Abstract
Background
Chronic urticaria (CU) is a common disease, characterized by the development of wheals, angioedema, or both. CU reduces quality of life and can also cause emotional distress. Studies addressing depression and anxiety in such patients are rare in the literature. The aim of this study was to determine the relationship between urticaria symptoms and depression and anxiety in patients with CU.
Material/Methods
The Hospital Anxiety-Depression Scale (HADS) was used to evaluate depression and anxiety in patients with CU. We included 50 patients with CU and a control group of 60 healthy volunteers. Urticaria activity score, medications, age, sex, comorbidities, occupation, and income of patients were recorded. Depression and anxiety scores were evaluated between the patient and the healthy groups.
Results
The HADS questionnaire showed that 24 (48%) subjects in the patient group had depressive symptoms and 24 (48%) had anxiety, and both of these conditions were significantly more frequent than in controls (p=0.002 and p=0.001). The mean anxiety and depression scores ±SD were 10.82±4.29 and 7.74±4.49 in the patient group and 6.42±3.02 and 4.85±3.26, in the control group respectively (p=0.001). The mean score of the UAS ±SD was 23.14±13.40 and a significant positive correlation between UAS and the anxiety and depression scores was observed (r=0.400; p=0.004 and r=0.373; p=0.004, respectively).
Conclusions
Our data demonstrated that depression and anxiety symptoms are more common in patients with CU than in the control group. Therefore, we should pay attention to the potential of mental comorbidities while managing patients with CU.
MeSH Keywords: Anxiety, Depression, Urticaria
Background
Urticaria is a disease characterized by the development of wheals, which usually disappear in 1–24 h. The clinical appearance of wheals is characterized by the sudden appearance of skin lesions and a central swelling (of variable size), which is associated with an itching or occasionally a burning sensation. Urticaria may occur with angioedema, the resolution of which can take up to 72 h and sometimes causes pain rather than an itching sensation [1]. Chronic urticaria (CU) is defined as the persistence of these skin lesions for more than 6 weeks [1]. The prevalence of CU in the general population has been estimated to range from 0.5% to 5% and has consequently been viewed as an important problem [2]. CU is classified into subtypes for clinical use as spontaneous (no specific eliciting factor involved) or inducible (specific eliciting factor involved) urticaria. Symptomatic dermographism, cold urticaria, delayed-pressure urticaria, solar urticaria, heat urticaria, vibratory angioedema, clinical urticaria, contact urticaria, and aquagenic urticaria are classified as chronic inducible urticaria [3].
Many studies have demonstrated that patients with CU have a poor quality of life (QoL) [4-6]. Depression and anxiety are the common psychiatric disorders found in CU patients, and these psychiatric disorders may in turn influence QoL [6,7]. Whether the presence of depression and anxiety prior to the onset of CU can make symptoms worse remains unclear.
There is an insufficient number of studies on depression and anxiety in CU. The aim of the present study was to evaluate depression and anxiety levels in patients with CU and to compare them with those of a control group.
Material and Methods
Patients and study design
Fifty patients over 18 years of age with CU were included in this study. The diagnoses and therapies for CU that they were receiving were appropriate according to recent urticaria guidelines [1]. Twelve (24%) of the patients had both chronic inducible urticaria and chronic spontaneous urticaria. None of the patients had only chronic inducible urticaria. We excluded patients who had a diagnosed psychiatric disorder, a cognitive impairment due to a current cerebral or psychotic illness, a malignant or a central nervous system disease, or received glucocorticoid therapy for at least 1 month. We excluded these patients because all of these diseases and glucocorticoids can affect mood and can cause higher anxiety and depression levels. According to the guidelines, the urticaria activity score for 7 days (UAS7), which ranges from 0 to 42, was used to assess the disease activity in CU patients [1].
UAS7, disease duration, medications, age, gender, marital status, comorbidities, occupation, and income were recorded. Sixty healthy volunteers were included in the study as a control group. There was no significant demographic difference between the patient group and the healthy group. The levels of depression and anxiety were assessed with the Hospital Anxiety and Depression Scale (HADS), which is a self-administered questionnaire developed to identify depression and anxiety symptoms in patient and out-patient populations, not to diagnose psychiatric disorders [8].
In this study, the 14-items HADS questionnaire for anxiety and depression, as validated by Aydemir et al. was used [9]. Seven items measure anxiety and 7 items measure depression. Each item on the scale is scored from 0 to 3, so the total score ranges between 0 and 21, and anxiety-depression increases as the score increases. According to Aydemir et al., the anxiety subscale cut-off score is 10 or more, and the depression subscale cut-off score is 7 or more [9].
