Abstract
Background
Sepsis-induced lung injury is associated with high mortality. The present investigation evaluated the protective effect of hesperidin against sepsis-induced lung injury and also postulates the possible mechanism of its action.
Material/Methods
Lung injury was induced by sepsis in all animals, in which sepsis was produced by cecal ligation and puncture (CLP). Animals were treated with hesperidin 10 and 20 mg/kg i.v. 30 min after the surgery. Oxygenation index and lung injury score were determined and levels of pro-inflammatory mediators and markers of oxidative stress were also estimated in the lung tissues. Moreover, expression of caspase-3, B-cell lymphoma (Bcl-2), Toll-like receptor 4 (TLR4), heat-stable protein 70 (Hsp70) and myeloid differentiation primary response 88 (MyD88) protein was estimated by Western blot assay and immunofluorescence assay.
Results
Hesperidin attenuated the partial pressure of arterial oxygen/fraction of inspired oxygen (PaO2/FiO2) ratio and lung injury score in CLP-induced lung injury mice. There was a significant (p<0.01) decrease in the level of pro-inflammatory mediators in the lung tissue of CLP-induced lung injury mice. Moreover, markers of oxidative stress were attenuated in the hesperidin-treated group. Treatment with hesperidin attenuated the expression of caspase-3, Bcl-2, TLR4, Hsp70, and MyD88 protein in the lung tissue of CLP-induced lung injury mice.
Conclusions
Hesperidin protects against lung injury by attenuating the Hsp70/TLR4/MyD88 pathway in CLP-induced lung injury mice.
MeSH Keywords: Hesperidin, Lung Injury, Sepsis
Background
Sepsis is a systemic inflammation that occurs due to infection that causes multiple organ dysfunctions, including acute respiratory distress syndrome (ARDS) [1]. Type I alveolar epithelial cells are destroyed due to inflammation, but the exact pathogenesis of it not known. However, apoptosis is reported to have role in the development of lung injury [2]. The sepsis-induced lung injury mortality rate is very high and thus it is important to develop more therapeutic interventions for the management of lung injury [3]. Heat shock proteins (HSPs) are involved in the regulation of cellular homeostasis [4]. HSPs activate the process of apoptosis by stimulating adaptive and innate immunity [5]. HSP70 is involved in a number of cell process and is a highly inducible protein of the HSPs family. Overexpression of HSP70 is reported to enhance the resistance to hypoxia-induced lung and heart injury [6]. Moreover, Toll-like receptors (TLRs) have also been reported to have roles in bleomycin- or hypoxia-induced injury [7]. The literature reveals that the LTRs pathway is involved in immunity, inflammation, and microbial challenge-like events [8].
In the last few decades, molecules from natural sources have been reported to be useful in the management of several disorders as an alternative medicine. Hesperidin is a flavonoid isolated from plants belonging to families Lamiaceae and Betulaceae [9]. The literature reveals that hesperidin attenuates apoptosis, hypotension, oxidative stress, and inflammation [10–12]. Moreover, it protects against neuronal injury by ameliorating oxidative stress and inflammation [12]. Hesperidin is also reported to have antimicrobial, anticancer, antiarthritic, antihypercholesterolemic, and antiatherogenic activity [13–15]. A study reported that hesperidin attenuate pancreatitis by inhibiting the TLR4/IRAK1/NF-κB pathway [16]. Thus, the present investigation evaluated the beneficial effects of hesperidin against sepsis-induced lung injury.
Material and Methods
Animal
Male albino mice (20–25 g) were procured from Shanghai Medical College, Shanghai, China. Mice were housed under standard conditions as per our institution’s guidelines (humidity: 60±5%; temperature: 24±3°C) with a 12-h light/dark cycle and free access to standard chow diet and tap water. All the protocols of the study were approved by the Institutional Animal Care and Use Committee of Second Military Medical University, China (IACUC/SMMU/2017/08) and the study followed the guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC) for experimentation and animal use.
