Abstract
Introduction
Chronic granulomatous disease (CGD) is a genetic disorder in which phagocyte dysfunction leads to recurrent infection. Persistent pulmonary infections sometimes require thoracic surgical intervention. We reviewed our 25-year experience to identify outcomes and prognostic factors associated with thoracic surgery in these patients.
Methods
A retrospective single-institution review of all patients with CGD from 1990 through 2015 was performed. Univariate analysis identified prognostic variables to include in a Cox model. Overall survival was estimated by the Kaplan-Meier method.
Results
We identified 258 patients who had 2221 admissions (both scheduled and emergent). During the period examined, 51 thoracic operations were performed in 13.6 % (35/258) of patients and 2.3 % (35/2221) of overall admissions. Patients undergoing surgery did not have statistically significant differences in disease genotype compared to those that did not require surgery. Pathogens were identified from 67 % (34/51) of specimens. Complications occurred in 27 % (14/51), including 10 % (5/51) with wound and 12 % (6/51) with pulmonary infections. Mortality at 30 and 90 days was 0 and 6 % (3/51), respectively. Overall survival probabilities were 75 and 62 % at 5- and 10-year follow-up (median potential follow-up: 16.5 years), respectively. Undergoing thoracic surgery was associated with an increased hazard ratio for death of 3.71 (p < 0.0001). Both chest wall resection and EBL > 500 mL were negative prognostic factors (p < 0.05).
Conclusions
A minority of CGD patients required thoracic surgery for infections refractory to antibiotic or antifungal therapy. Patients who had these operations had significant morbidity and relatively poor long-term survival, particularly in the cases of chest wall resection or significant blood loss.
Keywords: Empyema, lung abscess, pulmonary resection, chronic granulomatous disease
Introduction
Chronic granulomatous disease (CGD) is a rare inherited genetic disorder that adversely affects the immune system and results in recurrent infections and inflammatory complications. In the USA, CGD has an incidence of at least one in 250,000 births [1–3]. It is a disorder of white blood cell oxidative metabolism affecting phagocytic cells causing failure to clear bacteria and fungi despite normal cell numbers [1–3]. It is caused by mutations in any of the five phagocyte NADPH oxidase genes: CYBB encoding gp91phox is on the X chromosome and accounts for about 65 % of North American cases, while genes encoding p22 phox, p47 phox, p67 phox, and p40 phox are all autosomal recessive and account for the remainder [3–5].
CGD patients generally contract infections with a narrow spectrum of microorganisms including Staphylococcus aureus, Burkholderia cepacia complex, Serratia marsescens, Nocardia species, and Aspergillus species [3]. CGD patients also develop extensive inflammatory lesions especially in hollow viscera [3]. Since its first description in 1954 as a fatal condition of early childhood, the combination of prophylactic antibiotics, antifungals, and interferon-γ has increased the median survival well into adulthood [6–8]. Despite these improvements, morbidity and mortality remain high.
Lung infections are the most common causes of morbidity and mortality in CGD [2, 9]. Many patients develop chronic bilateral pulmonary infiltrates, pulmonary fibrosis, or pulmonary calcifications associated with restrictive lung disease [10]. Thoracic surgery is occasionally required for pulmonary infections in CGD; however, only one multi-case series exists, describing 31 thoracic interventions in 19 patients between 1974 and 1990 [11].
Patients with CGD undergoing surgery are generally at a higher risk of complications than immunocompetent patients undergoing routine thoracic operations. In one series of ten children with CGD who underwent surgery, 30 % experienced wound dehiscence [12]. In a separate series of patients undergoing colonic resections, 75 % (3/4) of patients receiving a colostomy developed pyoderma gangrenosum, two of whom required additional surgical interventions [13]. In a retrospective study of 52 surgical treatments of hepatic abscesses, the complication rate was 56 %. Patients had persistent liver abscess in 42 % (22/52) of cases and wound dehiscence in 13 % of cases (7/52) [14].
