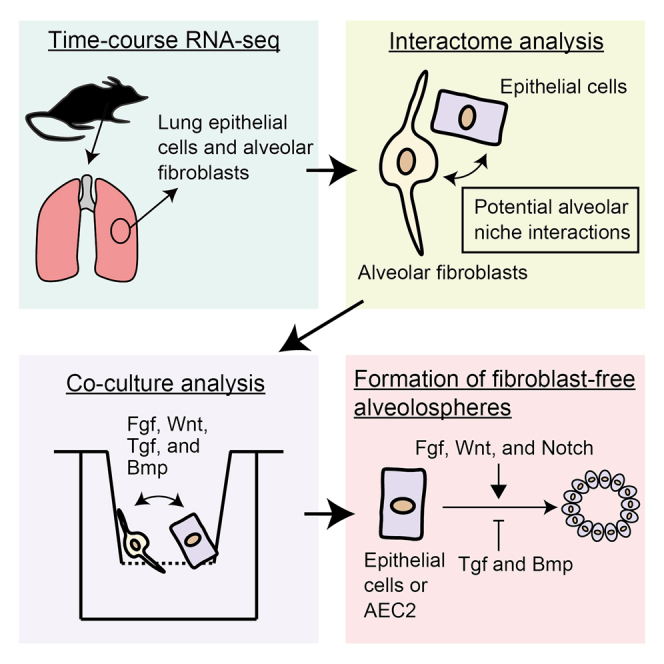
Summary
Lung epithelial cells and fibroblasts are key cell populations in lung development. Fibroblasts support type 2 alveolar epithelial cells (AEC2) in the developing and mature lung. However, fibroblast-AEC2 interactions have not been clearly described. We addressed this in the present study by time course serial analysis of gene expression sequencing (SAGE-seq) of epithelial cells and fibroblasts of developing and mature murine lungs. We identified lung fibroblast-epithelial interactions that potentially regulate alveologenesis and are mediated by fibroblast-expressed ligands and epithelial cell surface receptors. In the epithelial-fibroblast co-culture alveolosphere formation assay, single intervention against fibroblast-expressed ligand or associated signaling cascades promoted or inhibited alveolosphere growth. Adding the ligand-associated molecules fibroblast growth factor 7 and Notch ligand and inhibitors of bone morphogenetic protein 4, transforming growth factor β, and glycogen synthase kinase-3β to the culture medium enabled fibroblast-free alveolosphere formation. The results revealed the essential factors regulating fibroblast-AEC2 interactions.
Subject Areas: Molecular Interaction, Developmental Biology, Transcriptomics
Graphical Abstract
Highlights
-
•
Lung fibroblast-epithelial interactions regulating alveologenesis were analyzed
-
•
The interactions were assessed in fibroblast-epithelial co-culture organoid assays
-
•
A specific combination of molecules enabled fibroblast-free alveolosphere formation
-
•
The spheres were passageable and could be used to study lung development and biology
Molecular Interaction; Developmental Biology; Transcriptomics
Introduction
The lungs consist of at least 40–60 different cell types that form a complex three-dimensional structure, with branched epithelial walls surrounded by interstitium and a vascular network (McQualter and Bertoncello, 2012). The airway epithelium is composed of ciliated, secretory (club and goblet), and basal cells (Barkauskas et al., 2017). In contrast, the alveolar epithelium where gas exchange takes place comprises two cell types—type 2 alveolar epithelial cells (AEC2) that secrete surfactant proteins and are considered as tissue stem cells and type 1 alveolar epithelial cells (AEC1) that form a thin wall for gas exchange (Barkauskas et al., 2013). In alveoli, fibroblasts are in close contact with and are thought to support AEC2 maintenance, constituting an alveolar stem cell niche (Hogan et al., 2014).
