Introduction
There is compelling evidence that children exposed to high levels of prenatal stress are at risk for a host of adverse health outcomes [1, 2, 3, 4, 5, 6, 7]. Prenatal maternal stress (PNMS) increases fetal exposure to stress biomarkers (e.g., cortisol and pro-inflammatory cytokines) and may alter the development of critical biological systems (e.g., the endocrine, immune, and nervous systems) [8, 9, 10]. This confers lasting susceptibility to health complications in the child [2, 4, 5, 6] and potentially transgenerational risks through epigenetic programming [8, 9, 11]. There is also accumulating evidence that the child’s biological sex may modify the effect of PNMS on child health [12, 13, 14, 15]. Consequently, many scholars have underscored the need to evaluate for sex-dependent effects in PNMS programming models [12, 13, 15, 16, 17, 18].
The potential sex-dependent nature of PNMS programming is intuitive given well-established sex differences in human embryonic and fetal development [19, 20]. The placenta is sexually dimorphic both in terms of its biological makeup [15, 20] and its adaptations within the intrauterine environment [12, 15, 16, 20, 21]. Female and male fetuses generally employ different evolutionary strategies to optimize fitness. Males invest heavily in growth but are less adaptable to environmental challenges in utero. In contrast, female fetuses are more responsive to environmental stress signals and are better able to adapt to prenatal insults (e.g., deficient nutrient supply) [13, 15, 16, 20, 21]. The female fetus is also better protected from inflammatory processes that could compromise viability [13, 16]. Thus, females are better positioned to survive suboptimal intrauterine conditions, whereas males are more susceptible to severe consequences of intrauterine adversity (e.g., mortality or pervasive disability) [12]. However, the female viability advantage appears to come with increased vulnerability to less severe but lasting health complications (e.g., anxiety and depression) [12, 13, 14]. PNMS programming of HPA-axis sensitivity and internalizing behaviors in females may also promote adaptive caregiving behaviors that increase survival in the next generation [17].
In sum, PNMS is hypothesized to alter embryonic and placental functioning, contributing to lifespan health complications. There is accumulating evidence for sex differences in PNMS programming models and strong biological plausibility given the sexually dimorphic nature of prenatal development.
The purpose of this paper is to review the recent literature evaluating sex-dependent effects of PNMS on child health outcomes. Clarifying the nature of sex-dependent PNMS programming may lead to a stronger understanding of lifelong disease processes and facilitate tailored intervention approaches. We conceptualized “stress” broadly, including psychological distress (e.g., perceived stress and psychopathology symptoms), challenging life events (e.g., daily hassles and natural disasters), and established stress biomarkers (e.g., maternal cortisol). For clarity, we distinguish between two types of sex-dependent associations. Sex-specific effects indicate that the PNMS➔outcome association is significant for one sex but not the other. Importantly, a sex-specific association does not provide evidence that the PNMS➔outcome association was significantly stronger in one sex than the other. Sex interactions, in contrast, indicate that the magnitude of the PNMS➔outcome association differs significantly for female and male offspring.
Method
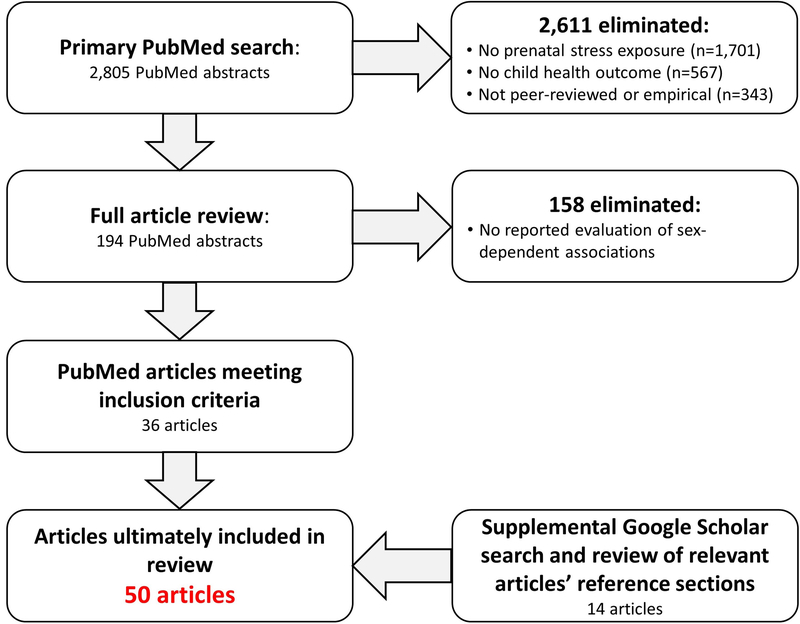
We searched PubMed on January 18, 2018, for articles evaluating associations between PNMS and child health outcomes. We limited the results to human-subject studies published in English between January 2015 and December 2017 (see online supplement for search criteria). The primary search returned 2805 abstracts (Fig. 1), which were screened by the authors and included if they:
Measured prenatal maternal stress as an exposure variable
Measured some form of offspring health or development as an outcome, with no restrictions on offspring age at the time of outcome ascertainment
Described an empirical study published in a refereed journal
Reported evaluating whether PNMS programming effects were sex-dependent
Fig. 1.
Literature review search strategy results
We found additional articles by conducting a secondary Google Scholar search (using the same search criteria) and by reviewing reference lists of relevant papers.
Results
We identified 50 peer-reviewed articles meeting the inclusion criteria (Table 1). The majority of included articles reported at least one sex-dependent association (k = 35; 70%), either PNMSxsex interactions (k = 17; 34%) or sex-specific effects (k = 18; 36%). Most studies had modest sample sizes (Mdn = 205.5, first quartile = 107.2, third quartile = 465.0), and it is likely that most were not optimally powered to detect sex differences [71, 72]. Studies reporting sex-dependent associations (either sex-specific associations or interactions) had a similar median sample size (Mdn = 216) compared to those that did not (Mdn = 194): W = 247, p = .75. Similarly, studies finding significant sex interactions had a similar median sample size (Mdn = 212) compared to those that did not (Mdn = 199): W = 304, p = .64.
Table 1.
