Abstract
Limited information is available regarding epigenomic events mediating initiation and progression of tobacco-induced lung cancers. In this study, we established an in vitro system to examine epigenomic effects of cigarette smoke in respiratory epithelia. Normal human small airway epithelial cells and cdk-4/hTERT-immortalized human bronchial epithelial cells (HBEC) were cultured in normal media with or without cigarette smoke condensate (CSC) for up to 9 months under potentially relevant exposure conditions. Western blot analysis showed that CSC mediated dose- and time- dependent diminution of H4K16Ac and H4K20Me3, while increasing relative levels of H3K27Me3; these histone alterations coincided with decreased DNA methyltransferase 1 (DNMT1) and increased DNMT3b expression. Pyrosequencing and quantitative RT-PCR experiments revealed time-dependent hypomethylation of D4Z4, NBL2, and LINE-1 repetitive DNA sequences; up-regulation of H19, IGF2, MAGE-A1, and MAGE-A3; activation of Wnt signaling; and hypermethylation of tumor suppressor genes such as RASSF1A and RAR-β, which are frequently silenced in human lung cancers. Array-based DNA methylation profiling identified additional novel DNA methylation targets in soft-agar clones derived from CSC-exposed HBEC; a CSC gene expression signature was also identified in these cells. Progressive genomic hypomethylation and locoregional DNA hypermethylation induced by CSC coincided with a dramatic increase in soft-agar clonogenicity. Collectively, these data indicate that cigarette smoke induces ‘cancer-associated’ epigenomic alterations in cultured respiratory epithelia. This in vitro model may prove useful for delineating early epigenetic mechanisms regulating gene expression during pulmonary carcinogenesis.
Keywords: tobacco smoke, lung cancer, epigenetics, respiratory epithelial cells
Introduction
Mounting evidence implicates aberrant expression/activity of epigenetic regulators of gene expression in the initiation and progression of lung cancers, the majority of which are directly attributable to cigarette smoking (Lee et al., 2005; D’Alessio and Szyf, 2006; Lin et al., 2007; Schrump et al., 2007). For example, increased DNA methyltransferase (DNMT) expression coincides with progression to malignancy in murine pulmonary adenomas induced by tobacco carcinogens (Belinsky et al., 1996). Over-expression of DNMT1 and DNMT3b, as well as methyl-binding domain 2, correlates with hypermethylation of tumor suppressor genes in tobacco-induced lung cancers, and diminished survival of patients with these neoplasms (Kim et al., 2006; Lin et al., 2007; Xing et al., 2008). Aberrant activities of histone lysine methyl transferases and histone deacetylases enhance oncogenic transformation of bronchial epithelial cells (Watanabe et al., 2008), and induce global alterations in the histone code, which correlate with advanced stage of disease and poor prognosis in lung cancer patients (Barlesi et al., 2007; Van Den et al., 2008; Seligson et al., 2009). Over-expression of polycomb proteins such as Bmi 1 and Ezh2 facilitates epigenetic silencing of tumor suppressor genes, and enhances stem cell signaling in lung cancer cells (Dovey et al., 2008; Vrzalikova et al., 2008; Hussain et al., 2009; McCabe et al., 2009).
Epigenetic alterations during malignant transformation seem analogous to those regulating gene expression during gametogenesis and stem cell development (Simpson et al., 2005; Mathews et al., 2009). For example, tumor suppressor genes such as p16 and p19/ARF, which mediate replicative senescence and apoptosis in response to oncogene signaling (Agherbi et al., 2009), are frequently observed to be silenced in cancer cells exhibiting coordinate de-repression of germ-line restricted genes such as NY-ESO-1 and MAGE family members, several of which physically interact and suppress p53-mediated apoptosis (Cho et al., 2006; Monte et al., 2006; Yang et al., 2007). These observations suggest that epigenetic perturbations in lung cancer cells are not merely manifestations of random stochastic events, but instead reflect strong selective pressure to reactivate/maintain stem cell gene expression during multistage pulmonary carcinogenesis.
Despite the unequivocal association between cigarette smoking and lung cancer, the epigenetic mechanisms by which tobacco smoke initiates and promotes pulmonary carcinogenesis have not been fully delineated. In particular, epigenetic events associated with initiation of tobacco-induced lung cancers have not as yet been elucidated. This study was undertaken to characterize epigenomic alterations in cultured human respiratory epithelial cells mediated by cigarette smoke.
Results
Growth inhibitory effects of cigarette smoke condensate in cultured cells
Preliminary experiments were initiated to examine the effects of cigarette smoke condensate (CSC) in cultured respiratory epithelia and lung cancer cells to define appropriate exposure conditions for subsequent studies. As shown in Figure 1a, CSC mediated dose-dependent growth inhibition, which coincided with morphologic changes in cultured respiratory epithelia; after CSC exposure, cdk-4/h-Tert immortalized human bronchial epithelial cells (HBEC), and to a lesser extent normal human small airway epithelial cells (SAEC) became less polygonal and appeared more elongated and rectangular in shape. Interestingly, under these exposure conditions, the effects of CSC were less apparent in fully transformed A549 lung cancer cells.
Figure 1.
(a) Morphology and proliferation of SAEC, HBEC, and A549 cells exposed to CSC. Dose-dependent alterations in cell morphology seemed to coincide with growth inhibition after CSC exposure. CSC-mediated morphologic changes and growth inhibition in A549 lung cancer cells were considerably less than those observed in short-term SAEC or immortalized HBEC. (b) Upper panel: Western blot analysis of H4K16 acetylation and H4K20 trimethylation in HBEC after exposure to CSC. A dose-dependent decrease in H4K16 acetylation was observed within 4 h of CSC exposure; this phenomenon persisted after exposures of 24 and 48 h. No appreciable change in H4K20 trimethylation was observed after short-term CSC exposure. Lower panel: Immunoflourescence analysis of acetylated H4K16 and trimethylated H4K20 expression in HBEC exposed to CSC for 3 days. The results reveal a decrease in H4K16 acetylation in HBEC without significant reduction in H4K20 trimethylation in HBEC exposed to 1% CSC. These results are consistent with western blot data in upper panel. (c) Western blot analysis of H4K16Ac, and H4K20Me3 and total H4 levels in cultured NHBE and SAEC exposed to CSC for 72 h. Short-term CSC exposure seemed to decrease H4K16 acetylation, without altering H2K20Me3 in both types of normal respiratory epithelia. (d) Western blot analysis of histone alterations as well as DNMT and EZH2 expression in HBEC exposed to CSC. Densitometry analysis revealed time-dependent decreases in H4K16 acetylation and H4K20 trimethylation without appreciable alterations in total histone H4 levels. These alterations coincided with a relative increase in H3K27 trimethylation as well as a modest increase in EZH2, the polycomb protein mediating this repressive mark. These phenomena, which were evident after 5-month CSC exposure, coincided with a decrease in DNMT1:DNMT3b protein ratios.
‘Cancer-associated’ histone alterations mediated by CSC
Malignant transformation is associated with global loss of monoacetylated H4K16 as well as decreased H4K20me3 levels (Fraga et al., 2005; Van Den et al., 2008). As such, western blot experiments were performed to ascertain whether CSC exposure could induce these histone changes in respiratory epithelial cells. Briefly, SAEC and HBEC were cultured in normal media with or without CSC at various concentrations and exposure durations. As shown in Figure 1b (upper panel), within 24 h of exposure, CSC mediated a dose- dependent decrease in H4K16Ac levels, without appreciably changing total H4 expression in HBEC. A similar phenomenon was observed after 24 or 48 h CSC exposure. Densitometry analysis revealed no appreciable change in H4K20Me3 levels under these exposure conditions. Additional analysis using indirect immunofluorescence techniques (Figure 1b, lower panel) confirmed results of aforementioned western blot experiments. Short-term CSC exposure mediated dose-dependent decreases in H4K16Ac levels without appearing to alter H4K20Me3 levels in short-term normal human bronchial epithelial (NHBE) cells as well as SAEC (Figure 1c), suggesting that the acute effects of CSC exposure on the histone code were not unique to immortalized HBEC.
Additional experiments were performed to examine the effects of prolonged CSC exposures in immortalized HBEC. Representative results of this analysis are depicted in Figure 1d. Relatively low concentrations of CSC (1%) mediated time-dependent decreases in H4K16Ac, as well as H4K20me3 levels; reduced levels of H4K16Ac—but not H4K20Me3—were observed in HBEC 5 days after initiation of CSC exposure. Decreased H4K20Me3 levels were evident in HBEC 1 month after initiation of CSC exposure. More prolonged exposure to 1% CSC virtually abolished H4K16Ac, and dramatically reduced H4K20Me3 levels without significantly diminishing total H4 levels in HBEC; these histone alterations coincided with a 2.6- fold relative increase in H3K27Me3, an 80% reduction in DNMT1, as well as ~2–3-fold increase in EZH2 and DNMT3b levels, respectively.