Given the improvement in the treatment of CGD and advances in surgical techniques, we reviewed our experience with thoracic surgical management of CGD over time. We sought to describe the subset of CGD patients who required thoracic surgery for pulmonary infections, their surgical morbidity, long-term outcomes, and prognostic factors.
Methods
Subjects and Data Collection
Patients with CGD admitted to the NIH between January 1, 1990 and January 1, 2015 on natural history or treatment protocols of the National Institute of Allergy and Infectious Disease were identified. All patients or their guardians signed consents for participation in research activities. Demographic data included age, sex, race/ethnicity, affected CGD gene, and status at last visit to the NIH. The diagnosis of CGD was confirmed by either nitroblue tetrazolium reduction or dihydrorhodamine oxidation, depending on the year of diagnosis. These patients were cross-referenced against all NIH clinical center patients who underwent thoracic surgery. Only operative procedures were included; bronchoscopic or interventional radiology procedures were excluded.
Operative and perioperative details were collected through a manual review of the electronic and paper medical records by two authors (PF and HQ). Activity of NADPH oxidase and superoxide production levels were obtained as previously described [15]. Dates of last follow-up or death were obtained from the medical and CGD protocol records.
Statistical Analysis
Overall survival was calculated from the date of first thoracic procedure until the date of death or last follow-up and was estimated by the Kaplan-Meier method. Univariate analysis was first used in order to identify prognostic variables to include in a Cox proportional hazards model. Features examined for their potential association with survival included gender, age at diagnosis, presence of comorbidities, presence of previous diagnosis of pneumonia, presence of previous infections, preoperative treatment with interferon gamma or steroids, type of resection (anatomic or nonanatomic, chest wall, or non-chest wall), urgency of procedure, preoperative erythrocyte sedimentation rate, preoperative white blood cell (WBC) count, preoperative C reactive protein (CRP), preoperative albumin, preoperative temperature, and intra-operative estimated blood loss (EBL). The need for a second operation was evaluated for its association with survival as a time-varying covariate, based on the time until the second procedure, if performed, using Cox proportional hazards modeling. Similarly, a Cox model with time to surgery as a time-varying covariate was used to assess the impact of thoracic surgery on overall survival in the full cohort of patients with CGD. Analyses were performed using SAS version 9.3 (SAS Institute Cary, NC) and the R statistical computing environment [16]. All p values are two-tailed and are presented without adjustment for multiple comparisons.
Results
Patient Characteristics and Demographics
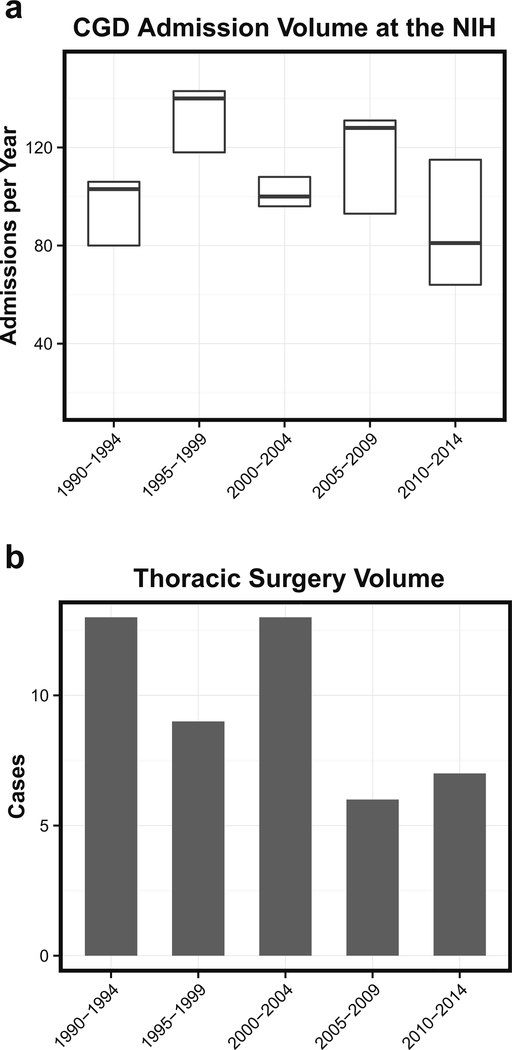
During the 25-year study period, there were 51 thoracic operations performed on 13.6 % (35/258) of patients with CGD, for an overall rate of 2.3 % (35/2221) per admission. The annual number of admissions did not significantly change over the time period, but this number reflects both protocol-driven and clinically driven admissions (Fig. 1a). Thoracic surgery was more frequent during the first 5-year time period, 1990 to 1994, but the rate was relatively stable over the last 20 years (Fig. 1b).
