of HES solution rather than goal-directed therapy. Some have even used the colloid solutions as first-line fluid therapy beginning from the initiation of anesthesia itself.[10,11] It is neither likely in clinical practice nor recommended that HES solutions including tetrastarch be used as a first-line intravenous fluid therapy in the absence of hypovolemia.
In addition, most of the earlier trials evaluating effect of HES solutions on AKI have used serum creatinine and glomerular filtration rate for diagnosing the renal dysfunction. One of the greatest drawbacks of serum creatinine for diagnosing AKI is its late and non-specific increase following renal injury or dysfunction.[12] Urinary neutrophil gelatinase-associated lipocalin (NGAL) is a biomarker validated for detecting AKI early on because it is released within 6 h of renal injury.[13,14] There is scanty literature evaluating postoperative AKI with urinary NGAL as the diagnostic criteria, following intraoperative use of HES solutions.[10,11,15]
Against this background, this study compared postoperative AKI diagnosed using urinary NGAL, following the intraoperative use of SVV-guided tetrastarch (6% HES 130/0.4) or Ringer's lactate in patients scheduled for orthopedic surgery with anticipated blood loss.
Materials and Method
This prospective randomized double-blinded trial was undertaken after Institutional Ethics Committee's approval (in meeting held on 20.10.2015) and informed written consent from all participating patients. It is retrospectively registered with Clinical Trial Registry of India (CTRI/2016/07/007114). It was conducted from November 2015 to April 2017.
Adult patients scheduled for major orthopedic surgery under general anesthesia with anticipated blood loss >200–300 mL were included. Those <18 years or >65 years, or with body mass index <15 or >40 kg/m2, preoperative renal dysfunction, history of receiving renal replacement therapy, transplanted kidney, significant dysrhythmia, cardiac failure, stroke, coronary heart disease, or preoperative hemoglobin <8 g% were excluded.
The primary objective was to evaluate and compare incidence of early postoperative AKI using urinary NGAL as a marker, following intraoperative SVV-guided tetrastarch or Ringer's lactate therapy. The secondary objective was to compare between the two fluid intervention therapies the incidence of early postoperative AKI as per Kidney Disease Improving Global Outcomes (KDIGO) guidelines using serum creatinine and/or urine output as diagnostic criteria.[12]
A computer-generated random number table was used for randomizing patients to one of two groups according to intervention fluid received. Patients were unaware of group allocation, as was the microbiologist who assayed the urine samples for NGAL, making this a double-blinded trial.
In the operating room, monitoring including oscillometric non-invasive blood pressure, lead II electrocardiography, pulse oximetry, and capnography were instituted. An intravenous line was established and infusion of Ringer's lactate (5 mL/kg) before induction was given. Following morphine 0.1–0.15 mg/kg i.v., radial artery cannulation was done with a 20-G arterial cannula. A dedicated transducer (Flo Trac™; Edwards Lifesciences, Dominican Republic) was connected to the radial arterial line on one side and to Vigileo™ system (Edwards Lifesciences LLC, USA) on the other. This system enables the continuous monitoring of stroke volume and cardiac output by pulse contour analysis, without requirement of external calibration. The SVV was calculated automatically by the monitoring system as the variation in beat-to-beat stroke volume from the mean value during the most recent 20 s data: SVV = (SVmax− SVmin)/SVmean
Following establishment of optimal arterial waveform, anesthesia was induced with propofol (1–2.5 mg/kg i.v.), while vecuronium (0.1 mg/kg i.v.) was used to facilitate endotracheal intubation. Mechanical ventilation was initiated with tidal volume of 8 mL/kg ideal body weight and respiratory rate of 10/min. Respiratory rate was titrated to maintain end tidal carbon dioxide level of 35–40 mmHg. Anesthesia was maintained using isoflurane at minimum alveolar concentration of 1 ± 0.1. Inspired oxygen concentration was initiated at 0.3 and adjusted intraoperatively to maintain SpO2 ≥95%. At the end of surgery, neuromuscular blockade was reversed with glycopyrrolate (0.01 mg/kg i.v.) and neostigmine (0.05 mg/kg i.v.).