DNA methylation changes mediated by CSC
Additional experiments were performed to ascertain whether CSC exposure altered global DNA methylation status in HBEC. Initial pyrosequencing experiments focused on NBL2-seq3 and D4Z4 DNA repeats, as well as LINE-1 elements, as these have been shown to be demethylated in cancer cells exhibiting decreased levels of H4K16Ac and H4K20Me3 (Fraga et al., 2005). Preliminary analysis showed modest demethylation of these sequences in untreated immortalized HBEC relative to primary cultures of SAEC or NHBE cells, although not to the extent observed in A549 lung cancer cells (Figure 2a). Subsequent experiments revealed a dose-dependent demethylation of NBL2 and D4Z4 subtelomeric DNA repeats, and to a lesser extent, LINE-1 elements in HBEC after 5-month continuous CSC exposure (Figure 2b).
Figure 2.
Pyrosequencing analysis of methylation status in repetitive DNA sequences before and after CSC exposure. (a) Pyrosequencing analysis of NBL2, D4Z4, and LINE-1 methylation in untreated respiratory epithelia and A549 lung cancer cells. Relative to SAEC and NHBE, immortalized HBEC exhibited somewhat reduced methylation in these DNA repeats. This phenomenon was even more pronounced in A549 lung cancer cells. (b) Pyrosequencing analysis of NBL2, D4Z4, and LINE-1 sequences in HBEC exposed to CSC for 5 months. A dose-dependent decrease in DNA methylation was observed, suggestive of global DNA demethylation mediated by cigarette smoke. (c) Left panel: Quantitative RT–PCR and correlative RT–PCR analysis of imprinted and cancer-testis gene expression in HBEC after CSC exposure (upper and lower panels, respectively). The results of RT–PCR depicted as fold change relative to untreated HBEC. CSC dose-dependently increased expression of H19 and IGF-2 imprinted loci, as well as MAGE-A1 and MAGE-A3. No increase in NY-ESO-1 expression was observed. The results of RT–PCR analysis were consistent with qRT–PCR data. Right panel: Pyrosequencing analysis of genomic DNA and cDNA pertaining to IGF2 and H19 in control HBEC as well as HBEC exposed to CSC for 5 months. Genotyping revealed polymorphisms that could distinguish between maternal and paternal alleles of IGF2 and H19. After prolonged CSC exposure, only the maternally imprinted IGF2 and the paternally imprinted H19 loci were expressed. These results suggest that enhanced expression of these loci was attributable to mono-allelic up-regulation rather than loss of imprinting. (d) qRT–PCR with representative RT–PCR, and pyrosequencing analyses of tumor suppressor gene loci in HBEC after prolonged CSC exposure (upper and lower panels, respectively). A progressive, dose-dependent increase in promoter methylation was observed in RASSF1A and RAR-β promoter regions. No apparent hypermethylation was observed in several tumor suppressor genes such as p16, E-cadherin, DAP kinase, and MGMT, which are frequently silenced in tobacco-associated lung cancers by epigenetic mechanisms. DAP kinase expression in untreated and CSC-exposed HBEC was exceedingly low. Dkk-1 was not methylated, but was repressed, a phenomenon possibly attributable to polycomb repressor complexes (Hussain et al., 2009). Diminution of RASSF1A expression coincided with hypermethylation of the RASSF1A promoter. Hypermethylation of RAR-β seemed insufficient to silence this gene under exposure conditions used for these experiments.
Quantitative RT-PCR experiments were performed to ascertain whether hypomethylation of the aforementioned repetitive DNA sequences coincided with activation of imprinted loci, as well as cancer-testis-X chromosome (CT-X) genes that are frequently derepressed in primary lung cancers through epigenetic mechanisms. As shown in Figure 2c (left panel), CSC mediated dose-dependent up-regulation of IGF2 and H19 imprinted loci, as well as several CT-X genes, including MAGE-A1 and MAGE-A3. Interestingly, NY-ESO-1 (cancer-testis antigen 1), a CT-X gene frequently de-repressed in lung cancer cells (Schrump et al., 2007), did not seem to be activated in HBEC by CSC under these exposure conditions.
Additional experiments were performed to determine whether enhanced expression of IGF2 and H19 was due to mono-allelic up-regulation of these loci, or loss of imprinting. Genomic sequencing (Figure 2c, right panel) revealed polymorphisms that could distinguish maternal and paternal IGF2 and H19 alleles in HBEC; additional pyrosequencing experiments indicated that only the paternal IGF2 and the maternal H19 alleles were expressed in untreated as well as CSC-exposed HBEC. These data suggested that increased IGF2 and H19 expression in HBEC after 5-month CSC exposure was due to mono-allelic up-regulation of the normally expressed alleles, rather than loss of imprinting. These findings are consistent with the data reported by Kaplan et al. (2003) after exposure of NHBE to cigarette smoke.
Additional qRT-PCR and pyrosequencing experiments were performed to examine whether CSC exposures sufficient to induce global DNA demethyla-tion also mediated repression of tumor suppressor genes typically silenced in human lung cancers by DNA hypermethylation mechanisms. As shown in Figure 2d, CSC exposure did not appear to alter expression or methylation status of p16, E-cadherin, DAP kinase, or MGMT. However, CSC mediated dose-dependent diminution of RASSF1A as well as Dickkopf-1 (Dkk-1) expression; repression of RASSF1A, but not Dkk-1, coincided with promoter hypermethylation. The results pertaining to Dkk-1 repression in HBEC after CSC exposure were consistent with recent data showing that cigarette smoke induces polycomb-mediated repression of Dkk-1 in lung cancer cells (Hussain et al., 2009). Interestingly, CSC mediated dose-dependent hypermethylation without appreciably diminishing expression of RAR-β, which is frequently silenced through epigenetic mechanisms in tobacco-associated lung cancers (Toyooka et al., 2003, 2006).
Activation of Wnt signaling by CSC
As CSC exposure silenced Dkk-1, additional experiments were performed using focused qRT-PCR arrays to further examine the effects of cigarette smoke on Wnt signaling in HBEC. The results of this analysis are summarized in Figure 3. Prolonged CSC exposure dramatically enhanced expression of several Wnt ligands implicated in invasion and metastasis of cancer cells as well as maintenance of cancer stem cells, including Wnt 10a, Wnt 6, Wnt 5a, and Wnt 2 (Huang et al., 2005; Le et al., 2005; Katoh and Katoh, 2007; Huang and He, 2008), and up-regulated downstream targets of Wnt signaling, including FOXN1 and TCF7. In addition, CSC exposure markedly decreased expression of several Wnt antagonists, including SFRP1 and Dkk-1(Winn et al., 2006; Katoh and Katoh, 2007). Whereas epigenetic mechanisms may have contributed to enhanced Wnt signaling perhaps through repression of SFRP1 and Dkk1, which are known to be silenced in human lung cancers by DNA methylation and polycomb repressor complexes (Licchesi et al., 2008; Hussain et al., 2009), the data do not exclude the possibility that perturbation of Wnt signaling was in part a manifestation of cellular response to oxidative stress (Hoogeboom and Burgering, 2009) (Figure 3b).
Figure 3.
(a) The results of Wnt superarray analysis showing marked up-regulation of a variety of Wnt ligands and down-regulation of antagonists of Wnt signaling including SFRP1 and Dkk-1. (b) The results of superarrays showing CSC-mediated changes in expression levels of numerous genes regulating cellular response to oxidative stress.
Clonogenicity and tumorigenicity of HBEC after CSC exposure
Additional experiments were performed to ascertain whether CSC exposure was sufficient to transform HBEC. As shown in Figures 4a and b, CSC exposure dramatically increased soft-agar clonogenicity of HBEC in a time-dependent manner, a phenomenon that coincided with progressive demethylation of NBL2 and D4Z4, and hypermethylation of RASSF1A and RAR-β, but not p16, DAPK, E-cadherin, or CDH13 (Figure 4c). Five soft-agar clones arising spontaneously from control HBEC and ~30 clones derived from HBEC exposed to CSC for 9 months were expanded for further analysis. Pyrosequencing experiments indicated that methylation profiles in spontaneous and CSC-derived clones reflected those observed in untreated and CSC-exposed cell populations, respectively (Figure 4d). The fact that consistent methylation profiles were observed in CSC-derived relative to control clones strongly suggests that enhanced clonogenic potential of CSC-treated HBEC was a manifestation of selection pressure mediated by CSC on the HBEC epigenome, rather than outgrowth of cells exhibiting aberrant DNA methylation already present in untreated pooled HBEC. Despite the dramatic increase in soft-agar clonogenicity of CSC-treated HBEC, no tumors were observed in 100 nude or NOD.SCId\iL-2Rγ null mice inoculated in contralateral flanks with control HBEC and one of the six CSC-derived soft-agar clones (data not shown).