Lung development begins at around embryonic day 9.0 (E9.0) in mice, and maturation of alveoli starts at around E16.5 and continues to around postnatal day 30 (P30) (Herriges and Morrisey, 2014). In the developing and mature lung, fibroblasts interact with and support the differentiation and maturation of epithelial cells (McCulley et al., 2015); thus, fibroblast-epithelial cell interactions are important in alveologenesis and AEC2 maintenance. Indeed, alveolospheres—an organoid derived from AEC2—can be generated by co-culturing AEC2 with lung mesenchymal cells (Barkauskas et al., 2013); alveolosphere cultures have been used to investigate fibroblast-AEC2 interactions in vitro (Zepp et al., 2017). However, the molecular mechanisms of fibroblast-AEC2 interactions and the factors critical for alveolosphere formation are not known.
To investigate fibroblast-AEC2 interactions, we carried out a time course serial analysis of gene expression sequencing (SAGE-seq) of lung epithelial cells and fibroblasts during alveologenesis and in the mature state. We demonstrate that these interactions are mediated by pairs of fibroblast ligands and their cognate epithelial receptors. Moreover, the results of our in vitro alveolosphere formation assay revealed a set of ligand-associated factors that are required for fibroblast-free alveolosphere formation.
Results
Transcriptional Changes during Alveologenesis and in Mature Lungs
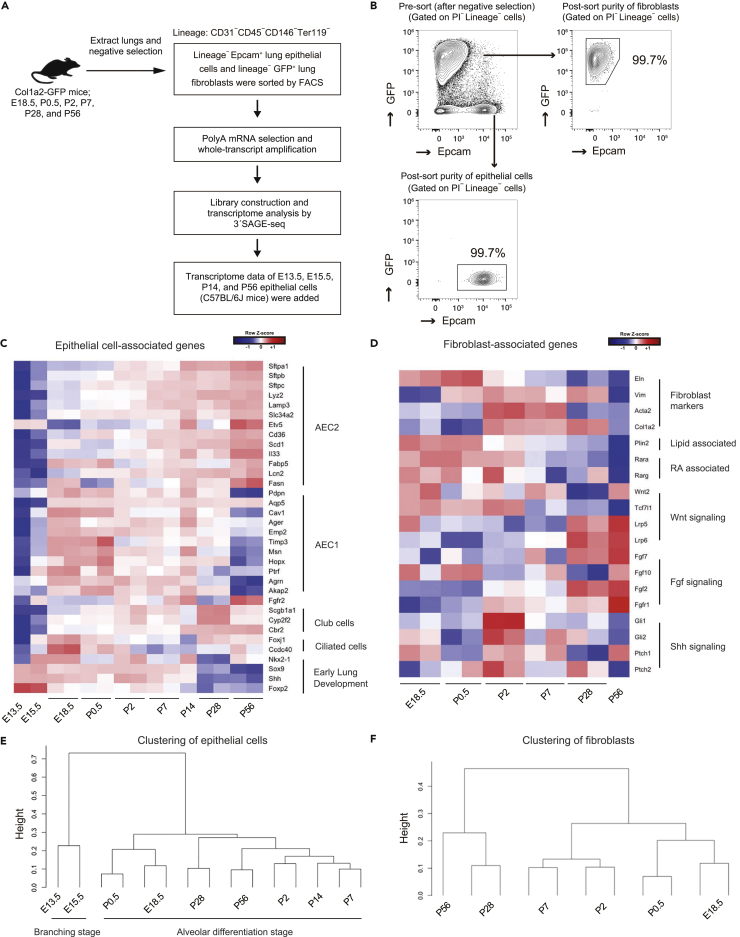
To clarify fibroblast-epithelial interactions during alveologenesis and in mature lungs, we performed a time course transcriptome analysis of epithelial cells and fibroblasts in developing and mature murine lungs. We purified lineage (CD31, CD45, CD146 and Ter119)− Epcam+ lung epithelial cells and lineage– GFP+ fibroblasts from E18.5, P0.5, P2, P7, P28, and P56 (fibroblasts only) Col1a2-GFP mice for SAGE-seq analysis (Figures 1A and 1B). We performed flow cytometry and immunohistochemical analyses of Col1a2-GFP mice at different developmental stages to analyze the characteristics of the lineage– GFP+ population. GFP+ cells were present in alveolar walls as well as in peribronchiolar and perivascular regions in Col1a2-GFP mice (Tsukui et al., 2013) at the examined time points and were negative for CD31, CD45, Epcam, or Ter119 (Figures S1A and S1B). Peribronchiolar and perivascular GFP+ cells were co-labeled with α-SMA+ smooth muscle cells (Figure S1B) (De Val et al., 2002). Since we depleted CD146+ smooth muscle cells before cell sorting, the analyzed GFP+ CD146− population comprised alveolar fibroblasts, including Pdgfra+ and Pdgfra− cells (Figures S1C and S1D). No distinct GFP+ Pdgfrb+ CD146− mesenchymal population was isolated by flow cytometry (Figure S1C). Transcriptome data for E13.5, E15.5, P14, and P56 epithelial cells of C57BL/6J mice were also included in the analysis.