Fig. 1.
a Boxplot showing CGD admission volumes during the study period. Thick lines represent median and white boxes represent interquartile ranges. b Barchart showing number of thoracic surgeries performed on CGD patients during the study period
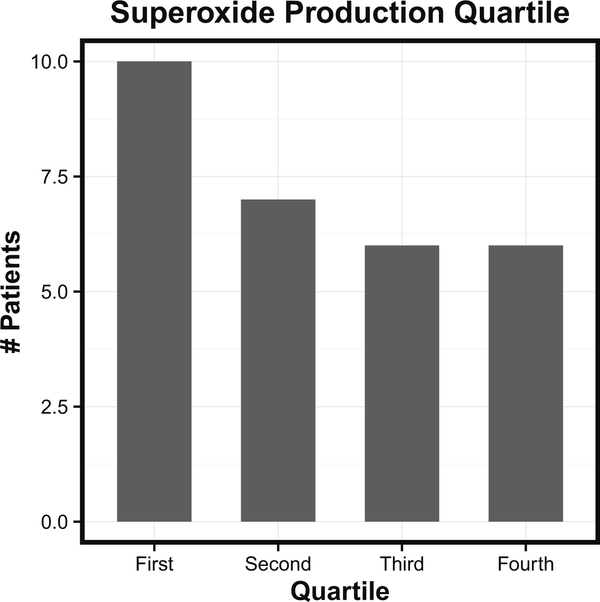
Among CGD patients followed at the NIH between 1990 and 2015, 65.9 % were known to have the gp91 X-linked mutation. Among the patients requiring thoracic surgery, 91 % were male and 80 % had X-linked disease (Table 1). Genotype of those undergoing surgery was compared to the overall NIH CGD cohort, and X-linked disease was associated with a weak, but not statistically significant association with thoracic surgery. The remaining patients undergoing thoracic surgery had autosomal recessive p47phox, p22 phox, or p67 phox deficiencies with frequencies of 14, 2.8, and 2.8 %, respectively. The low incidence of the p47 phox mutation likely reflects the previously documented improved prognosis of p47phox deficiency due to residual NADPH oxidase activity in these patients [15]. Previously, the amount of residual superoxide production by neutrophils was shown to predict survival in the NIH CGD cohort—subjects in the lowest quartile of superoxide production were at significantly greater risk of death than those in the third and fourth quartiles [15]. Among 29 patients undergoing surgery for whom residual superoxide production level was available, there was a trend toward increased representation in the bottom quartile of production; however, this did not reach statistical significance (Fig. 2).
Table 1.