Figure 4.
(a, b) Clonogenic potential of HBECs after prolonged CSC exposure. Soft-agar assay showing a time-dependent increase in clonogenic potential of HBEC after prolonged CSC treatment. (c) Pyrosequencing analysis of DNA methylation within repetitive DNA sequences and tumor suppressor genes in HBEC after prolonged CSC exposure. A progressive time-dependent increase in RASSF1A and RARβ promoter methylation was observed without an apparent increase in methylation of p16, DAPK, E-cadherin, or CDH13. Increased clonogenic potential also coincided with a time-dependent decrease in methylation status within DNA repeats, particularly NBL2 and D4Z4. (d) Pyrosequencing analysis of DNA methylation in soft-agar clones emerging spontaneously from untreated HBEC and representative soft-agar clones derived from HBEC exposed to CSC for 9 months. The CSC-exposed clones showed markedly diminished methylation status within NBL2 and D4Z4, findings which were consistent with results observed in bulk cultures. Once again, there was no change in methylation status within p16, DAPK, E-cadherin, H-cadherin, or MGMT. However, clones derived from CSC-exposed HBEC exhibited markedly increased methylation status of RASSF1A and RARβ. These data suggest that CSC induced alterations, which contributed to enhanced clonogenicity rather than selecting for outgrowth of cells exhibiting aberrant methylation profiles in bulk untreated HBEC.
Array analysis of DNA methylation and gene expression mediated by CSC
Illumina array techniques were used to more comprehensively examine DNA methylation and gene expression profiles in HBEC exposed to CSC. Briefly, sodium bisulfite-modified DNA samples derived from untreated and CSC-exposed HBEC, as well as soft-agar clones derived from control and CSC-exposed HBEC, were analyzed using the Illumina Infinium methylation assays that measure DNA methylation at over 27 000 CpG loci distributed among > 14K genes. Also profiled were bisulfite-modified DNAs derived from several established lung cancer lines. Partial results of this analysis are depicted in Figure 5. Short-term (5 days) CSC exposure had negligible effects in SAEC and HBECs (data not shown). However, using an absolute value DiffScore >25, which corresponded to P<0.05, 48 targets appeared to become demethylated, whereas 56 targets exhibited increased methylation in HBEC after 9-month CSC exposure (Figure 5a; Table 1). The Illumina methylation assays were non-informative for global methylation, but rather provided locus-specific methylation measurements; however, pyrosequencing data were consistent with global genomic demethylation occurring in the context of locoregional hypermethylation. Overall, methylation profiles in spontaneous soft-agar clones derived from untreated HBEC were virtually identical to those observed in pooled, control HBEC. Furthermore, methylation changes observed in soft-agar clones from CSC-exposed HBEC expanded under CSC exposure were remarkably similar to those observed in the same clones expanded in the absence of CSC. These findings strongly suggest that DNA methylation changes induced by prolonged CSC treatment were quite stable and heritable. Several CpG targets, including RASSF1A, LOX, and CALCA, that exhibited DNA hypermethylation in HBEC after CSC exposure were also noted to be methylated in cultured lung cancer lines; in contrast, other targets such as MEG-3, HOXA9, TFF-2, and GNAS that were methylated in several cultured as well as primary lung cancers did not appear to be methylated in HBEC after CSC exposure (data not shown). These results suggest that either more prolonged CSC exposures, or cigarette smoke components not present in the condensates are necessary to further shift DNA methylation profiles in HBEC toward those typically observed in cultured non-small cell lung cancers.
Figure 5.
(a) Illumina array analysis of DNA methylation in control bulk HBEC, control spontaneous soft-agar clones, and soft-agar clones derived from HBEC exposed to CSC for 9 months. Treatment groups were as follows—1: control untreated HBEC; 2: control spontaneous soft-agar clones; 3: CSC-derived soft-agar clones expanded in the absence of CSC; 4: CSC-derived soft-agar clones expanded in the presence of CSC; 5: pooled HBEC exposed to CSC for 9 months. Each lane pertains to an individual clone or pooled sample. Spontaneous clones exhibited methylation profiles remarkably similar to those observed in untreated HBEC cells. In contrast, CSC-exposed clones showed considerable alterations in methylation status across numerous gene loci. Additional short-term CSC exposure did not seem to further alter methylation profiles in CSC-derived soft-agar colonies. Heat map represents a differential score >25 for genes hypo- or hypermethylated by CSC treatment. These data are summarized in Table 1. (b) Quantitative RT–PCR analysis of HSPB1 expression in untreated control and CSC-treated HBEC, as well as spontaneous and CSC-derived soft-agar clones. Data from two independent experiments revealed that CSC exposure diminished HSPB1 expression in HBEC. Interestingly, HSPB1 expression in spontaneous soft-agar clones was decreased relative to untreated controls, albeit to a lesser extent than CSC-treated HBEC. (c) Pyrosequencing analysis of HSPB1 promoter methylation in untreated and pooled CSC-treated HBEC, as well as spontaneous and CSC-derived soft-agar clones. CSC exposure mediated a progressive increase in HSPB1 promoter methylation. No apparent increase in HSPB1 promoter methylation was observed despite diminished HSPB1 expression in spontaneous clones relative to untreated HBEC controls. (d) ChIP analysis showing decreased H3K9Ac (activation mark) with increased H3K27Me3 (repression mark) within the HSPB1 promoter in HBEC exposed to CSC. These findings are consistent with CSC-mediated down-regulation of HSPB1. (e) Quantitative RT–PCR analysis of HSPB1 expression in a panel of immortalized respiratory epithelia and lung cancer lines. The results are depicted as fold relative to SAEC. HSPB1 expression in immortalized respiratory epithelia (HBEC and BEAS) and numerous lung cancer lines was lower than that observed in SAEC.
Table 1.