Figure 1.
Time Series Global Transcriptome Analysis of Epithelial Cells and Fibroblasts during Alveologenesis
(A) Experimental scheme of transcriptomic analysis of E18.5, P0.5, P2, P7, P28, and P56 lung epithelial cells and fibroblasts (n = 2 animals except for P56 fibroblasts [n = 1]). FACS, fluorescence-activated cell sorting.
(B) Gating scheme for lung epithelial cells and fibroblasts and purity of cells after cell sorting. Representative plots of P56 mice are shown.
(C) Heatmap of selected AEC2, AEC1, and club cell markers and early lung development-associated genes.
(D) Heatmap of selected fibroblast markers and genes associated with lipids; retinoic acids; and Wnt, Fgf, and Shh signaling.
(E and F) Hierarchical clustering by dendrogram of epithelial cells (E) and fibroblasts (F) based on their transcriptome.
See also Figures S1 and S2, and Tables S7 and S8.
We first analyzed the transcriptome of epithelial cells (Figure 1C) and fibroblasts (Figure 1D) to evaluate transcriptional changes during alveologenesis and in mature lungs. In epithelial cells, the expression of AEC2 marker genes (Treutlein et al., 2014), such as Sftpa1, Sftpb, and Sftpc, as well as club and ciliated cell marker genes (Treutlein et al., 2014) increased in a time-dependent manner (Figure 1C). In contrast, the expression of genes known to be associated with early lung development, such as Sox9 and Foxp2 (Hogan et al., 2014), decreased over time (Figure 1C). The levels of AEC1 marker genes (Treutlein et al., 2014) peaked at E18.5–P0.5 before gradually decreasing (Figure 1C). A qPCR analysis revealed trends in the expression of AEC1/AEC2 markers that were similar to those observed by SAGE-seq analysis (Figures S2A and S2B). Hierarchical clustering of epithelial cells based on their transcriptome revealed that E13.5 and E15.5 epithelial cells clustered separately from other epithelial cells (Figure 1E). These results suggest that the transcriptome data reflected the development and maturation of epithelial cells.
The expression levels of the fibroblast marker genes Eln, Vim, Acta2, and Col1a2 (Tsukui et al., 2013) as well as Plin2, which encodes a protein that delivers lipids within the fibroblast cytosol to adjacent AEC2 (Schultz et al., 2002), were elevated throughout lung development but were especially high in the perinatal period or saccular stage (E17.5–P5) (Figure 1D). Retinoic acid signaling-associated transcription factors Rara and Rarg (McGowan et al., 1995) were highly expressed at this stage (E18.5–P2) (Figure 1D). Wnt receptors Lrp5 and Lrp6 were highly expressed in the mature state, but Tcf7l1, a downstream gene of Wnt signaling (Shy et al., 2013), was gradually downregulated as the lungs matured (Figure 1D). Fibroblast growth factor (Fgf) ligands and receptor (Fgfr1) were highly expressed in the mature lungs (Figure 1D); this corresponded with epithelial expression of Fgfr2 (Figure 1C), suggesting an important role for Fgf signaling at this stage. Interestingly, Sonic hedgehog (Shh) signaling-associated genes were specifically upregulated in P2 (Figure 1D), which coincided with epithelial upregulation of Shh at P2 (Figure 1C) (Peng et al., 2015). Hierarchical clustering of fibroblasts based on their transcriptome showed that P28 (the end of alveologenesis) and P56 (mature state) clustered separately from the other developmental stages (Figure 1F).