Depression and anxiety scores were compared between the patient and the healthy groups. Also, in the patient group, UAS7 scores and depression and anxiety scores were compared.
All the patients and healthy volunteers provided written informed consent for participation in the study and to answer the questionnaire. The study was approved by the Ethics Committee of Mustafa Kemal University, Tayfur Ata Sokmen Medical School.
Statistics analysis
In the statistical analysis, categorical variables were given as numbers and percentages, and continuous variables were presented as mean ± standard deviation (SD), as in the descriptive analyses. Continuity correction chi-square tests were used for the comparison of categorical variables between groups. The conformity of continuous variables to normal distribution was evaluated using visual (histogram and probability graphs) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk tests). Normality analysis showed that all continuous variables for all groups did not conform to normal distribution. An independent-samples t test was used for the comparison of data which were normally distributed for the variables. The Mann-Whitney U test was used for comparison of data that were not normally distributed for the variables. Analyses for each variable are presented in individual tables. The relationship between UAS7 and HAD anxiety, depression and total scores were evaluated using Spearman’s correlation analysis. P<0.05 was considered statistically significant.
Results
The demographic characteristics of the patients with CU and the healthy volunteers included in the study are given in Table 1. A total of 50 patients and 60 healthy volunteers (as a control group) were included in the study. Twenty-two (44%) of the patients received single doses of antihistamines, 24 (48%) received increased doses of antihistamines, 1 (2%) received ranitidine, montelukast, and single doses of antihistamines, 2 (4%) received omalizumab, and 1 (2%) received omalizumab plus increased doses of antihistamine. No patient had only chronic inducible urticaria. Twelve (24%) patients had both chronic inducible urticaria and chronic spontaneous urticaria.
Table 1.
Demographic characteristics of patients with CU and controls.
| Characteristic | Finding | P value | |
|---|---|---|---|
| Patients | Controls | ||
| Age (mean ±SD) (y) | 38.28±13.90 | 37.10±13.28 | 0.544* |
| Female, No. (%) | 35 (70.0%) | 41 (68.3%) | 1.000** |
| Marital status, No. (%) | 0.990*** | ||
| Single | 11 (22.0%) | 13 (21.7%) | |
| Married | 38 (76.0%) | 46 (76.6%) | |
| Widow | 1 (2.0%) | 1 (1.7%) | |
| Years of education No. (%) (y) | 0.997*** | ||
| Primary | 15 (30.0%) | 18 (30%) | |
| High school | 17 (34.0%) | 20 (33.3%) | |
| University | 18 (36.0%) | 22 (36.7%) | |
UAS7 – urticaria activity score for 7 days.
Mann-Whitney U test;
Continuity Correction Chi-Square Test;
Pearson Chi-Square Test.
The mean ±SD age of these patients was 38.2±13.9 years, and 35 (70.0%) patients were women. Thirty-eight (76%) of the patients were married and 18 (36%) had graduated from university (Table 1). The mean ±SD duration of CU disease and UAS7 were 23.94±25.01 months and 23.14±13.40, respectively. There was no statistically significant difference between the patient group and the control group in terms of demographic findings (Table 1).
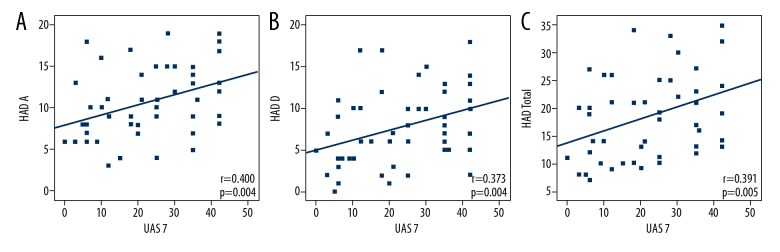
The mean HADS depression scores for the CU and healthy groups were 7.74±4.49 and 4.85±3.26, respectively. The mean HADS anxiety scores for the CU and healthy groups were 10.82±4.29 and 6.42±3.02, respectively. There were significant differences between the groups in terms of the HADS anxiety and depression scores (p=0.001). The patient group had higher levels of depression and anxiety (Table 2). When the cut-off points for anxiety and depression were set at 10 and 7, respectively, 24 (48%) patients had anxiety, and 24 (48%) patients had depression symptoms. There was a significant positive correlation between UAS and anxiety, as well as depression and the total (anxiety plus depression) scores (r=0.400; p=0.004 and r=0.373; p=0.004, r=0.391; p=0.005 respectively) (Figure 1).