Characteristics of Articles Included in the Review
| Article | N | Prenatal Maternal Stress Exposure Variable | Timing of Exposure Measurement | Child Health Outcome Categories |
|---|---|---|---|---|
| NO SEX-DEPENDENT EFFECTa (k=15; 30%) | ||||
| Kingsbury et al. 2016 | 9,166 | Life Events; Psychological: Perceived impact of events |
18 weeks gestations | Emotional/Behavioral: Depression/anxiety symptoms |
| O'Donnell et al. 2017b | 6,969 & 425 | Psychological: Anxiety & depression | 18 and 32 weeks gestation | Cognitive Development; Emotional/Behavioral: ADHD/conduct symptoms |
| Gaillard et al. 2016 | 1,116 | Biological: C-reactive protein plasma level | Second trimester | Physical: Body composition |
| Mansell et al. 2016 | 481 | Psychological: Perceived stress & depression | 28 weeks gestation | Physical: Neural/nervous system development |
| Giesbrecht et al. 2015 | 291 | Biological: Maternal cortisol and salivary alpha-amylase; Psychological: Depression and anxiety |
22 weeks and 32 weeks gestation | Physical: Fetal/neonatal health |
| Koutra et al. 2017 | 288 | Psychological: Anxiety & depression | 28-32 weeks gestation | Physical: Motor development; Cognitive Development; Emotional/Behavioral: ADHD/conduct symptoms & broad psychopathology measures |
| Lee et al. 2017 | 199 | Life Events | M=28.4 weeks gestation | Physical: Respiratory health |
| Rash et al. 2015 | 194 | Biological: Salivary cortisol; Psychological: Depression |
14 & 32 weeks gestation | Physical: Neural/nervous system development |
| Simcock et al. 2016 | 130 | Natural Disaster; Psychological: Perceived impact of events & related symptoms |
Any time during pregnancy | Physical: Motor development |
| Liu et al. 2016 | 111 | Natural Disaster; Psychological: Perceived impact of events & related symptoms |
Various times in pregnancy | Physical: Body composition |
| Dancause et al. 2015 | 106 | Natural Disaster; Psychological: Perceived impact of events & related symptoms |
Various times in pregnancy | Physical: Body composition |
| Davis et al. 2017 | 91 | Biological: Plasma cortisol | 19 and 31 weeks gestation | Physical: Neural/nervous system development; Cognitive Development |
| Braithwaite et al. 2016 | 88 | Psychological: Depression; Biological: Cortisol reactivity |
Second or third trimester | Physical: Neural/nervous system development |
| Entringer et al. 2017 | 67 | Biological: Cumulative cortisol production | Repeated throughout pregnancy | Physical: Body composition |
| St-Hilaire et al. 2015 | 54 | Natural Disaster; Psychological: Perceived impact of events |
Any time during pregnancy | Emotional/Behavioral: Eating disorder symptoms |
| SIGNIFICANT SEX INTERACTIONc (k=17; 34%) | ||||
| Quarini et al. 2016 | 7,959 | Psychological: Depression | 18 and 32 weeks gestation | Emotional/Behavioral: Depression/anxiety symptoms |
| Gilman et al. 2016 | 1,258 | Biological: Inflammatory cytokines | Late 2nd or early 3rd trimester | Emotional/Behavioral: Depression/anxiety symptoms |
| Bendiksen et al. 2015 | 1,195 | Psychological: Anxiety & depression | 17 & 30 weeks gestation | Emotional/Behavioral: ADHD/conduct symptoms |
| Maselko et al. 2016 | 885 | Psychological: Depression | Third trimester | Emotional/Behavioral: Broad psychopathology measures |
| Bandoli et al. 2016 | 344 | Psychological: Depression | 32 weeks gestation | Physical: Motor development; Cognitive Development |
| Fineberg et al. 2016 | 301 | Life Events; Psychological: Anxiety, negative affect |
Second trimester | Emotional/Behavioral: Schizophrenia spectrum disorders |
| Giesbrecht et al. 2017 | 236 | Biological: Cortisol; Psychological: Distress |
14 weeks and 32 weeks | Physical: Neural/nervous system development |
| Braithwaite, Pickles et al. 2017 | 216 | Biological: Cortisol | Measured at 32 weeks | Emotional: Temperament |
| Kaitz et al. 2015 | 212 | Psychological: Anxiety | Third Trimester | Physical: Fetal/neonatal health |
| Doyle et al. 2015 | 125 | Psychological: Negative mood; Biological: Cortisol, diastolic blood pressure, immune markers |
Second and Third Trimester | Physical: Neural/nervous system development |
| Simcock, Elgbeili et al. 2017 | 121 | Natural Disaster; Psychological: Perceived impact of events & related symptoms |
General Pregnancy (Timing measured) | Emotional/Behavioral: Temperament |
| Simcock, Laplante et al. 2017 | 115 | Natural Disaster; Psychological: Perceived impact of events & related symptoms |
General Pregnancy (Timing measured) | Cognitive Development |
| Yong Ping et al. 2015 | 94 | Natural Disaster; Psychological: Perceived impact of events & related symptoms |
General Pregnancy | Physical: Neural/nervous system development |
| Braithwaite, Murphy et al. 2017 | 88 | Biological: Cortisol and salivary alpha amylase; Psychological: Depression |
27 weeks gestation | Emotional/Behavioral: Temperament |
| Braithwaite et al. 2015 | 57 | Psychological: Depression; Biological: Cortisol |
2nd or 3rd trimester | Physical: Neural/nervous system development |
| Lebel et al. 2016 | 52 | Psychological: Depression | Measured in each trimester | Physical: Neural/nervous system development |
| Vangeel et al. 2017 | 45 | Psychological: Anxiety | Measuring in each trimester | Physical: Neural/nervous system development |
| SEX-SPECIFIC EFFECTd (k=18; 36%) | ||||
| Zhu et al. 2015 | 1,765 | Life Events | Cumulative: Prenatal Report | Emotional/Behavioral: ADHD/conduct symptoms |
| Herbison et al. 2017 | 1,214 | Life Events | 18 & 34 weeks gestation | Emotional/Behavioral: Depression/anxiety symptoms |
| Frith et al. 2015 | 1,041 | Morning cortisol concentration | 28-32 weeks gestation | Physical: Fetal/neonatal health |
| Pickles et al. 2017 | 813 | Psychological: Anxiety, depression | 1st and 2nd trimester | Emotional/Behavioral: Broad psychopathology measures |
| Lee et al. 2016 | 765 | Life Events | Cumulative: Prenatal report | Physical: Respiratory health |
| Rosa et al. 2016 | 417 | Life Events | 2nd or 3rd trimester | Physical: Respiratory health |
| Stinson et al. 2015 | 262 | Biological: Cortisol | 3rd trimester | Physical: Adult cardiovascular health |
| Soe et al. 2016 | 258 | Psychological: Depression | 2nd trimester | Physical: Neural/nervous system development; Emotional/Behavioral: Broad psychopathology measures; |
| Plamondon et al. 2015 | 236 | Life Events; Psychological: Anxiety, depression |
Cumulative: Prenatal Report | Cognitive Development |
| Wen et al. 2017 | 235 | Psychological: Depression | Measured at 26 weeks | Physical: Neural/nervous system development |
| Edwards et al. 2016 | 196 | Psychological: Depression | Cumulative: Prenatal report | Emotional/Behavioral: Broad psychopathology measures; Physical: Fetal/neonatal |
| Gholipoor et al. 2017 | 158 | Psychological: Perceived stress; Life events |
Any time during pregnancy | Physical: Neural/nervous system development |
| Stroud et al. 2016 | 153 | Psychological: Depression | Any time during pregnancy or within 3 months of conception | Physical: Neural/nervous system development |
| Moss et al. 2017 | 145 | Natural Disaster; Psychological: Perceived impact of events & related symptoms |
Pregnancy | Cognitive Development; Physical: Motor development |
| Simcock, Kildea et al. 2017 | 130 | Natural Disaster; Psychological: Perceived impact of events & related symptoms |
General Pregnancy (Timing measured) | Cognitive Development |
| Mina et al. 2015 | 93 | Psychological: Anxiety and depression; Biological: Cortisol |
17 and 28 weeks of gestation | Physical: Neural/nervous system development |
| van den Heuvel et al. 2015 | 90 | Psychological: Anxiety | 20.5 weeks gestation | Emotional/Behavioral: Temperament |
| Kim et al. 2017 | 49 | Biological: Cortisol; Psychological: Anxiety, depression |
5 times throughout pregnancy | Physical: Neural/nervous system development Emotional/Behavioral: Broad psychopathology measures |
Articles evaluating sex-dependent associations between prenatal stress and child health outcomes but found no evidence of sex-dependence
This article included two studies and sample sizes for both are listed.