Chemicals
Hesperidin was procured from Sigma Aldrich, USA. ELISA kits for IL-6, IL-1β, and TNF-α were purchased from Lifespan Biosciences, Inc., WA, USA. iNOS, NO, SOD, and MDA kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China).
Experiments
Lung injury was induced by sepsis in all animals, in which sepsis was produced by cecal ligation and puncture (CLP). All the mice were fasted overnight and anesthetized by pentobarbital (30 mg/kg, i.p.). The cecum was exposed to perform laparotomy. An 18-gauge needle was used twice to puncture below the ileocecal valve to ligate the cecum. The abdominal cavity was closed after returning the cecum. However, in the sham-operated group, only laparotomy was performed, without ligating the cecum. All animals were separated into 4 different groups: the control group, the negative control group, and the hesperidin 10 and 20 mg/kg groups (hesperidin 10 and 20 mg/kg, respectively, i.v. 30 min after surgery).
Assessment of PaO2/FiO2 ratio
All the animals were anesthetized 1 day after CLP. A 20-gauge catheter was used for endotracheal intubation in the mice and pure oxygen (7 ml/kg) was given later by mechanical ventilation. PaO2 analysis was done on arterial blood samples using a GEM Premier 3000 gas analyzer (Instrumentation Laboratory, Italy) after ventilating the animal for 15 min. The oxygenation index is expressed as PaO2/FiO2. Lungs were isolated from the animals, weighed, and cleaned of blood and water with filter paper. Later, lungs were dried by keeping them in an oven for 2 days, after which the dry weight was measured.
Isolation of lung
All the animals were sacrificed by cervical dislocation method and lungs were isolated from each animal. Lobes were separated out from the isolated lung and 5-μm sections were prepared for histochemical analysis. The other lobes were homogenized with 0.9% saline solution and protein was extracted.
Histopathological analysis
All the animals were sacrificed and lungs were isolated. Isolated lungs were fixed in formalin (10%) for 3 days. Slices of pulmonary lobes were seeded in the paraffin and a microtome was used to make 3-μm sections. Hematoxylin and eosin was used to stain the lung tissue sections. Using a scale of 0–3, we assessed infiltration of inflammatory cells, necrosis, interstitial edema, and hemorrhage.
Determination of pro-inflammatory mediators
ELISA assay was used for the estimation IL-6, IL-1β, and TNF-α in the serum as per the instructions of the manufacturer. We used a nitrite/nitrate colorimetric assay kit to estimate the level of nitrite in the serum of CLP-induced lung injury mice.
Determination of markers of oxidative stress
Levels of oxidative stress markers iNOS, NO, SOD, and MDA in the lung tissue homogenate were estimated using the kits and methods according to the instructions of the manufacturer.
Western blot
Isolated lungs were homogenized with 62.5 mM Tris buffer and RIPA lysis buffer was used to lyse the cells. BCA Protein Assay was used to estimate the concentration of protein in the lysate, and proteins were separated by ready-made Tris-HCl gel (12%). Proteins were transferred onto nitrocellulose membranes and incubated overnight with caspase-3 (1: 500), Bcl-2 (1: 1000), TLR4 (1: 1000), Hsp70 (1: 1000), and β-Actin antibody (Santa Cruz Biotechnology Inc., Santa Cruz, USA). HRP-conjugated goat anti-rabbit IgG antibody was used to incubate the membrane and a chemiluminescence Lumi GLO detection kit was used for the detection of signals.
Immunofluorescence assay
Isolated lungs were sliced and incubated for overnight at 4°C with MyD88 and TLR4 antibodies (Santa Cruz, California, USA) for immunofluorescence staining. Thereafter, PBS was used to wash the lung tissue and fluorescein-labeled secondary antibody was incubated with the lung tissue for 1 h. DAPI (5 μg/ml) was used for the staining of cell nuclei. A fluorescence microscope was used to capture immunostained images.