Table 2.
Anxiety and depression scores of patients with CU and control groups.
| CU group (n=50) | Control group (n=60) | P value | |
|---|---|---|---|
| HAD-A (mean ±SD) | 10.82±4.29 | 6.42±3.02 | 0.001* |
| HAD-A ≤10 (No. (%)) | 26 (52%) | 56 (93.3%) | 0.001** |
| HAD-A >10 (No. (%)) | 24 (48%) | 4 (6.7%) | |
| HAD-D (mean ±SD) | 7.74±4.49 | 4.85±3.26 | 0.001* |
| HAD-D ≤7 (No. (%)) | 26 (52%) | 49 (81.7%) | 0.002** |
| HAD-D >7 (No. (%)) | 24 (48%) | 11 (18.3%) | |
| HAD-T (mean±SD) | 18.76±7.82 | 11.27±5.36 | 0.001* |
HAD-A – Hospital Anxiety-Depression Scale Anxiety score; HAD-D – Hospital Anxiety-Depression Scale Depression score; HAD-T – Hospital Anxiety-Depression Scale Total score.
Independent-Samples t Tests;
Continuity Correction Chi-Square Tests.
Figure 1.
(A) Scatter plots of correlation between UAS 7 and anxiety subscore of HADS (B) between UAS 7 and depression subscore of HADS (C) between UAS 7 and total scores of HADS. HADS – Hospital Anxiety Depression Scale; UAS7 – urticaria activity score for 7 days.
We compared women and men in the patient group regarding anxiety, depression, and total anxiety and depression scores and found no significant differences. In the patient group, the mean anxiety, depression, and total anxiety-depression scores ±SD were 11.23±4.32, 7.49±4.40, and 19.00±7.69 in women and 9.87±4.21, 8.33±4.78, and 18.20±8.36 in the men, respectively (p=0.308, 0.546, and 0.744, respectively). All patients had urticaria and 18 patients had angioedema with urticaria. The mean anxiety, depression, and total anxiety-depression scores ±SD were 10.56±4.53, 7.61±4.82, and 18.17±8.36 in patients with angioedema and 10.97±4.21, 7.81±4.37, and 19.09±7.61 in patients without angioedema, respectively (p=0.747, 0.881, and 0.692, respectively). No patients had only chronic inducible urticaria. Twelve (24%) patients had both chronic inducible urticaria and chronic spontaneous urticaria. We found no significant differences in regarding anxiety, depression, and total scores between CU patients with or without inducible urticaria (p>0.05).
There was no significant correlation between age and anxiety, and depression and total (anxiety plus depression) scores (r=−0.025; p=0.864, r=0.120; p=0.405, r=0.095; p=0.511, respectively). Also, we found no significant difference between disease duration and anxiety, depression, and the total (anxiety plus depression) scores (r=−0.213; p=0.137, r=0.202; p=0.159, r=0.241; p=0.091, respectively).
Discussion
The results of our study show that patients with CU had higher levels of depression and anxiety when compared with the healthy group. We also found that UAS7 had an important effect on both anxiety and depression. In the literature, there are many studies about psychiatric comorbidity and QoL in chronic idiopathic urticaria (CIU), but fewer studies that specifically address anxiety and depression in patients with CU. An early study by Sheehan et al. found that patients with chronic urticaria had more depressive symptomatology than the controls, but the difference was not statistically significant. By using the Speilberger state-trait anxiety inventory, they found there were no significant differences between patients with pruritus or urticaria and the controls. However, their group consisted of just 34 patients, which is not a large group, and it was conducted in the 1990s [10]. Sperber et al. performed a psychological assessment (symptom checklist 90) to 19 patients with CIU and found that the patients with urticaria had significantly higher scores on somatization, obsessive-compulsive, interpersonal sensitivity, depression and anxiety scales when compared to the control group [11]. These results are similar to ours; but their sample size was small.
Engin et al. showed that CIU patients frequently suffered from depression and anxiety and that such patients had impaired QoL. They used the Beck Depression Inventory (BDI), the Beck Anxiety Inventory (BAI) and the World Health Organization QoL Assessment-Brief (WHOQOL-BREF) to evaluate levels of depression, anxiety, and QoL, respectively. Also, after Spearman correlation analysis, they found that BDI, BAI, and all domains of WHOQOL-BREF were unrelated to age, duration of illness, and UAS in the CIU group [6].
