Abstract
Background:
Chaetogaster limnaei is an annelid symbiotically associated with lymnaeid snails as Galba truncatula. This association is considered a preventive mechanism against trematode miracidia infection, including Fasciola hepatica. The objective of this study was to determine the effectiveness of Chaetogaster limnaei as a natural controller of Fasciola hepatica in laboratory conditions.
Procedures:
Fascsola hepatica miracidia were inoculated in parallel into snails carrying Chaetogaster limnaei and snails without the annelid. The degree of infection was measured after 40 days of exposure. Furthermore, the number of annelids per snail was quantified, as well as the ability of Chaetogaster limnaei to devour miracidia at different times of exposure.
Results:
An effective 70% natural control of Fasciola hepatica in Galba truncatula was observed. The carrying capacity of the snail was established to be of 10.6 ± 1 annelids. Chaetogaster limnaei is a predator of Fasciola hepatica devouring an average of 3.79 ± 0.21 miracidia. The results of these experiments have a potential value as a control measure against fascioliasis in the environment.
Keywords: Chaetogaster limnaei, Fasciola hepatica, Galba truncatula, miracidia, natural control
INTRODUCTION
Fasciola hepatica (Linnaeus, 1758) the main etiologic agent of fasciolosis in the world is the only known in America. Fasciolosis is a parasitic zoonosis of global concern. The WHO estimates that between 2.4 million[1,2] and 17 million[3] people are affected and 180 million are at risk of contracting the disease.[4] Fasciolosis is also an important veterinary parasitic disease with negative economic repercussions in the livestock industry. Direct losses are represented by cases of livestock death and meat confiscation. Indirect losses, caused by subclinical conditions, are higher[5] and they refer to reduced growth rates, reduced milk and meat production, adverse effects on the quantity and quality of wool, interference with fertility and fecundity, greater receptivity to other infections, and/or expensive treatment cost.[6]
The human and animal fascioliasis has not decreased despite the availability of a wide range of antiparasitic drugs. The prevention of this disease does not entirely consider the biological factors involved.[7,8] In order to fight a multi-host parasite as F. hepatica is crucial to understand the relationships between its larval stages with other organisms that take part in the completion of its lifecycle. In addition, more environment-friendly preventive measures against fascioliasis are necessary. At present only the application of chemicals is available and extensively applied in breeding zones such as the Andean area of Cusco (Peru), considered as hyperendemic for this parasitic disease.[9] In fact, new biotopes of the snail Galba truncatula (Müller, 1774), the most common intermediate host in the lifecycle of F. hepatica in Europe, were reported in this region.[10] Therefore, this zoonosis has spread worldwide and needs more effective counteractive measures.
The F. hepatica lifecycle starts when an infected definitive host (a mammal) spreads thousands of F. hepatica eggs through its feces into the environment. In adequate conditions, these eggs release a high number of miracidia in the water. The miracidium, a free-swimming ciliated larva, seeks out and penetrates a lymnaeid snail host (including the genera Galba, Fossaria and Pseudosuccinea) in which it develops asexually into sporocysts, rediae until around 500 cercariae.[7,11] The cercariae release from the snail and encyst as metacercariae (infective larva) on aquatic vegetation. Humans and other mammals acquire the infection usually by eating freshwater plants.
How stop this lifecycle naturally? An alternative can derive from Chaetogaster limnaei (von Baer, 1827). Lymnaeid snails as G. truncatula are also optimal hosts for C. limnaei annelids with whom they develop a symbiotic relationship. The snail provides shelter and locomotion while the annelid is able or not to offer protection against miracidia infection. A confirmed symbiotic mutualism between G. truncatula and C. limnaei could interrupt the F. hepatica lifecycle.
C. limnaei is a segmented annelid that lives in fresh water and moist soil.[12] This worm has an unusual symbiotic lifestyle: living in the mantle cavity of lymnaeid snails, feeding from small and ectosymbiont fauna as rotifers and algae. However, C. limnaei also lives in renal tissue of host snails, feeding on kidney cells and larval trematodes.[13] These different lifestyles allowed the hypothesis that there are two different subspecies of C. limnaei occupying two different locations in the snail (ectosymbionts and endosymbionts).[13] Based on these observations, two subspecies were established: C. limnaei limnaei (ectosymbiont) that lives between the shell and the body of the snail and C. limnaei vaghini (endosymbiont) that is parasitic.[14]
To estimate the effectiveness of C. limnaei as a controller of F. hepatica in laboratory conditions, two different biotopes of G. truncatula (carrier and non-carrier of C. limnaei) were localized in breeding areas. The subspecies of C. limnaei were identified by anatomic characteristics. The collected snails were maintained in bioterium. Then, miracidia of F. hepatica obtained in vitro were inoculated into these snails to quantify infection degree after 40 days of exposure. Additionally, the number of annelids as well as the number of devoured miracidia were quantified at different times of experimental infection.
METHODS
G. truncatula snails carriers of C. limnaei were collected from La Joya area; along the Pitumayo River, in Anta, Cusco, Peru; located at 13° 28’ 51.0” LS, 72° 16’ 44.9” LW at an altitude of 3268 m. G. truncatula snails noncarriers of C. limnaei were collected from the Rumitabla stream; in San Jeronimo, Cusco, Peru; located at 13° 32’ 21.9” LS, 71° 52’ 15.6” LW, at an altitude of 3324 m. The snails were maintained in aquariums under laboratory conditions. Since naturally infected snails show gigantism,[15,16,17] the large snails were separated.
Morphological characteristics allowed the C. limnaei subspecies identification of six annelids following the next taxonomic keys:[13,14] Four segments of the ectosymbiont C. limnaei exhibit a representative media of setae: Segment two: 18.7, Segment six: 16.3, Segment seven: 19.2, Segment eight: 20.1, corresponding to C. limnaei limnaei. Meanwhile, the ectosymbiont C. limnaei shows a fewer number of setae: Segment two: 12.4, Segment six: 12.0, Segment seven: 13.1, Segment eight: 12.9, corresponding to C. limnaei vaghini.
F. hepatica miracidia were obtained in vitro at 26°C in the presence of light after 12 days of incubation.[18] The miracidia were distributed in a number of 10 per well (Nunc MaxiSorp®– ELISA plates). 30 snails carrier and 30 non-carriers of C. limnaei were individually exposed to 10 miracidia for 15 min. The two snail groups were maintained for 40 days post-infection in aquarium.[10] Afterward, the snails were dissected by squash on a Petri dish[19] and analyzed under stereomicroscope observation to confirm F. hepatica infection in the snail's tissues,[20] as well to determine the degree of development of larval stages.
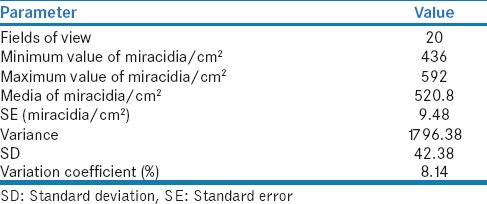
Same age miracidia hatch at about the same time if the incubation conditions are identical.[18] Before hatching, the miracidia density was estimated after 11 incubation days.[18] Their holder Petri dish was placed on a millimeter sheet of paper to count the number of embryonated eggs per area. The embryonated eggs carry a well-differentiated miracidium larva in contrast to the non-embryonated eggs as the stereomicroscope observation revealed.[18]20 randomly selected areas of 25 mm2 were registered.
Successively, 15 snails carrying C. limnaei were divided into three groups of 5 individuals (Group I, II and III). Each group was placed in the Petri dish containing 1.5 h hatched miracidia for 5, 10, and 15 min respectively. After miracidia exposure, the snails were dissected by squash technique.[19] The number of C. limnaei hosted by snail were quantified under stereomicroscope observation. Subsequently, each C. limnaei was isolated and observed under a light microscope to quantify the number of miracidia inside each annelid's stomach.
Statistical analysis
Correlations between the number of setae to identify C. limnaei subspecies were calculated by the Pearson's r coefficient. The miracidia density was analyzed by univariate statistics. The infected snails by F. hepatica were compared using Chi-square test. The eaten miracidia in three different times were tested by one-way ANOVA using the package SPSS v. 19.0 for Windows (SPSS, Inc, Chicago, IL).
RESULTS
The number of setae in the segments two [Figure 1], six, seven, and eight [Figure 2] of C. limaei [Table 1] were counted by microscopic observation. These average number of setae are closer to the ectosymbiont subspecies of C. limnaei (Pearson's r = 0.982, P = 0.05) than the endosymbiont subspecies (Pearson's r = 0.944, P = 0.05). So, there is statistical evidence to assume that this subspecies is C. limnaei limnaei.
Figure 1.
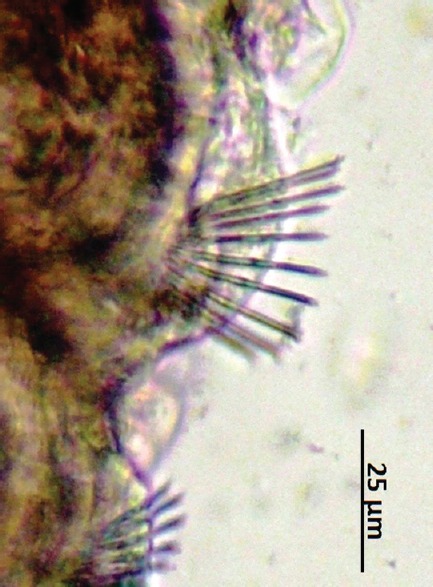
Segment two of Chaetogaster limnaei
Figure 2.
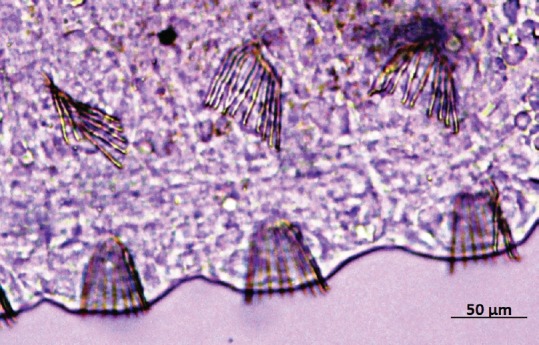
Segments six, seven, and eight of Chaetogaster limnaei
Table 1.
Identification of sub-species Chaetogaster limnaei by the number of setae

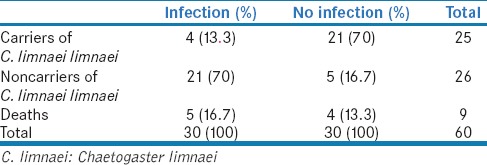
The effectiveness of C. limnaei as a controller of F. hepatica are shown in Table 2. It was found that C. limnaei limnaei prevents the F. hepatica infection in 70% in G. truncatula against 16.7% of infection. The chi-square test indicates that C. limnaei is a natural controller of F. hepatica with high confidence (P < 0.01) in this experimental infection.
Table 2.
Fasciola hepatica infection in snails Galba truncatula carriers and noncarriers of Chaetogaster limnaei limnaei
Besides, the miracidia density and other parameters were measured [Table 3] in order to estimate the number of eaten miracidia by C. limnaei limnaei per unit time.
Table 3.
Number of Fasciola hepatica miracidia per unit area
During the real-time observation of the experimental infection [Videos 1–3], it was verified that C. limnaei limnaei is able to eat F. hepatica miracidia [Figure 3] which were quantifiable after sacrificing the snails and isolating each annelid [Table 4]. Furthermore miracidia larvae, F. hepatica eggs also were devoured but in reduced proportion [Figure 4].
Figure 3.
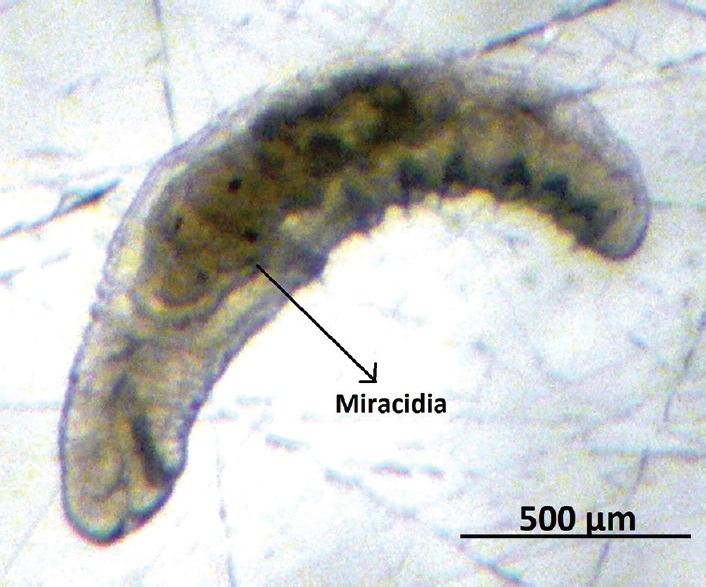
Chaetogaster limnaei limnaei contains miracidia inside its stomach
Table 4.
Quantification of Chaetogaster limnaei and Fasciola hepatica miracidia in experimental infections of Galba truncatula at different times of exposure
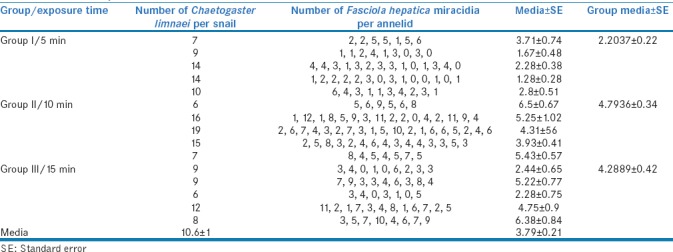
Figure 4.

Chaetogaster limnaei limnaei contains a Fasciola hepatica egg inside its stomach
Regarding the C. limnaei ability to devour miracidia at different times of exposure, the one-way ANOVA (F = 17.7, P = 0.000) confirmed a significant difference in at least one group. The post hoc analysis by Tukey's (P = 0.000) confirmed a significant difference in the number of devoured miracidia after 5 min of exposure with respect the other two groups. Meanwhile, there is not a significant difference between 10 and 15 min.
DISCUSSION
C. limnaei limnaei did not allow the F. hepatica development with a 70% effectiveness in G. truncatula snails. This result matches with Rodgers et al. (2005),[21] in the United States, who reported an 85% protection in snail carriers of C. limnaei using Schistosoma mansoni miracidia and Biomphalaria glabrata snails. Meanwhile, Ibrahim (2007),[22] in Germany, after studying the C. limnaei prevalence in lymnaeid snails, concluded that C. limnaei have implications for the biological control of trematodes. Nevertheless, three important limitations observed in this control: (i) body size, (ii) time of effective protection and (iii) location of C. limnaei limnaei on the snail body, will be discussed.
The body size of C. limnaei limnaei (about 200 μm) cannot devour mature trematode larvae as sporocysts (550 μm), rediae (3 mm), or cercariae (270 μm) of F. hepatica[8] Moreover, sporocysts and rediae develop only inside the intermediate snail. The endosymbiont C. limnaei vaghini is perhaps a larger controller for these internal larvae as reported by Rajasekariah (1978).[23] Despite the size disadvantage, it is remarkable the C. limnaei limnaei ability to move and catch the swimming miracidia [Videos 1–3] as well as few eggs of F. hepatica. [Figure 4] This natural protection has confirmed a mutualistic relationship between C. limnaei limnaei and G. truncatula.
To figure out the time limitation of the protection provided by C. limnaei limnaei three groups of snails were exposed in a liquid media containing 520.8 ± 9.48 miracidia/cm2. for 5, 10 and 15 min. The number of devoured miracidia by C. limnaei limaei were 2.2 ± 0.2 and 4.8 ± 0.3 after 5 and 10 min respectively. This positive correlation suggests an initial effective protection. However, after 15 min, the number of devoured miracidia lightly decreased to 4.3 ± 0.42. These results indicate that the natural control provided is maintained over time. In this experiment a notable 10 min post miracidia exposure. After that, the annelids probably got filled with miracidia [Figure 3]. Fortunately, to find such a high density of miracidia in the environment, where this larva tends to spread, is improbable.
Given that C. limnaei limnaei location is preferably between the mantle and the shell of the snail. The provided protection against miracidia infection is carried out mainly around the shell in a limited span. Consequently, miracidia penetration can still occur in ‘vulnerable areas’ such as the foot, head, and tentacles of the snail. This vulnerability can explain the 16,7 % of infection obtained in snails carrying C. limnaei limnaei. Nevertheless, the change of success of miracidia infection is reduced because the epithelial in these areas are thicker than the mantle epithelial.[16]
CONCLUSION
C. limnaei limnaei is a predator of F. hepatica miracidia. The association between this ectosymbiont annelid and G. truncatula snails has a potential value as a natural control against fascioliasis, with a 70% of effectiveness versus 16.7% of infection. Taking these results together, a clear implication for future studies involves the application of this natural control provided by C. limnaei limnaei as one more efficient ‘biological control’ in lymnaeid snails biotopes from regions with high fascioliasis prevalence.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on: www.tropicalparasitology.org
REFERENCES
- 1.Rim HJ, Farag HF, Sornmani S, Cross JH. Food-borne trematodes: Ignored or emerging?? Parasitol Today. 1994;10:207–9. doi:10.1016/0169-4758(94)90111-2. [Google Scholar]
- 2.Becerra-Rozo M. Considerations on sustainable strategies for the control of Fasciola hepatica in Latin America. Rev Colomb Cienc Pecu. 2001;14:28–35. [Google Scholar]
- 3.Hopkins DR. Homing in on Helminths. Am J Trop Med Hyg. 1992;46:626–34. doi: 10.4269/ajtmh.1992.46.626. doi:10.4269/ajtmh.1992.46.626. [DOI] [PubMed] [Google Scholar]
- 4.Kujawa M. Control of Foodborne Trematode Infections. WHO Technical Report Series 849. 157 Seiten, 4 Tabellen. World Health Organization, Geneva 1995. Preis: 26,– Sw.fr. Food Nahr. 1996;40:166–166. doi:10.1002/food.19960400342. [Google Scholar]
- 5.Valero MA, De Renzi M, Panova M, Garcia-Bodelon MA, Periago MV, Ordoñez D, et al. Crowding effect on adult growth, pre-patent period and egg shedding of Fasciola hepatica. Parasitology. 2006;133:453–63. doi: 10.1017/S003118200600059X. doi:10.1017/S003118200600059X. [DOI] [PubMed] [Google Scholar]
- 6.Rojo-Vázquez FA, Ferre-Pérez I. Fasciolosis. In: Cordero del Campillo M, Rojo-Vázquez FA, editors. Parasitología Veterinaria. 1st ed. Madrid: McGraw Hill Interamericana; 1999. pp. 260–272. [Google Scholar]