Fluid regimen
Patients of group HES received tetrastarch (6% HES 130/0.4) (Voluven®; Fresenius Kabi, Pune, India) and group RL, Ringer's lactate (Compound Sodium Lactate Injection IP; Abaris Healthcare, Mehsana, India) in volumes titrated by SVV. Whenever the SVV was >10% in supine or lateral position, or >14% in prone position, a bolus of 100 mL of the intervention fluid was infused over 2–4 min.[16,17] Fluid boluses were repeated every 5 min till SVV criteria were met. In case of failure of any response to bolus fluid, the wait period of 5 min was not adhered to. Unless accompanied by hemodynamic instability, SVV variations were treated only if sustained for at least a couple of minutes.
In addition, all patients were given an infusion of Ringer's lactate at a maintenance rate of 2 mL/kg/h throughout surgery using infusion pump (OT-701, JMS Co. Ltd., Japan).
Blood was transfused if loss exceeded the calculated “maximum allowable blood loss” for a target hematocrit of 24%. Mephenteramine boluses of 6 mg (i.v.) were used if fluid boluses failed to maintain a mean arterial pressure of >65 mmHg. These were recorded as hypotensive events.
Urine samples were collected for NGAL determination just before surgery, 2–3 h after surgery, and on first postoperative day (aimed at approximately 18 h after surgery). These were centrifuged and frozen at −80°C till assayed using Enzyme Linked Immunosorbent Assay (ELISA) kit (NGAL ELISA, BioVendor, Czech Republic) as per the manufacturer's instructions. The kit had a minimum detection level of 0.02 ng/mL. As previously documented in similar studies, urinary NGAL >100 ng/mL in either of the two postoperative samples taken 2–3 h or on first postoperative day was considered diagnostic of postoperative AKI.[10,11]
Early postoperative AKI (i.e. up to 48 h postoperatively) was also evaluated as per KDIGO criteria. For this, baseline serum creatinine value before surgery was noted and it was repeated on first 2 postoperative days. The KDIGO guidelines define AKI as an increase in serum creatinine ≥0.3 m% within 48 h or an increase to ≥1.5 times baseline which is known or presumed to have occurred within the prior 7 days, or urine output of <0.5 mL/kg/h for 6 h. The severity is graded as per the level of serum creatinine or urine output.[12]
We had planned that urine output would be considered for diagnosis of postoperative AKI in addition to serum creatinine only if estimates of hourly collection would be available in at least 32 patients for the first 2 postoperative days. Postoperative care in wards did not entail hourly urine output estimates. Thus, we have taken into consideration only serum creatinine, without urine output, for diagnosis of early postoperative AKI.[12]
Ancillary observations included incidence of late AKI (evaluated by increase in serum creatinine of ≥50% above preoperative value on the day of patient's discharge from hospital or 16th postoperative day, whichever was earlier). In addition, the volume of intervention fluid and Ringer's lactate used in both groups as background infusion and compensatory volume expansion; intraoperative blood transfusion, blood loss, and urine output; preoperative hemoglobin; demographic parameters; duration of surgery; and postoperative duration of hospitalization were noted. Intraoperative heart rate and cardiac index were recorded repeatedly: before induction of anesthesia (baseline), after intubation and initiation of mechanical ventilation, as well as change in position if any, followed by every 10 min intervals till the end of surgery. SVV was also recorded at all the above time points but not before induction of anesthesia because mechanical ventilation is an essential prerequisite for its measurement. The baseline for SVV accordingly was the value just after intubation and initiation of mechanical ventilation.
