Highlights
-
•
Rhodococcus equi infection primarily causes pneumonia but can disseminate to cause disease in virtually any human tissue.
-
•
Increasing recognition that this pathogen can cause disease in both immunocompetent and immunocompromised hosts.
-
•
Two cases of invasive R. equi infection at both ends of the spectrum in terms of susceptibility and severity of disease.
-
•
High index of suspicion in a broad range of settings and communication with microbiologist is essential for early diagnosis.
Keywords: Rhodococcus equi, Human immunodeficiency virus, Opportunistic infection, Cavitating pneumonia, Soft tissue infection
Abstract
Rhodococcus equi is a gram positive bacterium most commonly presenting clinically as pneumonia, however can disseminate to cause disease in virtually any human tissue. Although it is predominantly an opportunistic pathogen, a number of case series have described infection occurring among individuals with a normal immune system. We describe two cases of Rhodococcus equi infection which highlight the diversity of disease presentations of this rare organism.
Introduction
Rhodococcus equi is a zoonotic organism which is a frequent cause of disease in a number of animals, including being the most common cause of pneumonia in foals [1]. It is a non-motile gram positive coccoid or bacillary organism found in high numbers on dry surface soil of farming and livestock properties [1]. Despite being a well-recognized pathogen in veterinary medicine, it has been known also to cause human disease, particularly in immunocompromised hosts [2]. Currently, infection remains a rare occurrence even among immunocompromised individuals [3]. Although pneumonia accounts for roughly 80% of clinical manifestations, R. equi can also cause wound infection, isolated bacteremia and abscesses in virtually any organ system [4]. There is increasing recognition that this pathogen can cause disease in both immunocompetent and immunocompromised individuals, but little is known about the human immunological response to this pathogen, or deficits which may predispose to clinical infection [5]. Due to the rarity and heterogeneity of disease, treatment recommendations for R. equi infection remain largely anecdotal and are often complicated frequent disease recurrences and clinical treatment failures [2]. In addition, there are reports of increasing antimicrobial resistance, particularly to rifampin and macrolides, and ideal treatment regimens in resistant cases are not clear [2]. We present two cases of invasive R. equi infection at both ends of the spectrum in terms of immunological susceptibility and severity of disease.
Case 1
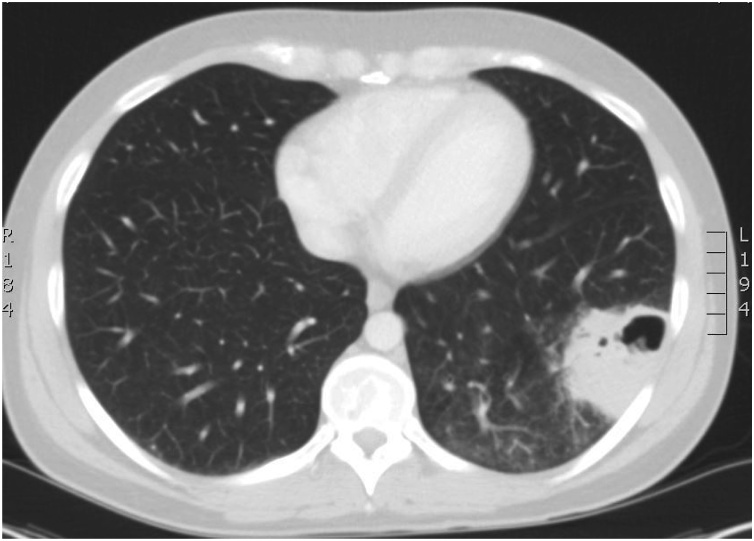
A 33 year-old human immunodeficiency virus (HIV)-positive male who lived on a property with cattle and horses presented to a regional hospital with one month of fever, dry cough, six-kilogram weight loss, sweats and anorexia. He was not on antiretroviral therapy (due to CD4 + T cell counts previously >350/mm3 consistent with clinical practice standards at that time) for five years and had failed to attend a recent clinic appointment. Initial blood tests revealed a CD4+ T cell count drop to 20/mm3, HIV viral load >100 000 copies/mL, and an abnormal liver profile (GGT 352 IU/L, ALT 540 IU/L, AST 403 IU/L, ALP 99 IU/L). A chest x-ray showed a left lower lobe cavitating lung lesion. Further imaging with computed tomography (CT) showed a five cm cavitating lesion (Fig. 1) with an irregular thickened wall in the left lower lobe with adjacent interstitial opacification. A CT guided lung aspirate and biopsy was performed and sent for routine bacterial, fungal and mycobacterial culture. Rod-like organisms were seen within one macrophage on tissue staining. Rhodococcus equi was subsequently grown from the lung aspirate and blood cultures. Intravenous (IV) meropenem 1 g three times daily and vancomycin 1.25 g twice daily therapy were initiated.