- 7.Quiroz-Romero H. Parasitología y enfermedades parasitarias de animales domésticos. 1st ed. Mexico: Limusa; 2003. [Google Scholar]
- 8.Carrada-Bravo T. Fasciola hepatica: Ciclo biológico y potencial biótico. Rev Latinoam Patol Clínica Med Lab. 2007;54:21–7. [Google Scholar]
- 9.Mas-Coma S. Epidemiology of fascioliasis in human endemic areas. J Helminthol. 2005;79:207–16. doi: 10.1079/joh2005296. [DOI] [PubMed] [Google Scholar]
- 10.Iturbe-Espinoza P, Muñiz Pareja F. Galba truncatula induced to infection with miracidia of Fasciola hepatica, collected in Huayllapampa, San jerónimo, Cusco, Peru. Neotropical Helminthol. 2012;6:211–7. [Google Scholar]
- 11.Mas-Coma S, Funatsu IR, Bargues MD. Fasciola hepatica and lymnaeid snails occurring at very high altitude in South America. Parasitology. 2001;123(Suppl):S115–27. doi: 10.1017/s0031182001008034. [DOI] [PubMed] [Google Scholar]
- 12.Storer T, Usigner R, Estebbins R, Nybakken J. Zoología General. 6th ed. New York: McGraw-Hill Book Company; 2003. [Google Scholar]
- 13.Gruffydd LD. Evidence for the existence of a new subspecies of Chaetogaster limnaei (Oligochaeta), in Britain. Proc Zool Soc Lond. 1965;146:175–96. doi:10.1111/j.1469-7998.1965.tb05208.x. [Google Scholar]
- 14.Patti A, Hochberg R, Litvaitis MK. Taxonomy and evolution of the Chaetogaster limnaei complex (Annelida: Oligochaeta) Integr Comp Biol. 2009;49(Suppl 1):E287. [Google Scholar]
- 15.Wilson RA, Denison J. The parasiticcastration and gigantism of Lymnaea truncatula infected with the larval stages of Fasciola hepatica. Z Parasitenkd Berl Ger. 1980;61:109–19. doi: 10.1007/BF00925458. [DOI] [PubMed] [Google Scholar]
- 16.Ginetsinskaya T. Trematodes: Their Life Cycles, Biology and Evolution. New Delhi: Amerind Publishing Co. Pvt. Ltd; 1988. [Google Scholar]
- 17.Dalton JP, editor. Fasciolosis. Dublin City University: CABI Publishing; 1999. [Google Scholar]
- 18.Iturbe-Espinoza P, Muñiz Pareja F. Development of eggs from Fasciola hepatica eggs isolated of gallbladder of sheep and cattle, exposed to light and darkness. Neotropical Helminthol. 2011;5:89–93. [Google Scholar]
- 19.Olazabal-Manso E, Morales-Monteagudo A, Serrano-Pérez H, Brito A. Fasciola hepatica metacercarie collection in Lymnaea cubensis, and parasite-host-relation in Winstar rats and Balb/c mice. Vet Méico. 1999;30:109–15. [Google Scholar]
- 20.Iturbe-Espinoza P, Muñiz Pareja F. Life cycle and biotic potential of Fasciola hepatica in Galba truncatula. Neotrop Helminthol. 2013;7:243–44. [Google Scholar]
- 21.Rodgers JK, Sandland GJ, Joyce SR, Minchella DJ. Multi-species interactions among a commensal (Chaetogaster limnaei limnaei), a parasite (Schistosoma mansoni), and an aquatic snail host (Biomphalaria glabrata)? J Parasitol. 2005;91:709–12. doi: 10.1645/GE-421R. doi:10.1645/GE-421R. [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim MM. Population dynamics of Chaetogaster limnaei (Oligochaeta: Naididae) in the field populations of freshwater snails and its implications as a potential regulator of trematode larvae community? Parasitol Res. 2007;101:25–33. doi: 10.1007/s00436-006-0436-0. doi:10.1007/s00436-006-0436-0. [DOI] [PubMed] [Google Scholar]
- 23.Rajasekariah GR. Chaetogaster limnaei K von Baer 1872 on Lymnaea tomentosa: Ingestion of Fasciola hepatica cercariae. Experientia. 1978;34:1458–9. doi: 10.1007/BF01932351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