Articles reporting significant stressxsex interactions in predicting child health outcomes
Articles reporting significant sex-specific associations between the exposure and outcome (a significant association in one sex but not the other)
For ease of presentation, we grouped the results into three major categories based on types of child health outcomes: physical health and development, cognitive development, and emotional/behavioral health (Table 2). Eight studies reported evaluating for sex-dependent associations with outcomes from multiple child health categories.
Table 2.
Evidence of Sexual Dimorphism by Outcome Category
| ka | Studies with sex-specific associationsb | Studies with sex interactionsc | |
|---|---|---|---|
| Physical Health & Development | 32 | ||
| Respiratory Health | 3 | k=2: Lee et al. 2016; Rosa et al. 2016 | None |
| Fetal/Neonatal Health | 4 | k=1: Frith et al. 2015 | k=1: Kaitz et al. 2015 |
| Body Composition | 4 | None | None |
| Motor Development | 4 | k=1: Moss et al. 2017 | k=1: Bandoli et al. 2016 |
| Adult Cardiovascular Health | 1 | k=1: Stinson et al. 2015 | None |
| Neural/Nervous System Development | 16 | k=6: Wen et al. 2017; Soe et al. 2016; Kim et al. 2017; Stroud et al. 2016; Gholipoor et al. 2017; Mina et al. 2015 | k=6: Lebel et al. 2016; Doyle et al. 2015; Yong Ping et al. 2015; Giesbrecht et al. 2017; Braithwaite et al. 2015; Vangeel et al. 2017 |
| Cognitive Development | 8 | k=2: Simcock, Kildea et al. 2017; Plamondon et al. 2015 | k=2: Simcock, Laplante et al. 2017; Bandoli et al. 2016 |
| Emotional/Behavioral Health | 19d | ||
| Temperament | 4 | k=1: van den Heuval et al. 2015 | k=3: Braithwaite, Pickles et al. 2017; Braithwaite, Murphy et al. 2017; Simcock, Elgbeili et al. 2017; |
| Broad Psychopathology Measures | 6 | k=4: Soe et al. 2016; Edwards & Hans 2016; Pickles et al. 2017; Kim et al. 2017 | k=1: Maselko et al. 2016 |
| Depression/Anxiety Symptoms | 5 | k=1: Herbison et al. 2017 | k=2: Quarini et al. 2016; Gilman et al. 2016 |
| ADHD/Conduct Symptoms | 4 | k=1: Zhu et al. 2015 | k=1: Bendiksen et al. 2015 |
| Eating Disorder Symptoms | 1 | None | None |
| Schizophrenia Spectrum Disorders | 1 | None | k=1: Fineberg et al. 2016 |
Number of studies within each child health outcome category with some studies measuring outcome variable from multiple categories
Indicates that the exposure→outcome association was significant in one sex but not the other,
Indicates that the effect among boys and girls differed significantly
The number of studies summed across the subcategories exceeds this total number (representing the total number of unique studies measuring Emotional/Behavioral Health outcomes) because some studies measured multiple Emotional/Behavioral Health outcomes across subcategories and are included in multiple rows
Physical Health and Development Outcomes
Thirty papers evaluated sex-dependent associations related to the child’s physical health or development. We further divided these papers into six subcategories.
Respiratory Health
Wright and colleagues published three papers focused on the effects of prenatal negative life events (PNLEs) on respiratory outcomes, with two reporting sex-dependent associations. Lee et al. found negative associations between PNLEs and several indicators of lung function at age 7 (N = 199), with no evidence of sex-dependent associations [28]. A second paper by this group (N = 765) showed a sex-dependent association whereby PNLEs were associated with increased odds of asthma at age 4 in boys but not girls. However, among girls, there was evidence of a combined effect of prenatal and postnatal stress on the odds of asthma [58•]. In the third study (N = 417), there was a sex-specific association whereby PNLEs were positively associated with risk for lifetime wheeze at age 4 among boys only [59].
Fetal/Neonatal Health
Four studies evaluated fetal/neonatal health outcomes with two reporting sex-dependent associations. Edwards et al. (N = 196) evaluated the association between prenatal maternal depression and a global measure of neonatal health problems (e.g., prematurity, low Apgar scores, low birth weight), with no evidence of sex-dependent associations [73]. Within the context of a randomized controlled trial (N = 1041), Frith et al. found that maternal prenatal salivary cortisol concentration was negatively associated with infant birth weight and head circumference only among male children whose mothers were randomized to the usual care condition [56]. Kaitz et al. (N = 212) reported significant interactions between maternal third trimester anxiety and child sex in predicting both third trimester fetal weight and birth weight. In both cases, boys weighed more than girls when exposed to prenatal anxiety, but there were no sex differences in its absence [45•]. Finally, Giesbrecht et al. (N = 291) reported that fetal sex appeared to have an effect on maternal prenatal stress physiology (salivary alpha-amylase and cortisol), which in turn was associated with child birth weight. However, there was no evidence that the strength of the association between maternal stress physiology and child birth weight differed by child sex [26].
Body Composition
Four studies evaluated child body composition with none finding sex-dependent associations. (Note, studies evaluating fetal weight and birth weight were included in the Fetal/Neonatal Health section.) In the Iowa Flood Study (N = 106), there was no evidence of sex-dependent associations between PNMS related to a flooding disaster and early childhood BMI and adiposity [32]. Similarly, data from the Quebec Ice Storm study (N = 111) showed no evidence of sex-dependent associations between disaster-related PNMS and child adiposity or BMI (ages 5–15) [31]. Data from Project Viva (N = 1116) provided no evidence of sex-dependent associations between maternal second trimester biomarkers of inflammation and child adiposity outcomes [24]. Finally, Entringer et al. (N = 67) found a positive association between maternal prenatal cortisol production and the rate of increase in infant percent body fat from 1 to 6 months of age, with no evidence of that the association was sex-dependent [35].
Motor Development
Four studies examined child motor development with two reporting sex-dependent associations. The Queensland Flood Study team reported associations between disaster-related PNMS and infant motor outcomes in two papers. In the first (N = 130), there was no evidence of sex-dependent associations between disaster-related PNMS and infant motor development [30]. In the second (N = 145), there was a near-significant interaction between maternal negative cognitive appraisal of flood-related stress and child sex predicting 16-month gross motor skills. Negative cognitive appraisal of the disaster was associated with poorer motor development among girls but not boys [66]. Bandoli et al. examined effects of prenatal alcohol exposure and maternal depression on child psychomotor development at 6 and 12 months of age (N = 344). In the group of infants with prenatal alcohol exposure, there was a significant interaction of maternal prenatal depressive symptoms and child sex in predicting motor development. Among alcohol-exposed girls, but not boys, maternal prenatal depressive symptoms were positively associated with 6-month motor deficits [41•]. Finally, in a prospective birth cohort study (N = 288), there was no evidence of sex-dependent associations between maternal psychopathology symptoms and child motor skills at age 4 [27].
Adult Cardiovascular Health
One study (N = 262) examined prenatal cortisol exposure and adult offspring coronary heart disease risk (mean age = 42) by evaluating data on risk factors such as diabetes and blood pressure. There was evidence of a sex-specific effect with increased cardiovascular risk associated with maternal cortisol only in female adult offspring [60].