Statistical analysis
All data are expressed as mean ±SEM (n=6). The statistical analysis was performed using one-way ANOVA. Post hoc comparison of means was carried out by Dunnett’s post hoc test (Gradpad prism 6.1., CA, USA) multiple comparisons. The level of statistical significance was set at P<0.05.
Result
Hesperidin attenuates the oxygenation index
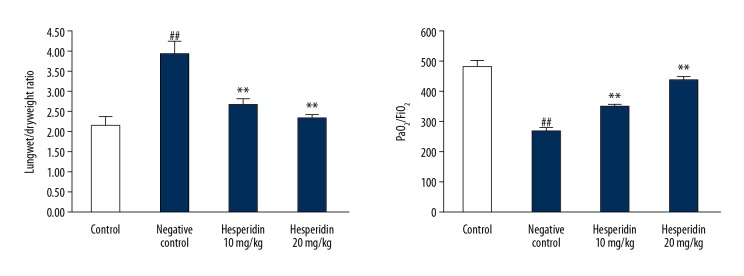
Figure 1 shows the effect of hesperidin on oxygenation index and wet/dry weight ratio of lungs in CLP-induced lung injured mice. Oxygenation index was estimated by determining PaO2/FiO2 ratio. The ratio of wet/dry weight of lungs was found to be significantly enhanced and the PaO2/FiO2 ratio was significantly (p<0.01) reduced in the negative control group compared to the control group. However, the oxygenation index (PaO2/FiO2 ratio) was significantly enhanced and the ratio of wet/dry weight of lungs was significantly reduced (p<0.01) in the hesperidin-treated group compared to the negative control group.
Figure 1.
Effect of hesperidin on oxygenation index and wet/dry weight ratio of lung in CLP-induced lung injury mice. Mean ±SEM (n=6). ## p<0.01 than control group. ** p<0.01 than negative control group.
Effect of hesperidin on histopathology of lung tissue
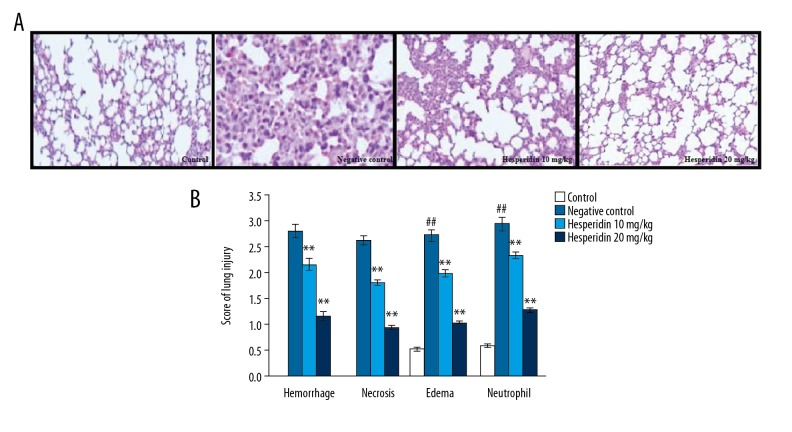
Effect of hesperidin on the histopathology of lung in CLP-induced lung injury mice was estimated by H&E staining. Treatment with hesperidin attenuated the histopathological changes in the CLP-induced lung injury tissue (Figure 2A). Lung tissue was observed by calculating the score of lung injury. There was a significant increase in the hemorrhage, necrosis, edema, and neutrophil scores in the lung tissues of the negative control group compared to the control group. However, treatment with hesperidin significantly (p<0.01) reduced the injury scores of hemorrhage, necrosis, edema, and neutrophil in the lung tissue compared to the negative control group (Figure 2B).
Figure 2.
Effect of hesperidin on the histopathology of lung in CLP-induced lung injury mice (A). Histopathology of lung tissue by H&E staining (100×). (B) Score of lung injury. Mean ±SEM (n=6). ## p<0.01 than control group. ** p<0.01 than negative control group.
Hesperidin attenuates pro-inflammatory mediators
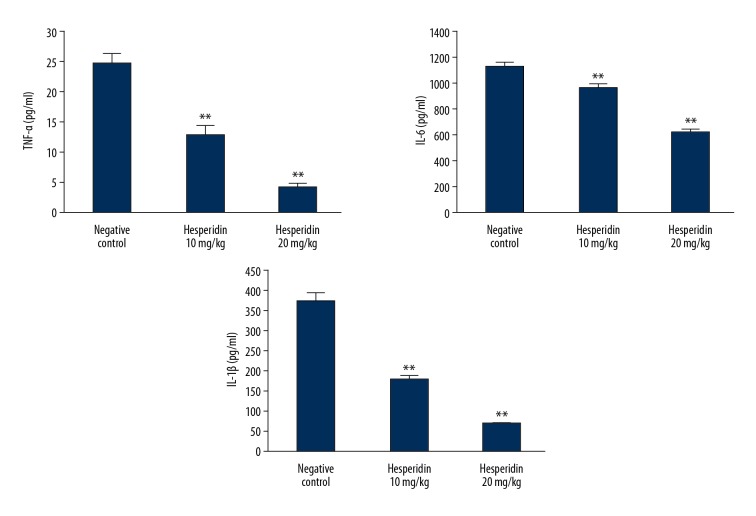
Effects of hesperidin on the level of pro-inflammatory mediator TNF-α, IL-6, and IL-1β in the lung tissue of CLP-induced lung injury mice is shown in Figure 3. Treatment with hesperidin significantly decreased (p<0.01) the levels of pro-inflammatory mediators compared to the negative control group.
Figure 3.
Effect of hesperidin on the level of pro-inflammatory mediator in the lung tissue of CLP-induced lung injury mice. Mean ±SEM (n=6). ** p<0.01 than negative control group.
Effect of hesperidin on markers of oxidative stress
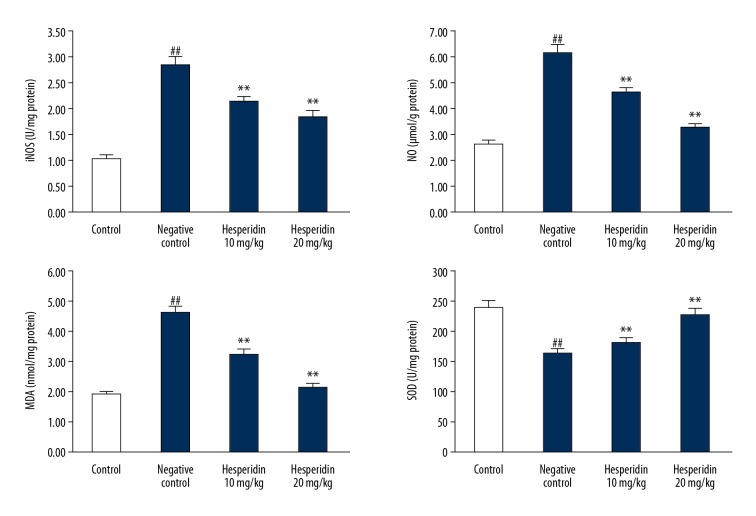
The effects of hesperidin on the markers of oxidative stress (iNOS, NO, MDA, and SOD) in the lung tissues of CLP-induced lung injury mice are shown in Figure 4. Levels of NO and MDA were significantly enhanced in the lung tissue homogenate of the negative control group compared to the control group. However, SOD activity was significantly (p<0.01) reduced and iNOS was significantly enhanced (p<0.01) in the lung tissues of the negative control group compared to the control group. Treatment with hesperidin attenuated the altered level of NO and MDA and activity of iNOS and SOD in the lung tissues of CLP-induced lung injury mice.
Figure 4.
Effect of hesperidin on markers of oxidative stress in lung tissue of CLP-induced lung injury mice. Mean ±SEM (n=6). ## p<0.01 than control group; * p<0.05, ** p<0.01 than negative control group.