Differentially methylated targets in CSC-derived soft-agar clones
| Hypomethylated in CSC-treated vs controls | Hypermethylated in CSC-treated vs controls | ||||
|---|---|---|---|---|---|
| Symbol | Illumina index | ABS (DiffScore) | Symbol | Illumina index | ABS (DiffScore) |
| ABCC6 | 2617 | 25.6 | ABHD7 | 13 946 | 25.3 |
| ADORA2A | 21 571 | 29.4 | ADAMTS17 | 7955 | 25.9 |
| ARR3 | 11 432 | 25.4 | C10orf59 | 6803 | 28.3 |
| ATP10A | 26 040 | 26.1 | Clord65 | 27 143 | 30.2 |
| BAGE | 7940 | 26.1 | C1QTNF1 | 8298 | 27.3 |
| BCNP1 | 6275 | 27.3 | C1QTNF1 | 24 812 | 28 |
| CCND1 | 990 | 27.6 | C2ord10 | 13 038 | 25.9 |
| CCND1 | 7397 | 28.2 | CALCA | 6269 | 26.1 |
| CCRL2 | 5667 | 26.1 | CCDC37 | 914 | 28.3 |
| CFLAR | 18 131 | 26.1 | CILP2 | 10 236 | 25.9 |
| CHRFAM7A | 6289 | 25.9 | CLSTN2 | 14 499 | 25.9 |
| DGCR2 | 16 686 | 26.4 | CPT1A | 19 599 | 28 |
| DNM1L | 27 322 | 28.3 | DOCK2 | 21 201 | 26.1 |
| DNTTIP2 | 22 772 | 26.1 | DRD1 | 16 186 | 25.6 |
| FAM105A | 23 585 | 25.2 | DRD5 | 9862 | 27.1 |
| FAM71C | 13 272 | 26.5 | FLJ13391 | 10 049 | 26.1 |
| FLJ31196 | 24 709 | 32 | GAD1 | 947 | 28 |
| FLJ38451 | 20 517 | 28 | GATA4 | 14 108 | 28 |
| FXN | 7155 | 27.3 | GRID2 | 14 015 | 29.4 |
| GHRH | 4495 | 26.1 | HSPB1 | 27 358 | 26.1 |
| GNAS | 25 261 | 28.4 | IGF2AS | 13 734 | 25.2 |
| GPR97 | 8320 | 30 | INA | 25 743 | 25.5 |
| HEM1 | 17 514 | 29.4 | INSR | 10 575 | 32.9 |
| HOXA9 | 26 483 | 27.1 | IRXL1 | 14 619 | 25.9 |
| IL1RAPL2 | 23 356 | 29.4 | KCNJ8 | 1234 | 30 |
| ITM2A | 10 794 | 26 | KCNJ8 | 2039 | 26.1 |
| MC2R | 11 493 | 25.4 | KIF1A | 21 303 | 30 |
| MEG3 | 25 815 | 29.4 | LOC221091 | 25 613 | 28 |
| MOBKL2A | 6887 | 28.3 | LOX | 22 807 | 30 |
| MSR1 | 1695 | 25.2 | MEGF11 | 26 400 | 31.5 |
| MYR8 | 14 385 | 27.6 | MOXD1 | 7539 | 30.2 |
| NMBR | 17 286 | 25.9 | NAALAD2 | 5511 | 27.1 |
| NOS3 | 3781 | 34.8 | NELL2 | 14911 | 25 |
| PPP3R2 | 15 807 | 26.3 | NKX2–2 | 17 058 | 25.9 |
| PRRG2 | 4235 | 28.3 | NMUR1 | 158 | 25.2 |
| RAB11FIP1 | 17 823 | 25.1 | PSCK6 | 20 210 | 26.1 |
| RLN1 | 20 739 | 25.9 | PLD5 | 12 552 | 30.2 |
| SLC26A3 | 22 266 | 28 | PNMA2 | 2174 | 28.3 |
| SUMF1 | 18 839 | 34.4 | PRG-3 | 16 937 | 26.1 |
| TBC1D21 | 12 658 | 25.9 | PRRX1 | 10 632 | 30 |
| TFF2 | 11 073 | 28.3 | PTGDR | 9454 | 26.3 |
| UNQ6411 | 16 017 | 34.8 | PTN | 11 461 | 27.1 |
| UROS | 19 336 | 26.1 | RARB | 26 099 | 28 |
| VHL | 16 908 | 27.1 | RASA4 | 10 198 | 26.1 |
| VHL | 24 046 | 27.6 | RASSF1 | 804 | 25.9 |
| XKRX | 11 593 | 27.1 | SLC26A11 | 24 809 | 28.3 |
| ZNF264 | 16 697 | 30.2 | SOCS1 | 6181 | 27.1 |
| ZNF467 | 23 709 | 28.3 | ST2GAL4 | 1242 | 25.9 |
| STON1 | 25 043 | 26.3 | |||
| TGFBR1 | 15 523 | 28 | |||
| TRPA1 | 1629 | 25.9 | |||
| TSSK3 | 21 138 | 26.3 | |||
| TUBB2B | 3481 | 26.3 | |||
| UBE2G1 | 21 711 | 25.9 | |||
| VAV3 | 34 624 | 30 | |||
| WNT3A | 1334 | 26.1 | |||
Abbreviation: CSC, cigarette smoke condensate.
A variety of CpG targets observed to become relatively hypo- or hypermethylated in HBEC by CSC exposure have not been typically associated with lung cancer development. For example, heat shock protein-binding protein 1 (HSPB1) and phospholipase D-5 seemed to become hypermethylated in HBEC after prolonged CSC exposure (Figure 5a; Table 1). Quantitative RT-PCR and pyrosequencing experiments revealed that CSC exposure diminished expression of HSPB1 coincident with increased methylation of the HSPB1 promoter (Figures 5b and c). This phenomenon was not observed for phospholipase D-5 (data not shown). Subsequent chromatin immunoprecipitation (ChIP) experiments revealed that CSC exposure diminished H3K9Ac, while increasing H3K27Me3 levels within the HSPB1 promoter (Figure 5d); no appreciable change in H4K16Ac level within the HSPB1 promoter was observed in these ChIP experiments (data not shown). Analysis of a panel of cultured respiratory epithelial lines revealed that HSPB1 levels in immortalized bronchial epithelial cells and numerous lung cancer lines appeared lower than those detected in SAEC. Interestingly, HSPB1 expression in spontaneous clones appeared lower than those observed in untreated controls (but higher than in CSC-treated cells/clones). Collectively, these data raise the possibility that HSPB1 may be a novel target of aberrant silencing by epigenetic mechanisms in cigarette smoke induced lung cancers. Whereas loss of intratumoral HSPB1 expression has been shown recently to correlate with diminished survival of lung cancer patients (Malusecka et al, 2008), the significance of our findings regarding CSC-mediated down-regulation of HSPB1 has not been firmly established.
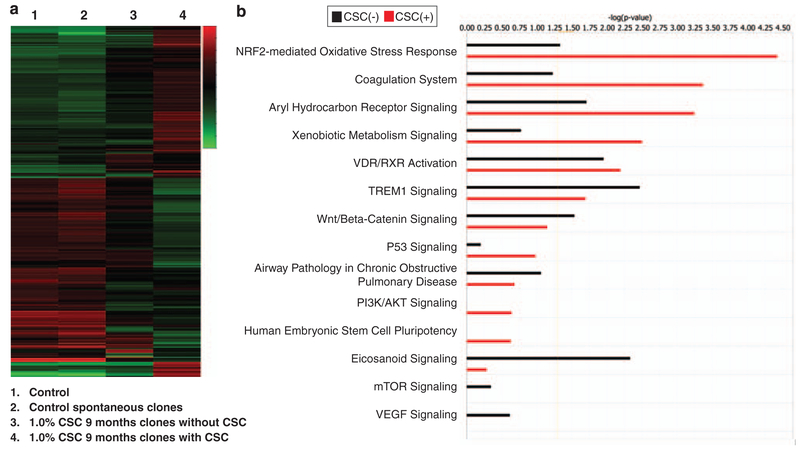
Additional correlative Illumina array experiments were performed to examine gene expression profiles mediated by CSC, using RNA harvested from cells providing DNA for the aforementioned DNA methylation arrays. The results of this analysis are depicted in Figure 6. Gene expression profiles in control spontaneous soft-agar clones were remarkably similar to those in pooled untreated HBEC. In contrast, gene expression profiles in six randomly selected soft-agar clones derived from HBEC exposed to CSC for 9 months (Figure 4d), and expanded in the absence of CSC were considerably different than those observed in controls, and seemed to represent stable sequelae of epigenetic (and presumably genetic) alterations induced by CSC. Profiles in CSC-derived soft-agar clones expanded in the absence of smoke were markedly different from those detected in the same clones after 6-day CSC exposure (Figure 6a, lanes 3 and 4). Using criteria of ≥ twofold relative to controls and P<0.05, 85 genes were up-regulated, whereas 109 genes were repressed in CSC-derived soft-agar clones expanded in the absence of CSC. Using similar criteria, 230 genes were induced, whereas 295 were repressed in these CSC-derived soft-agar clones expanded under CSC exposure. Interestingly, 44% of differentially modulated genes were up-regulated, whereas 56% were repressed in CSC-derived soft-agar clones expanded under either treatment condition. Ingenuity pathway analysis revealed that networks involving antigen presentation/immune response, organ development, tissue morphology/cancer, DNA replication/repair, and inflammation were preferentially modulated in CSC-derived soft-agar clones expanded in the absence of CSC. In contrast, networks preferentially modulated in these soft-agar clones expanded in the presence of CSC included cellular movement, small molecule biochemistry/gene expression, and genetic disorders (data available on request). Several pathways preferentially modulated in these clones are depicted in Figure 6b.
Figure 6.
(a) Illumina array analysis of gene expression in untreated HBEC, and spontaneous clones from control HBEC emerging from soft agar, as well as clones exposed to CSC and then expanded with or without additional tobacco smoke exposure. Gene expression profiles in untreated controls as well as spontaneous control clones were remarkably similar. Interestingly, array analysis revealed that gene expression profiles in clones derived from CSC-treated HBEC that were subsequently expanded without further CSC exposure were markedly altered; additional CSC exposure further modulated gene expression in HBEC, reflecting acute effects of CSC. (b) Ingenuity analysis of Illumina gene expression arrays revealing major pathways modulated in CSC-derived soft-agar clones expanded in the absence (CSC−) or presence (CSC+) of CSC.
Table 2 contains a list of the top 100 genes differentially modulated in CSC-derived soft-agar clones expanded in the absence or presence of CSC relative to control (spontaneous) soft-agar clones. Fifteen of the top 50 genes (30%) activated in CSC-derived clones expanded in the absence of CSC were among the top 50 genes activated in these clones after additional CSC exposure. In contrast, 23 of the 50 most repressed genes (46%) in CSC-derived soft-agar clones expanded in the absence of CSC were among the top 50 most repressed genes in these clones after CSC exposure. Although some exceptions were noted, the magnitude of repression of genes simultaneously down-regulated in CSC-derived soft-agar clones expanded in the absence or presence of CSC (that is TXNIP, DUSP23, VIM) was relatively comparable. In contrast, CSC seemed to enhance the magnitude of activation of genes such as SPANXB1, KLK6, and KYNU that were up-regulated in CSC-derived clones expanded under both treatment conditions. Overall, these data raise the possibility that repression was more stable than activation of gene expression in HBEC after prolonged CSC exposure.