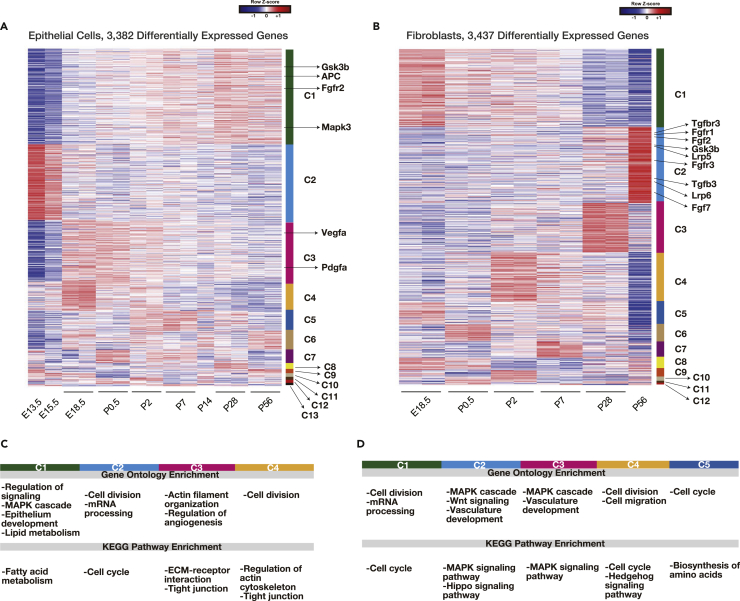
We next identified differentially expressed genes (DEGs; genes with adjusted p < 0.05, maximum expression >50) in the transcriptome data of epithelial cells (3,382 genes) and fibroblasts (3,437 genes). Using the CLICK method (Sharan et al., 2003), we identified 13 distinct clusters of DEGs (C1–C13; Figure 2A and Table S1) for epithelial cells and 12 for fibroblasts (C1–C12; Figure 2B and Table S1). Importantly, the levels of genes in epithelial cluster C1 increased with lung maturation and peaked at P28. Gene Ontology (GO) enrichment analysis (Ashburner et al., 2000) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis (Kanehisa et al., 2017) of epithelial C1 revealed that genes associated with lipid/fatty acid metabolism, epithelium development, and the mitogen-activated protein kinase (MAPK) cascade were highly enriched (Figure 2C and Table S2). Epithelial C1 genes annotated to the MAPK cascade (Table S2) included downstream factors (Mapk3) (Plotnikov et al., 2011) as well as those associated with Wnt signaling (Gsk3b and APC) (Volckaert and De Langhe, 2015) and Fgf signaling (Fgfr2) (Volckaert and De Langhe, 2015), suggesting important roles for Wnt/Fgf signaling in alveologenesis. In contrast, genes included in epithelial cluster C3 were highly expressed at E18.5–P2 (saccular stage) and included angiogenesis-associated genes, such as Vegfa and Pdgfa (Figure 2A and Table S2) (Chao et al., 2016). This suggests that genes regulating angiogenesis rather than epithelium maturation play important roles at the saccular as compared with the alveolar maturation stage (P5–P28). Genes included in epithelial clusters C2 and C4 were highly expressed at E13.5–E18.5 and included genes associated with cell division, which could reflect the high proliferative potential of epithelial cells during this period.
Figure 2.
Distinct DEG Clusters at Different Developmental Stages
(A and B) Heatmap of 3,382 DEGs in epithelial cells (A) and 3,437 DEGs in fibroblasts (B) after clustering. Cluster numbers are shown on the right.
(C and D) GO and KEGG pathway enrichment analyses for each epithelial (C) and fibroblast (D) cluster (Table S2).