Neural/Nervous System Development
Sixteen papers evaluated indicators of child neural and/or nervous system development with 12 reporting sex-dependent associations. Five evaluated effects of PNMS on neural structure and function, four providing evidence of sexually dimorphic programming effects in girls. Wen et al. (N = 235) reported a sex-specific association whereby prenatal depression was positively associated with greater right amygdala volume in 4.5-year-old girls, but not boys [63]. Data from the same study team (N = 258) showed that, among girls only, increases in maternal depressive symptoms from 26-week gestation to 3 months postpartum were associated with greater infant right frontal lobe activity and greater right frontal asymmetry at age 6 months and greater lower right frontal functional connectivity at age 18 months [61•]. Kim et al. (N = 49) found that, among girls only, prenatal exposure to maternal cortisol was associated with altered neural activity and connectivity at ages 6–9, which in turn mediated the association between prenatal cortisol and internalizing symptoms [70•]. Lebel et al. (N = 52) reported a significant interaction between child sex and second trimester maternal depressive symptoms in predicting cortical thickness in the right-middle temporal region. Maternal depression was negatively associated with cortical thickness with the association significantly stronger in girls than boys [52]. Finally, Davis et al. (N = 91) found significant associations between third trimester maternal cortisol concentrations and brain development, but no evidence of sex dependence [33].
Five studies evaluated associations between PNMS and child neuroendocrine functioning as indicated by cortisol concentrations, four indicating sex-dependent associations. Data from the Iowa Flood Study (N = 94) showed evidence of an interaction: There was a positive association between maternal flood-related subjective stress and child salivary cortisol reactivity (age 2.5 years) among girls, whereas the association was in the opposite direction among boys [49]. Giesbrecht et al. (N = 236) found multiple sex interactions. Less prominent cortisol awakening responses and flatter daytime cortisol slopes in pregnant mothers were associated with blunted cortisol response in infant girls, but the opposite was true in boys. Further, cortisol reactivity in girls differed depending on the combined levels of prenatal maternal distress and cortisol levels. Girls, but not boys, exposed to both low levels of prenatal maternal cortisol and high levels of maternal distress showed elevated stress reactivity at age 3 [43]. Stroud et al. (N = 153) reported a sex-specific effect whereby maternal prenatal major depressive disorder (MDD) was associated with increased infant salivary cortisol reactivity among girls but not boys. Further, among girls only, the positive association between prenatal maternal depressive symptoms on infant baseline cortisol levels was present at low but not high levels of methylation of the placental glucocorticoid gene HSD11B2. In contrast, prenatal MDD was associated with increased infant cortisol levels among boys only in combination with very low or high levels of expression of the placental serotonin gene SLC6A4 [65]. Gholipoor et al. (N = 158) found a sex-specific effect among young children (ages 0–2): Higher maternal prenatal perceived stress was positively associated with circulating levels of blood cortisol among girls but not boys [64]. Finally, Braithwaite (N = 88) found no evidence of associations—sex-dependent or otherwise—between maternal PNMS (depressive symptoms and salivary cortisol reactivity) and infant salivary cortisol reactivity in response to a standard pediatric inoculation [34].
Four papers described epigenetic studies evaluating associations between PNMS and the expression of genes involved in stress and emotion regulation, with three reporting sex-dependent associations. Braithwaite et al. (N = 57) reported an interaction such that maternal prenatal depressive symptoms were positively associated with buccal swab levels of DNA methylation of the NR3C1 glucocorticoid receptor gene in infant boys but not girls. But there was no evidence of sexually dimorphic effects of maternal prenatal cortisol in this study [51]. Similarly, Vangeel et al. (N = 45) found an interaction such that prenatal anxiety was positively associated with cord blood DNA methylation in the GABA-B receptor subunit 1 gene (GABBR1) in infant boys but not girls [53]. Mina et al. found sex-specific associations suggesting that infant girls might be particularly susceptible to the effects of prenatal maternal distress on placental mRNA levels of genes regulating fetal glucocorticoid exposure and placental growth [68]. Finally, data from the Barwon Infant Study (N = 481) showed no evidence of sex dependence in associations between PNMS and cord blood NR3C1 glucocorticoid receptor gene methylation [25].
Two papers describe studies evaluating other indicators of nervous system development. Doyle et al. (N = 125) reported several sex interactions in associations between both psychological (negative mood) and biological (salivary cortisol, blood pressure) markers of PNMS and indicators of fetal central and autonomic nervous system development (heart rate and movement). Findings generally showed that PNMS was associated with variable deviations from normal development in female fetuses, whereas PNMS was associated with consistently accelerated development in males [46]. In contrast, data from the Alberta Pregnancy Outcomes & Nutrition study (N = 194) found no evidence of sex-dependent associations when evaluating effects of both prenatal maternal cortisol and depressive symptoms on an indicator of offspring parasympathetic nervous system functioning (cardiac vagal control) at age 6 months [29].
Cognitive Development
Eight papers evaluated child cognitive development, with four reporting sex-dependent associations. Three papers reported data from the Queensland Flood Study. Simcock et al. (N = 115) found a sex interaction in the association between disaster-related PNMS and child problem solving at age 6 months, with girls having poorer problem solving than boys at high levels of prenatal stress [48]. In contrast, Moss et al. (N = 145) found no evidence of sex dependence in the association between PNMS and adolescent cognitive development [66]. In the final paper from this group (N = 130), there was a sex-specific effect whereby PNMS was associated with poorer theory of mind in 30-month-old girls but not boys [67].
Plamondon et al. (N = 236) showed that, among both sexes, PNLEs were associated with poorer working memory; however, there was a sex-specific effect whereby adaptive parenting behaviors buffered against the adverse effect of PNLEs among boys, but not girls [62]. Bandoli et al. also reported a sex interaction (N = 344): Prenatal depression, in conjunction with prenatal alcohol exposure, was associated with developmental deficits in 6-month-old girls, but not boys [41•]. In contrast, several studies provided no evidence of sex-dependent effects of PNMS on child cognitive development. Investigators from the Rhea Study (N = 288) found no evidence for sex dependence in the association between PNMS and child cognitive development at age 4 [27]. Davis et al. (N = 91) found no evidence of a sex-dependent association between maternal prenatal plasma cortisol concentrations and child (ages 6–9) cognitive functioning [33]. Finally, data from two independent cohorts (N = 6969 and N = 425) provided no evidence of sex dependence in an association between prenatal maternal anxiety and offspring working memory [23].
Emotional/Behavioral Health
Nineteen papers evaluated child emotional/behavioral health outcomes. For ease of interpretation, we grouped them into five subcategories.
Temperament
Four papers examined child temperament, all finding evidence of sex-dependent effects. Braithwaite et al. (N = 216) found a sex interaction such that 5-week-old girls exposed to maternal cortisol exhibited elevated negative emotionality, whereas cortisol-exposed boys exhibited attenuated negative emotionality [44•]. They then replicated this interaction effect in an independent sample (N = 88) of 2-month-old infants [50]. However, findings from the Queensland Flood Study (N = 121) showed an interaction with a contrasting pattern: disaster-related PNMS was associated with increased irritability in boys and was unrelated to irritability in girls [47]. Finally, data from the Prenatal Early Life Stress Project (N = 90) reported no sex dependence in the association between prenatal anxiety and infant temperament at 9–14 months; however, there was a sex-specific indirect effect of maternal prenatal mindfulness on infant self-regulation problems via prenatal maternal anxiety for boys only [69].
