Abstract
Lymphopenia is a marker of inferior survival in patients with various malignancies. However, the prognostic significance of lymphopenia in peripheral T-cell lymphoma (PTCL) is unclear. We analyzed the prognostic significance of lymphopenia in 826 patients with different types of PTCL and natural killer/T-cell lymphoma (NKTCL) from the International Peripheral T-cell Lymphoma Project. Lymphopenia was defined as an absolute lymphocyte count of less than 1,000 cells per microliter. The overall frequency of lymphopenia was 35.3%, ranging from 21.1% in ALK+ anaplastic large cell lymphoma (ALCL) to 47.5% in angioimmunoblastic T-cell lymphoma (AITL). Lymphopenia was independently associated with an inferior overall survival (OS) in patients with the lymphoma type of adult T-cell leukemia/lymphoma (ATLL), with a 2-year OS of 15% versus 40% for those without lymphopenia (P< 0.001). Lymphopenia was also an adverse predictor of survival in PTCL, not otherwise specified, but was associated with other unfavorable prognostic factors. A trend toward inferior survival for lymphopenic patients was also observed in AITL, ALK− ALCL and extranasal NKTCL lymphoma, whereas no difference in survival was found in nasal NKTCL, ALK+ ALCL, or enteropathy-associated T-cell lymphoma. In this study, lymphopenia was identified as a new adverse prognostic factor in the lymphoma type of ATLL.
Introduction
Peripheral T-cell lymphoma (PTCL) and natural killer/T-cell lymphoma (NKTCL) are an uncommon and heterogeneous group of disorders comprising approximately 5–20% of all non-Hodgkin lymphoma (NHL) in different parts of the world [1]. According to the current World Health Organization classification [1], PTCL is subclassified into several distinctive subtypes including PTCL, not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL), anaplastic large cell lymphoma (ALCL), adult T-cell leukemia/lymphoma (ATLL), nasal and extranasal NKTCL, enteropathy-associated T-cell lymphoma (EATL), as well as other rare entities. ALCL is further separated into the ALK+ and ALK− subtypes and, with the exception of ALK+ ALCL, patients with PTCL and NKTCL generally have a poor prognosis with standard chemotherapy [2]. However, some patients may benefit from more intensive treatment or the use of novel agents [3]. Although different clinical and pathological prognostic factors have been proposed for PTCL and NKTCL, the International Prognostic Index (IPI) for aggressive lymphoma is still the most widely used prognosticator in PTCL [4,5]. Its prognostic importance has been also confirmed in different entities, including PTCL-NOS [6], ALCL [7], ATLL [8], and NKTCL [9].
Lymphopenia has been recognized since 1970 as an adverse predictor of outcome in patients with advanced cancer [10]. More recently, lymphopenia was incorporated into a prognostic score as a negative predictor of survival in patients with advanced Hodgkin lymphoma [11]. Subsequently, other studies have demonstrated an adverse effect of lymphopenia on the survival of patients with aggressive NHL and diffuse large B-cell lymphoma (DLBCL) [12–17]. In PTCL, however, lymphopenia had no effect on the survival of patients with AITL [18–20] or PTCL-NOS [20]. Nevertheless, lymphopenia was identified as an independent predictor of survival in PTCL-NOS in two recent studies [21,22]. To our knowledge, lymphopenia has not been investigated as a prognostic marker in ATLL. Due to the rarity and heterogeneity of PTCL, most of these studies were underpowered to demonstrate whether lymphopenia represents an independent prognostic marker or is merely associated with more advanced disease or other unfavorable characteristics. Lymphopenia was defined as an absolute lymphocyte count (ALC) of less than 1,000 cells/μl in most of the previous studies [10,12,13,15–17,19–22].
The International Peripheral T-cell Lymphoma Project was undertaken as a large retrospective study of PTCL and NKTCL in North America, Europe, and Asia to provide better characterization of this group of lymphomas [2]. Our aim in this study was to assess the frequency and prognostic significance of lymphopenia in the more common entities in this cohort.
Methods
The International Peripheral T-cell Lymphoma Project included 22 institutions in North America, Europe, and Asia that participated in collection of the cases (Appendix 1). The study included 1,314 previously untreated patients who were diagnosed from January 1, 1990, to December 31, 2002 [2]. All cases were reviewed by four expert hematopathologists and a consensus diagnosis was reached in each case. A total of 826 patients with available clinical data and an ALC at the time of initial diagnosis were analyzed in this study, including 256 with PTCL-NOS, 200 with AITL, 109 with ALCL (57 ALK+ and 52 ALK-), 104 with the lymphoma type of ATLL, 81 with nasal NKTCL, 29 with extranasal NKTCL, and 47 with EATL. The other rare subtypes of PTCL and NKTCL were not included in this analysis. According to most previous studies, lymphopenia in this study was defined as an ALC of less than 1,000 cells/μl, unless otherwise specified.
Of the 126 cases with ATLL in the project, 104 with the lymphoma type of ATLL were included in this analysis. According to the classification of Shimoyama [23], these patients had an ALC of less than 4,000 cells/μl, elevated anti-human T-cell lymphotropic virus type I (HTLV) titer, and histologically proven lymphadenopathy. In six patients, the ALC was unknown, and 16 patients had the acute type of ATLL. Data on all patients with ATLL in the project were previously published [8].
Treatment outcome was determined by overall survival (OS) and failure-free survival (FFS). OS was defined as the time from diagnosis to death from any cause, with surviving patient follow-up being censored at the last contact date. FFS was defined as the time from diagnosis to first progression, relapse after response, or death from any cause. Follow up of patients not experiencing any of these events was censored at the date of last contact. OS and FFS were calculated by the method of Kaplan and Meier [24], and time-to-event distributions were compared using the log-rank test. Comparisons of clinical features and prognostic factors were performed with the chi-square test. Multivariate analysis was performed using the Cox proportional hazards regression model with stepwise selection [25].
Results
The overall frequency of lymphopenia in our cohort of 826 patients was 35.3%, ranging from 21.1% in ALK+ ALCL to 47.5% in AITL. The frequency of lymphopenia was 35.6% in the lymphoma type of ATLL, 35.3% in PTCLNOS, 25% in ALK- ALCL, 31.9% in EATL, 27.1% in nasal NKTCL, and 44.8% in extranasal NKTCL.
In ATLL, patients with an ALC of less than 1,000 cells/μl had a significantly inferior survival compared to those with an ALC of 1,000 cells/μl or more (2-year OS of 15% vs. 40%, P < 0.001; 2-year FFS of 8% vs. 30%, P = 0.0061; Fig. 1). In PTCL-NOS, the differences in survival by ALC were smaller but statistically significant (2-year OS of 43% vs. 53%, P = 0.034; 2-year FFS of 23% vs. 36%, P = 0.0062; Fig. 2). A trend toward inferior OS and/or FFS for patients with lymphopenia was also observed in AITL (P = 0.054 for OS, P = 0.30 for FFS), extranasal NKTCL (P = 0.072 for OS, P = 0.088 for FFS), and ALK- ALCL (P = 0.086 for OS, P = 0.23 for FFS), but no survival differences were observed in ALK+ ALCL, EATL, or nasal NKTCL (P > 0.10 for OS and FFS).
Figure 1.
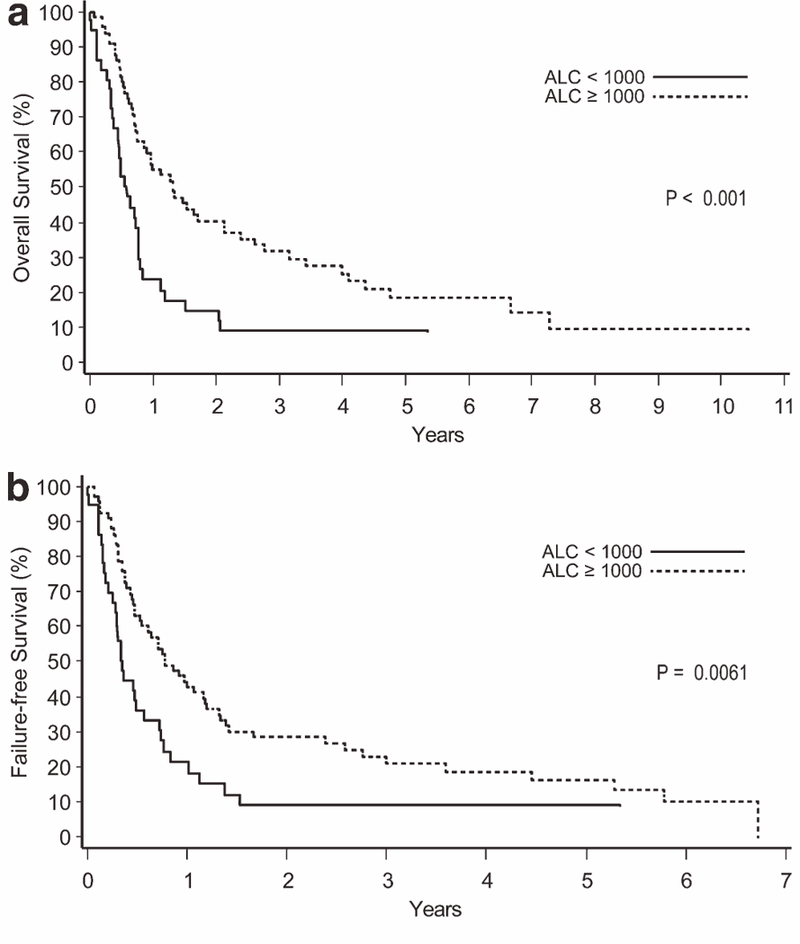
Overall survival (a) and failure-free survival (b) according to the absolute lymphocyte count (ALC) in the lymphoma type of adult T-cell leukemia/ lymphoma
Figure 2.
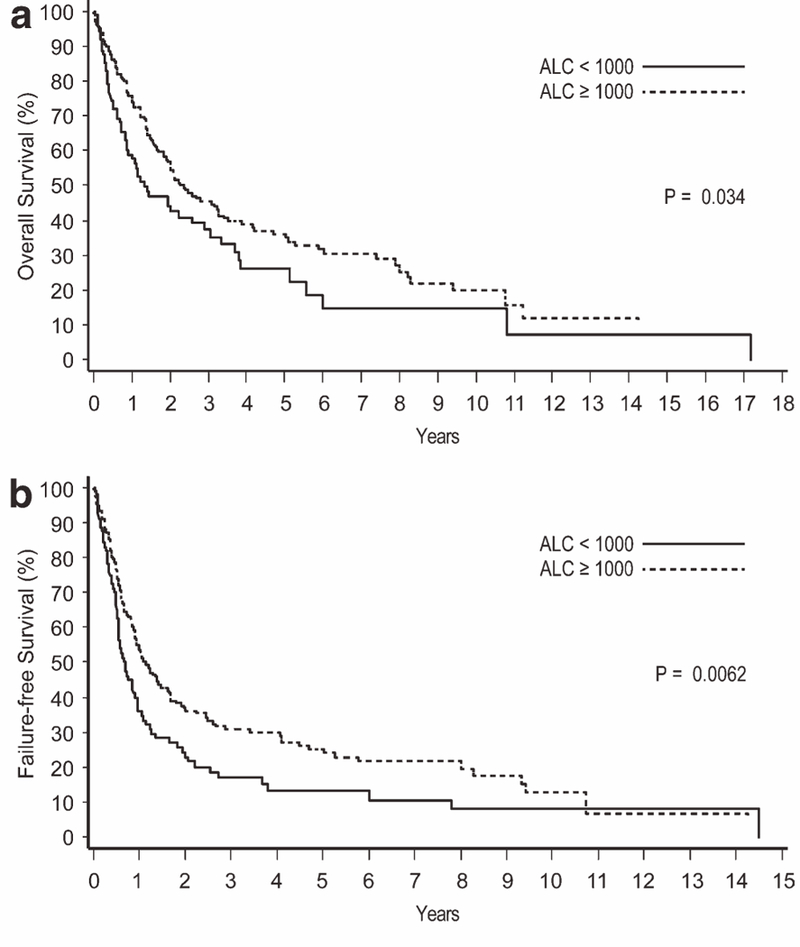
Overall survival (a) and failure-free survival (b) according to the absolute lymphocyte count (ALC) in peripheral T-cell lymphoma, not otherwise specified.
The chi-square test was used to assess the possible association of lymphopenia with the IPI score. Patients with lymphopenia were more likely to have high IPI scores (IPI 3–5) in PTCL-NOS (P = 0.004), AITL (P < 0.05), and extranasal NKTCL (P < 0.01). In ATLL, ALCL, EATL, and nasal NKTCL, lymphopenia was not associated with high IPI scores.
The Cox proportional hazards regression model was used to test whether lymphopenia was an independent predictor of survival while controlling for the IPI in ATLL, PTCL-NOS, and AITL (Table I). A high IPI score was a statistically significant predictor of OS and FFS in all three entities. In ATLL, lymphopenia was also predictive of survival with a hazard ratio (HR) of 2.37 for OS (P = 0.0003) and 1.93 for FFS (P = 0.004). In PTCL-NOS, the HR for lymphopenia was 1.25 for OS (P = 0.188) and 1.40 for FFS (P = 0.028). In AITL, lymphopenia was not an independent predictor of survival.
TABLE I.
Multivariate Analysis of Lymphopenia as a Predictor of Survival when Controlling for the International Prognostic Index (IPI) in Adult T-cell Leukemia/Lymphoma (ATLL), Peripheral T-cell Lymphoma, Not Otherwise Specified (PTCL-NOS), and Angioimmunoblastic T-cell Lymphoma (AITL)
| PTCL subtype | Hazard ratio | P value |
|---|---|---|
| ATLL OS | ||
| IPI 3–5 | 1.85 | 0.006 |
| Lymphopenia | 2.37 | 0.0003 |
| ATLL FFS | ||
| IPI 3–5 | 1.56 | 0.044 |
| Lymphopenia | 1.93 | 0.004 |
| PTCL-NOS OS | ||
| IPI 3–5 | 2.09 | 0.0001 |
| Lymphopenia | 1.25 | 0.188 |
| PTCL-NOS FFS | ||
| IPI 3–5 | 1.63 | 0.001 |
| Lymphopenia | 1.40 | 0.028 |
| AITL OS | ||
| IPI 3–5 | 1.57 | 0.013 |
| Lymphopenia | 1.30 | 0.152 |
| AITL FFS | ||
| IPI 3–5 | 1.59 | 0.006 |
| Lymphopenia | 1.09 | 0.605 |
We also compared the clinical characteristics of patients with ATLL and PTCL-NOS stratified by the ALC (Table II). In ATLL, patients with lymphopenia were more likely to have a normal serum lactate dehydrogenase (LDH) level (P = 0.007), but tended to have less than 150 × 109/l platelets (P = 0.061) in comparison with those without lymphopenia. In PTCL-NOS, patients with lymphopenia were more likely to be older than 60 years (P = 0.036), have B symptoms (P = 0.014), an elevated serum LDH (P = 0.012), less than 150 × 109/l platelets (P = 0.026), a hemoglobin less than 120 g/l (P = 0.006), an elevated beta-2 microglobulin level (P = 0.001), and high IPI scores (P = 0.004), whereas the association of lymphopenia with stage III/IV disease and performance status was of borderline significance (P = 0.06 and 0.056, respectively).
TABLE II.
Clinical Characteristics of Patients with Adult T-cell Leukemia/Lymphoma (ATLL) and Peripheral T-cell Lymphoma, Not Otherwise Specified (PTCL-NOS), According to the Absolute Lymphocyte Count (ALC)
| ATLL |
PTCL-NOS |
|||||
|---|---|---|---|---|---|---|
| ALC < 1000 N (%) | ALC ≥ 1000 N (%) | P | ALC < 1000 N (%) | ALC ≥ 1000 N (%) | P | |
| Age > 60 years | 25 (68) | 35 (52) | 0.15 | 53 (59) | 74 (45) | 0.036 |
| Male | 19 (51) | 36 (54) | 0.84 | 54 (60) | 108 (66) | 0.41 |
| B symptoms | 10 (27) | 17 (25) | 1.00 | 42 (47) | 51 (31) | 0.014 |
| Stage III/IV | 36 (97) | 57 (85) | 0.09 | 70 (79) | 111 (67) | 0.060 |
| Performance status ≥ 2 | 9 (24) | 13 (19) | 0.62 | 21 (24) | 23 (14) | 0.056 |
| Elevated serum LDH | 8 (22) | 33 (49) | 0.007 | 53 (60) | 69 (42) | 0.012 |
| Extranodal sites > 1 | 9 (24) | 23 (34) | 0.38 | 32 (36) | 47 (28) | 0.26 |
| Bone marrow involved | 4 (11) | 16 (24) | 0.13 | 21 (23) | 35 (21) | 0.75 |
| Largest mass ≥ 10 cm | 2 (6) | 6 (10) | 0.71 | 5 (7) | 7 (5) | 0.76 |
| Platelets < 150 × 109/l | 8 (22) | 5 (8) | 0.061 | 27 (30) | 29 (18) | 0.026 |
| Hemoglobin < 120 g/l | 13 (35) | 19 (28) | 0.51 | 42 (47) | 49 (30) | 0.006 |
| Elevated CRP | 24 (67) | 33 (53) | 0.21 | 28 (55) | 33 (48) | 0.47 |
| Elevated B2M | 3 (38) | 8 (35) | 1.00 | 24 (53) | 16 (23) | 0.001 |
| IPI score 3–5 | 16 (43) | 31 (46) | 0.84 | 43 (50) | 50 (31) | 0.004 |
LDH, lactate dehydrogenase; CRP, C-reactive protein; B2M, beta-2 microglobulin; IPI, International Prognostic Index.
To assess whether more profound lymphopenia results in more inferior OS, we set an ALC of less than 800 cells/μl as a new cut-off value for lymphopenia in ATLL, PTCL-NOS, and AITL. Patients with an ALC of less than 800 cells/μl had inferior OS compared with those with an ALC of 800 cells/μl or more in ATLL (P < 0.001), whereas no difference in OS was observed in PTCL-NOS and AITL (P > 0.10).
Discussion
Lymphopenia has been used as a prognostic indicator in patients with advanced-stage carcinoma, sarcoma, Hodgkin lymphoma and DLBCL [10–17]. The association of lymphopenia with inferior survival has also been demonstrated in acute myeloid and lymphoid leukemia, multiple myeloma, and in the transplantation setting of Hodgkin lymphoma and DLBCL [26–29]. In PTCL, lymphopenia was reported to have no prognostic significance in AITL [18–20], whereas three studies of PTCL-NOS had inconsistent results [20–22]. Most of these studies are retrospective, single-center studies that included relatively small numbers of patients without an external review of the diagnoses. In contrast, this study included large number of cases that were reviewed by expert hematopathologists and classified according to the World Health Organization classification.
The overall frequency of lymphopenia in PTCL and NKTCL was 35.3% (range, 21.1–47.5%) in our study, which is comparable to that reported in solid tumors and other lymphomas [12,13,15–17,20–22]. Interestingly, the lowest frequency of lymphopenia was found in ALK+ ALCL, which is prognostically the most favorable subtype of PTCL [2]. In contrast, the highest frequency of lymphopenia was observed in AITL, similar to previous reports [19,20]. AITL is characterized by advanced stage at presentation in about 80% the patients, with a marked paraneoplastic inflammatory response and significant immunosuppression secondary to the tumor [1,18], all of which may contribute to lymphopenia [12].
Our identification of lymphopenia as a strong and independent prognostic marker of inferior survival in the lymphoma type of ATLL represents a novel finding. ATLL is a peripheral T-cell malignancy caused by a retrovirus, HTLV-1 [1]. ATLL is endemic in several regions of the world, particularly southwestern Japan, the Caribbean basin, Peru, and parts of central Africa. ATLL can be divided into four clinical subtypes, including the smoldering, chronic, acute, and lymphoma types [23]. Adverse prognostic factors in ATLL include a poor performance status, high serum LDH level, age = 40 years, more than three involved sites, and hypercalcemia [30,31]. However, in our previous study, which included these same patients with the lymphoma type of ATLL, the IPI score was the only significant predictor of survival by multivariate analysis, whereas lymphopenia was not assessed [8]. Recently, a new risk model has been developed using some variables of the IPI (age, stage and performance status) and hypercalcemia for patients with predominantly the acute type of ATLL [32]. In this study, lymphopenia in ATLL was not associated with high IPI scores or with other parameters of advanced disease. Therefore, we hypothesize that the mechanism of the lymphopenia in ATLL may be different than in other types of lymphoma or solid tumors. Most likely, the lymphopenia is related to HTLV-1 infection and its lifelong persistence in CD4+ T-cells. HTLV-1 infection causes immunosuppression and increases the likelihood of opportunistic infections, even in patients without lymphoma, most probably due to impaired production of naive T lymphocytes [33]. Similar complications have been observed in patients with lymphoma related to another retrovirus that infects CD4+ T-cells, the human immunodeficiency virus. It has been shown that a low number of CD4+ T-cells is also associated with an inferior OS in human immunodeficiency virus-related B- and T-cell lymphomas [34,35]. As previously reported, the median OS of patients with the lymphoma type of ATLL in our cohort was only 0.8 years, and only 5% of the patients were in complete remission at the time of death [8]. Since most patients die with active disease, it is hard to distinguish lymphoma-related from treatment-related deaths. Nevertheless, further studies are needed to investigate the pathogenesis of lymphopenia in the lymphoma type of ATLL and the possible treatment implications for these patients.
Unlike ATLL, lymphopenia was not an independent predictor of OS in PTCL-NOS and was strongly associated with high IPI scores and other unfavorable prognostic factors, such as elevated serum beta-2 microglobulin and LDH levels, B symptoms, anemia, thrombocytopenia, and older age. In addition, patients with lymphopenia tended to have more advanced stage disease and a poor performance status. Although multivariate analysis showed the prognostic significance of lymphopenia in predicting FFS independent of the IPI (HR 1.40, P = 0.028), we conclude that lymphopenia in PTCL-NOS is most likely a reflection of other unfavorable characteristics in these patients. This conclusion is also supported by the finding that patients with an ALC of less than 800 cells/μl did not have inferior OS compared to those an ALC of 800 cells/μl or more, thus demonstrating the lack of association between the degree of lymphopenia and inferior OS in PTCL-NOS.
In conclusion, this study documents the frequency of lymphopenia in the major PTCL and NKTCL entities. Lymphopenia was associated with a significantly inferior survival only in patients with ATLL and PTCL-NOS. An ALC of less than 1,000 cells/μl appears to be an independent predictor of inferior survival in the lymphoma type of ATLL. In contrast, lymphopenia in PTCL-NOS was a less robust predictor of survival and was most likely related to other unfavorable prognostic factors.
Acknowledgments
The authors thank Martin Bast, Fred Ullrich, and James Anderson for the data collection and analysis. A complete list of study sites and physicians participating in the International Peripheral T-cell Lymphoma Project appears in Appendix 1.
Appendix
TABLE AI.
Participating Sites and Physicians
| Institution | Location | Physicians |
|---|---|---|
| British Columbia Cancer Agency | Vancouver, Canada | Kerry Savage, MD; Joseph Connors, MD; Randy Gascoyne, MD; Mukesh Chhanabhai, MD |
| National Cancer Institute | Bethesda, MD | Wyndham H. Wilson, MD; Elaine S. Jaffe, MD |
| University of Nebraska Medical Center | Omaha, NE | James Armitage, MD; Julie Vose, MD; Dennis Weisenburger, MD; James Anderson, PhD; Fred Ullrich, MS, Martin Bast, BS |
| Massachusetts General Hospital | Boston, MA | Ephraim Hochberg, MD; Agata Smogorzewska, MD; Nancy Harris, MD |
| Norris Cancer Center | Los Angeles, CA | Alexandra Levine, MD; Bharat Nathwani, MD |
| Arizona Health Sciences Center | Tucson, AZ | Thomas Miller, MD; Lisa Rimsza, MD |
| University of Barcelona Hospital | Barcelona, Spain | Emili Montserrat, MD; Armando Lopez-Guillermo, MD; Elias Campo, MD |
| Spanish National Cancer Center | Madrid, Spain | Marta Cuadros, MD; Javier Alvarez Ferreira, MD; Beatriz Martinez Delgado, MD |
| Norwegian Radium Hospital | Oslo, Norway | Harald Holte, MD; Jan Delabie, MD |
| University of Würzburg Hospital | Würzburg, Germany | Thomas Rüdiger, MD; Konrad Müller-Hermelink, MD; Peter Reimer, MD; Patrick Adam, MD |
| Nürnberg, Germany | Martin Wilhelm, MD | |
| Hamburg, Germany | Norbert Schmitz, MD | |
| Munich, Germany | Christoph Nerl, MD | |
| Saint Bartholomew’s Hospital | London, UK | Andrew Lister, MD; Andrew Norton, MD |
| University of Bologna Hospital | Bologna, Italy | Stefano Pileri, MD; Pier Luigi Zinzani, MD |
| University of Modena Hospital | Modena, Italy | Massimo Federico, MD; Monica Bellei, PhD |
| Centre Hospitalier Lyon-Sud | Lyon, France | Bertrand Coiffier, MD; Francoise Berger, MD |
| King Chulalongkorn Hospital | Bangkok, Thailand | Intragumtornchai Tanin, MD; Pongsak Wannakrairot, MD |
| Queen Mary Hospital | Hong Kong, China | Wing Y. Au, MD; Raymond Liang, MD; Florence Loong, MD |
| Singapore General Hospital | Singapore | Sandeep Rajan, MD; Ivy Sng, MD |
| National Cancer Center Hospital of Japan | Tokyo, Japan | Kensei Tobinai, MD; Yoshihiro Matsuno, MD |
| Aichi Cancer Center | Nagoya, Japan | Yasuo Morishima, MD; Shigeo Nakamura, MD; Masao Seto, MD |
| Okayama University Hospital | Okayama, Japan | Mitsune Tanimoto, MD; Tadashi Yoshino, MD |
| Fukuoka University Hospital | Fukuoka, Japan | Junji Suzumiya, MD; Koichi Ohshima, MD |
| Samsung Medical Center | Seoul, Korea | Won-Seog Kim, MD; Young-Hyeh Ko, MD |
Footnotes
Conflict of interest: Nothing to report.
References
- 1.Swerdlow S,Campo E,Harris N, et al. , editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed. Lyon: IARC Press; 2008. [Google Scholar]
- 2.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 2008;26:4124–4130. [DOI] [PubMed] [Google Scholar]
- 3.Foss FM, Zinzani PL, Vose JM, et al. Peripheral T-cell lymphoma. Blood 2011;117:6756–6767. [DOI] [PubMed] [Google Scholar]
- 4.Ship MA, Harrington DP, Anderson JR et al. A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med 1993;329:987–994. [DOI] [PubMed] [Google Scholar]
- 5.Ansell SM, Habermann TM, Kurtin PJ, et al. Predictive capacity of the International Prognostic Factor Index in patients with peripheral T-cell lymphoma. J Clin Oncol 1997;15:2296–2301. [DOI] [PubMed] [Google Scholar]
- 6.Weisenburger DD, Savage KJ, Harris NL, et al. Peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the International Peripheral T-cell Lymphoma Project. Blood 2011;117:3402–3408. [DOI] [PubMed] [Google Scholar]
- 7.Savage KJ, Harris NL, Vose JM, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK1 ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood 2008;111:5496–5504. [DOI] [PubMed] [Google Scholar]
- 8.Suzumiya J, Ohshima K, Tamura K, et al. The International Prognostic Index predicts outcome in aggressive adult T-cell leukemia/lymphoma: analysis of 126 patients from the International Peripheral T-cell Lymphoma Project. Ann Oncol 2009;20:715–721. [DOI] [PubMed] [Google Scholar]
- 9.Au WY, Weisenburger DD, Intragumtornchai T, et al. Clinical differences and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the Peripheral T-Cell Lymphoma Project. Blood 2009;113:3931–3937. [DOI] [PubMed] [Google Scholar]
- 10.Riesco A Five-year cancer cure: relation to total amount of peripheral lymphocytes and neutrophils. Cancer 1970;25:135–140. [DOI] [PubMed] [Google Scholar]
- 11.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med 1998;339:1506–1514. [DOI] [PubMed] [Google Scholar]
- 12.Ray-Coquard I, Cropet C, Van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res 2009;69:5383–5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim DH, Baek JH, Chae YS, et al. Absolute lymphocyte counts predicts response to chemotherapy and survival in diffuse large B-cell lymphoma. Leukemia 2007;21:2227–2230. [DOI] [PubMed] [Google Scholar]
- 14.Cox MC, Nofroni I, Ruco L, et al. Low absolute lymphocyte count is a poor prognostic factor in diffuse-large-B-cell-lymphoma. Leuk Lymphoma 2008;49:1745–1751. [DOI] [PubMed] [Google Scholar]
- 15.Oki Y, Yamamoto K, Kato H, et al. Low absolute lymphocyte count is a poor prognostic marker in patients with diffuse large B-cell lymphoma and suggests patients’ survival benefit from rituximab. Eur J Haematol 2008;81:448–453. [DOI] [PubMed] [Google Scholar]
- 16.Talaulikar D, Choudhury A, Shadbolt B, Brown M. Lymphocytopenia as a prognostic marker for diffuse large B cell lymphomas. Leuk Lymphoma 2008;49:959–964. [DOI] [PubMed] [Google Scholar]
- 17.Song MK, Chung JS, Seol YM, et al. Influence of low absolute lymphocyte count of patients with nongerminal center type diffuse large B-cell lymphoma with R-CHOP therapy. Ann Oncol 2010;21:140–144. [DOI] [PubMed] [Google Scholar]
- 18.Mourad N, Mounier N, Brière J, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood 2008;111:4463–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lachenal F, Berger F, Ghesquières H, et al. Angioimmunoblastic T-cell lymphoma: clinical and laboratory features at diagnosis in 77 patients. Medicine (Baltimore) 2007;86:282–292. [DOI] [PubMed] [Google Scholar]
- 20.Chihara D, Oki Y, Ine S, et al. Analysis of prognostic factors in peripheral T-cell lymphoma: prognostic value of serum albumin and mediastinal lymphadenopathy. Leuk Lymphoma 2009;50:1999–2004. [DOI] [PubMed] [Google Scholar]
- 21.Castillo JJ, Morales D, Quinones P, et al. Lymphopenia as a prognostic factor in patients with peripheral T-cell lymphoma, unspecified. Leuk Lymphoma 2010;51:1822–1828. [DOI] [PubMed] [Google Scholar]
- 22.Kim YR, Kim JS, Kim SJ, et al. Lymphopenia is an important prognostic factor in peripheral T-cell lymphoma (NOS) treated with anthracycline-containing chemotherapy. J Hematol Oncol 2011;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimoyama M Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87). Br J Haematol 1991;79:428–437. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc 1958;53:437–481. [Google Scholar]
- 25.Cox DR. Regression models and life tables. JR Stat Soc B 1972;34:187–220. [Google Scholar]
- 26.De Angulo G, Yuen C, Palla SL, et al. Absolute lymphocyte count is a novel prognostic indicator in ALL and AML: implications for risk stratification and future studies. Cancer 2008;112:407–415. [DOI] [PubMed] [Google Scholar]
- 27.Ege H, Gertz MA, Markovic SN, et al. Prediction of survival using absolute lymphocyte count for newly diagnosed patients with multiple myeloma: a retrospective study. Br J Haematol 2008;141:792–798. [DOI] [PubMed] [Google Scholar]
- 28.Bierman PJ, Lynch JC, Bociek RG, et al. The International Prognostic Factors Project score for advanced Hodgkin’s disease is useful for predicting outcome of autologous hematopoietic stem cell transplantation. Ann Oncol 2002;13:1370–1377. [DOI] [PubMed] [Google Scholar]
- 29.Porrata LF, Inwards DJ, Ansell SM, et al. New-onset lymphopenia assessed during routine follow-up is a risk factor for relapse postautologous peripheral blood hematopoietic stem cell transplantation in patients with diffuse large B-cell lymphoma. Biol Blood Marrow Transplant 2010;16:376–383. [DOI] [PubMed] [Google Scholar]
- 30.Shimoyama M, Takatsuki K, Araki K et al. Major prognostic factors of patients with adult T-cell leukemia-lymphoma: a cooperative study. Lymphoma Study Group (1984–1987). Leuk Res 1991;15:81–90. [DOI] [PubMed] [Google Scholar]
- 31.Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol 2009;27:453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips AA, Shapira I, Willim RD, et al. A critical analysis of prognostic factors in North American patients with human T-cell lymphotropic virus type-1-associated adult T-cell leukemia/lymphoma: a multicenter clinicopathologic experience and new prognostic score. Cancer 2010;116:3438–3446. [DOI] [PubMed] [Google Scholar]
- 33.Ji Yasunaga, Sakai T, Nosaka K, et al. Impaired production of naive T lymphocytes in human T-cell leukemia virus type I-infected individuals: its implications in the immunodeficient state. Blood 2007;97:3177–3183. [DOI] [PubMed] [Google Scholar]
- 34.Lim ST, Karim R, Tulpule A, et al. Prognostic factors in HIV-related diffuse large-cell lymphoma: Before versus after highly active antiretroviral therapy. J Clin Oncol 2005;23:8477–8482. [DOI] [PubMed] [Google Scholar]
- 35.Castillo JJ, Beltran BE, Bibas M, et al. Prognostic factors in patients with HIV-associated peripheral T-cell lymphoma: a multicenter study. Am J Hematol 2011;86:256–261. [DOI] [PubMed] [Google Scholar]