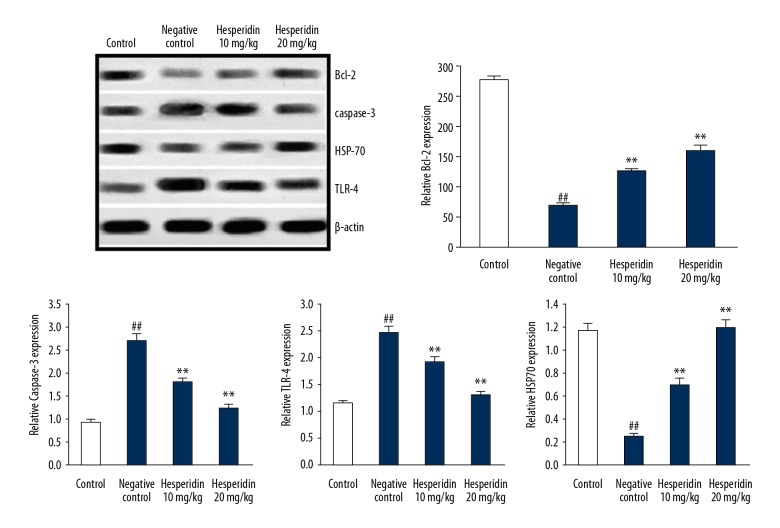
Hesperidin attenuated the expression of Bcl-2, caspase-3, TLR-4, and HSP70
Effects of hesperidin on the expression of Bcl-2, caspase-3, TLR-4, and HSP70 protein in the lung tissues of CLP-induced lung injury mice were determined by Western blot assay as shown in Figure 5. There was a significant (p<0.01) increase in the expression of TLR-4 and caspase-3 protein and decreased expression of Bcl-2 and HSP-70 protein in the lung tissues of the negative control group compared to the control group. However, treatment with hesperidin significantly attenuated the altered expression of Bcl-2, caspase-3, TLR-4 and HSP70 protein in the lung tissue of CLP-induced lung injury mice.
Figure 5.
Effect of hesperidin on the expression of Bcl-2, caspase-3, TLR-4, and HSP70 protein in the lung tissue of CLP-induced lung injury mice. Mean ±SEM (n=6). ## p<0.01 than control group; ** p<0.01 than negative control group.
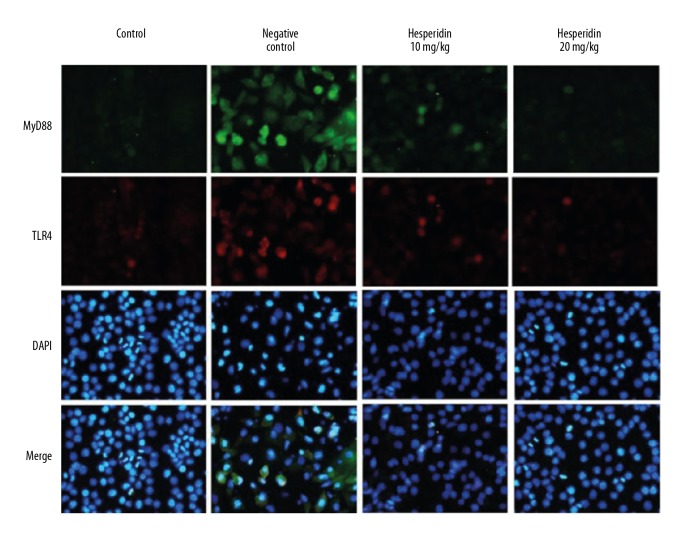
Hesperidin attenuated the expression of MyD88 and TLR4
Effects of hesperidin on the expression of MyD88 and TLR4 proteins in the lung tissue of CLP-induced lung injury mice by immunofluorescence assay are shown in Figure 6. It was observed that treatment with hesperidin significantly attenuated the altered level of expression of MyD88 and TLR4 proteins in the lung tissues of CLP-induced lung injury mice.
Figure 6.
Effect of hesperidin on the expression of MyD88 and TLR4 protein in the lung tissue of CLP-induced lung injury mice by immunofluorescence assay.