Broad Psychopathology Measures
Six studies evaluated broad measures of psychopathology—including symptoms from multiple psychiatric disorders—with five showing evidence of sex-dependent associations. Investigators from the Rhea Study (N = 288) found no evidence of sex dependence in the association between prenatal psychopathology symptoms and 4-year-old behavioral outcomes [27]. Data from the Growing Up in Singapore Towards Healthy Outcomes (GUSTO) cohort (N = 258) showed that maternal prenatal depressive symptoms were positively associated with both internalizing and externalizing symptoms at age 2 among girls, but only internalizing symptoms in boys [61•]. In a study of young African-American mothers and their offspring (N = 196), prenatal depressive symptoms were indirectly associated with increased child social/emotional problems at age 2 through postnatal maternal depressive symptoms and maternal parenting sensitivity, but this effect was significant only among boys [73]. Similarly, a community-based cohort study (N = 885) found a significant interaction between prenatal maternal depression and child sex in predicting a broad measure of social-emotional well-being at age 7: Prenatal maternal depression was associated with much higher levels of emotional-behavioral problems among boys and was not significant for girls [40]. Pickles et al. (N = 813) found that the adverse effects of prenatal pregnancy-specific anxiety on child internalizing and externalizing symptoms at age 3.5 were ameliorated by maternal-infant stroking. Interestingly, the buffering effect of infant stroking was only significant for girls [57•]. Kim et al. (N = 49) reported a positive association between maternal prenatal plasma cortisol concentrations and internalizing problems in girls only, with the effect mediated by changes in brain networks [70•].
Depression/Anxiety Symptoms
Five studies examined child depression and/or anxiety outcomes, with three finding sex-dependent associations. Using data from the Avon Longitudinal Study of Parents and Children (ALSPAC; N = 7959), Quarini et al. reported a significant sex interaction: Prenatal depression was associated with elevated odds of depression among 18-year-old girls, whereas there was a tendency for reduced odds for exposed boys. Prenatal depression was associated with increased odds of depression at age 12 regardless of child sex [37•]. In a second analysis from ALSPAC (N = 9166), maternal PNLEs were positively associated with offspring depressive symptoms and diagnosis at age 17–18, with no evidence of sex dependence [22•]. Using data from the Western Australian Pregnancy Cohort (N = 1214), Herbison et al. found that prenatal stress was positively associated with depression/anxiety symptoms at age 20 in male but not female offspring after adjusting for postnatal childhood stress trajectories [55]. In the Preschool ADHD study, Bendiksen et al. [39] found no evidence of sex dependence in the association between maternal prenatal anxiety and depression symptoms and early childhood anxiety symptoms [39]. Finally, in a case-control study (N = 1258), Gilman et al. evaluated the association between maternal inflammatory cytokines and odds of major depressive disorder (MDD) in adult offspring (mean age 39.7). There was a significant interaction whereby a higher ratio of maternal prenatal pro- to anti-inflammatory cytokines was associated with reduced odds of MDD in women and increased odds in men [38].
Attention Deficit/Hyperactivity and Conduct Symptoms
Four studies examined symptoms of attention deficit/hyperactivity disorder (ADHD) and/or conduct disorder, with two finding sex-dependent associations. Bendiksen et al. (N = 1195) found evidence of sex interactions: Maternal prenatal psychopathology symptoms were associated with increased odds of conduct symptoms in boys, but not girls [39]. Zhu et al. (N = 1765) found a sex-specific association: Severe PNLEs were associated with increased odds of clinical ADHD symptoms in preschool boys but not girls [54]. In the ALSPAC data (N = 6969), there was a significant interaction of maternal anxiety and a COMT genetic variation in predicting child ADHD symptoms at age 4, but no evidence of sex dependence [23]. Finally, in the Rhea Study (N = 288), maternal psychopathology symptoms were associated with child ADHD symptoms, but there was no evidence of sex dependence [27].
Eating Pathology
St-Hilaire et al. (N = 54) found no evidence of sex dependence in the association between disaster-related PNMS in the third trimester and eating disorder pathology. However, the study was under-powered for this purpose [36].
Schizophrenia Spectrum Disorders
Fineberg et al. (N = 301) evaluated odds of offspring schizophrenia spectrum disorders (SSD) and reported a significant interaction of prenatal daily life stressors and child sex. Among boys, PNMS was associated with significantly increased odds of SSD, whereas there was a trend for reduced odds among girls [42•].
Discussion
The fact that we identified 50 published articles evaluating the potential sex-dependent nature of PNMS effects on child health outcomes within a 3-year span is evidence that many researchers are heeding recommendations [12, 15, 16, 17, 20] to consider the role of the child’s biological sex in PNMS programming. Our findings reinforce this recommendation. The majority of articles reviewed found a sex-dependent association with at least one child outcome, and a substantial minority reported a significant PNMSxsex interaction. These results, in combination with strong biological evidence for sexually dimorphic prenatal development [12, 13, 15, 16, 19, 20, 21] and findings from prior reviews [12, 13, 14, 16, 17, 21], send a clear message that a child’s biological sex should always be considered in mixed-sex PNMS programming studies.
This is not to say that PNMS effects on child outcomes are invariably sex-dependent. In fact, we identified three large—and presumably well-powered—studies (Ns > 1000) that found evidence for PNMS programming but no sex-dependent associations [22•, 23, 24]. It is not yet clear why a child’s biological sex modifies PNMS programming in certain contexts and not others. Until we have a stronger understanding of these inconsistencies, we recommend that researchers conduct and report formal evaluations of sex dependencies and that journal reviewers and editors request these analyses if they are absent. Although many small-sample studies will not be optimally powered to detect sex-dependent effects, reporting descriptive statistics for outcome variables stratified by child sex could enable well-powered meta-analytic evaluations.
The strongest evidence for sex-dependent programming effects in this review came from studies evaluating offspring neural/nervous system development and temperament. Twelve of the 16 articles evaluating neural/nervous system development showed sex-dependent associations. Six reported significant PNMS*sex interactions. The preponderance of evidence suggests that girls may be particularly vulnerable to PNMS programming, altering neural structure, function, and neuroendocrine sensitivity in a manner that confers risk for anxiety and affective pathology [61•, 63, 70•]. One study found evidence that these neural sequelae may mediate PNMS effects on internalizing symptoms [70•].
All four studies evaluating programming effects on child temperament reported sex-dependent associations. Two reported significant sex interactions suggesting that PNMS is associated with elevated negative emotionality and reactivity in girls and decreased emotionality and reactivity in boys [44•, 50]. These findings are generally in keeping with preexisting scholarship [12, 14, 17] and theories positing a lasting PNMS-driven vulnerability to mood and anxiety problems in females and fear inhibition in males [12, 17]. A third study, however, found a significant interaction indicating increased infant irritability only in boys exposed to PNMS [47]. Thus, there are likely other factors (e.g., timing of exposure and outcome ascertainment) that may alter the sex-dependent nature of the association between PNMS and offspring temperament.
This review also found some indication of sexually dimorphic effects of PNMS on other child health outcomes. Among female offspring, there was greater evidence for positive associations between PNMS and early childhood difficulties with cognitive [48, 67] and motor development [41•, 66], and adulthood cardiovascular disease risk [60]. In contrast, we uncovered greater evidence for PNMS sensitivity in male offspring for early childhood wheezing/asthma [58•, 59], externalizing pathology [39, 54], and schizophrenia spectrum disorders [42•]. It is noteworthy that males have a greater risk in the general population for all of the adverse health outcomes showing male-biased PNMS programming effects in this review (early childhood asthma/wheezing [58•, 59, 74], externalizing pathology [39, 54, 75], and schizophrenia spectrum disorders [42•, 76]). It may be that PNMS contributes to, or exacerbates, vulnerabilities for these sexually dimorphic health conditions [77]. Alternatively, it is plausible that there was simply more power to detect associations between PNMS and these health conditions in males because the conditions are more common among males in the population.