Statistical analysis
Intergroup comparison was done using t-test for normally distributed quantitative data, Mann–Whitney U-test for non-normally distributed data, and Pearson's Chi-square or Fisher's test for qualitative parameters. For intergroup comparison of normally distributed repeated measures (cardiac index and SVV), general linear model of analysis of variance was used. For non-normally distributed data (i.e., urinary NGAL) Wilcoxon signed-rank test was used for intragroup comparison. Association of NGAL with early AKI was also evaluated using receiver operating characteristics (ROC) analysis. Because minimum detectable levels of NGAL were 0.02 ng/mL, all patients with undetectable values were assumed to have 0.019 ng/mL concentration for purposes of analysis. For statistical analysis of hemodynamic parameters, the readings were truncated beyond 2 h because after this time there was significant attrition of data due to completion of surgery in 7 of 19 (37%) and 5 of 19 (26%) patients of group RL and group HES, respectively. P < 0.05 was considered statistically significant. SPSS version 23.0 (IBM Corp) was used. Sample size: The standard deviation of urinary NGAL has been estimated to be 100 ng/mL.[10] At a significance level of 5% and a power of 80%, 32 patients are required to detect a 100% difference, that is, 100 ng/mL in urinary NGAL.[10,11] Thus, we enrolled a total of 40 patients, 20 in each group to accommodate possible dropouts.
Results
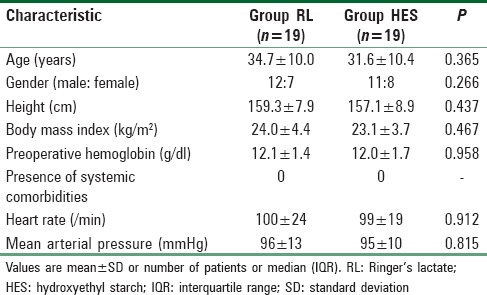
The final patient enrollment and inclusion are depicted in the CONSORT flowchart [Figure 1]. Preoperative patient characteristics were statistically similar between group RL and group HES (P > 0.05) [Table 1].
Figure 1.
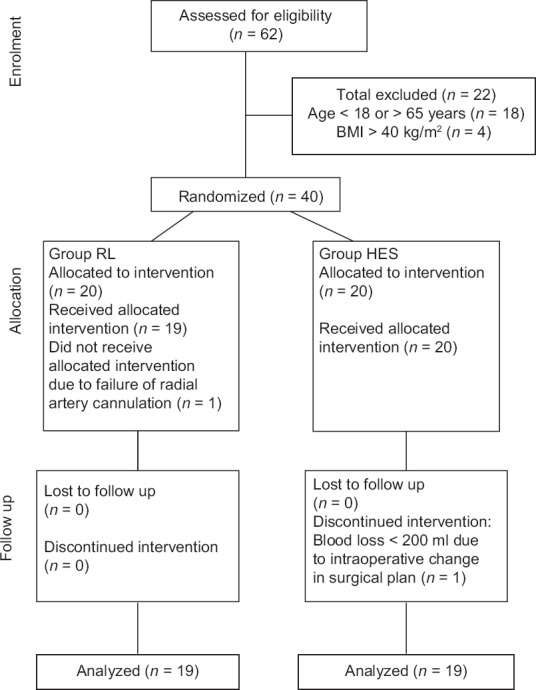
CONSORT flow diagram
Table 1.
Comparison of preoperative patient characteristics
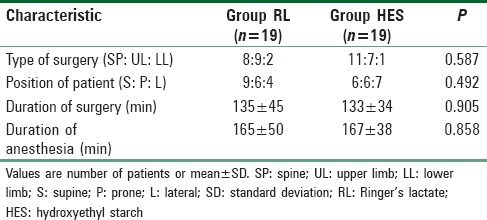
Distribution of nature of orthopedic procedure and proportion of patients undergoing surgery in supine, prone, or lateral position were statistically similar between both groups (P = 0.587 and 0.492, respectively) [Table 2]. The duration of surgery and anesthesia was also similar between both groups (P > 0.05) [Table 2].
Table 2.
Surgical details
None of the patients in either group had urinary NGAL levels >100 ng/mL. Urinary NGAL levels before and 2–3 h after surgery, as well as on first postoperative day were statistically similar between both groups (P > 0.05) [Table 3].