Fig. 1.
Computed Tomography (CT) chest of Rhodococcus equi cavitating pneumonia in an HIV positive male with Acquired Immunodeficiency Syndrome (AIDS) (Case 1).
He remained unwell with persistent fevers and progressive pancytopenia. He underwent a bone marrow aspirate and trephine that revealed a mildy hypercellular marrow with reactive changes and ample megakaryocytes; fungal and mycobacterial culture negative and no organisms were seen. Vancomycin was discontinued following concerns regarding drug-induced cytopenias, and the patient was temporarily supported with granulocyte-colony stimulating factor (G-CSF) 300 μg on alternate days. Oral ciprofloxacin 750 mg twice daily was added. Antiretroviral therapy was commenced and consisted of atazanavir 300 mg daily, ritonavir 100 mg daily and tenofovir/emtricitabine 300/200 mg daily six days after admission. Pulmonary consolidation worsened significantly after ART introduction, raising the possibility of an immune reconstitution inflammatory response. After an extended inpatient stay, he was discharged on home IV ertapenem 1 g daily, oral ciprofloxacin 750 mg twice daily, antiretroviral therapy, and prophylactic trimethoprim/sulfamethoxazole 80/400 mg daily and azithromycin 1 g weekly.
One month after discharge from hospital he was readmitted with headache, fevers and vomiting and a CT chest showed worsening left lower lobe consolidation and new multiple pulmonary nodules with cavitation of the larger nodules and a moderate left sided pleural effusion. His CRP had increased to 188 mg/L and his CD4+T cell count had increased to 210/mm3. A bronchoscopy and bronchoalveolar lavage (BAL) was performed which cultured Mycobacterium avium intracellulare. Combination therapy for Mycobacterium avium intracellulare infection included rifabutin 300 mg thrice weekly, ethambutol 800 mg daily, and clarithromycin 250 mg twice daily. Ertapenem was continued to make up three months total therapy for pulmonary R. equi infection before discontinuation; ciprofloxacin was continued for approximately two years. His respiratory symptoms improved and repeat CT chest six months after his original presentation to hospital showed significant improvement in left lower lobe consolidation and lymphadenopathy.
Case 2
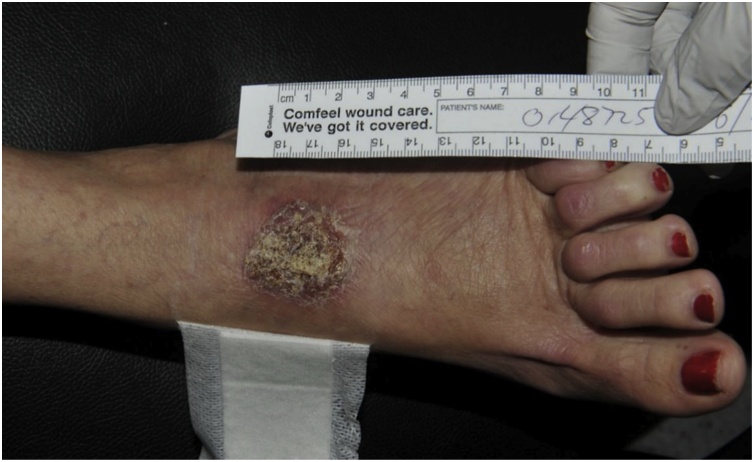
A 53 year-old female teacher developed a small area of superficial redness and blistering over the dorsum of her left foot. She lived in an urban setting without exposure to farming or any animals, including horses. The patient had no relevant past medical history, regular medications, had never smoked nor consumed alcohol. She did not use recreational drugs. Her skin lesion was attributed to a friction injury secondary to new footwear. The lesion (Fig. 2) enlarged with more prominent erythema and swelling, and began to ulcerate over a three week period. She was systemically well and had no other skin lesions or lymphadenopathy.