Findings from a couple of studies were notably consistent with the evolutionary perspective that male fetuses continue to prioritize growth and physical development in the presence of intrauterine adversity, whereas female fetuses modulate growth to improve viability [12, 16, 17]. Kaitz et al. found that male fetuses and neonates were larger than females only when exposed to PNMS [45•]. Likewise, Doyle et al. found that PNMS was associated with variable deviations from typical development in female fetuses and consistently accelerated development in male fetuses [46]. These results reinforce the potential value of evolutionary biology frameworks [12, 16, 17, 19] for understanding sex-dependent PNMS programming.
Limitations and Future Directions
This review included only studies that reported evaluating sex dependence in PNMS programming, likely providing a biased sample of the broader literature. Studies finding sex-dependent associations were probably more likely to report these analyses and be included in this review. Additionally, many studies included multiple PNMS exposure variables and child health outcomes, increasing the risk for type I errors. Thus, our results may overestimate the likelihood of sex-dependent associations. On the other hand, researchers typically power studies to detect main effects and not effect modification, meaning that if sex-dependent PNMS associations exist in the population, many studies will fail to detect them.
A critical future task, if we are to fully understand the nature of PNMS programming, is to explicate the processes that moderate and mediate effects on child health outcomes. This is challenging given the complexity of both the intrauterine and maternal environments and their interactions [16]. Detecting effect modification and mediation requires large sample sizes [71, 72]. Implementing studies that satisfy power projections while also optimizing the research design (e.g., repeated collection of biomarkers) would be challenging and costly. To further complicate matters, a number of studies have shown that PNMSxsex interactions are dependent on other factors, creating higher-order interactions. The most well-established of these factors is timing of exposure [77, 78], which was highlighted by many studies in this review [29, 36, 39, 43, 46, 49, 54, 66, 70•]. Finally, including child sex as a moderator may be insufficient: Evaluating PNMS programming may require entirely different models with different functional forms for girls and boys. Combining datasets using advancing data harmonization methods [79] may allow for increased statistical power and improved understanding of how child sex influences the hypothesized causal pathway from PNMS to offspring health outcomes.
Conclusions
This review found substantial—albeit somewhat inconsistent—evidence of sex-dependent prenatal maternal stress (PNMS) associations with child health outcomes in articles published between January 2015 and December 2017. There was strong evidence for sexual dimorphism in associations between PNMS and infant neural development and temperament, with girls appearing to be at elevated risk for profiles linked to anxiety and affective disorders. We hope these findings reinforce the importance of considering the role of the child’s biological sex when designing and evaluating PNMS programming studies.
Supplementary Material
Acknowledgements:
K12 HS 022990: National Institutes of Health/Agency for Healthcare Research and Quality (SMB); T32 MH 018921–25: National Institutes of Health/National Institute of Mental Health (SS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health of the Agency for Healthcare Research and Quality.
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health of the Agency for Healthcare Research and Quality.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Kim D, Bale T, Epperson C. Prenatal programming of mental illness: current understanding of relationship and mechanisms. Curr Psychiatry Rep. 2015;17:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stein A, Pearson R, Goodman S, Rapa E, Rahman A, McCallum M, et al. Effects of perinatal mental disorders on the fetus and child. Lancet. 2014;384:1800–19. [DOI] [PubMed] [Google Scholar]
- 3.Entringer S, Buss C, Wadhwa P. Prenatal stress, development, health and disease risk: a psychobiological perspective—2015 Curt Richter Award Paper. Psychoneuroendocrinology. 2015;62:366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flanigan C, Sheikh A, DunnGalvin A, Brew B, Almqvist C, Nwaru B. Prenatal maternal psychosocial stress and offspring’s asthma and allergic disease: a systematic review and meta-analysis. Clin Exp Allergy 2018;48(4):403–14. [DOI] [PubMed] [Google Scholar]
- 5.Accortt EE, Cheadle ACD, Dunkel SC. Prenatal depression and adverse birth outcomes: an updated systematic review. Matern Child Health J. 2015;19:1306–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearson J, Tarabulsy G, Bussières E-L. Foetal programming and cortisol secretion in early childhood: a meta-analysis of different programming variables. Infant Behav Dev. 2015;40:204–15. [DOI] [PubMed] [Google Scholar]
- 7.Graignic-Philippe R, Dayan J, Chokron S, Jacquet A-Y, Tordjman S. Effects of prenatal stress on fetal and child development: a critical literature review. Neurosci Biobehav Rev. 2014;43:137–62. [DOI] [PubMed] [Google Scholar]
- 8.Lewis A Depression in pregnancy and child development: understanding the mechanisms of transmission Psychopharmacol Pregnancy [Internet]. New York: Springer-Verlag Publishing; 2014. [cited 2015 Dec 9]. p. 47–65. [Google Scholar]
- 9.Beijers R, Buitelaar JK, de Weerth C. Mechanisms underlying the effects of prenatal psychosocial stress on child outcomes: beyond the HPA axis. Eur Child Adolesc Psychiatry. 2014;23:943–56. [DOI] [PubMed] [Google Scholar]
- 10.Coussons-Read M Effects of prenatal stress on pregnancy and human development: mechanisms and pathways. Obstet Med. 2013;6:527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao-Lei L, de Rooij S, King S, Matthews S, Metz G, Roseboom T, et al. Prenatal stress and epigenetics. Neurosci Biobehav Rev [Internet]. 2017;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12.Sandman C, Glynn L, Davis E. Is there a viability–vulnerability tradeoff? Sex differences in fetal programming. J Psychosom Res. 2013;75:327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiPietro J, Voegtline K. The gestational foundation of sex differences in development and vulnerability. Neuroscience. 2017;342:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpenter T, Grecian S, Reynolds R. Sex differences in early-life programming of the hypothalamic–pituitary–adrenal axis in humans suggest increased vulnerability in females: a systematic review. J Dev Orig Health Dis. 2017;8:244–55. [DOI] [PubMed] [Google Scholar]
- 15.Bale T Sex differences in prenatal epigenetic programing of stress pathways. Stress. 2011;14:348–56. [DOI] [PubMed] [Google Scholar]
- 16.Clifton V. Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31:S33–9. [DOI] [PubMed] [Google Scholar]
- 17.Glover V, Hill J. Sex differences in the programming effects of prenatal stress on psychopathology and stress responses: an evolutionary perspective. Physiol Behav. 2012;106:736–40. [DOI] [PubMed] [Google Scholar]
- 18.Bale T The placenta and neurodevelopment: sex differences in prenatal vulnerability. Dialogues Clin Neurosci. 2016;18:459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenfeld C Sex-specific placental responses in fetal development. Endocrinology. 2015;156:3422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabory A, Roseboom T, Moore T, Moore L, Junien C. Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics. Biol Sex Differ. 2013;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aiken C, Ozanne S. Sex differences in developmental programming models. Reproduction. 2013;145:R1–13. [DOI] [PubMed] [Google Scholar]
- 22.•.Kingsbury M, Weeks M, MacKinnon N, Evans J, Mahedy L, Dykxhoorn J, et al. Stressful life events during pregnancy and offspring depression: evidence from a prospective cohort study. J Am Acad Child Adolesc Psychiatry. 2016;55:709–16. [DOI] [PubMed] [Google Scholar]; This large study found no evidence of a sex-dependent association between prenatal stress and offspring depression in adolescence.