Patient demographics
| Overall referral | No. |
| Total number admissions 1990–2015 | 2221 |
| Total NIH CGD population 1990–2015 | 258 |
| Admissions per patient | 8.5 |
| Mean admissions per patient per year | 0.56 |
| Patients undergoing thoracic surgery | No. (%) |
| Total number of patients | 35 |
| Total number of thoracic surgeries | 51 |
| Male sex | 32 (91) |
| X-linked CGD | 28 (80) |
| Mean age at diagnosis | 3.68 years |
| Patients with at least one comorbidity | 17 (49) |
| Genetics | No. (%) |
| gp91phox (X-linked) | 28 (80) |
| p47 phox | 5 (14) |
| p22 phox | 1 (2.8) |
| p67 phox | 1 (2.8) |
| Previous inflammation or infectionsa | No. (%) |
| Total patients with a previous infection | 35 (100) |
| Pneumonia | 32 (91) |
| Liver abscess | 11 (31) |
| Abscess (other) | 9 (26) |
| Colitis | 7 (20) |
| Skin infection | 7 (20) |
| Osteomyelitis | 5 (14) |
| Rectal abscess | 5 (14) |
| Epididymitis | 3 (9) |
| Lymphadenitis | 3 (9) |
| Preoperative laboratory values | |
| Albumin (grams per liter) | 3.49 (1.9–5.5) |
| WBC (×109 per liter) | 8.22 (2.6–23) |
| Preoperative antibiotic treatmentb | No. (%)c |
| Two preoperative antibiotics treatment | 33 (67) |
| Three or more preoperative antibiotics treatment | 16 (33) |
| Preoperative antifungal agent | 41 (80) |
| Preoperative steroids | 19 (37) |
| Preoperative interferon gamma | 15 (29) |
| Trimethoprim/sulfamethoxazole | 29 (58) |
| Amphotericin preparation | 14 (28) |
| Posaconazole | 11 (22) |
| Voriconazole | 11 (22) |
| Ceftriaxone | 9 (18) |
| Levofloxacin | 7 (14) |
| Vancomycin | 7 (14) |
| Meropenem | 6 (12) |
| Itraconazole | 5 (10) |
| Anidulafungin | 4 (8) |
| Caspofungin | 4 (8) |
Only if occurred in >3 pts
Only if used in >3 cases
Percent of total cases
Fig. 2.
Numbers of patients undergoing thoracic surgery belonging to each quartile of residual superoxide production in neutrophils
Median age at diagnosis was 1 year, while median age at surgery was 15 years (2.5–42.4). The age at surgery may in part reflect the fact that the NIH Clinical Center does not admit children under 2 years. All patients had prior infections, and nearly all (91 %) patients had prior episodes of pneumonia.
Preoperatively, all patients were hospitalized with recalcitrant pulmonary infections. All patients were on antibiotic treatment prior to surgery. Nearly all (80 %) were also on antifungal treatment in the perioperative period (Table 1). Furthermore, the majority (67 %) of patients were treated with at least two antibiotic agents preoperatively while the remainder were treated with three or more agents. The most common preoperative antibiotic was trimethoprim/sulfamethoxazole (58 % of cases), which is the standard of care in CGD [3]. In contrast to other inherited immunodeficiencies, steroids are often utilized to aid in suppressing abnormal inflammation associated with CGD-associated infections. In 37 % (19/51) of the operations, patients were on preoperative steroid therapy. Similarly, interferon gamma is an agent with well-established utility as an immune stimulant in CGD and was utilized in 29 % (15/51) of cases. The mean preoperative leukocyte count was 8.22× 109 cells/L and albumin was 3.49g/L.
Operative Details
All operations were performed for pulmonary infections that were not responding adequately to medical management. Thirty-five patients underwent at least one operation: of these, 66 % (23/35), 26 % (9/35), 6 % (2/35), and 3 % (1/35) had one, two, three, and four operations, respectively (Table 2). The most frequent types of operation were nonanatomic wedge resections, segmentectomies, and chest wall resections. The only patient who required a fourth operation underwent a chest wall debridement due to wound complications. The most common approach was thoracotomy. VATS was first used in 2002 in this series and accounted for 10 % (5/51) of the cases.
Table 2.