Fig. 2.
Left foot Rhodococcus equi skin and soft tissue infection in an immunocompetent host (Case 2).
She presented to her primary care physician and was prescribed multiple courses of oral β-lactam antimicrobials including amoxicillin and cephalexin for a presumed bacterial soft tissue infection, without improvement. Initial wound swabs failed to culture a pathogen and a punch biopsy revealed only granulation tissue. At this stage, the lesion had formed a verruciform erythematous plaque. The patient was reviewed by a dermatologist who performed a repeat biopsy and culture of the lesion and commenced treatment with doxycycline 100 mg twice daily. Histopathology results showed an acute suppurative neutrophilic inflammatory process suggestive of infection, with subsequent culture growing Rhodococcus equi. Fungal and mycobacterial cultures, and Mycobacterium ulcerans PCR, were all negative. Her chest x-ray, full blood count, liver and renal function were normal, and she had a negative HIV antibody test. In addition, her lymphocyte subsets, neutrophil function and immunoglobulin levels were within normal range. Doxycycline was discontinued and she commenced on oral ciprofloxacin 500 mg twice daily however this was ceased prematurely due to the development of a right Achilles tendinopathy. She was then started on oral rifampin 600 mg daily and clarithromycin 500 mg twice daily. Despite this, her left foot lesion began to worsen and she was admitted to hospital for further management, including plastic surgery and dermatology consultation. Left foot magnetic resonance imaging (MRI) did not reveal evidence of osteomyelitis or abscess formation. A decision was made to surgically excise the lesion and to apply a split skin graft. Her antimicrobial therapy consisted of oral azithromycin 500 mg daily and rifampicin 600 mg daily on admission with a plan for three months of total therapy. At outpatient review, her skin graft had taken well with evidence of good wound healing.
Discussion
Rhodococcus equi is an aerobic acid-fast gram positive coccobacilliary organism described first in the lungs of foals with pyogranulomatous pneumonia [6]. The first case of human infection was described in 1967 where a young male with autoimmune hepatitis on a corticosteroid and 6-mercaptopurine who worked on a stockyard fell ill with a cavitating pneumonia [7]. Cases of Rhodococcus equi infection remained remarkably rare until the early 1980s where HIV-related immunosuppression and solid organ transplantation became more common [8]. Improved microbiological laboratory identification techniques during this time undoubtedly also played a role. Rhodococcus equi can be cultured from water and soil worldwide and exposure to farm soil, animals or manure has frequently been reported to cause human cases of disease by inhalation, ingestion or direct inoculation [9]. A thick walled cavitating pneumonia is common and can be complicated by lung abscess, empyema, pneumothorax and mediastinitis [10]. R. equi can also cause a wide spectrum of disease and has been cultured from a variety of human tissues including heart valves, cerebrospinal fluid, skin, lymph nodes, bone and peritoneal fluid [11].
The immunological response and individual susceptibility to R. equi infection remains largely unknown. Most infected individuals have some defect in their cell mediated immune response [4]. Poor outcomes occur in CD4+ and CD8 T+ cell deficient mice with R. equi infection [12]. Infection involves HIV-infected individuals (primarily in patients with CD4+ T cell count <100/mm3), organ transplant recipients and individuals receiving chemotherapy for a malignancy, corticosteroids or anti-TNF antibody therapy. Increased risk is seen in those with diabetes, excessive alcohol intake, chronic kidney disease, hematological malignancy, lung cancer, and sarcoidosis [2,4]. Disease in immunocompetent individuals has been documented. The largest reported case series of R. equi infection in immunocompetent hosts evaluated 19 patients with a broad spectrum of disease apart from skin or soft tissue infections [5]. Although milder localized disease represented a higher proportion of illness in this series, invasive severe infections were still observed.
Exposure to livestock or contaminated soil appears to be important as an epidemiological link to disease. However, this may not be necessary for acquisition of R. equi infection [5]. As demonstrated in a large case series, 9 out of 19 patients did not provide such a history, as in Case 2 [5]. Exposures are more evident when the patient is immunocompromised, as noted in numerous case series involving HIV infected individuals and solid organ transplant recipients [2]. There has been one documented case of human-to-human transmission of pulmonary R. equi infection in two HIV infected males living together [13].
Rhodococcus sp. belongs to the family Nocardiaeceae, order Actinomycetes that includes Nocardia sp. and Mycobacterium sp [3]. It grows optimally at 30°C and classically produces pale salmon-pink colonies on solid media. Microbiological identification using standard culture techniques can be troublesome with R. equi occasionally being misidentified as Nocardia. Moreover, because it is partially acid fast, it can also be mistaken for a Mycobacterium [14]. Given that patients with impaired cell mediated immunity are susceptible to infection from all of these organisms, co-infection is possible (as in Case 1) and high clinical suspicion, close observation and accurate laboratory identification is needed. Identification of Rhodococcus sp. using molecular techniques such as the 16S rRNA sequencing method provides accurate identification [15]. Quantitative PCR testing has been used in the veterinary medicine setting with success but is yet to become standard practice in humans [16]. MALDI-TOF has been used to provide rapid and accurate identification of R. equi. Ultimately, early communication with the microbiology laboratory regarding clinical suspicion is essential to expedite accurate diagnosis.
Treating R. equi infection is difficult, complicated by antimicrobial resistance, frequent treatment failures, and clinical relapses [2]. Little systematic data exists and treatment recommendations rely largely on animal studies and expert opinion. Available evidence suggests that combination therapy (at least two or three active agents) is superior to monotherapy [17]. This was indeed true in Case 2 where monotherapy led to treatment failure. Animal studies suggest that vancomycin, imipenem and rifampicin are most effective [18]. Bacterial clearance is dependent on the degree of immunocompromise and success of treatment often relies on immune reconstitution. A prolonged course of both intravenous and oral antimicrobial therapy is recommended [2]. Oral short course regimens have been used successfully in localised disease in immunocompetent individuals [5]. Surgical resection of local lesions should be considered early where treatment failure is likely and immune reconstitution unlikely.
Immune reconstitution inflammatory syndrome (IRIS) after introduction of antiretroviral therapy (ART) in HIV positive patents with Rhodococcus equi pneumonia is described [19]. Patient 1 had significant worsening of his pneumonia following the introduction of antiretroviral therapy, raising the question of possible IRIS. The role of corticosteroids or anti-inflammatory agents in the management of this is unclear.
Conclusion
Rhodococcus equi infection is a rare and sometimes elusive disease affecting mainly those with impaired cellular immunity. This bacterium is known to reside in high numbers in dry farm soil and animal manure, with this epidemiological exposure often being a clue to diagnosis. However, cases where infection occurs in immunocompetent hosts without typical exposure histories are being documented. In addition, the full spectrum of disease ranging from mild localised infection to disseminated multiorgan disease with bacteraemia has been observed in both immunocompetent and immunocompromised hosts. These cases highlight that a high index of suspicion in a broad range of clinical settings, together with early communication with the microbiology department, is essential for diagnosis and institution of effective treatment for R. equi infection.
Conflict of interest
None.
Acknowledgements
None.