- 23.O’Donnell K, Glover V, Lahti J, Lahti M, Edgar R, Räikkönen K, et al. Maternal prenatal anxiety and child COMT genotype predict working memory and symptoms of ADHD. PLoS ONE [Internet]. 2017. [cited 2018 May 28]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaillard R, Rifas-Shiman S, Perng W, Oken E, Gillman M. Maternal inflammation during pregnancy and childhood adiposity. Obesity. 2016;24:1320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansell T, Vuillermin P, Ponsonby A-L, Collier F, Saffery R, Ryan J. Maternal mental well-being during pregnancy and glucocorticoid receptor gene promoter methylation in the neonate. Dev Psychopathol. 2016;28:1421–30. [DOI] [PubMed] [Google Scholar]
- 26.Giesbrecht G, Campbell T, Letourneau N. Sexually dimorphic adaptations in basal maternal stress physiology during pregnancy and implications for fetal development. Psychoneuroendocrinology. 2015;56:168–78. [DOI] [PubMed] [Google Scholar]
- 27.Koutra K, Roumeliotaki T, Kyriklaki A, Kampouri M, Sarri K, Vassilaki M, et al. Maternal depression and personality traits in association with child neuropsychological and behavioral development in preschool years: mother-child cohort (Rhea Study) in Crete, Greece. J Affect Disord. 2017;217:89–98. [DOI] [PubMed] [Google Scholar]
- 28.Lee A, Chiu Y-H, Rosa M, Cohen S, Coull BA, Wright R, et al. Association of prenatal and early childhood stress with reduced lung function in 7-year-olds. Ann Allergy Asthma Immunol. 2017;119:153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rash J, Campbell T, Letourneau N, Giesbrecht G. Maternal cortisol during pregnancy is related to infant cardiac vagal control. Psychoneuroendocrinology. 2015;54:78–89. [DOI] [PubMed] [Google Scholar]
- 30.Simcock G, Kildea S, Elgbeili G, Laplante D, Stapleton H, Cobham V, et al. Age-related changes in the effects of stress in pregnancy on infant motor development by maternal report: the Queensland Flood Study. Dev Psychobiol. 2016;58:640–59. [DOI] [PubMed] [Google Scholar]
- 31.Liu G, Dancause K, Elgbeili G, Laplante D, King S. Disaster-related prenatal maternal stress explains increasing amounts of variance in body composition through childhood and adolescence: Project Ice Storm. Environ Res. 2016;150:1–7. [DOI] [PubMed] [Google Scholar]
- 32.Dancause K, Laplante D, Hart K, O’Hara M, Elgbeili G, Brunet A, et al. Prenatal stress due to a natural disaster predicts adiposity in childhood: the Iowa Flood Study. J Obes [Internet]. 2015. [cited 2018 May 29]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis E, Head K, Buss C, Sandman C. Prenatal maternal cortisol concentrations predict neurodevelopment in middle childhood. Psychoneuroendocrinology. 2017;75:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braithwaite E, Murphy S, Ramchandani P. Effects of prenatal depressive symptoms on maternal and infant cortisol reactivity. Arch Womens Ment Health. 2016;19:581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Entringer S, Buss C, Rasmussen J, Lindsay K, Gillen D, Cooper D, et al. Maternal cortisol during pregnancy and infant adiposity: a prospective investigation. J Clin Endocrinol Metab. 2017;102:1366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.St-Hilaire A, Steiger H, Liu A, Laplante D, Thaler L, Magill T, et al. A prospective study of effects of prenatal maternal stress on later eating-disorder manifestations in affected offspring: Preliminary indications based on the project ice storm cohort. Int J Eat Disord. 2015;48:512–6. [DOI] [PubMed] [Google Scholar]
- 37.•.Quarini C, Pearson R, Stein A, Ramchandani P, Lewis G, Evans J. Are female children more vulnerable to the long-term effects of maternal depression during pregnancy? J Affect Disord. 2016;189:329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study found that prenatal depression was associated with elevated odds of depression among 18-year-old girls, whereas there was a tendency for reduced odds for exposed boys.
- 38.Gilman SE, Cherkerzian S, Buka SL, Hahn J, Hornig M, Goldstein JM. Prenatal immune programming of the sex-dependent risk for major depression. Transl Psychiatry. 2016;6:e822, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bendiksen B, Aase H, Diep L, Svensson E, Friis S, Zeiner P. The associations between pre- and postnatal maternal symptoms of distress and preschooler’s symptoms of ADHD, oppositional defiant disorder, conduct disorder, and anxiety. J Atten Disord. 2015. [DOI] [PubMed] [Google Scholar]
- 40.Maselko J, Sikander S, Bangash O, Bhalotra S, Franz L, Ganga N, et al. Child mental health and maternal depression history in Pakistan. Soc Psychiatry Psychiatr Epidemiol. 2016;51:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.•.Bandoli G, Coles C, Kable J, Wertelecki W, Granovska I, Pashtepa A, et al. Assessing the independent and joint effects of unmedicated prenatal depressive symptoms and alcohol consumption in pregnancy and infant neurodevelopmental outcomes. Alcohol Clin Exp Res. 2016;40:1304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper found that, in combination with prenatal alcohol exposure, prenatal depression was associated with increased motor and cognitive development deficits in infant girls but not boys.
- 42.•.Fineberg A, Ellman L, Schaefer C, Maxwell S, Shen L, Chaudhury N, et al. Fetal exposure to maternal stress and risk for schizophrenia spectrum disorders among offspring: differential influences of fetal sex. Psychiatry Res. 2016;236:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study found that, among boys, prenatal stress was associated with significantly increased odds of schizophrenia spectrum disorders, whereas there was a trend for reduced odds among girls.
- 43.Giesbrecht G, Letourneau N, Campbell T. Sexually dimorphic and interactive effects of prenatal maternal cortisol and psychological distress on infant cortisol reactivity. Dev Psychopathol. 2017;29:805–18. [DOI] [PubMed] [Google Scholar]
- 44.•.Braithwaite E, Pickles A, Sharp H, Glover V, O’Donnell K, Tibu F, et al. Maternal prenatal cortisol predicts infant negative emotionality in a sex-dependent manner. Physiol Behav. 2017a;175:31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed infant girls exposed to maternal cortisol exhibited elevated negative emotionality, whereas cortisol-exposed boys exhibited attenuated negative emotionality.
- 45.•.Kaitz M, Mankuta D, Rokem A, Faraone S. Relation between maternal antenatal anxiety and infants’ weight depends on infants’ sex: a longitudinal study from late gestation to 1-month post birth. J Psychosom Res. 2015;79:620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper found that male fetuses and neonates weighed more than their female counterparts only when exposed to third trimester maternal anxiety.