Table 3.
Comparison of urinary neutrophil gelatinase-associated lipocalin levels
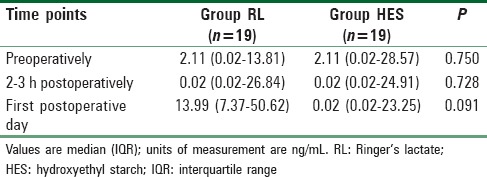
In group RL a significant increase in urinary NGAL was seen on the first postoperative day when compared with baseline (P = 0.027) [Table 3]. No such increase was seen in group HES postoperatively (P = 0.695) [Table 3].
The number of patients with postoperative urinary NGAL >50 ng/mL was clinically higher but statistically similar for group RL when compared with group HES (32% vs. 21%) (P = 0.461). The number of those with levels >25 ng/mL was also clinically greater but statistically similar for group RL when compared with group HES (47% vs. 32%) (P = 0.319).
Area under ROC curve for AKI versus NGAL assessed 2–3 h after surgery was 0.437 [95% confidence interval (CI): 0.229–0.644] (P = 0.571), and for NGAL measured on first postoperative day it was 0.511 (95% CI: 0.237–0.750) (P = 0.918).
The mean time of collection of urinary samples on the first postoperative day was 18.9 ± 1.5 h for group RL and 19.0 ± 1.8 for group HES (P > 0.770).
Postoperative acute kidney injury as per serum creatinine
The percentage of patients who developed early postoperative AKI as per derangement in serum creatinine was clinically higher though statistically similar, for group RL when compared with group HES (5/19 = 26% vs. 4/19 = 21%, respectively; P = 1.000).
The distribution of patients according to severity of AKI was statistically similar between both groups (P = 1.000). Of all patients with AKI, eight of nine had grade 1 of severity and the only patient with increased severity grade 2 (one of nine) belonged to group RL. The number of patients with late AKI was clinically greater for group RL when compared with group HES (four vs. one).
Hemodynamic stability
The SVV was lower in group HES signifying better maintained fluid balance when compared with group RL, and the comparison reached statistical significance at 40, 50, and 60 min after change in position of the patient (P < 0.05) [Figure 2].
Figure 2.
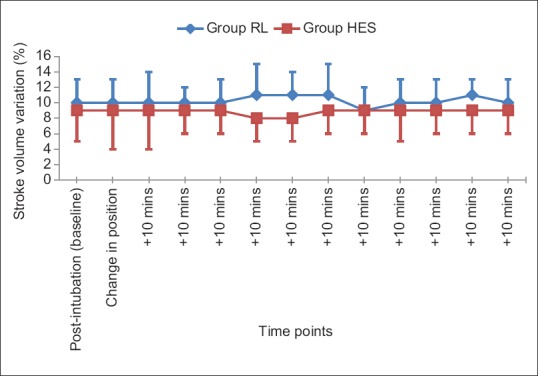
Comparison of trend of stroke volume variation
Intraoperative cardiac index was significantly higher for group HES when compared with group RL (P < 0.05) [Figure 3]. The heart rate was statistically similar between both groups at all observed time points. None of the patients in either group required vasopressor to maintain blood pressure.
Figure 3.