Table 2.
Genes most significantly induced/repressed in soft-agar clones expanded in the presence or absence of CSC
| Top 50 genes up-regulated in soft-agar clones without CSC vs control | Top 50 genes up-regulated in soft-agar clones with CSC vs control | Top 50 genes down-regulated in soft-agar clones without CSC vs control | Top 50 genes down-regulated in soft-agar clones with CSC vs control | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Symbol | (-CSC) | (+CSC) | Symbol | (+CSC) | (-CSC) | Symbol | (-CSC) | (+CSC) | Symbol | (+CSC) | (-CSC) |
| NUAK2 | 8.73 | 5.77 | KYNU | 42.39 | 3.42 | KRT5 | −2.63 | −3.5 | SPARC | −3.54 | −1.2 |
| FABP4 | 6.82 | 1.43 | GPX2 | 37.67 | 2.12 | BACE2 | −2.64 | −3.72 | ARMCX6 | −3.6 | −3.6 |
| SERPINA5 | 5.11 | 2.44 | S100A9 | 24.14 | 1.49 | EFEMP1 | −2.65 | −5.74 | CDH4 | −3.6 | −1.81 |
| ESM1 | 4.76 | −1.33 | ANXA10 | 22.24 | 1.29 | VLDLR | −2.73 | −4.01 | ITGA6 | −3.7 | −1.45 |
| TGM2 | 4.41 | 1.21 | IL8 | 19.58 | 2.84 | DDIT4 | −2.77 | −1.52 | G0S2 | −3.71 | −1.54 |
| EEF1A2 | 4.18 | 6.12 | CYP1B1 | 18.5 | −1.02 | CAMP | −2.8 | 1.28 | HNT | −3.71 | 2.73 |
| KLK5 | 4.12 | 13.02 | S100A8 | 17.83 | −1.6 | FAT2 | −2.84 | −1.65 | BACE2 | −3.72 | −2.64 |
| CDH2 | 3.9 | −1.06 | HSD17B2 | 13.72 | 1.01 | MYO5C | −2.87 | −3.06 | MARCH4 | −3.73 | 1.75 |
| ADAM19 | 3.46 | 1.73 | ALDH3A1 | 13.08 | −1.53 | CCNG2 | −2.88 | −2.63 | CD59 | −3.75 | −1.44 |
| TIMP2 | 3.44 | 1.93 | KLK5 | 13.02 | 4.12 | DLL3 | −2.89 | −2.92 | LMCD1 | −3.84 | −2.41 |
| KYNU | 3.42 | 42.39 | AKR1C3 | 11.74 | −2.2 | LXN | −2.98 | −1.73 | CTGF | −3.86 | −1.4 |
| CSF3 | 3.39 | 3.54 | AKR1C4 | 11.23 | −2.44 | RPL39L | −3.02 | −3.07 | LAMA3 | −3.9 | −1.37 |
| NTSR1 | 3.32 | 3.17 | PTGES | 10.61 | 3.03 | PTGS2 | −3.06 | −1.94 | CAV1 | −4.01 | −1.46 |
| PDPN | 3.3 | 1.03 | SPANXB1 | 10.15 | 2.37 | TMEM47 | −3.06 | −3.06 | VLDLR | −4.01 | −2.73 |
| MRGPRX4 | 3.25 | −1.07 | SPANXC | 9.72 | 2.22 | UST | −3.16 | −1.97 | CDC42EP5 | −4.04 | −3.82 |
| KLK6 | 3.21 | 8.95 | GDF15 | 9.67 | 1.36 | ANGPTL4 | −3.32 | −4.1 | DBN1 | −4.08 | −1.71 |
| VGLL1 | 3.2 | 1.53 | MGC59937 | 9.64 | 2.27 | SLC2A3 | −3.35 | −2.43 | ANGPTL4 | −4.1 | −3.32 |
| PTGES | 3.03 | 10.61 | AKR1B10 | 9.3 | −1.47 | NRCAM | −3.37 | −1.44 | F3 | −4.13 | −1.52 |
| CD83 | 3.02 | 1.95 | KLK6 | 8.95 | 3.21 | MGMT | −3.41 | −2.71 | PRSS23 | −4.25 | 1.07 |
| TNFRSF10D | 2.99 | 1.88 | TSPAN7 | 8.73 | −1.29 | COL8A1 | −3.44 | −4.29 | SRPX | −4.25 | −1.91 |
| GFPT2 | 2.93 | 3.04 | DHRS3 | 8.24 | 2.14 | GJA1 | −3.59 | −2.49 | COL8A1 | −4.29 | −3.44 |
| XYLT1 | 2.86 | −3.01 | S100P | 8.05 | −1.33 | ARMCX6 | −3.6 | −3.6 | AOX1 | −4.34 | −2.14 |
| IL8 | 2.84 | 19.58 | GCNT3 | 7.87 | 2.62 | PTHLH | −3.6 | −4.85 | CA9 | −4.42 | −4.44 |
| MAL2 | 2.79 | 5.03 | CYP1A1 | 6.86 | 1.3 | CRIP2 | −3.68 | −2.7 | TXNIP | −4.61 | −5.13 |
| SLC19A1 | 2.75 | 1.74 | FTH1 | 6.81 | −1.33 | CKMT1B | −3.77 | −2.43 | ADAMTS6 | −4.62 | −1.26 |
| HNT | 2.73 | −3.71 | PIR | 6.21 | −1.01 | CDC42EP5 | −3.82 | −4.04 | TMEM16D | −4.62 | −4.07 |
| CGI-96 | 2.73 | 2.33 | EEF1A2 | 6.12 | 4.18 | Clorf24 | −3.82 | −2.63 | ARHGDIB | −4.63 | 1.33 |
| STARD1O | 2.72 | 5.83 | SPANXE | 6.09 | 1.94 | CLCA2 | −4.01 | −2.01 | BNIP3 | −4.7 | −4.58 |
| E2F2 | 2.66 | 2.52 | OLR1 | 5.92 | 1.33 | FBN2 | −4.02 | −8.92 | PLOD2 | −4.72 | −1.33 |
| LTB | 2.66 | 2.62 | STARD10 | 5.83 | 2.72 | CBS | −4.04 | −2.04 | EMP3 | −4.84 | −4.98 |
| MDM2 | 2.66 | 2.08 | UGT1A6 | 5.82 | 1.08 | TMEM16D | −4.07 | −4.62 | FLRT2 | −4.85 | 1.09 |
| FAM43A | 2.63 | 4.45 | NUAK2 | 5.77 | 8.73 | STOM | −4.22 | −2.44 | PTHLH | −4.85 | −3.6 |
| GCNT3 | 2.62 | 7.87 | CSF2 | 5.72 | 2.24 | HSPB1 | −4.32 | −2.22 | SLC38A5 | −5.04 | −5.12 |
| TFAP2C | 2.6 | 4.26 | KLK9 | 5.27 | 1.53 | CA9 | −4.44 | −4.42 | PCDH7 | −5.14 | −1.96 |
| SEMA4D | 2.53 | 1.72 | ABCC3 | 5.15 | −1.93 | SNCA | −4.55 | −3.07 | AEBP1 | −5.22 | −5.16 |
| RAB3IL1 | 2.53 | 2.98 | MAL2 | 5.03 | 2.79 | BNIP3 | −4.58 | −4.7 | GSPT2 | −5.72 | −5.35 |
| CATSPER1 | 2.5 | 1.72 | FTL | 4.67 | 1.46 | CKMT1A | −4.94 | −2.51 | EFEMP1 | −5.74 | −2.65 |
| CYP26B1 | 2.46 | 1.37 | GCLM | 4.62 | 1.35 | PLD5 | −4.96 | −9.07 | DKK1 | −5.88 | −1.42 |
| C14orfl47 | 2.46 | 4.28 | IMPA2 | 4.55 | 1.5 | EMP3 | −4.98 | −4.84 | CYR61 | −5.93 | −1.58 |
| APOBEC3B | 2.44 | 4.11 | FAM43A | 4.45 | 2.63 | SLC38A5 | −5.12 | −5.04 | LPHN2 | −6.34 | −6.14 |
| POLDIP3 | 2.42 | 2.9 | KRT23 | 4.35 | −1.99 | TXNIP | −5.13 | −4.61 | KCNMA1 | −6.46 | −1.88 |
| LHX1 | 2.42 | 2.4 | CLIC3 | 4.32 | 1.28 | AEBP1 | −5.16 | −5.22 | MFAP5 | −6.51 | 1.44 |
| SH3KBP1 | 2.41 | 2.82 | IL13RA2 | 4.3 | −1.22 | NDRG1 | −5.24 | −3.16 | KRT14 | −6.89 | −6.41 |
| SERPINA1 | 2.41 | 1.95 | C14orfl47 | 4.28 | 2.46 | GSPT2 | −5.35 | −5.72 | THBS1 | −7.78 | −1.42 |
| SPANXB1 | 2.37 | 10.15 | HMOX1 | 4.28 | 1.23 | LPHN2 | −6.14 | −6.34 | SERPINE1 | −8.71 | −1.45 |
| PACSIN2 | 2.37 | 2.58 | TFAP2C | 4.26 | 2.6 | KRT14 | −6.41 | −6.89 | FBN2 | −8.92 | −4.02 |
| PLCG2 | 2.36 | 2.79 | APOBEC3B | 4.11 | 2.44 | DUSP23 | −11.23 | −10.46 | PLD5 | −9.07 | −4.96 |
| ITM2C | 2.32 | 1.59 | G6PD | 4.01 | 1.14 | GJB2 | −13.17 | −2.13 | DUSP23 | −10.46 | −11.23 |
| HOXB5 | 2.32 | 1.17 | ZP3 | 3.91 | 1.4 | PRSS3 | −13.37 | −11.11 | PRSS3 | −11.11 | −13.37 |
| KHDRBS3 | 2.32 | 1.58 | EPHX1 | 3.87 | 1.48 | VIM | −17.34 | −36.44 | VIM | −36.44 | −17.34 |
Abbreviation: CSC, cigarette smoke condensate.
Discussion
A number of studies have been performed to examine the effects of individual components of tobacco smoke, such as nicotine or NNK in cultured respiratory epithelia (West et al., 2003; Ho et al., 2005; Jin et al., 2008; Wu et al., 2009). Although informative, these studies are inconclusive as the effects of single tobacco components are unlikely to represent those mediated by the complex mixture of organic as well as inorganic carcinogens detected in tobacco smoke (Smith et al., 2003; Shin et al., 2009). Indeed, genetic responses of cultured respiratory epithelia to tobacco carcinogens vary considerably depending on which components from air/liquid interphase such as nicotine, cadmium, and NNK, or vapor phase such as formaldehyde or ethyl carbamate are used for the exposures (Sexton et al., 2008). Additional studies using CSCs have produced variable results depending on the type of condensates and treatment conditions used for the experiments, including supplementation of culture media with S9 microsomal fractions to enhance activation of tobacco carcinogens in respiratory epithelial cells (Jorgensen et al., 2004; Fields et al., 2005).
In this study, we focused our efforts on characterizing the epigenomic effects of CSC in SAEC and HBEC, acknowledging that neither was perfect for this analysis. For example, SAEC, which theoretically contain true target cells of tobacco carcinogens, spontaneously senesce after several passages in culture. On the other hand, immortalization may have subtly perturbed chromatin structure, and altered susceptibility of HBEC to tobacco carcinogens.
Despite these potential limitations, our experiments yielded several interesting and potentially relevant findings. For example, CSC exposure dramatically decreased acetylation of H4K16, and subsequently diminished H4K20 trimethylation in cultured respiratory epithelia. These histone modifications have been observed in several malignancies, including lung cancers (Fraga et al., 2005; Van Den et al., 2008). Given the highly dynamic nature of histone modifications in response to environmental stimuli including tobacco carcinogens (Hussain et al., 2009; Jensen et al., 2009; Zhou et al., 2009), it is not surprising that modulation of the histone code seemed to precede aberrant DNA methylation typically observed in lung cancers (Toyooka et al., 2003, 2006). Moreover, our data suggest that demethylation of repetitive DNA sequences, mono-allelic up-regulation of H19 and IGF2, as well as de-repression of several CT-X genes such as MAGE-A1, MAGE-A3, and SPANXB1, precedes DNA methylation-mediated silencing of tumor suppressor genes, such as p16, MGMT, DAPK, E-cadherin, and cdh13 during tobacco-induced pulmonary carcinogenesis. These findings suggest that global DNA demethylation is an early event during—not a consequence of—malignant transformation. Interestingly, progressive global DNA demethylation with consistent hypermethylation of RASSF1A-a tumor suppressor frequently silenced by epigenetic mechanisms in tobacco-induced lung cancers (Richter et al., 2009), and activation of Wnt signaling coincided with increased clonogenicity of HBEC. The fact that CSC-exposed HBEC were not tumorigenic in murine hosts suggests that additional epigenetic alterations that silence other tumor suppressors or activate stem cell genes such as Oct4, SOX, and NANOG-a phenomenon not observed in our present studies (data not shown), may be necessary to fully transform these cells.
Despite the fact that DNA methylation and gene expression profiling could readily discriminate between control and CSC-exposed HBEC, little correlation was observed between DNA methylation and gene expression profiles for any given treatment condition. These discrepancies may be attributable to the fact that many of the CpG targets on the methylation arrays are not within promoter regions; furthermore, modulation of gene expression by CSC may occur through mechanisms independent of DNA methylation (Kang et al., 2007; Hussain et al., 2009). Overall, these findings are consistent with data indicating that hypermethylation of CpG islands in cancer cells frequently involves genes, which typically do not mediate proliferation or tumorigenicity (Keshet et al., 2006), as well as a recent report showing no correlation between DNA methylation and gene expression profiles in follicular lymphomas (Killian et al., 2009).
The results of our studies vary considerably from those reported by Damiani et al. (2008), who observed that the tobacco carcinogens methyl-nitrosourea and benzo (a) pyrene-diolepoxide increased DNMT1 levels, and induced hypermethylation of E-cadherin, CDH13, proto-cadherin 10, GATA5, and PAX5 in HBEC; these epigenetic alterations coincided with enhanced soft-agar clonogenicity, but not tumorigenicity in nude mice. Notably, in Damiani’s model as well as ours, p16 was neither silenced nor hypermethylated by carcinogen exposure, despite the fact that inactivation of this tumor suppressor gene is believed to be a frequent, early event during tobacco-induced pulmonary carcinogenesis (Toyooka et al., 2006). In our experiments, CSC exposure seemed insufficient to inactivate E-cadherin and CDH13, yet mediated progressive DNA hypermethylation of RASSF1A and RAR-β, which are known to be targets of aberrant DNA methylation in human lung cancers (Licchesi et al., 2008; Seng et al., 2008); these alterations seemed to coincide with a markedly increased DNMT3b/DNMT1 protein ratio. Discrepancies regarding results reported by Damiani et al. (2008) and our current findings may be attributable (at least in part) to exposure conditions including the use of purified, activated carcinogens, serum supplementation and avoidance of oxidative stress by Damiani et al. (2008) rather than CSC, serum-free media and oxidative stress used for our experiments.
From a clinical biomarker discovery perspective, the predictive value of the model described in this manuscript seems to be relatively low regarding identification of differentially methylated genetic targets during cigarette smoke-induced pulmonary carcinogenesis. Conceivably, additional CSC exposures will induce epigenetic alterations in HBEC more typically observed in lung cancer cells; these experiments are ongoing in our laboratory. Despite potential limitations, this model may enable investigation of a variety of epigenetic mechanisms contributing to tobacco-induced lung cancers, and evaluation of novel agents for the treatment and prevention of these neoplasms.
Materials and methods
Cell culture
All lung cancer cell lines were obtained from American Type Culture Collection (Manassas, VA, USA) and maintained in RPMI media supplemented with 10% FBS and 10mM glultamic acid. SAEC and NHBE were obtained from Lonza, Inc. (Frederick, MD, USA), and cultured in appropriate media (SAGM and BEBM, respectively) according to vendor instructions. HBEC were generously provided by John D Minna (U-T Southwestern, Dallas, TX, USA), and cultured in complete Keratinocyte-SFM media (Invitrogen, Carlsbad, CA, USA) supplemented with 5 μg/l epidermal growth factor and 50 mg/l bovine pituitary extract. Cell proliferation was assessed by MTT techniques.
Generation of CSCs and cell line exposure
CSCs were generated from Kentucky 2R4F research cigarettes (University of Kentucky, Tobacco and Health Research Institute, Lexington, KY, USA) using a Borgwaldt-LM1 smoking machine (Richmond, VA, USA) and standard Federal Trade Commission smoking conditions (35 ml puff volume, 2.0 s duration, and 1 puff/min, 9 puffs/cigarette). The smoke condensates were trapped on Cambridge glass fiber filters, weighed, and dissolved in K-SFM, RPMI, SAGM, and BEBM media, respectively, at a concentration of 1 mg/ml, which was defined as 10% CSC (Narayan et al., 2004).