Discussion
Sepsis a cause of lung injury that has a high mortality rate [17]. Thus, the present investigation evaluated the protective effect of hesperidin against sepsis-induced lung injury and also explored the possible molecular mechanism of its action. Effects of hesperidin were evaluated by determining oxygenation index, wet/dry weight ratio, and injury score from histopathological changes in the lung tissue of CLP-induced lung injury mice. ELISA, Western blot, and immunofluorescence assays were also performed to estimate the effect of hesperidin on the lung tissue of CLP-induced lung injury mice.
Inflammation and oxidative stress are major causes of organ injury due to sepsis [18]. Pro-inflammatory mediators such as Il-6, IL-1β, and TNF-α and oxidative stress enhance the process of apoptosis by enhancing the activity of caspase-3 enzyme in lung tissues [19]. Our study reveals that treatment with hesperidin attenuated the altered level of markers of oxidative stress and pro-inflammatory mediators in the CLP-induced lung injury tissues in a dose-dependent manner. HSP70 is also reported to have anti-inflammatory activity and has the potential to protect against cell injury [20]. Expression of HSP70 was reported to be reduced in sepsis-induced lung injury tissues [21] and results of the present investigation reveal that the expression of HSP70 significantly enhanced in hesperidin-treated group compared to the negative control group in a dose-dependent manner.
It was reported that in lung injury the level of pro-inflammatory mediators were enhanced by stimulation of the TLR4/MyD88 pathway [22]. Moreover, TLR4 also enhances the production of reactive oxygen species, which further enhances the oxidative stress in lung tissue [23]. Oxidative stress and inflammatory cytokines cause organ injury in sepsis [4]. Data from Western blot and immunofluorescence assays suggest that the expression of TLR4/MyD88 protein in the CLP-induced lung injury tissue was attenuated in the hesperidin-treated group in a dose-dependent manner.
Conclusions
We found that hesperidin protects against lung injury by attenuating the TLR4/MyD88/HSP70 pathway. Levels of pro-inflammatory mediators and markers of oxidative stress were also significantly ameliorated in the lung tissue of CLP-induced lung injury mice. Our results show that hesperidin has potential utility in clinical management of lung injury.
Footnotes
Source of support: Shanghai Pudong New Area Key Specialty Project [No: PWZzK2017-05] and the Innovation Funds for Development of Science and Technology in Pu Dong New Area [No: PKJ2017-Y22]
Conflict of interest
None.
References
- 1.Wang Y, Wang X, Zhang L, Zhang R. Alleviation of acute lung injury in rats with sepsis by resveratrol via the phosphatidylinositol 3-kinase/nuclear factor-erythroid 2 related factor 2/heme oxygenase-1 (PI3K/Nrf2/HO-1) pathway. Med Sci Monit. 2018;24:3604–11. doi: 10.12659/MSM.910245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Riccio V, van Tuyl M, Post M. Apoptosis in lung development and neonatal lung injury. Pediatr Res. 2004;55(2):183–89. doi: 10.1203/01.PDR.0000103930.93849.B2. [DOI] [PubMed] [Google Scholar]
- 3.Perl M, Lomas-Neira J, Venet F, et al. Pathogenesis of indirect (secondary) acute lung injury. Exp Rev Respir Med. 2011;5(1):115–26. doi: 10.1586/ers.10.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koliński T, Marek-Trzonkowska N, Trzonkowski P, Siebert J. Heat shock proteins (HSPs) in the homeostasis of regulatory T cells (Tregs) Cent Eur J Immunol. 2016;41(3):317–23. doi: 10.5114/ceji.2016.63133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colaco CA, Bailey CR, Walker KB, Keeble J. Heat shock proteins: Stimulators of innate and acquired immunity. Biomed Res Int. 2013;2013 doi: 10.1155/2013/461230. 461230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Zhang X, Shan P, et al. A Protective Hsp70-TLR4 pathway in lethal oxidant lung injury. J Immunol. 