References
- 1.Cohen N.D. Rhodococcus equi foal pneumonia. Vet Clin North Am Equine Pract. 2014;30(3):609–622. doi: 10.1016/j.cveq.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Yamshchikov A.V., Schuetz A., Lyon G.M. Rhodococcus equi infection. Lancet Infect Dis. 2010;10(5):350–359. doi: 10.1016/S1473-3099(10)70068-2. [DOI] [PubMed] [Google Scholar]
- 3.Prescott J.F. Rhodococcus equi: an animal and human pathogen. Clin Microbiol Rev. 1991;4(1):20–34. doi: 10.1128/cmr.4.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinstock D.M., Brown A.E. Rhodococcus equi: an emerging pathogen. Clin Infect Dis. 2002;34(10):1379–1385. doi: 10.1086/340259. [DOI] [PubMed] [Google Scholar]
- 5.Kedlaya I., Ing M.B., Wong S.S. Rhodococcus equi infections in immunocompetent hosts: case report and review. Clin Infect Dis. 2001;32(3):E39–E46. doi: 10.1086/318520. [DOI] [PubMed] [Google Scholar]
- 6.Magnusson H. Spezilfi sche infektiose pneumonie beim fohlen: ein neuer eitererreger beim pferd. Arch Wiss Prakt Tierheilkd. 1923;50:22–38. [Google Scholar]
- 7.Golub B., Falk G., Spink W.W. Lung abscess due to Corynebacterium equi. Report of first human infection. Ann Intern Med. 1967;66(6):1174–1177. doi: 10.7326/0003-4819-66-6-1174. [DOI] [PubMed] [Google Scholar]
- 8.Torres-Tortosa M. Prognosis and clinical evaluation of infection caused by Rhodococcus equi in HIV-infected patients: a multicenter study of 67 cases. Chest. 2003;123(6):1970–1976. doi: 10.1378/chest.123.6.1970. [DOI] [PubMed] [Google Scholar]
- 9.Vazquez-Boland J.A. Rhodococcus equi: the many facets of a pathogenic actinomycete. Vet Microbiol. 2013;167(1–2):9–33. doi: 10.1016/j.vetmic.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Muntaner L. Radiologic features of Rhodococcus equi pneumonia in AIDS. Eur J Radiol. 1997;24(1):66–70. doi: 10.1016/s0720-048x(96)01022-4. [DOI] [PubMed] [Google Scholar]
- 11.Antinori S. Disseminated Rhodococcus equi infection initially presenting as foot mycetoma in an HIV-positive patient. AIDS. 1992;6(7):740–742. doi: 10.1097/00002030-199207000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Kanaly S.T., Hines S.A., Palmer G.H. Failure of pulmonary clearance of Rhodococcus equi infection in CD4+ T-lymphocyte-deficient transgenic mice. Infect Immun. 1993;61(11):4929–4932. doi: 10.1128/iai.61.11.4929-4932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arlotti M. Rhodococcus equi infection in HIV-positive subjects: a retrospective analysis of 24 cases. Scand J Infect Dis. 1996;28(5):463–467. doi: 10.3109/00365549609037941. [DOI] [PubMed] [Google Scholar]
- 14.Walsh R.D., Schoch P.E., Cunha B.A. Rhodococcus. Infect Control Hosp Epidemiol. 1993;14(5):282–287. doi: 10.1086/646738. [DOI] [PubMed] [Google Scholar]
- 15.Bharadwaj R. Clinical impact of the use of 16S rRNA sequencing method for the identification of "difficult-to-identify" bacteria in immunocompromised hosts. Transpl Infect Dis. 2012;14(2):206–212. doi: 10.1111/j.1399-3062.2011.00687.x. [DOI] [PubMed] [Google Scholar]
- 16.Shaw S.D. Estimating the Sensitivity and Specificity of Real-Time Quantitative PCR of Fecal Samples for Diagnosis of Rhodococcus equi Pneumonia in Foals. J Vet Intern Med. 2015;29(6):1712–1717. doi: 10.1111/jvim.13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tse K.C. Rhodococcus lung abscess complicating kidney transplantation: successful management by combination antibiotic therapy. Transpl Infect Dis. 2008;10(1):44–47. doi: 10.1111/j.1399-3062.2007.00231.x. [DOI] [PubMed] [Google Scholar]
- 18.Nordmann P., Kerestedjian J.J., Ronco E. Therapy of Rhodococcus equi disseminated infections in nude mice. Antimicrob Agents Chemother. 1992;36(6):1244–1248. doi: 10.1128/aac.36.6.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferretti F. Disseminated Rhodococcus equi infection in HIV infection despite highly active antiretroviral therapy. BMC Infect Dis. 2011;11:343. doi: 10.1186/1471-2334-11-343. [DOI] [PMC free article] [PubMed] [Google Scholar]