Operative details
| Characteristics | |
| Total number of thoracic operations | 51 |
| Mean operations per patient | 1.4 |
| Mean age at first surgery (min, max) | 17.41 years (2.5, 42.4) |
| Type of operation | No. (%) |
| First operation | 35 |
| Thoracotomy | 32 (90) |
| VATS | 3 (10) |
| Bilobectomy | 2 (6) |
| Lobectomy | 6 (17) |
| With wedge | 1 |
| Wedge | 9 (26) |
| Multi-wedge | 4 |
| Segmentectomy | 6 (17) |
| Multi-segment | 2 |
| Chest wall resection | 6 (17) |
| With associated structures | 1 |
| Biopsy | 6 (14) |
| Pleurodesis | 1 (3) |
| Patients with more than one operation | 12 |
| Second operation | 12 |
| Thoracotomy | 10 (83) |
| VATS | 2 (17) |
| Pneumonectomy | 1 (8) |
| Wedge | 3 (25) |
| Segmentectomy | 3 (25) |
| Chest wall resection | 2 (17) |
| Biopsy | 2 (17) |
| Decortication | 1 (8) |
| Third operation | 3 |
| Thoracotomy | 3 (100) |
| Chest wall resection | 1 (33) |
| Decortication | 1 (33) |
| Segmentectomy | 1 (33) |
| Fourth operation | 1 |
| Thoracotomy | 1 (100) |
| Chest wall debridement | 1 (100) |
Operations were associated with significant morbidity (Table 3). Median estimated blood loss was approximately 100 mL (mean 500 mL). Median postoperative length of stay in the intensive care unit was 4 days (mean 12.3). Twenty-seven percent of cases (14/51) had operative complications with 12 % (6/51) experiencing recurrent pulmonary infection and respiratory failure. No mortalities occurred in the first 30 postoperative days, but there was a 6 % (3/51) mortality rate at 90 days. At the time of analysis, 37 % (13/35) of patients had expired; pulmonary infection was the primary cause of death in 53 % (7/13) of these cases.
Table 3.
Postoperative outcomes
| Growth on Micro | No. (%) |
| Positive growth | 34 (67) |
| Negative result | 17 (33) |
| Aspergillus species | 34 (45) |
| Penicllium Species | 5 (17) |
| Paecilomyces species | 3 (10) |
| Trichosporon species | 2 (7) |
| Complications | No. (%) |
| Total | 14 (27) |
| Recurrent pulmonary infection / Respiratory failure | 6 (12) |
| Wound complication | 5 (10) |
| Vascular injury | 2 (4) |
| Median EBL | 100 mL |
| Median ICU LOS | 4.0 days |
| Mortality | No. (%) |
| 30 days | 0 |
| 90 days | 6 (3/51) |
| Median potential follow-up | 16.5 years |
| Median survival | Not reached |
| Patients expired at time of analysis | 12 (34) |
| Pulmonary cause of death | 7 (58) |
Microbial analysis results were identified in all cases. Thirty-three percent (17/51) of specimens had no growth (Table 3). Of the 17 that did not grow in microbial culture, pathology analysis revealed fungus by histology or staining in 47 % (8/17) cases. Fungus was identified by either pathologic or microbial culture in a total of 80 % (41/51) of cases. There were ten cases in which fungus was identified by pathology only, and there were six cases in which fungus was identified by microbial culture alone. In microbial culture, the most common species isolated were Aspergillus species in 60 % (18/ 51), Penicillium species in 10 % (5/51), Paecilomyces species in 6 % (3/51), and both Trichosporon and Nocardia species in 4 % (2/51). Some cultures had multiple species present.
Pathology reports were additionally examined for histologic changes associated with CGD. Lung or chest wall tissue granulomas or granulomatous changes were almost universally present, mentioned in 94 % (48/51) of pathology reports. Necrotizing granulomatous tissue was present in 41 % (21/51) of cases.