See also Figure S3.
The expression levels of genes in fibroblast clusters C2 and C3 tended to increase with lung maturation, peaking at P56 and P28, respectively (Figure 2B). These clusters included genes associated with the MAPK cascade and Wnt and Hippo signaling (Figure 2D and Table S2). Fibroblast C2 genes annotated to the MAPK cascade (Table S2) included Gsk3b, Fgf2, Fgfr1, Fgfr3, Tgfb3, and Tgfbr3, suggesting that Wnt, Fgf, and Tgf-β signaling pathways are activated in fibroblasts. Fibroblast C2 genes were associated with Wnt signaling (Table S2) and included Lrp5 and Lrp6 (Volckaert and De Langhe, 2015). These results suggest that Wnt/Fgf/Tgf-β signaling-associated genes were enriched in fibroblasts in alveologenesis and in the mature state. Genes included in fibroblast clusters C1, C4, and C5 were highly expressed at E18.5–P2 and included those associated with cell division, which could reflect the high proliferative potential of fibroblasts during these periods as compared with later stages. Collectively, our transcriptome analysis revealed significant changes in transcriptional profiles throughout lung development and identified several signaling pathways that may regulate alveologenesis and AEC2 maintenance.
Signaling Pathways Activated during Alveologenesis and in Mature Lungs
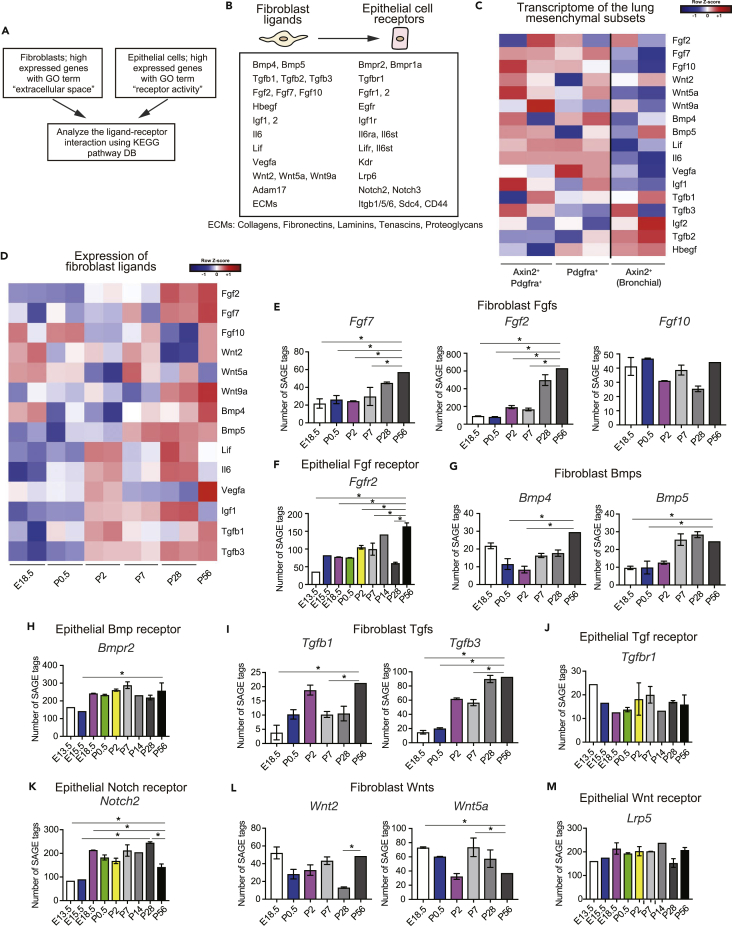
To identify lung fibroblast-epithelial interactions that potentially regulate alveologenesis and homeostasis of the alveolar stem cell niche, we examined genes expressed in fibroblasts and epithelial cells encoding extracellular molecules and cell surface receptor molecules, respectively (Figure 3A). We then reconstructed fibroblast-epithelial interactions according to interaction information provided by the KEGG database (Figures 3A and 3B; