Abstract
The cytotoxic T-cell and natural killer (NK)-cell lymphomas and related disorders are important but relatively rare lymphoid neoplasms that frequently are a challenge for practicing pathologists. This selective review, based on a meeting of the International Lymphoma Study Group, briefly reviews T-cell and NK-cell development and addresses questions related to the importance of precise cell lineage (αβ-type T cell, γδ T cell, or NK cell), the implications of Epstein-Barr virus infection, the significance of anatomic location including nodal disease, and the question of further categorization of enteropathy-associated T-cell lymphomas. Finally, developments subsequent to the 2008 World Health Organization Classification, including the recognition of indolent NK-cell and T-cell disorders of the gastrointestinal tract are presented.
Keywords: cytotoxic T-cell lymphoma, γδ T cells, natural killer cells, enteropathy-associated T-cell lymphoma
Cytotoxic lymphomas are T-cell or natural killer (NK)-cell neoplasms that express 1 or more cytotoxic markers, such as TIA1, granzyme B, or perforin. Although most are CD8+, some are CD4+, such as ALK+ anaplastic large cell lymphoma and some nodal cytotoxic T-cell lymphomas, and others are CD4− and CD8−, such as hepatosplenic T-cell lymphomas (HSTCL) or type I enteropathy-associated T-cell lymphomas (EATL).1 They occur mostly at extranodal sites and are generally very aggressive (Table 1). Although sharing many features, they include many different entities and require a multiparameter diagnostic approach.
TABLE 1.
Major Cytotoxic T-cell and NK-cell Lymphomas Recognized in the 2008 WHO Classification*
| Entity | Predominant Presentation | T-cell or NK-cell Type | Cytotoxic Phenotype† |
EBV Status |
|---|---|---|---|---|
| T-cell large granular lymphocytic leukemia | Leukemic/disseminated | αβ T cells (rarely γδ T cells) | Activated | Negative |
| Chronic lymphoproliferative disorders of NK cells | Leukemic/disseminated | NK cells | Activated | Negative |
| Aggressive NK-cell leukemia | Leukemic/disseminated | NK cells | Activated | Positive |
| Systemic EBV+ T-cell lymphoproliferative disease of childhood | Systemic | αβ T cells | Activated | Positive |
| Extranodal NK/T-cell lymphoma, nasal-type | Extranodal (upper aerodigestive tract, skin, soft tissue, GI tract, testis, etc.) | NK cells > > γδ T cells > αβ T cells | Activated | Positive |
| EATL | Extranodal (small intestine, other) | αβ, γδ or TCR-silent T cells | Activated | Negative |
| HSTCL | Extranodal (liver, spleen) | γδ T cells > > αβ T cells | Nonactivated | Negative |
| Subcutaneous panniculitis-like T-cell lymphoma | Skin | αβ T cells | Activated | Negative |
| Primary cutaneous CD30+ T-cell lymphoproliferative disorders | Skin | αβ or TCR-silent T cells; γδ T cells in subset of lymphomatoid papulosis | Activated | Negative |
| Primary cutaneous γδ T-cell lymphoma | Skin | γδ T cells | Activated | Negative |
| Primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma (provisional entity) | Skin | αβ T cells | Variable | Negative |
| HV-like lymphoma | Skin | αβ or γδ T cells or NK cells | Activated | Positive |
| PTCL, NOS (subset) | Nodal or extranodal | αβ, γδ or TCR-silent T cells | Variable | Variable |
| ALK+ anaplastic large cell lymphoma (most) | Nodal (extranodal) | αβ or TCR-silent T cells | Activated | Negative |
| ALK-anaplastic large cell lymphoma (many) | Nodal (extranodal) | αβ or TCR-silent T cells | Activated | Negative |
Some cases of mycosis fungoides, especially in advanced stages, may express cytotoxic markers.
Activated cytotoxic phenotype: expression of perforin and/or granzyme B in addition to TIA1; nonactivated cytotoxic phenotype: expression of TIA1 only. ALK indicates anaplastic lymphoma kinase.
The classification of T-cell lymphomas is difficult because of the histopathologic and phenotypic heterogeneity within distinct lymphoma entities, overlapping features between different entities, and the importance of anatomic location in defining disease entities. Whereas B-cell lymphomas are classified to a great extent on the basis of the normal B-cell counterpart they most closely resemble, an analogous approach to T-cell lymphoma classification is currently impossible. Very little reliance is put, for example, on CD4 versus CD8 expression in T-cell lymphomas, as many T-cell lymphoma types can be either CD4+ or CD8+ without clinical significance. In addition, determining the precise phenotype of neoplastic T cells can be difficult because of the presence of many admixed reactive T cells. More recently, growing attention has been paid to the distinction of T-cell neoplasms that express the αβ T-cell receptor (TCR) from those that express the γδ TCR.
There are many questions and controversies related to cytotoxic T/NK-cell lymphomas. When is it and is it not important to distinguish neoplasms of T-cell versus NK-cell origin? Among T-cell neoplasms, when is it important whether they express the γδ TCR, the αβ TCR, both, or neither? What does it mean to find Epstein-Barr virus (EBV) infection in the neoplastic cells in a T/NK-cell neoplasm and when should you look for it? Many of the cytotoxic T-cell and NK-cell neoplasms are named on the basis of the site where they most often occur, but can one recognize similar cases at other sites? And what should they be called? Are primary nodal cases distinct or should some be grouped with another specific category? Among extranodal lymphomas, there is growing interest in more clearly segregating type I from type II EATL and to recognize indolent T-cell and NK-cell proliferations in the gastrointestinal (GI) tract.
In this position paper, we begin with a review of normal cytotoxic T-cell development and diversity and the tools we can use to recognize them. Then we discuss each of the controversies and questions. Finally, we conclude with a pragmatic approach to the diagnosis and classification of potential cytotoxic T-cell neoplasms. It should be recognized that this is neither a comprehensive review of all T/NK-cell neoplasms nor does it discuss all of the cytotoxic T/NK-cell neoplasms in detail.
T-CELL AND NK-CELL DEVELOPMENT WITH AN EMPHASIS ON γδ AND OTHER CYTOTOXIC T CELLS
T-cell immunity includes a dominant component that is a part of the adaptive immune system (“conventional” T cells that recognize peptide antigens presented by MHC molecules) and a significant second component that includes “unconventional” cytotoxic CD8+ T cells, γδ T lymphocytes, and NK cells, which are part of the innate immune system (Fig. 1).2,3 Other T cells “straddle the fence” between these 2 systems.4 Innate immunity forms the first line of defense and involves barrier defenses at mucosal and cutaneous sites. Innate immune cells do not need to encounter antigen in the context of the major histocompatibility complex. The immune response they initiate is independent of antigen-presenting cells, is not target-specific, and is not associated with immunologic memory, in contrast to the adaptive immune response, which is antigen-specific and includes a significant component of memory T cells.
FIGURE 1.

Basic T-cell development is illustrated emphasizing the major T-cell and NK-cell subsets, and the distinction of the innate from the adaptive immune system. Some more specific T-cell subsets such as the CD4+ T follicular helper cells or cytotoxic CD4+ cells are not illustrated. CLP indicates common lymphoid precursor; DN, double negative (CD4−, CD8−); DP, double positive (CD4+, CD8+).
In the maturation process, T lymphocytes rearrange their TCR genes, to produce TCRs, which are expressed on the surface of T lymphocytes in association with the CD3 complex. TCR is a heterodimer composed, like immunoglobulins, of 2 different protein chains, each containing a variable and constant region. In 95% of T lymphocytes, the TCR is composed of an α and β chain, whereas in 5% it consists of a γ and δ chain.3 NK cells do not rearrange the TCR genes nor do they have a complete TCR complex, but they do express the ∊ chain of CD3 in their cytoplasm (and therefore are CD3∊+ in paraffin section immunohistochemical stains).
γδ T cells develop in the bone marrow from CD4−/CD8− “double negative” thymic precursors. Compared with αβ T cells, the developmental process is less dependent on thymic microenvironment signals, and specific subsets of γδ T cells can originate extrathymically.2,5 Similar to other nonconventional T cells, γδ T cells detect conserved nonpeptide antigens, which are upregulated by cells under stress.6 When activated, γδ T cells appear large and granular and can display 1 or more NK-associated surface molecules (CD56, CD16, CD57) and cytotoxic makers.2 On the basis of their distribution, γδ T cells are classified as lymphoid tissue-associated or intra-epithelial.2,6,7 The latter are much less diverse than those that populate the lymphoid tissues and frequently express site-specific invariant or closely related γδ TCRs. γδ T cells account for 15% of T cells in the spleen, 2% to 4% in lymph nodes, 1% in the thymus cortex, 3% to 5% in the thymic medulla, and 5% in peripheral blood.2 Two major subpopulations of γδ T cells, vδ1 and vδ2, are recognized on the basis of differences in the delta V gene usage. The majority of peripheral blood γδ T cells in healthy individuals express vγ9vδ2 TCRs, which recognize small phosphorylated antigens.2,6 Vδ2 T cells are prevalent in the tonsils, interfollicular areas of lymph nodes, and skin; vδ1 T cells predominate in almost all other sites, including the spleen and the intestine.7 Vδ1 T cells maintain the phenotype of naive T cells, whereas vδ2 T cells express CD45RO and act as antigen-presenting and memory cells.2,7
NK cells and a subset of CD8+ T cells are professional killer cells based on their cytolytic machinery, with killing of their targets mediated predominantly by perforin and granzymes.8 Recently, a more direct role for CD4+ T cells in cell-mediated immunity has been suggested. In particular, class II restricted CD4+ cytolytic T cells may also contribute to protective responses against viral and bacterial infections and antitumor responses.9
HOW IS TCR EXPRESSION DETECTED;IS IT IMPORTANT IN THE CATEGORIZATION OF T-CELL LYMPHOMAS;WHAT ARE ITS IMPLICATIONS WITHIN SPECIFIC ENTITIES; AND SHOULD ANY NEW CATEGORIES BE DEFINED ON THE BASIS OF THEIR TCR EXPRESSION?
Until recently, expression of the γδ TCR in tissues could only be assessed by flow cytometry or immunohistochemistry using frozen sections. As a consequence, in routine formalin-fixed paraffin-embedded material the γδ phenotype was, sometimes incorrectly, extrapolated from the negativity for αβ TCR (recognized by βF1 antibody). Monoclonal antibodies detecting the constant region of the TCRγ chain (CγM1) or TCRδ chain (TCRδ1) in paraffin sections have now become commercially available, allowing for positive identification of γδ T cells. With the use of these antibodies, the majority of T-cell lymphomas can be assigned to one or the other lineage (αβ or γδ); however, a subset of cases is either TCR silent (both βF1 and TCR γδ negative) or dual TCR positive.10 Although some cases reported as TCR silent may represent false-negative staining because of technical difficulties with the use of the antibodies or problems with tissue fixation, such a pattern has in fact been recorded in up to 20% of peripheral T-cell lymphomas (PTCLs) on frozen sections and/or flow cytometry.10 Some T-cell lymphomas become TCR silent during their evolution.25 Some authors have grouped lymphomas with coexistent αβ TCR and γδ TCR expression together with those of γδ derivation.11
As normal γδ T cells have a restricted pattern of distribution (predominantly in the skin, mucosal sites, and splenic red pulp), T-cell lymphomas of γδ lineage not unexpectedly also show preferential occurrence in these sites. However, demonstration of a γδ lineage does not necessarily define a specific entity, and many types of T-cell lymphomas may express γδ TCR in a variable proportion of cases (Table 1). Currently only 2 lymphoma types, both cutaneous, mandate evaluation of TCR expression. Subcutaneous panniculitis-like T-cell lymphoma must be of αβ TCR type.13,14 Cases formerly considered panniculitis-like T-cell lymphoma that have γδ TCR expression, and that often extend into the dermis, are now diagnosed as primary cutaneous γδ T-cell lymphoma (PCGDTCL).15,16 A diagnosis of PCGDTCL requires demonstration of γδ TCR expression, although some also include cases that express both γδ and αβ TCR.11,4 Although considered a distinct entity, PCGDTCLs are heterogenous, exhibiting a wide spectrum of histologic and clinical manifestations.15,16 They may resemble mycosis fungoides, have a pagetoid reticulosis-like pattern with epidermal necrosis, or present as plaques or tumors, including some with prominent subcutaneous involvement. PCGDTCL is considered a very aggressive neoplasm, due in part to an increased risk for a hemophagocytic syndrome, although variation in clinical outcome is described. Tumors with involvement restricted to superficial sites appear to have a more indolent clinical course, as do mycosis fungoides-like lesions with or without a panniculitis-like presentation.15,16 Recently, it has been suggested that some cases of PCGDTCL, even if involving subcutaneous tissue, may not be aggressive, although some reported cases are based solely on a negative action βF1 stain, which is insufficient to infer expression of γδ TCR.16–20 Furthermore, rare cases of γδ TCR+ mycosis fungoides ( ± αβ TCR) are still reported.11 It is possible that one pathologist’s less aggressive MF-like PCGDTCL is another’s MF with γδ TCR expression. Less controversial, and of even greater importance, about one third of the cases of type D lymphomatoid papulosis, which may be confused with primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma, and almost 50% of hydroa vacciniforme–like (HV) lymphomas have been reported to express the γδ TCR but have a clinical course unlike PCGDTCL and should not be diagnosed as such.11,21,22
In contrast to cutaneous T-cell lymphomas, the recognition of an αβ versus γδ phenotype may be less significant within other categories of T-cell lymphomas, as is most clearly established for HSTCL. Although HSTCL is most commonly of γδ derivation, a subset shows an αβ phenotype. Both subtypes are similar in morphology, clinical behavior, and cytogenetic abnormalities,23,24 although HSTCLs of αβ phenotype are relatively more common in female individuals and have an older age distribution.24 Furthermore, the gene expression profiles of αβ and γδ HSTCLs are similar, emphasizing the apparent lack of significance of TCR subtype expression in this disease.23
Most other T-cell lymphomas expressing the γδ TCR present in extranodal sites, most commonly the GI tract (including some EATLs), and less commonly the upper aerodigestive tract (including some extranodal NK/T-cell lymphomas [ENKTLs], nasal type), central nervous system, orbit, and lung.25–29 Although most such extranodal lymphomas express cytotoxic molecules, evidence to suggest that a γδ phenotype denotes a distinct entity is lacking.27–29 HV-like lymphoma is another neoplasm in which diverse phenotypes have been shown, including αβ TCR+, γδ TCR, and NK-cell types.30 Although there are some clinical differences between cases of T-cell versus NK-cell origin, there have been too few γδ TCR+ cases to assess whether they have any distinctive findings. Although the predominant occurrence of γδ T-cell lymphomas at mucocutaneous sites raises the possibility that they might be grouped under a single umbrella, γδ T-cell lymphomas are clearly heterogenous.25,27 Those arising in the skin are usually of Vδ2 type, whereas the GI cases and HSTCLs are usually of Vδ1 type.26,28,31
WHAT IS THE SIGNIFICANCE OF EBV POSITIVITY IN THE CATEGORIZATION OF T-CELL LYMPHOMAS?
The presence of EBV is best detected by in situ hybridization (ISH) for EBV early RNA (EBER), which is present in virtually all latently infected cells.32 Immunostaining for EBV-associated proteins (eg, LMP-l) may be of use in determining the type of latency but is significantly less sensitive than EBER ISH. Polymerase chain reaction studies are not useful because they may give positive results from bystander EBV+ B cells. One should distinguish cases in which EBV is present in virtually all (> 50%) neoplastic cells (when the lymphoma is considered EBV-associated) from cases in which the EBV is only present in a small proportion of the neoplastic cells or in admixed bystander B cells, such as occurs in angioimmunoblastic T-cell lymphoma.
There are a number of T/NK-cell lymphomas in which EBV positivity is the expectation and its absence either excludes the diagnosis or at least calls for caution (Table 1). ENKTL, nasal type, shows a near-constant association with EBV independent of patient ethnicity, and thus absence of EBV should make one question the diagnosis.29,32,33 Similarly, aggressive NK-cell leukemia, a rare neoplasm occurring more commonly in Asians, is also highly associated with EBV.34 Other uniformly EBV+ T/NK-cell neoplasms include HV-like lymphoma and systemic EBV+ T-cell lymphoproliferative disease of childhood.22,30,35 EBV may also be found in other T-cell lymphomas, even including rare cases of HSTCL.32,36–40 It is therefore recommended to include EBER ISH in the workup of cytotoxic T/NK-cell lymphomas.
The major site where EBV in an T/NK-cell lymphoma causes diagnostic difficulties is the GI tract, wherein it is uncertain whether all EBV+ cases should be categorized as ENKTL, nasal type. ENKTL, nasal type, presents in the GI tract in 2% to 7% of cases, can morphologically and phenotypically mimic other extranodal cytotoxic lymphomas, is not uniformly CD56+, and can be of T-cell origin.29,34,41 However, a moderate number of aggressive GI EBV+ T-cell lymphomas not categorized as ENKTL, nasal type, have been reported, predominantly from East Asian countries and Mexico.26,42–48 These lymphomas have been categorized as type II EATL or EATL of unspecified type42,46,47 or specifically have been described as not being associated with enteropathy or simply not further specified.43–45,47,48 Many may represent ENKTL with findings of angiocentricity or with CD56 expression43,48,49; however, others have features suggestive of some other types of lymphoma, such as striking epitheliotropism,50 CD4 positivity,29,48,51 or associated celiac disease-type changes or frank celiac disease.26,42,43,46,47,52–55, Although it may be impossible to make a definite assessment in individual cases, the vast majority of cases of EATL are EBV−,27,42 so that EBER positivity should raise the alternative possibility of an ENKTL. EBV positivity also should exclude the indolent T-cell or NK-cell lymphoproliferative diseases of the GI tract that are discussed below, or in words from an editorial written about one of these indolent disorders, “Strong EBV positivity...should indeed send chills down one’s spine….”56
The other major area in which the presence of EBV raises diagnostic questions is the skin, which is home to several types of EBV− cytotoxic cutaneous T-cell lymphomas and several types of EBV+ lymphomas including HV-like lymphoma and ENKTL, nasal type, which presents with cutaneous disease in 3% to 13% of cases (Table 1).29,30,57,58 Two cases of “difficult-to-categorize” EBV+ cutaneous T-cell lymphomas that did well when treated with chemotherapy and/or radiation have also been reported, suggesting that EBV positivity by itself should not be taken as pathognomonic of one of the classic EBV+ T/NK-cell lymphomas.59 Nonetheless, some authors have reported that cases of ENKTL, nasal type, restricted to the skin do better than other ENKTLs.60,61 One should also keep in mind mucocutaneous ulcer, a T-cell-rich lesion in which EBV+ atypical B cells are seen.57
Although PCGDTCL has traditionally been considered EBV−,62,63 recent reports have described rare EBV+ cases including 2/8 in one series.25,64 In addition, at least 1 EBV+ γδ T-cell lymphoma has been reported to have features of both PCGDTCL and ENKTL.65 Criteria often cited for the distinction of ENKTL from PCGDTCL are not absolute, ranging from features seen in a minority of ENKTLs (such as lack of angiocentricity or a γδ T-cell origin) to features that are actually common to both (such as a cytotoxic phenotype). Conversely, EBV− cutaneous ENKTLs have been reported, raising further diagnostic confusion.39 In practical terms, we would caution against a definitive diagnosis of PCGDTCL when EBV is positive.
HOW IMPORTANT IS ANATOMIC LOCATION IN THE CATEGORIZATION OF CYTOTOXIC T/NK-CELL LYMPHOMAS?
The majority of mature T/NK-cell lymphomas exhibiting a cytotoxic phenotype occur in extranodal locations, with several distinct entities having a predilection for specific extranodal sites, which is sometimes reflected in their names (eg, ENKTL, “nasal type”; “hepatosplenic” T-cell lymphoma, and several of the cutaneous lymphomas). Thus, when dealing with a cytotoxic T/NK-cell lymphoma, the localization of the disease is often a first clue to the most likely diagnosis. However, certain extranodal lymphoma entities defined by their predilection for certain sites may primarily affect other organs. For example, ENKTL, nasal type, not uncommonly presents in other parts of the upper aerodigestive tract, the GI tract, skin, or testis and, at least secondarily, can involve lymph nodes.29,66,67 EATL, which typically presents in the small intestine may also present elsewhere in the GI tract or with mesenteric or even distant nodal or extranodal involvement. When the location is atypical, establishing the diagnosis requires that the lesion otherwise fulfills all other criteria of the entity in consideration. When dealing with a cytotoxic lymphoma in an anatomic site that is typical for a specific entity, such as the nose (ENKTL, nasal type), liver (HSTL), or small intestine (EATL), one can be more liberal in accepting some morphologic and/or phenotypic variation. Some cytotoxic T/NK-cell neoplasms do show significant phenotypic variation. For example, ENKTL, nasal type, may be CD45−, CD56−, CXCL13+, IRF4/MUM1+, OCT2+, and/or CD30+, with many of these phenotypic features raising the possibility of other lymphoma types.29 Conversely, a variety of cytotoxic T-cell lymphomas can occur at sites well known to be home for specific lymphoma types, for example, not all cytotoxic T-cell lymphomas in the liver are of hepatosplenic type, or in the intestine of EATL type. Although often CD4+, anaplastic large cell lymphoma is a usually cytotoxic neoplasm that can present in the GI tract or other extranodal sites.68–72
As listed in Table 1, 6 of the 14 major cytotoxic T/NK-cell lymphomas recognized in the World Health Organization (WHO) classification primarily arise in the skin and are a clinically diverse group of neoplasms.19 In addition, a small subset of mycosis fungoides/Sézary syndrome can also express cytotoxic markers. Although associated with an increased risk for disease progression or transformation, the presence of cytotoxic markers in this setting is not associated with other distinctive features, and the cases are not segregated on the basis of this phenotype.73,74
Anatomic location can aid in recognizing several distinctive cytotoxic T/NK-cell lymphomas/lymphoproliferative diseases that are unexpectedly indolent. The uncommon indolent CD8+ cytotoxic cutaneous T-cell lymphoproliferative diseases of the ear, face, and other acral sites show slowly progressive skin lesions despite histologic features worrisome for an aggressive T-cell lymphoma. It has even been suggested that these might be a phenotypic variant of primary cutaneous CD4+ small-medium T-cell lymphomas, but their morphologies are clearly different.75–81 The lesions comprise a monoclonal nonepidermotropic proliferation of monomorphous medium-sized CD3+ CD8+ cells with a cytotoxic T-cell immunophenotype (TIA1+, granzyme B−) and low proliferation fraction. Although often considered a “lymphoma,” some authors refer to these simply as a lymphoproliferative disease. An important differential diagnosis is primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma, which may involve the ear, again highlighting how anatomic location can be a clue but does not provide a diagnosis.82,83
A superficial T-cell or NK-cell infiltrate of the GI tract should raise the possibilities of “NK-cell enteropathy”/”lymphomatoid gastropathy” or “indolent T-cell lymphoproliferative disease of the GI tract” (Fig. 2), diagnoses with very different clinical implications compared with the aggressive NK-cell or T-cell lymphomas with which they are often confused. The former lesions demonstrate superficial ulceration, edema, and hemorrhage. They consist of a mucosal infiltrate of intermediate to large cells with irregular nuclei, which may show infiltration into the glandular epithelium. There is no angioinvasion or necrosis, except possibly in areas of ulceration. They have an EBV− cytotoxic NK-cell phenotype (cCD3+, surface CD3−, CD5−, CD4/CD8−, CD56+, TCR−, and lack clonal TCR rearrangement) and a Ki-67 proliferation fraction of about 25%. The lesions persist without progression or show spontaneous regression with or without anti-Helicobacter pylori therapy, sometimes with recurrences.84–86 In most cases, even aggressive therapy does not prevent disease recurrence, but the patients do well.
FIGURE 2.
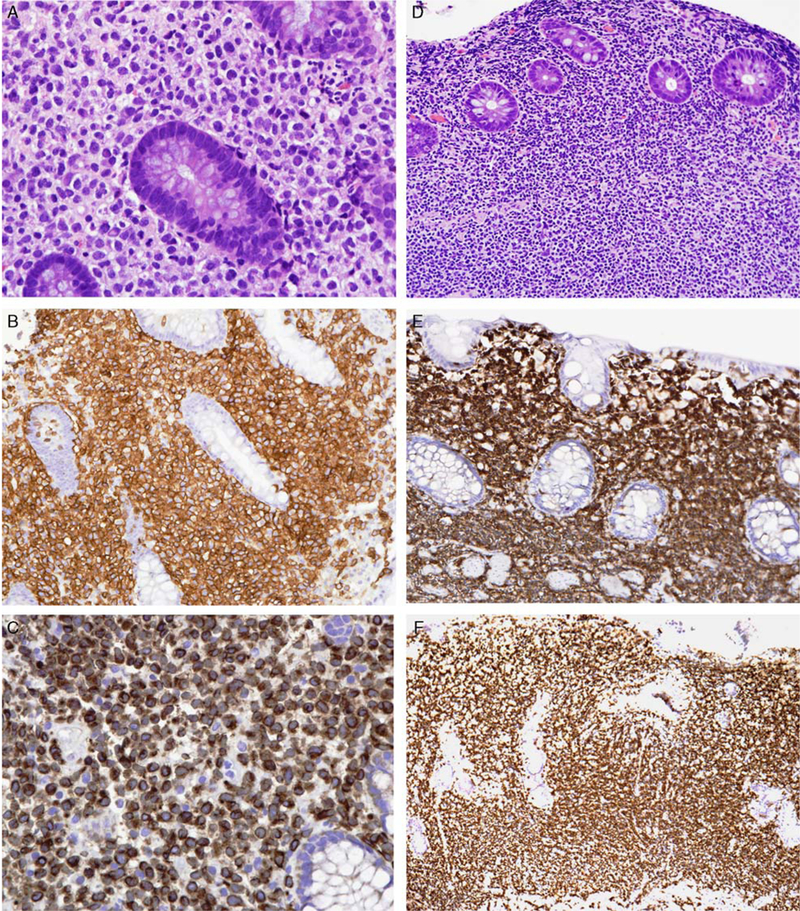
Indolent NK-cell or T-cell disorders of the GI tract. A, NK-cell enteropathy involving the colon. Medium-sized cells with clear cytoplasm and irregular nuclei surround the glands. The atypical cells are positive for CD56 (B) and express cytoplasmic CD3 (C). D, Indolent T-cell lymphoproliferative disease. Small lymphoid cells without cytologic atypia fill the lamina propria. The cells are positive for CD8 (E) and TIA1 (F), but negative for granzyme B (not shown).
Indolent T-cell lymphoproliferative disease of the GI tract presents with shallow ulcers or multiple small polyps. It is characterized by a mucosal and occasionally submucosal, usually nonepitheliotropic, nondestructive, monoclonal EBV− T-cell infiltrate composed mostly of monotonous small lymphocytes without significant cytologic atypia.87 The recently reported cases are mostly CD8+ with a nonactivated cytotoxic phenotype, similar to the CD8+ T-cell lesions of the ear and other acral sites. Many of the similar-appearing cases reported earlier are CD4+. The cells express αβ TCR and have a low Ki-67 index (≤ 10%). Most patients do well but have persistent disease, even if they have been treated with chemotherapy. Rare cases can show peripheral blood involvement,87 extraintestinal involvement, or transformation.88,89 The relationship of these cases to those of NK-cell origin remains to be determined.
IS THERE A DISTINCTIVE TYPE OF NODAL CYTOTOXIC T/NK-CELL LYMPHOMA?
The issue of whether there are 1 or more distinct types of nodal cytotoxic T-cell lymphomas among the heterogenous PTCL, not otherwise specified (NOS) category is controversial, with some proposing such an addition to the WHO classification.1,32,90 Cytotoxic PTCL, NOS are reported to have a distinctive gene expression profile, may be either CD4+ or CD8+, and include cases of both γδ and αβ TCR type.1,32,90,91 Like other cytotoxic T-cell lymphomas, nodal PTCL, NOS with a cytotoxic phenotype are also aggressive neoplasms.32,90 However, many of these “nodal” lymphomas also have concurrent extranodal involvement, and many features of specific types of extranodal cytotoxic T-cell lymphomas often including EBV positivity, emphasizing that site alone cannot dictate a diagnosis and that clinical staging is important. A recent study looking at EBV+ and EBV− nodal cytotoxic PTCL made the point that the EBV+ group was more likely to be CD8+ and clinically distinct from extranasal ENKTL (and by their definition CD56−) and that the absence of CD5 but not EBV status was an independent adverse prognostic factor.1 Another confounding issue is that, as T-cell lymphomas may have numerous admixed reactive T cells (including of cytotoxic type), the definition of a nodal cytotoxic T-cell lymphoma is often based on the presence of >30% positive tumor cells1 or documenting a cytotoxic phenotype among the proliferating cells.90
Other potentially distinctive types of nodal cytotoxic T-cell lymphomas also have been described. Lymphoepithelioid cell (Lennert) lymphoma is a rare variant of PTCL, NOS characterized by a proliferation of small/ medium-sized atypical lymphocytes in association with an infiltrate of epithelioid histiocytes that is reported to have a nonactivated cytotoxic phenotype.72,92 Most cases are CD8+,93 although uncommonly, morphologically similar tumors can have a T follicular helper phenotype.94 Another group of nodal CD8+ cytotoxic T-cell lymphomas composed of larger cells, with or without EBV, displays massive necrosis or apoptosis, is accompanied by disseminated intravascular coagulation or hemophagocytic syndrome and pursues a very aggressive or fulminant course.72,95,96 When associated with EBV, there is overlap with the systemic EBV+ T/NK-cell lymphomas seen mainly in children. Some of these cases have also been reported as an aggressive variant of Lennert lymphoma (EBV+ in 2/3).97
HOW SHOULD WE DEAL WITH INTESTINAL T/NK-CELL LYMPHOMAS—DO THE 2 TYPES OF EATL NEED A DIVORCE?
Should Type II EATL be Considered a Separate Entity?
EATL is a highly aggressive lymphoma defined as an intestinal tumor of intraepithelial T lymphocytes, associated with at least some degree of enteropathic changes. Two subtypes are recognized: type I (classical) and type II (Fig. 3). In whites, type II EATL is much less common than type I, accounting for only 10% to 20% of all EATLs, but EATL in Asians is almost exclusively of type II. Although the similarities in clinical presentation (ulcerative growth in small bowel with frequent perforation), certain pathologic findings (such as dense transmural infiltration, ulceration, perforation, and epitheliotropic growth), and genetic changes (including gain in 9q31-qter or loss in 16q12) argue for placing type I and type II EATL under the same umbrella group of “EATL,” there are many differences in epidemiologic, clinical, histologic, immunophenotypic, and genetic findings (Table 2). Because of these differences, there is a growing consensus that type II EATL should be segregated and ultimately renamed. It also must be remembered that not all intestinal T-cell lymphomas represent EATL.
FIGURE 3.

A, Type I EATL. Large pleomorphic lymphoma cells, often with admixed eosinophils and histiocytes. B, Type II EATL. Monotonous population of small cells. C, Type I EATL. CD8−. D, Type I EATL. CD56−. E, Type II EATL. CD8+. F, Type II EATL. CD56+.
TABLE 2.
Comparison Between Type I and Type II EATL
| Type I EATL | Type II EATL | |
|---|---|---|
| Epidemiology | Occurs predominantly in whites, with a higher frequency in Northern Europe, where celiac disease is more prevalent. It is extremely rare in Asians. | Less common than type I EATL in whites, and represents practically the exclusive type of EATL in Asians, Hispanics and indigenous populations in the Americas. There are currently no data as to whether the absolute incidence of type II EATL is higher in the latter populations. |
| Association with celiac disease | Strong association | Usually no evidence of celiac disease |
| HLA phenotype | Association with HLA DQ2 or DQ8 phenotype | Incidence of HLA DQ2 or DQ8 phenotype similar to the general population. |
| Common histologic features | Usually polymorphic-appearing, with many large lymphoid cells, and frequent admixture of eosinophils and histiocytes. Epitheliotropism common. Necrosis is common. | Monomorphic infiltrate of small or medium-sized lymphoma cells, with few admixed inflammatory cells. Epitheliotropism common. Usually no coagulative necrosis except beneath ulcer. |
| “Enteropathic” changes in surrounding or distant mucosa | Histologic features of celiac disease: villous atrophy, crypt hyperplasia, intraepithelial lymphocytosis, and increased lymphocytes and plasma cells in lamina propria | Very focal or extensive areas with epitheliotropism of small lymphocytes, usually in the absence of overt villous atrophy and crypt hyperplasia. |
| Usual immunophenotype of lymphoma | CD3+, CD5−, CD4−, CD8−/+, CD56− TIA1+, granzyme B+, MATK−, CD30+/−. | CD3+, CD5−, CD4−, CD8+, CD56+, TIA1+, granzyme B+, MATK+, CD30−. |
| Immunophenotype of intraepithelial lymphocytes in surrounding or distant mucosa | Often aberrant (eg, CD5−), and is usually concordant with that of the lymphomatous component | Often aberrant (e.g. CD5- or extensively CD56+), and can be concordant or discordant with that of the lymphomatous component |
| Chromosomal imbalances | + 9q31-qter or −16q12 Frequent +1q32-q41 and +5q34-q35* |
+ 9q31-qter or −16q12 Frequent +8q24 (with amplification of MYC locus)* |
Neither finding is specific with ~20% to 30% of each found in the other type of EATL.100
What Is the Most Appropriate Name for Type II EATL?
Names that have been suggested for type II EATL include monomorphic CD56+ intestinal T-cell lymphoma (used in the 2010 WHO GI tumor monograph),98 monomorphic intestinal T-cell lymphoma,27 and epitheliotropic intestinal T-cell lymphoma or the more “unwieldy” epitheliotropic nonenteropathic intestinal T-cell lymphoma.99 Some features stressed in some of the names such as “monomorphic” are quite subjective, and some cases of type II EATL are not completely monomorphic, whereas type I EATL may appear “monotonous.” In addition, CD56 is negative in 6% to 13% of type II EATL.27,42,99 In contrast, “epitheliotropic” features are found also in type I EATL and other types of T/NK-cell lymphomas. The WHO classification is currently being updated, and choice of a new name for type II EATL is therefore deferred at this time.
Diagnostic Criteria for Type II EATL
The main diagnostic features of type II EATL include: (1) evidence of intestinal involvement, with epitheliotropism if the overlying mucosa can be evaluated in the biopsy or resection specimen, (2) dense infiltrates of monotonous atypical small to medium-sized lymphoma cells, without extensive tumor necrosis, (3) appropriate T-cell immunophenotype as listed in Table 2, although deviation in 1 or 2 markers is acceptable if other features are compatible, and (4) EBV− (see discussion above). Whereas some report a predominance of γδ TCR+ cases,27 others report a predominance of αβ TCR+ cases.99 The finding of intraepithelial lymphocytosis in the surrounding or distant mucosa is further supportive but not essential for diagnosis, because this feature can be missed on sampling and cannot be assessed in limited biopsies. Although diffuse enteropathic findings characteristic of celiac disease are not expected, the literature reports a varying frequency of villous blunting and crypt hyperplasia.27,28,42 Gain in 8q34 involving MYC is found in 73% of type II EATL but in only 27% of type I EATL.100 In limited biopsies, definitive distinction from type I EATL or other types of T-cell or NK-cell lymphomas (such as ENKTL, PTCL, NOS, and indolent T-cell lymphoproliferative disease of the GI tract) may not be possible.
CONCLUSIONS
Although recognition of a cytotoxic phenotype in T/NK-cell lymphomas is often straightforward, their classification may be problematic, and in some cases definitive categorization may be impossible. Most are aggressive neoplasms; however, there are some exceptions that are important to recognize as they require different management. The next revision of the WHO classification of hematopoietic and lymphoid tumors will undoubtedly recognize some of these more recently described lesions (Table 3). Use of a multiparameter approach is critical, with the individual features varying in their degree of importance in different circumstances. The relative frequency of γδ versus αβ TCR+ cases varies between different lymphoma types, and in most circumstances, the nature of the TCR is not a defining feature and is not associated with specific clinical implications. Even in the skin, where a γδ TCR origin has the greatest implications, not all abnormal lymphoid proliferations of γδ T cells should be categorized as PCGDTCL. Furthermore, although differences have been reported for some lymphomas on the basis of a T-cell versus NK-cell origin, in many other situations both clinically, and on the basis of gene expression profiling,12 even this distinction does not seem to matter.
TABLE 3.
Recently Recognized Cytotoxic T-cell and NK-cell Lesions Not Included in the 2008 WHO Classification
| Indolent CD8+ cytotoxic cutaneous T-cell lymphoproliferative diseases of the ear, face, and other acral sites |
| NK-cell enteropathy/lymphomatoid gastropathy |
| Indolent T-cell lymphoproliferative disease of the GI tract |
| Breast implant–associated ALK− anaplastic large cell lymphoma |
Anatomic location provides a very important clue for the diagnosis of some specific entities and is a critical diagnostic component for others, but by itself does not define any lymphoma type. Location can, however, be used to exclude certain diagnoses, for instance, cutaneous lymphomas require initial restriction to a cutaneous site. Whether nodal cytotoxic T-cell lymphomas should be segregated as a distinct entity remains controversial, in part because they frequently show concurrent involvement of extranodal sites. In the face of EBV positivity, strong consideration must be given to one of the lymphomas known to be EBV+, even if some of the other features may not be completely typical. Nevertheless, some often ill-defined cytotoxic lymphomas may be EBV+ but not reflect one of the typically EBV+ neoplasms. Finally, type II EATL needs to be segregated from type I EATL, even though the 2 intestinal lymphomas share some important features, and some of the “distinguishing” features are not at all absolute.
ACKNOWLEDGMENTS
The authors thank those who also specifically reviewed the manuscript in detail: E. Campo, J. Chan, L. Quintanilla-Fend, R. Gascoyne, P. Kluin, M. Piris, A. Rosenwald, R. Siebert, and H. Stein. Additional current/emeritus members of the ILSG include: A. Chott, R. Dalla-Favera, G. Delsol, B. Falini, T. Grogan, P. Isaacson, Y-H. Ko, S. Nakamura, L. Rimsza, and T. Yoshino.
Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Footnotes
Conflicts of Interest
This manuscript is based on discussions held at the 2011 meeting of the International Lymphoma Study Group, Pittsburgh, PA.
REFERENCES
- 1.Kato S, Takahashi E, Asano N, et al. Nodal cytotoxic molecule (CM)-positive Epstein-Barr virus (EBV)-associated peripheral T cell lymphoma (PTCL): a clinicopathological study of 26 cases. Histopathology. 2012;61:186–199. [DOI] [PubMed] [Google Scholar]
- 2.Tripodo C, Iannitto E, Florena AM, et al. Gamma-delta T-cell lymphomas. Nat Rev Clin Oncol. 2009;6:707–717. [DOI] [PubMed] [Google Scholar]
- 3.Kindt TJ, Goldsby RA, Osborne BA. Kuby Immunology. New York: W.H. Freeman & Co; 2007. [Google Scholar]
- 4.Wencker M, Turchinovich G, Di Marco Barros R, et al. Innate-like T cells straddle innate and adaptive immunity by altering antigen-receptor responsiveness. Nat Immunol. 2014;15:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciofani M, Knowles GC, Wiest DL, et al. Stage-specific and differential notch dependency at the alphabeta and gammadelta T lineage bifurcation. Immunity. 2006;25:105–116. [DOI] [PubMed] [Google Scholar]
- 6.Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–478. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien RL, Born WK. gammadelta T cell subsets: a link between TCR and function? Semin Immunol. 2010;22:193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8(+) T cells. Nat Rev Immunol. 2011;11:645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown DM. Cytolytic CD4 cells: direct mediators in infectious disease and malignancy. Cell Immunol. 2010;262:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaulard P, Bourquelot P, Kanavaros P, et al. Expression of the alpha/beta and gamma/delta T-cell receptors in 57 cases of peripheral T-cell lymphomas. Identification of a subset of gamma/delta T-cell lymphomas. Am J Pathol. 1990;137:617–628. [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Pinilla SM, Ortiz-Romero PL, Monsalvez V, et al. TCR-gamma expression in primary cutaneous T-cell lymphomas. Am J Surg Pathol. 2013;37:375–384. [DOI] [PubMed] [Google Scholar]
- 12.Iqbal J, Weisenburger DD, Chowdhury A, et al. Natural killer cell lymphoma shares strikingly similar molecular features with a group of non-hepatosplenic gammadelta T-cell lymphoma and is highly sensitive to a novel aurora kinase A inhibitor in vitro. Leukemia. 2011;25:348–358. [DOI] [PubMed] [Google Scholar]
- 13.Willemze R, Jansen PM, Cerroni L, et al. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008;111:838–845. [DOI] [PubMed] [Google Scholar]
- 14.Jaffe ES, Gaulard P, Ralfkiaer E, et al. Subcutaneous panniculitis-like T-cell lymphoma In: Swerdlow SH, Campo E, Harris NL, et al. , eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008:294–295. [Google Scholar]
- 15.Toro JR, Liewehr DJ, Pabby N, et al. Gamma-delta T-cell phenotype is associated with significantly decreased survival in cutaneous T-cell lymphoma. Blood. 2003;101:3407–3412. [DOI] [PubMed] [Google Scholar]
- 16.Guitart J, Weisenburger DD, Subtil A, et al. Cutaneous gammadelta T-cell lymphomas: a spectrum of presentations with overlap with other cytotoxic lymphomas. Am J Surg Pathol. 2012;36:1656–1665. [DOI] [PubMed] [Google Scholar]
- 17.Endly DC, Weenig RH, Peters MS, et al. Indolent course of cutaneous gamma-delta T-cell lymphoma. J Cutan Pathol. 2013;40:896–902. [DOI] [PubMed] [Google Scholar]
- 18.Magro CM, Wang X. Indolent primary cutaneous gamma/delta T-cell lymphoma localized to the subcutaneous panniculus and its association with atypical lymphocytic lobular panniculitis. Am J Clin Pathol. 2012;138:50–56. [DOI] [PubMed] [Google Scholar]
- 19.Quintanilla-Martinez L, Jansen PM, Kinney MC, et al. Non-mycosis fungoides cutaneous T-cell lymphomas: report of the 2011 Society for Hematopathology/European Association for Haematopathology workshop. Am J Clin Pathol. 2013;139:491–514. [DOI] [PubMed] [Google Scholar]
- 20.Attygalle AD, Cabecadas J, Gaulard P, et al. Peripheral T-cell and NK-cell lymphomas and their mimics; taking a step forward - report on the lymphoma workshop of the XVIth meeting of the European Association for Haematopathology and the Society for Hematopathology. Histopathology. 2014;64:171–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kempf W, Kazakov DV, Scharer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37:1–13. [DOI] [PubMed] [Google Scholar]
- 22.Kimura H, Ito Y, Kawabe S, et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood. 2012;119:673–686. [DOI] [PubMed] [Google Scholar]
- 23.Travert M, Huang Y, de Leval L, et al. Molecular features of hepatosplenic T-cell lymphoma unravels potential novel therapeutic targets. Blood. 2012;119:5795–5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macon WR, Levy NB, Kurtin PJ, et al. Hepatosplenic alphabeta T-cell lymphomas: a report of 14 cases and comparison with hepatosplenic gammadelta T-cell lymphomas. Am J Surg Pathol. 2001;25:285–296. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Herrera A, Song JY, Chuang SS, et al. Nonhepatosplenic gammadelta T-cell lymphomas represent a spectrum of aggressive cytotoxic T-cell lymphomas with a mainly extranodal presentation. Am J Surg Pathol. 2011;35:1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnulf B, Copie-Bergman C, Delfau-Larue MH, et al. Non-hepatosplenic gammadelta T-cell lymphoma: a subset of cytotoxic lymphomas with mucosal or skin localization. Blood. 1998; 91:1723–1731. [PubMed] [Google Scholar]
- 27.Chan JK, Chan AC, Cheuk W, et al. Type II enteropathy-associated T-cell lymphoma: a distinct aggressive lymphoma with frequent gammadelta T-cell receptor expression. Am J Surg Pathol. 2011;35:1557–1569. [DOI] [PubMed] [Google Scholar]
- 28.Wilson AL, Swerdlow SH, Przybylski GK, et al. Intestinal gammadelta T-cell lymphomas are most frequently of type II enteropathy-associated T-cell type. Hum Pathol. 2013;44:1131–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pongpruttipan T, Sukpanichnant S, Assanasen T, et al. Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and alphabeta, gammadelta, and alphabeta/gammadelta T-cell origin: a comprehensive clinicopathologic and phenotypic study. Am J Surg Pathol. 2012;36:481–499. [DOI] [PubMed] [Google Scholar]
- 30.Quintanilla-Martinez L, Ridaura C, Nagl F, et al. Hydroa vacciniforme-like lymphoma: a chronic EBV+ lymphoproliferative disorder with risk to develop a systemic lymphoma. Blood. 2013;122:3101–3110. [DOI] [PubMed] [Google Scholar]
- 31.Przybylski GK, Wu H, Macon WR, et al. Hepatosplenic and subcutaneous panniculitis-like gamma/delta T cell lymphomas are derived from different Vdelta subsets of gamma/delta T lymphocytes. J Mol Diagn. 2000;2:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rezk SA, Weiss LM. Epstein-Barr virus-associated lymphoproliferative disorders. Hum Pathol. 2007;38:1293–1304. [DOI] [PubMed] [Google Scholar]
- 33.Li S, Feng X, Li T, et al. Extranodal NK/T-cell lymphoma, nasal type: a report of 73 cases at MD Anderson Cancer Center. Am J Surg Pathol. 2013;37:14–23. [DOI] [PubMed] [Google Scholar]
- 34.Mori N, Yamashita Y, Tsuzuki T, et al. Lymphomatous features of aggressive NK cell leukaemia/lymphoma with massive necrosis, haemophagocytosis and EB virus infection. Histopathology. 2000;37:363–371. [DOI] [PubMed] [Google Scholar]
- 35.Quintanilla-Martinez L, Kimura H, Jaffe ES. EBV+ T-cell lymphoproliferative disorders of childhood In: Swerdlow SH, Campo E, Harris NL, et al. , eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008:278–280. [Google Scholar]
- 36.Ohshima K, Suzumiya J, Sugihara M, et al. Clinical, immunohistochemical and phenotypic features of aggressive nodal cytotoxic lymphomas, including alpha/beta, gamma/delta T-cell and natural killer cell types. Virchows Arch. 1999;435:92–100. [DOI] [PubMed] [Google Scholar]
- 37.Anagnostopoulos I, Hummel M, Stein H. Frequent presence of latent Epstein-Barr virus infection in peripheral T cell lymphomas. A review. Leuk Lymphoma. 1995;19:1–12. [DOI] [PubMed] [Google Scholar]
- 38.Zhou XG, Hamilton-Dutoit SJ, Yan QH, et al. High frequency of Epstein-Barr virus in Chinese peripheral T-cell lymphoma. Histopathology. 1994;24:115–122. [DOI] [PubMed] [Google Scholar]
- 39.Ohshima K, Liu Q, Koga T, et al. Classification of cell lineage and anatomical site, and prognosis of extranodal T-cell lymphoma—natural killer cell, cytotoxic T lymphocyte, and non-NK/ CTL types. Virchows Arch. 2002;440:425–435. [DOI] [PubMed] [Google Scholar]
- 40.Belhadj K, Reyes F, Farcet JP, et al. Hepatosplenic gammadelta T-cell lymphoma is a rare clinicopathologic entity with poor outcome: report on a series of 21 patients. Blood. 2003;102:4261–4269. [DOI] [PubMed] [Google Scholar]
- 41.Tan SY, Nakamura S, Tan HC, et al. Diagnosis of type II enteropathy-associated T-cell lymphoma should be limited to EBER — cases. Am J Hematol. 2012;87:E129–E130. [DOI] [PubMed] [Google Scholar]
- 42.Tse E, Gill H, Loong F, et al. Type II enteropathy-associated T-cell lymphoma: a multicenter analysis from the Asia Lymphoma Study Group. Am J Hematol. 2012;87:663–668. [DOI] [PubMed] [Google Scholar]
- 43.Mitarnun W, Saechan V, Pradutkanchana J, et al. Epstein-Barr virus-associated peripheral T-cell lymphoma with gastrointestinal tract involvement. J Med Assoc Thai. 2003;86:816–828. [PubMed] [Google Scholar]
- 44.Lavergne A, Brocheriou I, Delfau MH, et al. Primary intestinal gamma-delta T-cell lymphoma with evidence of Epstein-Barr virus. Histopathology. 1998;32:271–276. [DOI] [PubMed] [Google Scholar]
- 45.de Bruin PC, Jiwa NM, Oudejans JJ, et al. Epstein-Barr virus in primary gastrointestinal T cell lymphomas. Association with gluten-sensitive enteropathy, pathological features, and immune-phenotype. Am J Pathol. 1995;146:861–867. [PMC free article] [PubMed] [Google Scholar]
- 46.Pan L, Diss TC, Peng H, et al. Epstein-Barr virus (EBV) in enteropathy-associated T-cell lymphoma (EATL). J Pathol. 1993;170:137–143. [DOI] [PubMed] [Google Scholar]
- 47.Quintanilla-Martinez L, Lome-Maldonado C, Ott G, et al. Primary non-Hodgkin’s lymphoma of the intestine: high prevalence of Epstein-Barr virus in Mexican lymphomas as compared with European cases. Blood. 1997;89:644–651. [PubMed] [Google Scholar]
- 48.Zhang WY, Li GD, Liu WP, et al. Features of intestinal T-cell lymphomas in Chinese population without evidence of celiac disease and their close association with Epstein-Barr virus infection. Chin Med J. 2005;118:1542–1548. [PubMed] [Google Scholar]
- 49.Quintanilla-Martinez L, Lome-Maldonado C, Ott G, et al. Primary intestinal non-Hodgkin’s lymphoma and Epstein-Barr virus: high frequency of EBV-infection in T-cell lymphomas of Mexican origin. Leuk Lymphoma. 1998;30:111–121. [DOI] [PubMed] [Google Scholar]
- 50.Chuang SS, Jung YC. Natural killer cell lymphoma of small intestine with features of enteropathy but lack of association with celiac disease. Hum Pathol. 2004;35:639–642. [DOI] [PubMed] [Google Scholar]
- 51.de Bruin PC, Connolly CE, Oudejans JJ, et al. Enteropathy-associated T-cell lymphomas have a cytotoxic T-cell phenotype. Histopathology. 1997;31:313–317. [DOI] [PubMed] [Google Scholar]
- 52.Walsh SV, Egan LJ, Connolly CE, et al. Enteropathy-associated T-cell lymphoma in the West of Ireland: low-frequency of Epstein-Barr virus in these tumors. Mod Pathol. 1995;8: 753–757. [PubMed] [Google Scholar]
- 53.Korbjuhn P, Anagnostopoulos I, Hummel M, et al. Frequent latent Epstein-Barr virus infection of neoplastic T cells and bystander B cells in human immunodeficiency virus-negative European peripheral pleomorphic T-cell lymphomas. Blood. 1993;82:217–223. [PubMed] [Google Scholar]
- 54.Isaacson PG. Intestinal lymphoma and enteropathy. J Pathol. 1995;177:111–113. [DOI] [PubMed] [Google Scholar]
- 55.Ilyas M, Niedobitek G, Agathanggelou A, et al. Non-Hodgkin’s lymphoma, coeliac disease, and Epstein-Barr virus: a study of 13 cases of enteropathy-associated T- and B-cell lymphoma. J Pathol. 1995;177:115–122. [DOI] [PubMed] [Google Scholar]
- 56.Porcu P, Caligiuri M. A sheep in wolf’s clothing. Blood. 2011;117:1438–1439. [DOI] [PubMed] [Google Scholar]
- 57.Dojcinov SD, Venkataraman G, Raffeld M, et al. EBV positive mucocutaneous ulcer—a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34: 405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Novelli M, Merlino C, Ponti R, et al. Epstein-Barr virus in cutaneous T-cell lymphomas: evaluation of the viral presence and significance in skin and peripheral blood. J Invest Dermatol. 2009;129:1556–1561. [DOI] [PubMed] [Google Scholar]
- 59.Park S, Lee DY, Kim WS, et al. Primary cutaneous Epstein-Barr virus-associated T-cell lymphoproliferative disorder-2 cases with unusual, prolonged clinical course. Am J Dermatopathol. 2010;32:832–836. [DOI] [PubMed] [Google Scholar]
- 60.Choi YL, Park JH, Namkung JH, et al. Extranodal NK/T-cell lymphoma with cutaneous involvement:’nasal’ vs. ‘nasal-type’ subgroups-a retrospective study of 18 patients. Br J Dermatol. 2009;160:333–337. [DOI] [PubMed] [Google Scholar]
- 61.Yu JB, Zuo Z, Tang Y, et al. Extranodal nasal-type natural killer/T-cell lymphoma of the skin: a clinicopathologic study of 16 cases in China. Hum Pathol. 2009;40:807–816. [DOI] [PubMed] [Google Scholar]
- 62.Gaulard P, Berti E, Willemze R, et al. Primary cutaneous peripheral T-cell lymphomas, rare subtypes In: Swerdlow SH, Campo E, Harris NL, et al. , eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008:302–305. [Google Scholar]
- 63.Toro JR, Beaty M, Sorbara L, et al. gamma delta T-cell lymphoma of the skin: a clinical, microscopic, and molecular study. Arch Dermatol. 2000;136:1024–1032. [DOI] [PubMed] [Google Scholar]
- 64.Caudron A, Bouaziz JD, Battistella M, et al. Two atypical cases of cutaneous gamma/delta T-cell lymphomas. Dermatology. 2011;222:297–303. [DOI] [PubMed] [Google Scholar]
- 65.Yu WW, Hsieh PP, Chuang SS. Cutaneous EBV-positive gammadelta T-cell lymphoma vs. extranodal NK/T-cell lymphoma: a case report and literature review. J Cutan Pathol. 2013;40:310–316. [DOI] [PubMed] [Google Scholar]
- 66.Vazquez A, Khan MN, Blake DM, et al. Extranodal natural killer/T-Cell lymphoma: a population-based comparison of sinonasal and extranasal disease. Laryngoscope. 2014;124:888–895. [DOI] [PubMed] [Google Scholar]
- 67.Kim SJ, Jung HA, Chuang SS, et al. Extranodal natural killer/T-cell lymphoma involving the gastrointestinal tract: analysis of clinical features and outcomes from the Asia Lymphoma study group. J Hematol Oncol. 2013;6:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carey MJ, Medeiros LJ, Roepke JE, et al. Primary anaplastic large cell lymphoma of the small intestine. Am J Clin Pathol. 1999;112:696–701. [DOI] [PubMed] [Google Scholar]