In contrast to that study, UAS7 had an important effect on both anxiety and depression in our study.
Staubach’s study showed that CU patients with psychiatric disorders and psychiatric comorbidities (i.e., depression, anxiety, and somatoform disorders) had worse QoL, and that the severity of the psychiatric disease was correlated with worse QoL [7]. A study of children with CIU found that internalizing problems, somatic complaints, anxiety and depressed scores were significantly higher in children with CIU, and also found there was no correlation between the severity and duration of the illness and the patient’s psychological status [12]. In Altınoz’s study, anxiety and depression scores were compared among 3 groups: patients with CSU, patients with alopecia areata, and a healthy control group. Anxiety and depression scores in the patient group with CSU were found to be significantly higher than those of the healthy control group.
Symptoms and thoughts of anger, and situations that cause anger were significantly more common in the group with CSU compared to the alopecia areata group and the healthy group [13].
In a systematic review of psychosocial factors and chronic spontaneous urticaria, which consisted of 22 papers, a prevalence of comorbidity between psychosocial factors and CSU was demonstrated [14].
Mast cells play the main role in chronic urticaria, but the role of allergens as triggers has received scant attention. A study examining IgE sensitization and allergy in 128 adults with chronic urticaria found that only 46.7% were IgE-sensitized [15]. Most findings up to now highlight the complex nature of the pathogenesis of chronic urticaria, which has many features in addition to the release of histamine from mast cells [16,17].
There has been confusion regarding the psychological components of CU. Many practitioners accept that psychosocial factors are possible contributors to the exacerbation of symptoms in CSU, but some experts largely deny the involvement of psychological parameters in the onset of the condition. Many allergists suggest that psychological factors play an important role in the pathogenesis of this condition [18].
In a recent study, Bozo et al. showed that CU patients with high trait anxiety scores reported more depressive symptoms, and the ones who use more problem-focused coping strategies reported fewer depressive symptoms [19]. In a study from 5 European countries, CU patients showed a worse health-related quality of life compared to patients with overall psoriasis, and CU patients also showed a higher risk of anxiety, depression, and sleep difficulties and greater health care resource use compared to overall psoriasis patients. The overall activity impairment was noticeably greater in CU patients than in overall psoriasis patients (P=0.001), but the impact on work was not significantly different [20]. Ograczyk et al. found that the CU patients had significantly higher anxiety levels than those of the control group, as in our study [21]. In a questionnaire survey, Gattey et al. also showed that common symptoms were pruritus, disturbed sleep, and anxiety in chronic spontaneous urticaria patients [22].
Here, we demonstrated that depression and anxiety symptoms were more common in patients with CU than in the control group. In this study we only evaluated the effect of urticaria on anxiety and depression, so we excluded the patients who had received glucocorticoid therapy for at least 1 month because glucocorticoids can affect mood. Generally, glucocorticoids are prescribed when the symptoms were severe or uncontrolled in urticaria. If we had not excluded these patients, the anxiety and depression scores might have been higher. We also excluded patients who had a diagnosed psychiatric disorder, a cognitive impairment due to a current cerebral or psychotic illness, and those with a malignant or a central nervous system disease, as all of these diseases can affect mood and cause higher anxiety and depression levels.
Our study has a few limitations. The cohort size was not large and the study was conducted in a single center. On the other hand, this study specifically addressed depression and anxiety in CU and our cohort was heterogeneous.
Conclusions
Because of the complex nature of CU, curative treatment is impossible at present.