Outcomes
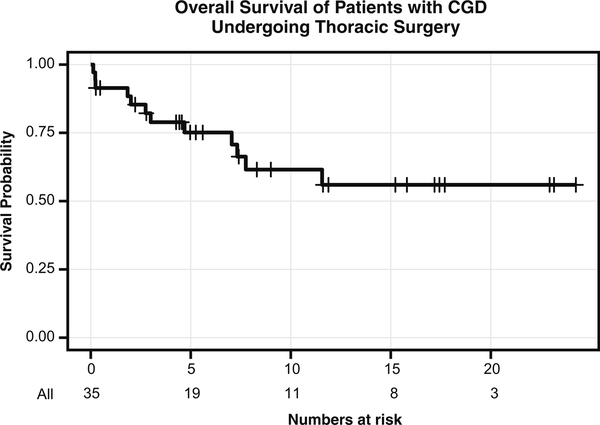
We evaluated the overall survival of patients in our cohort requiring thoracic surgery. For the entire NIH cohort of 258 patients, 44 had expired at time of analysis, 13 of whom had had thoracic surgery (30 %). Of those who had expired, the median age at death was 24.5 years. For the 35 patients who had undergone thoracic surgery, 12 had expired at the time of analysis; similarly, the median age at death for this group was 25.5 years (Fig. 3). Using a Cox model with time to operation as a time-varying covariate, among all patients with CGD, having a thoracic surgery was associated with an increased hazard ratio for death of 3.71 (95 % confidence interval (CI) 1.89,7.27; p < 0.0001). For patients undergoing surgery, median survival after the first operation had not been reached. The 5-year overall survival probability for the thoracic surgery subgroup was 75.1 % (95 % CI 56.1–86.8 %), and the 10-year overall survival probability was 61.6 % (95 % CI 40.4–77.1 %), with a median potential follow-up of 16.5 years. At the time of analysis, 34 % (12/35) of the patients had expired and pulmonary infection was a primary cause of mortality in 58 % (7/12). Among the 35 patients that had at least one surgery, a Cox model with time to second operation as a time-varying covariate demonstrated that a second operation was unassociated with survival (p = 0.68).
Fig. 3.
Kaplan-Meier curve demonstrating survival probability of all CGD patients that underwent thoracic surgery at the NIH during the study period
Univariate analysis identified a small number of variables that were associated with mortality. The only variables that were univariate negative prognostic factors were chest wall resection and estimated blood loss greater than 500 mL (p<0.05). Initially, both parameters were included in a Cox model, but only chest wall resection remained as a negative prognostic factor (HR=4.24; 95 % CI on HR 1.24–14.54; p=0.022.
Discussion
We identified all patients with CGD who required thoracic surgery for recalcitrant pulmonary infections over 25 years at one institution. No similar series of outcomes and survival after thoracic surgery has been reported in over 20 years [2, 9, 17]. During that time, antibiotics, antifungal drugs, utilization of interferon gamma, steroids, and bone marrow transplantations have significantly improved long-term outcomes for patients with CGD [2]. Furthermore, gene therapy provides promise as a future potentially definitive treatment of the disease [18]. However, the important role for thoracic surgery in the management of pulmonary infections in CGD patients appears to persist. This series presents the only description of the rate at which patients received thoracic surgical interventions for pulmonary infections in CGD.
There is a paucity of recent literature discussing the role of surgery for clearance or diagnosis of infections in patients with immunodeficiencies. We searched PubMed for “Thoracic Surgery” and “Chronic Granulomatous Disease,” “Common Variable Immunodeficiency,” and “X-Linked Agammaglobulinemia” and found no multi-patient reports. The role of thoracic surgery in the management of mycobacterial pulmonary processes in immunocompetent patients is better documented. A recent paper reported an operative mortality rate of 2.6 % and morbidity rate of 11.7 % in 265 operations on 236 patients with nontuberculous mycobacterial disease of the lung [19]. Our perioperative complications appear to be roughly consistent with this report, although the age of the mycobacterial patients was much greater than the ages of our CGD patients.
Substantial effort has focused on identifying the specific pathogens to which CGD patients are most susceptible. The finding of Aspergillus species as the predominant organisms in these severe lung infections is also consistent with previous work, as Aspergillus infection of the lung is a major cause of CGD mortality [2]. An older series reported Aspergillus in 16 % of CGD-related infections [20]. While S. aureus is known to cause infection complications in CGD patients, it is a relatively rare cause of pneumonia [2]. Accordingly, S. Aureus was not identified on any of the surgical microbial cultures in this series. The finding that 33 % of surgical cultures did not yield diagnostic growth is consistent with the fact that the organism load is typically low in CGD and further complicated by the exuberant inflammatory responses which lead to tissue granuloma formation [13, 21, 22]. This rate of failure to grow pathogens is consistent with previous series of thoracic diagnostic procedures in immunocompromised children, which found a definitive diagnosis in only 50 % (25/50) of patients [23].
Postoperative thoracic wound infections are relatively rare even in operations performed for infection clearance; however, 10 % of this group had wound complications. This finding is consistent with the previously observed rates of wound complications after surgery for CGD compared to other infectious diseases. In contrast, the series examining thoracic surgery in nontuberculous mycobacterial disease identified only two instances of wound infections in 265 operations [19]. Previous series of CGD patients have reported rates of wound complications ranging from 20 to 40 %, likely reflecting the unique inflammatory defect in CGD that leads to exuberant granulation tissue formation and wound dehiscence [11, 12]. It is relatively unique to CGD that wound dehiscence is treated most effectively with systemic corticosteroids, which rapidly reverse it.
The rate of 90-day postoperative mortality was 6 % (3/51) per case but occurred in 9 % (3/35) of patients. The only previous report on thoracic surgery in CGD reported a mortality rate of 15 % (3/19) during 20 months of follow-up [11]. Given that no patients in our series died within 30 days, this mortality may reflect the underlying disease severity among our patients with recalcitrant pulmonary infections. Median survival after initial thoracic surgery was not reached. Five-year overall survival was 75 %, and 10-year overall survival was 62 %; however, we do not know whether thoracic surgery influenced this survival. Antimicrobial therapy likely played an important role as well.
Blood loss greater than 500 mL and chest wall resection were associated with worse outcomes, with only chest wall resection remaining as an independent factor when both were considered in a Cox model. These factors likely reflected the underlying disease severity and may not be readily modifiable. For example, patients requiring chest wall resection likely have more extensive and invasive infection, such as Aspergillus species, and may not be amenable to a lesser resection. Additionally, increased blood loss is usually related to more complicated procedures.
Conclusion
CGD is a rare genetic disorder that results in refractory pulmonary infections that occasionally require thoracic surgical treatment. Among patients undergoing thoracic surgery, 91 % were male and 80 % had X-linked disease; however, the genotype distribution did not show a statistically significant difference compared to the overall NIH CGD patient cohort. There was a substantial number of surgical microbial specimens that did not yield growth; however, fungus was identified by either pathologic staining or microbial culture in the vast majority of cases. CGD patients undergoing thoracic surgery had overall survival probabilities of 75.1 and 61.6 % at 5 and 10 years, respectively, as well as a significantly increased hazard ratio of death compared to the entire NIH CGD patient population. The surgical risk was further increased for patients who required a chest wall resection or had significant intraoperative blood loss. Unfortunately, thoracic surgery remains an infrequent but occasionally necessary intervention in patients with CGD. This series sets a benchmark for the surgical treatment of pulmonary infections in patients with CGD.
Acknowledgments
The authors would like to thank the Laboratory of Clinical Infectious Disease at the National Institute of Allergy and Infectious Disease for their assistance and collaboration in this work. This work supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Compliance with Ethical Standards All patients or their guardians signed consents for participation in research activities.