- 69.Ross CW, Hanson CA, Schnitzer B. CD30 (Ki-1)-positive, anaplastic large cell lymphoma mimicking gastrointestinal carcinoma. Cancer. 1992;70:2517–2523. [DOI] [PubMed] [Google Scholar]
- 70.Sun J, Zhou JL, Chen J, et al. Clinicopathologic features of gastrointestinal tract involvement of anaplastic large cell lymphoma. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2012;34:228–233. [DOI] [PubMed] [Google Scholar]
- 71.ten Berge RL, Oudejans JJ, Ossenkoppele GJ, et al. ALK expression in extranodal anaplastic large cell lymphoma favours systemic disease with (primary) nodal involvement and a good prognosis and occurs before dissemination. J Clin Pathol. 2000;53:445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kagami Y, Suzuki R, Taji H, et al. Nodal cytotoxic lymphoma spectrum: a clinicopathologic study of 66 patients. Am J Surg Pathol. 1999;23:1184–1200. [DOI] [PubMed] [Google Scholar]
- 73.Vermeer MH, Geelen FA, Kummer JA, et al. Expression of cytotoxic proteins by neoplastic T cells in mycosis fungoides increases with progression from plaque stage to tumor stage disease. Am J Pathol. 1999;154:1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aung PP, Climent F, Muzzafar T, et al. Immunophenotypic shift of CD4 and CD8 antigen expression in primary cutaneous T-cell lymphomas: a clinicopathologic study of three cases. J Cutan Pathol. 2014;41:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petrella T, Maubec E, Cornillet-Lefebvre P, et al. Indolent CD8-positive lymphoid proliferation of the ear: a distinct primary cutaneous T-cell lymphoma? Am J Surg Pathol. 2007;31: 1887–1892. [DOI] [PubMed] [Google Scholar]
- 76.Beltraminelli H, Mullegger R, Cerroni L. Indolent CD8+ lymphoid proliferation of the ear: a phenotypic variant of the small-medium pleomorphic cutaneous T-cell lymphoma? J Cutan Pathol. 2010;37:81–84. [DOI] [PubMed] [Google Scholar]
- 77.Suchak R, O’Connor S, McNamara C, et al. Indolent CD8-positive lymphoid proliferation on the face: part of the spectrum of primary cutaneous small-/medium-sized pleomorphic T-cell lymphoma or a distinct entity? J Cutan Pathol. 2010;37:977–981. [DOI] [PubMed] [Google Scholar]
- 78.Swick BL, Baum CL, Venkat AP, et al. Indolent CD8 + lymphoid proliferation of the ear: report of two cases and review of the literature. J Cutan Pathol. 2011;38:209–215. [DOI] [PubMed] [Google Scholar]
- 79.Zeng W, Nava VE, Cohen P, et al. Indolent CD8-positive T-cell lymphoid proliferation of the ear: a report of two cases. J Cutan Pathol. 2012;39:696–700. [DOI] [PubMed] [Google Scholar]
- 80.Kempf W, Kazakov DV, Cozzio A, et al. Primary cutaneous CDS(+) small- to medium-sized lymphoproliferative disorder in extrafacial sites: clinicopathologic features and concept on their classification. Am J Dermatopathol. 2013;35:159–166. [DOI] [PubMed] [Google Scholar]
- 81.Greenblatt D, Ally M, Child F, et al. Indolent CDS(+) lymphoid proliferation of acral sites: a clinicopathologic study of six patients with some atypical features. J Cutan Pathol. 2013; 40:248–258. [DOI] [PubMed] [Google Scholar]
- 82.Fika Z, Karkos PD, Badran K, et al. Primary cutaneous aggressive epidermotropic CD8 positive cytotoxic T-cell lymphoma of the ear. J Laryngol Otol. 2007;121:503–505. [DOI] [PubMed] [Google Scholar]
- 83.Gormley RH, Hess SD, Anand D, et al. Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma. J Am Acad Dermatol. 2010;62:300–307. [DOI] [PubMed] [Google Scholar]
- 84.Mansoor A, Pittaluga S, Beck PL, et al. NK-cell enteropathy: a benign NK-cell lymphoproliferative disease mimicking intestinal lymphoma: clinicopathologic features and follow-up in a unique case series. Blood. 2011;117:1447–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takeuchi K, Yokoyama M, Ishizawa S, et al. Lymphomatoid gastropathy: a distinct clinicopathologic entity of self-limited pseudomalignant NK-cell proliferation. Blood. 2010;116: 5631–5637. [DOI] [PubMed] [Google Scholar]
- 86.Terai T, Sugimoto M, Uozaki H, et al. Lymphomatoidgastropathy mimicking extranodal NK/T cell lymphoma, nasal type: a case report. World J Gastroenterol. 2012;18:2140–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Perry AM, Warnke RA, Hu Q, et al. Indolent T-cell lymphoproliferative disease of the gastrointestinal tract. Blood. 2013; 122:3599–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Margolskee E, Jobanputra V, Lewis SK, et al. Indolent small intestinal CD4+ T-cell lymphoma is a distinct entity with unique biologic and clinical features. PLoS One. 2013;8:e68343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carbonnel F, d’Almagne H, Lavergne A, et al. The clinicopathological features of extensive small intestinal CD4 T cell infiltration. Gut. 1999;45:662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Asano N, Suzuki R, Kagami Y, et al. Clinicopathologic and prognostic significance of cytotoxic molecule expression in nodal peripheral T-cell lymphoma, unspecified. Am J Surg Pathol. 2005;29:1284–1293. [DOI] [PubMed] [Google Scholar]
- 91.Iqbal J, Weisenburger DD, Greiner TC, et al. Molecular signatures to improve diagnosis in peripheral T-cell lymphoma and prognostication in angioimmunoblastic T-cell lymphoma. Blood. 2010;115:1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hartmann S, Agostinelli C, Klapper W, et al. Revising the historical collection of epithelioid cell-rich lymphomas of the Kiel Lymph Node Registry: what is Lennert’s lymphoma nowadays? Histopathology. 2011;59:1173–1182. [DOI] [PubMed] [Google Scholar]
- 93.Geissinger E, Odenwald T, Lee SS, et al. Nodal peripheral T-cell lymphomas and, in particular, their lymphoepithelioid (Lennert’s) variant are often derived from CD8(+) cytotoxic T-cells. Virchows Arch. 2004;445:334–343. [DOI] [PubMed] [Google Scholar]
- 94.Rodriguez-Pinilla SM, Atienza L, Murillo C, et al. Peripheral T-cell lymphoma with follicular T-cell markers. Am J Surg Pathol. 2008;32:1787–1799. [DOI] [PubMed] [Google Scholar]
- 95.Kagami Y, Sobue R, Ito N, et al. Cytotoxic large T-cell lymphoma with fulminant clinical course, CD8+ and CD56- phenotype, and its relation to Epstein-Barr virus: a report of two cases. Int J Hematol. 1999;70:105–111. [PubMed] [Google Scholar]
- 96.Mukai HY, Hasegawa Y, Kojima H, et al. Nodal CD8 positive cytotoxic T-cell lymphoma: a distinct clinicopathological entity. Mod Pathol. 2002;15:1131–1139. [DOI] [PubMed] [Google Scholar]
- 97.Kitamura A, Yamashita Y, Sato Y, et al. Aggressive Lennert’s lymphoma: report of three cases in comparison to non-aggressive Lennert’s lymphoma. Pathol Int. 2005;55:626–631. [DOI] [PubMed] [Google Scholar]
- 98.Müller-Hermelink H, Delabie J, Ko Y, et al. T-cell lymphoma of the small intestine In: Bosman F, Carneiro F, Hruban R, et al. , eds. WHO Classification of Tumours of the Digestive System. Lyon: IARC; 2010:112–114. [Google Scholar]
- 99.Tan SY, Chuang SS, Tang T, et al. Type II EATL (epitheliotropic intestinal T-cell lymphoma): a neoplasm of intra-epithelial T-cells with predominant CD8alphaalpha phenotype. Leukemia. 2013; 27:1688–1696. [DOI] [PubMed] [Google Scholar]
- 100.Isaacson PG, Chott A, Ott G, et al. Enteropathy-associated T-cell lymphoma In: Swerdlow SH, Campo E, Harris NL, et al. , eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008:289–291. [Google Scholar]