CSC treatments
NHBE, SAEC, HBEC, and A549 cells were plated in six-well plates or 10 cm2 culture dishes in appropriate normal media with or without CSC in the absence of microsomal supplementation. Media and CSC were changed daily. At appropriate times, cells were harvested, and processed for further analysis.
Genomic DNA isolation, bisulfite conversion, and pyrosequencing
Genomic DNA was isolated from cells using the DNeasy Blood and Tissue kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. A total of 0.5 mg of DNA was used for bisulfite conversion by using EpiTect Bisulfite kit (Qiagen). Bisulfite-modified DNA was dissolved in 30 μl H2O, and 2 μl of DNA template was used for pyrosequencing PCR amplification. Dispensation order pyrosequencing reactions and data analysis were performed using the Pyromark MD pyrosequencer with software provided by Biotage (Charlottesville, VA, USA). p16, MGMT, and Line-1 pyrosequencing primers were obtained from Biotage; additional pyrosequencing primer sequences are included in Supplementary Table 1.
RNA extraction and quantitative real-time RT-PCR
Submitted as Supplementary Information.
Western blot
Immunoblotting of non-histone proteins was performed as described (Hussain et al., 2009); western blot analysis of histones was performed according to Millipore protocols, with minor modifications (detailed methods submitted as Supplementary Information). Antibodies used for immunoblotting included H4K16Ac (Abcam ab1762 Cambridge, MA, USA), H4K20Me3 (Upstate 07–463 Billerica, MA, USA), H3K27Me3 (Upstate 07–449), total H3 (Upstate 05–499), and total H4 (Abcam ab10158), DNMT1 (Abcam ab16632–100), DNMT3b (Cell Signal, 2161s, Boston, MA, USA), and EZH2 (BD, 612667, Woburn, MA, USA). Western blot signals were detected with the appropriate secondary-HRP-conjugated antibodies and ECL reagent (Amersham, Pittsburgh, PA, USA), followed by densitometric analysis.
RT-PCR superarrays
Wnt signaling RT-PCR superarrays (PAH-043A) and Human Stress and Toxicity Pathway Finder (PAH-003A) were obtained from SABiosciences (Frederick, MD, USA). Full details of these methods are submitted as Supplementary Information.
Chromatin immunoprecipitation
ChIP was performed using reagents and protocols contained in the Millipore 17–295 ChIP kit (Billeria, MA, USA), and ChlP-grade antibodies recognizing H3K9Ac (ab4441–50; Abcam) or H3K27me3 (07–449; Millipore, Billerica, MA, USA). Full methods and primer sequences for ChIP PCR are submitted as Supplementary Information.
Illumina methylation array
Genomic DNA was extracted from cells using Qiagen DNeasy kit (Qiagen, Duesseldorf, Germany). Purified DNA was bisulfite treated with EpiTect Bisulfite kit (Qiagen). DNA methylation levels of bisulfite converted DNAs were measured using Illumina Infinium or GoldenGate methylation assays (Illumina, San Diego, CA, USA) according to the manufacturer’s protocol. Methylation and differential methylation analyses were performed in BeadStudio (Illumina). In group comparisons, differentially methylated targets were determined based on abs (DiffScore) > 25, as described earlier (Killian et al., 2009).
Illumina gene expression array
A total of 200 ng of total RNA was amplified and cRNA was labeled with biotin using Illumina TotalPrep RNA amplification kit (Ambion, Austin, TX, USA); 750 ng of biotin labeled cRNA was hybridized to Sentrix BeadChip Array for Gene Expression HumanRef-8 V2 (Illumina) and incubated at 58 °C for 16–20 h in an Illumina hybridization oven with rocker speed at 5. Beadchips were washed and stained according to Illumina’s protocol. Arrays were scanned by Illumina chip scanner, and images analyzed by Bead Studio (Illumina). Data were exported and processed using Genespring GX 7.31 (Agilent, Santa Clara, CA, USA). Differentially regulated genes between TSC-treated and control cells were identified by statistical significance P<0.05 and fold change >1.5. Significantly regulated genes were subjected to further analysis using Ingenuity Pathway software (Ingenuity, Redwood City, CA, USA).
Soft-agar assays
Submitted as Supplementary Information.
Murine xenograft experiments
Submitted as Supplementary Information.
Supplementary Material
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Conflict of interest
The authors declare no conflict of interest.
References
- Agherbi H, Gaussmann-Wenger A, Verthuy C, Chasson L, Serrano M, Djabali M. (2009). Polycomb mediated epigenetic silencing and replication timing at the INK4a/ARF locus during senescence. PLoS One 4: e5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlesi F, Giaccone G, Gallegos-Ruiz MI, Loundou A, Span SW, Lefesvre P et al. (2007). Global histone modifications predict prognosis of resected non small-cell lung cancer. J Clin Oncol 25: 4358–4364. [DOI] [PubMed] [Google Scholar]
- Belinsky SA, Nikula KJ, Baylin SB, Issa JP. (1996). Increased cytosine DNA-methyltransferase activity is target-cell-specific and an early event in lung cancer. Proc Natl Acad Sci USA 93: 4045–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Caballero OL, Gnjatic S, Andrade VC, Colleoni GW, Vettore AL et al. (2006). Physical interaction of two cancer-testis antigens, MAGE-C1 (CT7) and NY-ESO-1 (CT6). Cancer Immun 6: 12. [PubMed] [Google Scholar]
- D’Alessio AC, Szyf M. (2006). Epigenetic tête-á-tête: the bilateral relationship between chromatin modifications and DNA methylation. Biochem Cell Biol 84: 463–476. [DOI] [PubMed] [Google Scholar]
- Damiani LA, Yingling CM, Leng S, Romo PE, Nakamura J, Belinsky SA. (2008). Carcinogen-induced gene promoter hypermethylation is mediated by DNMT1 and causal for transformation of immortalized bronchial epithelial cells. Cancer Res 68: 9005–9014. [DOI] [PubMed] [Google Scholar]
- Dovey JS, Zacharek SJ, Kim CF, Lees JA. (2008). Bmi1 is critical for lung tumorigenesis and bronchioalveolar stem cell expansion. Proc Natl Acad Sci USA 105: 11857–11862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields WR, Leonard RM, Odom PS, Nordskog BK, Ogden MW, Doolittle DJ. (2005). Gene expression in normal human bronchial epithelial (NHBE) cells following in vitro exposure to cigarette smoke condensate. Toxicol Sci 86: 84–91. [DOI] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Villar-Garea A, Boix-Chornet M, Espada J, Schotta G et al. (2005). Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet 37: 391–400. [DOI] [PubMed] [Google Scholar]
- Ho YS, Chen CH, Wang YJ, Pestell RG, Albanese C, Chen RJ et al. (2005). Tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) induces cell proliferation in normal human bronchial epithelial cells through NFkappaB activation and cyclin D1 up-regulation. Toxicol Appl Pharmacol 205: 133–148. [DOI] [PubMed] [Google Scholar]
- Hoogeboom D, Burgering BM. (2009). Should I stay or should I go: beta-catenin decides under stress. Biochim Biophys Acta 1796: 63–74. [DOI] [PubMed] [Google Scholar]
- Huang CL, Liu D, Nakano J, Ishikawa S, Kontani K, Yokomise H et al. (2005). Wnt5a expression is associated with the tumor proliferation and the stromal vascular endothelial growth factor—an expression in non-small-cell lung cancer. J Clin Oncol 23: 8765–8773. [DOI] [PubMed] [Google Scholar]
- Huang H, He X. (2008). Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol 20: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M, Rao M, Humphries AE, Hong JA, Liu F, Yang M et al. (2009). Tobacco smoke induces polycomb-mediated repression of Dickkopf-1 in lung cancer cells. Cancer Res 69: 3570–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TJ, Wozniak RJ, Eblin KE, Wnek SM, Gandolfi AJ, Futscher BW. (2009). Epigenetic mediated transcriptional activation of WNT5A participates in arsenical-associated malignant transformation. Toxicol Appl Pharmacol 235: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, Menter DG, Mao L, Hong WK, Lee HY. (2008). Survivin expression in normal human bronchial epithelial cells: an early and critical step in tumorigenesis induced by tobacco exposure. Carcinogenesis 29: 1614–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen ED, Dozmorov I, Frank MB, Centola M, Albino AP. (2004). Global gene expression analysis of human bronchial epithelial cells treated with tobacco condensates. Cell Cycle 3: 1154–1168. [PubMed] [Google Scholar]