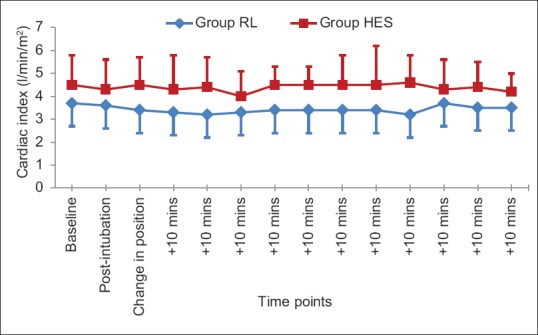
Comparison of trend of intraoperative cardiac index
Ancillary observations
The volume of intervention fluid was significantly higher for group RL when compared with group HES (1211 ± 758 vs. 689 ± 394 mL; P = 0.013). Intraoperative hourly urine output was slightly higher, though statistically similar, for group RL when compared with group HES [120 (111–169) vs. 106 (86–145) mL/h; P = 0.102]. The total volume of Ringer's lactate used for baseline infusion and compensatory volume expansion was statistically similar between both groups (group RL: 480 ± 200 mL vs. group HES: 475 ± 189 mL) (P = 0.936). Intraoperative blood loss [700 (450–950) vs. 700 (560–1150) mL; P = 0.761] and units of blood transfused [1 (1–1.25) vs. 1 (1–1.5); P = 0.892] were statistically similar between group RL and group HES. The duration of postoperative hospitalization was statistically similar between group RL and group HES (8 ± 4 and 9 ± 4 days, respectively) (P = 0.393).
Discussion
The issue of renal safety with HES solutions used intraoperatively continues to excite debate.[6] This trial was conducted in major orthopedic surgery to evaluate postoperative AKI using urinary NGAL level, following the use of intraoperative SVV-guided tetrastarch (6%, 130/0.4 Voluven) versus Ringer's lactate.
The salient observations of our study were that none of the patients developed postoperative urinary NGAL >100 ng/mL, while incidence of AKI diagnosed by serum creatinine was clinically lesser with tetrastarch when compared with Ringer's lactate, though statistically similar (21% vs. 26%). Efficacy for volume expansion and hemodynamic stability was significantly better maintained with tetrastarch when compared with Ringer's lactate.
AKI is common in perioperative period and carries a significant morbidity and mortality,[12] yet it is not very frequently addressed. Incidence of AKI in hospitalized patients ranges from 2% to 18%, while postoperative AKI is seen in 1%–31% of surgical patients.[18,19,20]
Our observation of lack of postoperative AKI with tetrastarch based on urinary NGAL >100 ng/mL is similar to the two earlier studies comparing tetrastarch with normal saline intraoperatively.[10,11]
The unanticipated finding of our study is the clinical trend, although insignificant, toward higher propensity for postoperative renal dysfunction with Ringer's lactate rather than tetrastarch. Ringer's lactate was associated with a significant increase in postoperative urinary NGAL, a change not evidenced with tetrastarch. In addition, the number of patients with postoperative AKI diagnosed using serum creatinine as per KDIGO criteria, those with urinary NGAL >50 ng/mL, and with “late” AKI were clinically greater following Ringer's lactate when compared with tetrastarch. In addition, while all patients who developed AKI had severity of grade 1, the only patient with grade 2 had received Ringer's lactate. To conclusively prove or disprove such an association of worse postoperative renal functions with Ringer's lactate when compared with tetrastarch, an adequately sized future trial will have to include 876 patients per group at power of 80% and alpha error of 5% based on our reported incidence of 26% and 21% of AKI with the two fluids, respectively.
This surprising clinical trend of worse renal functions following intraoperative Ringer's lactate is in contrast to earlier publications.[10,11,15] We hypothesize it to be a result of the SVV goal-directed therapy rather than use of predetermined, fixed volumes of the fluids in previous studies.[10,11,15] SVV-guided therapy had led to more efficacious volume expansion with tetrastarch as indicated by the higher cardiac index and lower SVV, despite requirement of smaller volumes. This could have contributed to trend of better renal functions and lesser postoperative AKI with tetrastarch. Because current recommendations encourage the use of small volumes of newer starch solutions during intraoperative period,[5] use of SVV is highly relevant to intraoperative use of HES solutions.