References
- 1.Kume A, Dinauer MC. Gene therapy for chronic granulomatous disease. J Lab Clin Med. 2000;135(2):122–8. [DOI] [PubMed] [Google Scholar]
- 2.Marciano BE, Spalding C, Fitzgerald A, Mann D, Brown T, Osgood S, et al. Common severe infections in chronic granulomatous disease. Clin Infect Dis. 2015;60(8):1176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holland SM, Gallin JI. Disorders of granulocytes and monocytes In: Kasper D, Fauci A, Hauser S, Longo D, Jameson JL, Loscalzo J, editors. Harrison’s principles of internal medicine, 19e. New York, NY: McGraw-Hill Education; 2015. [Google Scholar]
- 4.Matute JD, Arias AA, Wright NA, Wrobel I, Waterhouse CC, Li XJ, et al. A new genetic subgroup of chronic granulomatous disease with autosomal recessive mutations in p40 phox and selective defects in neutrophil NADPH oxidase activity. Blood. 2009;114(15):3309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roos D, Kuhns DB, Maddalena A, Roesler J, Lopez JA, Ariga T, et al. Hematologically important mutations: X-linked chronic granulomatous disease (third update). Blood Cells Mol Dis. 2010;45(3): 246–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holland SM. Chronic granulomatous disease. Hematol Oncol Clin North Am. 2013;27(1):89–99. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martire B, Rondelli R, Soresina A, Pignata C, Broccoletti T, Finocchi A, et al. Clinical features, long-term follow-up and outcome of a large cohort of patients with chronic granulomatous disease: an Italian multicenter study. Clin Immunol. 2008;126(2):155–64. [DOI] [PubMed] [Google Scholar]
- 8.Bridges RA, Berendes H, Good RA. A fatal granulomatous disease of childhood; the clinical, pathological, and laboratory features of a new syndrome. AMA J Dis Child. 1959;97(4):387–408. [PubMed] [Google Scholar]
- 9.van den Berg JM, van Koppen E, Ahlin A, Belohradsky BH, Bernatowska E, Corbeel L, et al. Chronic granulomatous disease: the European experience. PLoS One. 2009;4(4):e5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fishman JA. Pulmonary infection in immunocompromised hosts In: Grippi MA, Elias JA, Fishman JA, Kotloff RM, Pack AI, Senior RM, et al. , editors. Fishman’s pulmonary diseases and disorders. New York, NY: McGraw-Hill Education; 2015. [Google Scholar]
- 11.Pogrebniak HW, Gallin JI, Malech HL, Baker AR, Moskaluk CA, Travis WD, et al. Surgical management of pulmonary infections in chronic granulomatous disease of childhood. Ann Thorac Surg. 1993;55(4):844–9. [DOI] [PubMed] [Google Scholar]
- 12.Eckert JW, Abramson SL, Starke J, Brandt ML. The surgical implications of chronic granulomatous disease. Am J Surg. 1995;169(3):320–3. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez-Downing MM, Kamal N, Inchauste SM, Khangura SK, Malech HL, Holland SM, et al. The role of surgery in the management of patients with refractory chronic granulomatous disease colitis. Dis Colon Rectum. 2013;56(5):609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lublin M, Bartlett DL, Danforth DN, Kauffman H, Gallin JI, Malech HL, et al. Hepatic abscess in patients with chronic granulomatous disease. Ann Surg. 2002;235(3):383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhns DB, Alvord WG, Heller T, Feld JJ, Pike KM, Marciano BE, et al. Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med. 2010;363(27):2600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R Development Core Team. R. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 17.Jones LB, McGrogan P, Flood TJ, Gennery AR, Morton L, Thrasher A, et al. Special article: chronic granulomatous disease in the United Kingdom and Ireland: a comprehensive national patient-based registry. Clin Exp Immunol. 2008;152(2):211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang EM, Malech HL. Gene therapy for chronic granulomatous disease. Methods Enzymol. 2012;507:125–54. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell JD, Bishop A, Cafaro A, Weyant MJ, Pomerantz M. Anatomic lung resection for nontuberculous mycobacterial disease. Ann Thorac Surg. 2008;85(6):1887–92. discussion 92–3. [DOI] [PubMed] [Google Scholar]
- 20.Mouy R, Fischer A, Vilmer E, Seger R, Griscelli C. Incidence, severity, and prevention of infections in chronic granulomatous disease. J Pediatr. 1989;114(4 Pt 1):555–60. [DOI] [PubMed] [Google Scholar]
- 21.Winkelstein JA, Marino MC, Johnston RB Jr, Boyle J, Curnutte J, Gallin JI, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine. 2000;79(3):155–69. [DOI] [PubMed] [Google Scholar]
- 22.Moskaluk CA, Pogrebniak HW, Pass HI, Gallin JI, Travis WD. Surgical pathology of the lung in chronic granulomatous disease. Am J Clin Pathol. 1994;102(5):684–91. [DOI] [PubMed] [Google Scholar]
- 23.Naiditch JA, Barsness KA, Rothstein DH. The utility of surgical lung biopsy in immunocompromised children. J Pediatr. 2013;162(1):133–6. e1. [DOI] [PubMed] [Google Scholar]