- Kang Y, Hong JA, Chen GA, Nguyen DM, Schrump DS. (2007). Dynamic transcriptional regulatory complexes including BORIS, CTCF and Sp1 modulate NY-ESO-1 expression in lung cancer cells. Oncogene 26: 4394–4403. [DOI] [PubMed] [Google Scholar]
- Kaplan R, Luettich K, Heguy A, Hackett NR, Harvey BG, Crystal RG. (2003). Monoallelic up-regulation of the imprinted H19 gene in airway epithelium of phenotypically normal cigarette smokers. Cancer Res 63: 1475–1482. [PubMed] [Google Scholar]
- Katoh M, Katoh M. (2007). WNT signaling pathway and stem cell signaling network. Clin Cancer Res 13: 4042–4045. [DOI] [PubMed] [Google Scholar]
- Keshet I, Schlesinger Y, Farkash S, Rand E, Hecht M, Segal E et al. (2006). Evidence for an instructive mechanism of de novo methylation in cancer cells. Nat Genet 38: 149–153. [DOI] [PubMed] [Google Scholar]
- Killian JK, Bilke S, Davis S, Walker RL, Killian MS, Jaeger EB et al. (2009). Large-scale profiling of archival lymph nodes reveals pervasive remodeling of the follicular lymphoma methylome. Cancer Res 69: 758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kwon YM, Kim JS, Han J, Shim YM, Park J et al. (2006). Elevated mRNA levels of DNA methyltransferase-1 as an independent prognostic factor in primary nonsmall cell lung cancer. Cancer 107: 1042–1049. [DOI] [PubMed] [Google Scholar]
- Le FN, Rivat C, De WO, Bruyneel E, Mareel M, Dale T et al. (2005). The proinvasive activity of Wnt-2 is mediated through a non-canonical Wnt pathway coupled to GSK-3beta and c-Jun/AP-1 signaling. FASEB J 19: 144–146. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Jeon HS, Jang JS, Park SH, Lee GY, Lee BH et al. (2005). DNMT3B polymorphisms and risk of primary lung cancer. Carcinogenesis 26: 403–409. [DOI] [PubMed] [Google Scholar]
- Licchesi JD, Westra WH, Hooker CM, Machida EO, Baylin SB, Herman JG. (2008). Epigenetic alteration of Wnt pathway antagonists in progressive glandular neoplasia of the lung. Carcinogenesis 29: 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RK, Hsu HS, Chang JW, Chen CY, Chen JT, Wang YC. (2007). Alteration of DNA methyltransferases contributes to 5’CpG methylation and poor prognosis in lung cancer. Lung Cancer 55: 205–213. [DOI] [PubMed] [Google Scholar]
- Malusecka E, Krzyzowska-Gruca S, Gawrychowski J, Fiszer-Kierz-kowska A, Kolosza Z, Krawczyk Z. (2008). Stress proteins HSP27 and HSP70i predict survival in non-small cell lung carcinoma. Anticancer Res 28: 501–506. [PubMed] [Google Scholar]
- Mathews LA, Crea F, Farrar WL. (2009). Epigenetic gene regulation in stem cells and correlation to cancer. Differentiation 78: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe MT, Lee EK, Vertino PM. (2009). A multifactorial signature of DNA sequence and polycomb binding predicts aberrant CpG island methylation. Cancer Res 69: 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte M, Simonatto M, Peche LY, Bublik DR, Gobessi S, Pierotti MA et al. (2006). MAGE-A tumor antigens target p53 transactivation function through histone deacetylase recruitment and confer resistance to chemotherapeutic agents. Proc Natl Acad Sci USA 103: 11160–11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan S, Jaiswal AS, Kang D, Srivastava P, Das GM, Gairola CG. (2004). Cigarette smoke condensate-induced transformation of normal human breast epithelial cells in vitro. Oncogene 23: 5880–5889. [DOI] [PubMed] [Google Scholar]
- Richter AM, Pfeifer GP, Dammann RH. (2009). The RASSF proteins in cancer; from epigenetic silencing to functional characterization. Biochim Biophys Acta 1796: 114–128. [DOI] [PubMed] [Google Scholar]
- Schrump DS, Hong JA, Nguyen DM. (2007). Utilization of chromatin remodeling agents for lung cancer therapy. Cancer J 13: 56–64. [DOI] [PubMed] [Google Scholar]
- Seligson DB, Horvath S, McBrian MA, Mah V, Yu H, Tze S et al. (2009). Global levels of histone modifications predict prognosis in different cancers. Am J Pathol 174: 1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seng TJ, Currey N, Cooper WA, Lee CS, Chan C, Horvath L et al. (2008). DLEC1 and MLH1 promoter methylation are associated with poor prognosis in non-small cell lung carcinoma. Br J Cancer 99: 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton K, Balharry D, BeruBe KA. (2008). Genomic biomarkers of pulmonary exposure to tobacco smoke components. Pharmacogenet Genomics 18: 853–860. [DOI] [PubMed] [Google Scholar]
- Shin HJ, Sohn HO, Han JH, Park CH, Lee HS, Lee DW et al. (2009). Effect of cigarette filters on the chemical composition and in vitro biological activity of cigarette mainstream smoke. Food Chem Toxicol 47: 192–197. [DOI] [PubMed] [Google Scholar]
- Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. (2005). Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer 5: 615–625. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Perfetti TA, Garg R, Hansch C. (2003). IARC carcinogens reported in cigarette mainstream smoke and their calculated log P values. Food Chem Toxicol 41: 807–817. [DOI] [PubMed] [Google Scholar]
- Toyooka S, Maruyama R, Toyooka KO, McLerran D, Feng Z, Fukuyama Y et al. (2003). Smoke exposure, histologic type and geography-related differences in the methylation profiles of nonsmall cell lung cancer. Int J Cancer 103: 153–160. [DOI] [PubMed] [Google Scholar]
- Toyooka S, Tokumo M, Shigematsu H, Matsuo K, Asano H, Tomii K et al. (2006). Mutational and epigenetic evidence for independent pathways for lung adenocarcinomas arising in smokers and never smokers. Cancer Res 66: 1371–1375. [DOI] [PubMed] [Google Scholar]
- Van Den BA, Brambilla E, Moro-Sibilot D, Lantuejoul S, Brambilla C, Eymin B et al. (2008). Loss of histone H4K20 trimethylation occurs in preneoplasia and influences prognosis of non-small cell lung cancer. Clin Cancer Res 14: 7237–7245. [DOI] [PubMed] [Google Scholar]
- Vrzalikova K, Skarda J, Ehrmann J, Murray PG, Fridman E, Kopolovic J et al. (2008). Prognostic value of Bmi-1 oncoprotein expression in NSCLC patients: a tissue microarray study. J Cancer Res Clin Oncol 134: 1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Soejima K, Yasuda H, Kawada I, Nakachi I, Yoda S et al. (2008). Deregulation of histone lysine methyltransferases contributes to oncogenic transformation of human bronchoepithelial cells. Cancer Cell Int 8: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM et al. (2003). Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest 111: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn RA, Van SM, Hammond M, Rodriguez K, Crossno JT Jr, Heasley LE et al. (2006). Antitumorigenic effect of Wnt 7a and Fzd 9 in non-small cell lung cancer cells is mediated through ERK-5-dependent activation of peroxisome proliferator-activated receptor gamma. J Biol Chem 281: 26943–26950. [DOI] [PubMed] [Google Scholar]
- Wu JP, Chang LW, Yao HT, Chang H, Tsai HT, Tsai MH et al. (2009). Involvement of oxidative stress and activation of aryl hydrocarbon receptor in elevation of CYP1A1 expression and activity in lung cells and tissues by arsenic: an in vitro and in vivo study. Toxicol Sci 107: 385–393. [DOI] [PubMed] [Google Scholar]
- Xing J, Stewart DJ, Gu J, Lu C, Spitz MR, Wu X. (2008). Expression of methylation-related genes is associated with overall survival in patients with non-small cell lung cancer. Br J Cancer 98: 1716–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, O’Herrin SM, Wu J, Reagan-Shaw S, Ma Y, Bhat KM et al. (2007). MAGE-A, mMage-b, and MAGE-C proteins form complexes with KAP1 and suppress p53-dependent apoptosis in MAGE-positive cell lines. Cancer Res 67: 9954–9962. [DOI] [PubMed] [Google Scholar]
- Zhou X, Li Q, Arita A, Sun H, Costa M. (2009). Effects of nickel, chromate, and arsenite on histone 3 lysine methylation. Toxicol Appl Pharmacol 236: 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.