We assessed urinary NGAL early at 2–3 h after surgery and then approximately 18 h postoperatively keeping in mind its earlier documented temporal trend. Almost all earlier studies evaluating urinary NGAL in postoperative AKI document its utility for discriminating patients with AKI when assessed early after surgery (30 min to 4 h),[10,11,15] and for up to 18 h.[21,22] At 24 h postoperatively, the discrimination was seen to be lost.[21,22]
Although we used a cut-off value of urinary NGAL >100 ng/mL to diagnose AKI, the ideal level could be debated about. Previous studies evaluating urinary NGAL as a diagnostic marker for postoperative AKI have demonstrated variable cut-offs.[21,22] Using ROC analyses, cut-offs varying from 25 to 250 ng/mL in different kinds of surgery at various postoperative times ranging from 2 to 18 h have been advocated. Although we had not planned originally to evaluate urinary NGAL as a predictor for AKI because its utility is well-established, our observations depicted otherwise. Hence, we also assessed the association between urinary NGAL and AKI and it was noted to be insignifiacnt in this group of patients. NGAL is perhaps the most widely evaluated marker for AKI.[23] It is a small protein normally secreted in very small amounts by various body cells. Under conditions of renal tubular stress, it is upregulated and secreted in increased amounts in primarily the thick ascending limb and collecting duct of the nephron. It has shown to be a good predictor in pediatric and cardiac surgical patients, as well as in critically ill and emergency room setting.[23] However, it is documented that NGAL elevation is witnessed with intrinsic renal dysfunction and not rapidly reversible volume-dependent derangement.[24] In addition, it depends on the duration of azotemia. The lack of raised NGAL even in the presence of deranged serum creatinine or urine output with prerenal azotemia may explain the results of our study. Notwithstanding the apparent success of urinary NGAL to predict AKI in certain subsets of patients, its utility in non-cardiac surgical patients may thus need further validation.
Based on our observations, we conclude that neither tetrastarch nor Ringer's lactate results in early postoperative AKI, when using urinary NGAL level of >100 ng/mL as a cut-off to diagnose postoperative AKI. There is, however, an unexpected insignificant trend of better postoperative renal function associated with tetrastarch when compared with Ringer's lactate. This may be a result of more efficacious fluid optimization seen with tetrastarch when compared with Ringer's lactate when using SVV-guided intraoperative fluid therapy.
We thus recommend that using SVV-guided tetrastarch may be preferred to Ringer's lactate in patients undergoing major orthopedic surgery under general anesthesia, due to the significantly better intravenous expansion efficacy (lesser volumes required), higher cardiac index, and an insignificant trend toward better postoperative renal function.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Patel A, Waheed U, Brett SJ. Randomised trials of 6% tetrastarch (hydroxyethyl starch 130/0.4 or 0.42) for severe sepsis reporting mortality: Systematic review and meta-analysis. Intensive Care Med. 2013;39:811–22. doi: 10.1007/s00134-013-2863-6. [DOI] [PubMed] [Google Scholar]
- 2.Zarychanski R, Abou-Setta AM, Turgeon AF, Houston BL, McIntyre L, Marshall JC, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: A systematic review and meta-analysis. JAMA. 2013;309:678–88. doi: 10.1001/jama.2013.430. [DOI] [PubMed] [Google Scholar]
- 3.Gattas DJ, Dan A, Myburgh J, Billot L, Lo S, Finfer S, et al. Fluid resuscitation with 6% hydroxyethyl starch (130/0.4 and 130/0.42) in acutely ill patients: Systematic review of effects on mortality and treatment with renal replacement therapy. Intensive Care Med. 2013;39:558–68. doi: 10.1007/s00134-013-2840-0. [DOI] [PubMed] [Google Scholar]
- 4.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 5.Ghijselings I, Rex S. Hydroxyethyl starches in the perioperative period. A review on the efficacy and safety of starch solutions. Acta Anaesthesiol Belg. 2014;65:9–22. [PubMed] [Google Scholar]
- 6.Heßler M, Arnemann PH, Ertmer C. To use or not to use hydroxyethyl starch in intraoperative care: Are we ready to answer the ‘Gretchen question’? Curr Opin Anaesthesiol. 2015;28:370–7. doi: 10.1097/ACO.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder B, Barbeito A, Bar-Yosef S, Mark JB. Cardiovascular monitoring. In: Miller RD, Cohen NH, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Young WL, editors. Miller's Anesthesia. 8th ed. Canada: Elsevier; 2015. [Google Scholar]
- 8.Marx G, Schindler AW, Mosch C, Albers J, Bauer M, Gnass I, et al. Intravascular volume therapy in adults: Guidelines from the Association of the Scientific Medical Societies in Germany. Eur J Anaesthesiol. 2016;33:488–521. doi: 10.1097/EJA.0000000000000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng K, Li J, Cheng H, Ji FH. Goal-directed fluid therapy based on stroke volume variations improves fluid management and gastrointestinal perfusion in patients undergoing major orthopedic surgery. Med Princ Pract. 2014;23:413–20. doi: 10.1159/000363573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kancir AS, Johansen JK, Ekeloef NP, Pedersen EB. The effect of 6% hydroxyethyl starch 130/0.4 on renal function, arterial blood pressure, and vasoactive hormones during radical prostatectomy: A randomized controlled trial. Anesth Analg. 2015;120:608–18. doi: 10.1213/ANE.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 11.Kancir AS, Pleckaitiene L, Hansen TB, Ekeløf NP, Pedersen EB. Lack of nephrotoxicity by 6% hydroxyethyl starch 130/0.4 during hip arthroplasty: A randomized controlled trial. Anesthesiology. 2014;121:948–58. doi: 10.1097/ALN.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2:1–138. [Google Scholar]
- 13.Haase M, Story DA, Haase-Fielitz A. Renal injury in the elderly: Diagnosis, biomarkers and prevention. Best Pract Res Clin Anaesthesiol. 2011;25:401–12. doi: 10.1016/j.bpa.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Singer E, Markó L, Paragas N, Barasch J, Dragun D, Müller DN, et al. Neutrophil gelatinase-associated lipocalin: Pathophysiology and clinical applications. Acta Physiol (Oxf) 2013;207:663–72. doi: 10.1111/apha.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Yu Y, Jia J, Yu W, Xu R, Geng L, et al. Administration of HES in elderly patients undergoing hip arthroplasty under spinal anesthesia is not associated with an increase in renal injury. BMC Anesthesiol. 2017;17:29. doi: 10.1186/s12871-017-0320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manecke GR. Edwards floTrac sensor and vigileo monitor: Easy, accurate, reliable cardiac output assessment using the arterial pulse wave. Expert Rev Med Devices. 2005;2:523–7. doi: 10.1586/17434440.2.5.523. [DOI] [PubMed] [Google Scholar]
- 17.Biais M, Bernard O, Ha JC, Degryse C, Sztark F. Abilities of pulse pressure variations and stroke volume variations to predict fluid responsiveness in prone position during scoliosis surgery. Br J Anaesth. 2010;104:407–13. doi: 10.1093/bja/aeq031. [DOI] [PubMed] [Google Scholar]
- 18.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–66. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 19.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–6. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 20.Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, et al. Acute kidney injury: An increasing global concern. Lancet. 2013;382:170–9. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 21.Wagener G, Jan M, Kim M, Mori K, Barasch JM, Sladen RN, et al. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006;105:485–91. doi: 10.1097/00000542-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Wagener G, Minhaz M, Mattis FA, Kim M, Emond JC, Lee HT, et al. Urinary neutrophil gelatinase-associated lipocalin as a marker of acute kidney injury after orthotopic liver transplantation. Nephrol Dial Transplant. 2011;26:1717–23. doi: 10.1093/ndt/gfq770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schrezenmeier EV, Barasch J, Budde K, Westhoff T, Schmidt-Ott KM. Biomarkers in acute kidney injury – Pathophysiological basis and clinical performance. Acta Physiol (Oxf) 2017;219:554–72. doi: 10.1111/apha.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singer E, Elger A, Elitok S, Kettritz R, Nickolas TL, Barasch J, et al. Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney Int. 2011;80:405–14. doi: 10.1038/ki.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]