- 46.Doyle C, Werner E, Feng T, Lee S, Altemus M, Isler J, et al. Pregnancy distress gets under fetal skin: maternal ambulatory assessment & sex differences in prenatal development. Dev Psychobiol. 2015;57:607–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simcock G, Elgbeili G, Laplante D, Kildea S, Cobham V, Stapleton H, et al. The effects of prenatal maternal stress on early temperament: the 2011 Queensland Flood Study. J Dev Behav Pediatr. 2017c;38:310–21. [DOI] [PubMed] [Google Scholar]
- 48.Simcock G, Laplante D, Elgbeili G, Kildea S, Cobham V, Stapleton H, et al. Infant neurodevelopment is affected by prenatal maternal stress: the QF2011 Queensland Flood study. Infancy. 2017a;22:282–302. [DOI] [PubMed] [Google Scholar]
- 49.Yong Ping E, Laplante D, Elgbeili G, Hillerer K, Brunet A, O’Hara M, et al. Prenatal maternal stress predicts stress reactivity at 2½ years of age: the Iowa Flood Study. Psychoneuroendocrinology. 2015;56:62–78. [DOI] [PubMed] [Google Scholar]
- 50.Braithwaite E, Murphy S, Ramchandani P, Hill J. Associations between biological markers of prenatal stress and infant negative emotionality are specific to sex. Psychoneuroendocrinology. 2017b;86:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braithwaite E, Kundakovic M, Ramchandani P, Murphy S, Champagne F. Maternal prenatal depressive symptoms predict infant NR3C1 1F and BDNF IV DNA methylation. Epigenetics. 2015;10:408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lebel C, Walton M, Letourneau N, Giesbrecht G, Kaplan B, Dewey D. Prepartum and postpartum maternal depressive symptoms are related to children’s brain structure in preschool. Biol Psychiatry. 2016;80:859–68. [DOI] [PubMed] [Google Scholar]
- 53.Vangeel E, Pishva E, Hompes T, van den Hove D, Lambrechts D, Allegaert K, et al. Newborn genome-wide DNA methylation in association with pregnancy anxiety reveals a potential role for GABBR1. Clin Epigenetics. 2017;9:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu P, Hao J-H, Tao R-X, Huang K, Jiang X-M, Zhu Y-D, et al. Sex-specific and time-dependent effects of prenatal stress on the early behavioral symptoms of ADHD: a longitudinal study in China. Eur Child Adolesc Psychiatry. 2015;24:1139–47. [DOI] [PubMed] [Google Scholar]
- 55.Herbison C, Allen K, Robinson M, Newnham J, Pennell C. The impact of life stress on adult depression and anxiety is dependent on gender and timing of exposure. Dev Psychopathol. 2017;29:1443–54. [DOI] [PubMed] [Google Scholar]
- 56.Frith A, Naved R, Persson L, Frongillo E. Early prenatal food supplementation ameliorates the negative association of maternal stress with birth size in a randomised trial. Matern Child Nutr. 2015;11:537–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.•.Pickles A, Sharp H, Hellier J, Hill J. Prenatal anxiety, maternal stroking in infancy, and symptoms of emotional and behavioral disorders at 3.5 years. Eur Child Adolesc Psychiatry. 2017;26:325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study found that prenatal pregnancy-specific anxiety was associated with increased internalizing and externalizing symptoms in preschool boys and girls, but that maternal-infant stroking buffered against this effect only in girls.
- 58.•.Lee A, Chiu Y-H, Rosa M, Jara C, Wright R, Coull B, et al. Prenatal and postnatal stress and asthma in children: temporal-and sex-specific associations. J Allergy Clin Immunol. 2016;138:740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper found that prenatal stress was associated with increased risk for asthma in preschoolers among boys but not girls and that prenatal and postnatal stress in combination was predictive of asthma in girls.
- 59.Rosa M, Just A, Ortiz M, Schnaas L, Svensson K, Wright R, et al. Prenatal and postnatal stress and wheeze in Mexican children: sex-specific differences. Ann Allergy Asthma Immunol. 2016;116:306–312.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stinson L, Stroud L, Buka S, Eaton C, Lu B, Niaura R, et al. Prospective evaluation of associations between prenatal cortisol and adulthood coronary heart disease risk: the New England family study. Psychosom Med. 2015;77:237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.•.Soe N, Wen D, Poh J, Li Y, Broekman B, Chen H, et al. Pre- and post-natal maternal depressive symptoms in relation with infant frontal function, connectivity, and behaviors. PLOS ONE. 2016;11:e0152991. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper showed that, among girls only, increases in depressive symptoms from 32-week gestation to 3 months postpartum was associated with greater frontal lobe activity and right frontal asymmetry in infant.
- 62.Plamondon A, Akbari E, Atkinson L, Steiner M, Meaney M, Fleming A. Spatial working memory and attention skills are predicted by maternal stress during pregnancy. Early Hum Dev. 2015;91:23–9. [DOI] [PubMed] [Google Scholar]
- 63.Wen D, Poh J, Ni S, Chong Y-S, Chen H, Kwek K, et al. Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Transl Psychiatry. 2017;7:e1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gholipoor R, Saboory E, Ghazavi A, Kiyani A, Roshan-Milani S, Mohammadi S, et al. Prenatal stress potentiates febrile seizure and leads to long-lasting increase in cortisol blood levels in children under 2years old. Epilepsy Behav. 2017;72:22–7. [DOI] [PubMed] [Google Scholar]
- 65.Stroud L, Papandonatos G, Parade S, Salisbury A, Phipps M, Lester B, et al. Prenatal major depressive disorder, placenta glucocorticoid and serotonergic signaling, and infant cortisol response. Psychosom Med. 2016;78:979–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moss K, Simcock G, Cobham V, Kildea S, Elgbeili G, Laplante D, et al. A potential psychological mechanism linking disaster-related prenatal maternal stress with child cognitive and motor development at 16 months: the QF2011 Queensland Flood Study. Dev Psychol. 2017;53:629–41. [DOI] [PubMed] [Google Scholar]
- 67.Simcock G, Kildea S, Elgbeili G, Laplante DP, Cobham V, King S. Prenatal maternal stress shapes children’s theory of mind: the QF2011 Queensland Flood Study. J Dev Orig Health Dis. 2017b;8:483–92. [DOI] [PubMed] [Google Scholar]
- 68.Mina T, Räikkönen K, Riley S, Norman J, Reynolds R. Maternal distress associates with placental genes regulating fetal glucocorticoid exposure and IGF2: role of obesity and sex. Psychoneuroendocrinology. 2015;59:112–22. [DOI] [PubMed] [Google Scholar]
- 69.van den Heuvel M, Johannes M, Henrichs J, Van den Bergh B. Maternal mindfulness during pregnancy and infant socio-emotional development and temperament: the mediating role of maternal anxiety. Early Hum Dev. 2015;91:103–8. [DOI] [PubMed] [Google Scholar]
- 70.•.Kim D-J, Davis E, Sandman C, Sporns O, O’Donnell B, Buss C, et al. Prenatal maternal cortisol has sex-specific associations with child brain network properties. Cereb Cortex. 2017;27:5230–41. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper showed that, among girls only, prenatal exposure to maternal cortisol was associated with brain network patterns that mediated the association between prenatal cortisol and internalizing symptoms in childhood.
- 71.VanderWeele T Explanation in causal inference: methods for mediation and interaction. Oxford: Oxford University Press; 2015. [Google Scholar]
- 72.VanderWeele T, Knol M. A tutorial on interaction. Epidemiol Methods. 2014;3:33–72. [Google Scholar]
- 73.Edwards R, Hans S. Prenatal depressive symptoms and toddler behavior problems: the role of maternal sensitivity and child sex. Child Psychiatry Hum Dev. 2016;47:696–707. [DOI] [PubMed] [Google Scholar]
- 74.Geier D, Kern J, Geier M. Demographic and neonatal risk factors for childhood asthma in the USA. J Matern Fetal Neonatal Med [Internet]. 2017;Published online ahead of print. [DOI] [PubMed] [Google Scholar]
- 75.Arnett A, Pennington B, Willcutt E, DeFries J, Olson R. Sex differences in ADHD symptom severity. J Child Psychol Psychiatry. 2015;56:632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abel K, Drake R, Goldstein J. Sex differences in schizophrenia. Int Rev Psychiatry. 2010;22:417–28. [DOI] [PubMed] [Google Scholar]
- 77.Bale T, Epperson C. Sex differences and stress across the lifespan. Nat Neurosci. 2015;18:1413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davis E, Sandman C. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81:131–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown CH, Sloboda Z, Faggiano F, Teasdale B, Keller F, Burkhart G, et al. Methods for synthesizing findings on moderation effects across multiple randomized trials. Prev Sci. 2013;14:144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.