2013;191(3):1393–403. doi: 10.4049/jimmunol.1300052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ioannou S, Voulgarelis M. Toll-like receptors, tissue injury, and tumourigenesis. Med Inflamm. 2010;2010 doi: 10.1155/2010/581837. 581837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2000. 2014;64(1):57–80. doi: 10.1111/prd.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: An overview. ScientificWorldJournal. 2013;2013 doi: 10.1155/2013/162750. 162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia R, Sheng X, Xu X, et al. Hesperidin induces apoptosis and G0/G1 arrest in human non-small cell lung cancer A549 cells. Int J Mol Med. 2018;41(1):464–72. doi: 10.3892/ijmm.2017.3250. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M, Jokura H, Hashizume K, et al. Hesperidin metabolite hesperetin-7-O-glucuronide, but not hesperetin-3′-O-glucuronide, exerts hypotensive, vasodilatory, and anti-inflammatory activities. Food Funct. 2013;4(9):1346–51. doi: 10.1039/c3fo60030k. [DOI] [PubMed] [Google Scholar]
- 12.Parhiz H, Roohbakhsh A, Soltani F, et al. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother Res. 2015;29(3):323–31. doi: 10.1002/ptr.5256. [DOI] [PubMed] [Google Scholar]
- 13.Lee K-A, Lee S-H, Lee Y-J, et al. Hesperidin induces apoptosis by inhibiting Sp1 and its regulatory protein in MSTO-211H cells. Biomol Ther (Seoul) 2012;20(3):273–79. doi: 10.4062/biomolther.2012.20.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abuelsaad AS, Mohamed I, Allam G, et al. Antimicrobial and immunomodulating activities of hesperidin and ellagic acid against diarrheic Aeromonas hydrophila in a murine model. Life Sci. 2013;93(20):714–22. doi: 10.1016/j.lfs.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Milenkovic D, Deval C, Dubray C, et al. Hesperidin displays relevant role in the nutrigenomic effect of orange juice on blood leukocytes in human volunteers: A randomized controlled cross-over study. PLoS One. 2011;6(11):e26669. doi: 10.1371/journal.pone.0026669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li M, Guo Z, Shao H, Qin B. Therapeutic effect of hesperidin on severe acute pancreatitis in rats and its mechanism. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2017;29(10):921–25. doi: 10.3760/cma.j.issn.2095-4352.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Varisco BM. The pharmacology of acute lung injury in sepsis. Adv Pharmacol Sci. 2011;2011 doi: 10.1155/2011/254619. 254619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zarbock A, Gomez H, Kellum JA. Sepsis-induced AKI revisited: Pathophysiology, prevention and future therapies. Curr Opin Crit Care. 2014;20(6):588–95. doi: 10.1097/MCC.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mittal M, Siddiqui MR, Tran K, et al. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126–67. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borges TJ, Wieten L, van Herwijnen MJC, et al. The anti-inflammatory mechanisms of Hsp70. Front Immunol. 2012;3:95. doi: 10.3389/fimmu.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bromberg Z, Raj N, Goloubinoff P, Deutschman CS, Weiss YG. Enhanced expression of 70-kilodalton heat shock protein limits cell division in a sepsis-induced model of acute respiratory distress syndrome. Crit Care Med. 2008;36(1):246–55. doi: 10.1097/01.CCM.0000295473.56522.EF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin X, Kong J, Wu Q, et al. Effect of TLR4/MyD88 signaling pathway on expression of IL-1β and TNF-α in synovial fibroblasts from temporomandibular joint exposed to lipopolysaccharide. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/329405. 329405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katare PB, Bagul PK, Dinda AK, Banerjee SK. Toll-like receptor 4 inhibition improves oxidative stress and mitochondrial health in isoproterenol-induced cardiac hypertrophy in rats. Front Immunol. 2017;8:719. doi: 10.3389/fimmu.2017.00719. [DOI] [PMC free article] [PubMed] [Google Scholar]