However, it is clear that this disease causes impaired QoL. In this study, we found that depression and anxiety scores were higher in CU patients than in controls, and effective treatment of CU must address this problem. Studies involving larger cohorts are needed.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Zuberbier T, Aberer W, Asero R, et al. The EAACI/GA2LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: The 2013 revision 180 and update. Allergy. 2014;69(7):868–87. doi: 10.1111/all.12313. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270–77. doi: 10.1016/j.jaci.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 3.Zuberbier T, Aberer W, Asero R, et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393–414. doi: 10.1111/all.13397. [DOI] [PubMed] [Google Scholar]
- 4.Weldon DR. Quality of life in patients with urticaria. Allergy Asthma Proc. 2006;27:96–99. [PubMed] [Google Scholar]
- 5.Baiardini I, Giardini A, Pasquali M, et al. Quality of life and patients’ satisfaction in chronic urticaria and respiratory allergy. Allergy. 2003;58:621–23. doi: 10.1034/j.1398-9995.2003.00091.x. [DOI] [PubMed] [Google Scholar]
- 6.Engin B, Uguz F, Yilmaz E, et al. The levels of depression, anxiety and quality of life in patients with chronic idiopathic urticaria. J Eur Acad Dermatol Venereol. 2008;22:36–40. doi: 10.1111/j.1468-3083.2007.02324.x. [DOI] [PubMed] [Google Scholar]
- 7.Staubach P, Eckhardt-Henn A, Dechene M, et al. Quality of life in patients with chronic urticaria is differentially impaired and determined by psychiatric comorbidity. Br J Dermatol. 2006;154:294–98. doi: 10.1111/j.1365-2133.2005.06976.x. [DOI] [PubMed] [Google Scholar]
- 8.Zigmond AS, Snaith PR. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 9.Aydemir Ö, Güvenir T, Küey L, Kültür S. [Validity and reliability of the Turkish version 196 of Hospital anxiety and depression scale]. Türk Psikiyatri Dergisi. 1997;8:280–87. [in Turkish] [Google Scholar]
- 10.Sheehan-Dare RA, Henderson MJ, Cotterill JA. Anxiety and depression in patients with chronic urticaria and generalized pruritus. Br J Dermatol. 1990;123:769–74. doi: 10.1111/j.1365-2133.1990.tb04195.x. [DOI] [PubMed] [Google Scholar]
- 11.Sperber J, Shaw J, Bruce S. Psychological components and the role of adjunct interventions in chronic idiopathic urticaria. Psychother Psychosom. 1989;51(3):135–41. doi: 10.1159/000288147. [DOI] [PubMed] [Google Scholar]
- 12.Hergüner S, Kiliç G, Karakoç S, et al. Levels of depression, anxiety and behavioural problems and frequency of psychiatric disorders in children with chronic idiopathic urticaria. Br J Dermatol. 2011;164(6):1342–47. doi: 10.1111/j.1365-2133.2010.10138.x. [DOI] [PubMed] [Google Scholar]
- 13.Altinoz AE, Taskintuna N, Altinoz ST, Ceran S. A cohort study of the relationship between anger and chronic spontaneous urticaria. Adv Ther. 2014;31(9):1000–7. doi: 10.1007/s12325-014-0152-6. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Shoshan M, Blinderman I, Raz A. Psychosocial factors and chronic spontaneous urticaria: A systematic review. Allergy. 2013;68:131–41. doi: 10.1111/all.12068. [DOI] [PubMed] [Google Scholar]
- 15.Augey F, Gunera-Saad N, Bensaid B, et al. Chronic spontaneous urticaria is not an allergic disease. Eur J Dermatol. 2011;21:349–53. doi: 10.1684/ejd.2011.1285. [DOI] [PubMed] [Google Scholar]
- 16.Greaves MW. Chronic urticaria. N Engl J Med. 1995;332:1767–72. doi: 10.1056/NEJM199506293322608. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan AP. Clinical practice. Chronic urticaria and angioedema. N Engl J Med. 2002;346:175–79. doi: 10.1056/NEJMcp011186. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Shoshan M, Clarke A, Raz A. Psychosocial factors and the pathogenesis of chronic hives: A survey of Canadian physicians. J Allergy Therapy. 2012;68:131–41. [Google Scholar]
- 19.Bozo O, Demirtepe-Saygılı D, Güneş S, et al. Does problem focused coping buffer the effects of trait anxiety on depressive symptoms of chronic urticaria patients? J Gen Psychol. 2018;145(1):64–78. doi: 10.1080/00221309.2017.1420622. [DOI] [PubMed] [Google Scholar]
- 20.Balp MM, Khalil S, Tian H, et al. Burden of chronic urticaria relative to psoriasis in 5 European countries. J Eur Acad Dermatol Venereol. 2018;32(2):282–90. doi: 10.1111/jdv.14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ograczyk A, Miniszewska J, Pietrzak A, Zalewska-Janowska A. Sense of coherence as a protective factor in chronic urticaria. Postepy Dermatol Alergol. 2017;34(2):168–73. doi: 10.5114/ada.2017.67084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gattey N, Bahrani B, Hull PR. Chronic spontaneous urticaria: a questionnaire survey. J Cutan Med Surg. 2016;20(3):241–43. doi: 10.1177/1203475415623777. [DOI] [PubMed] [Google Scholar]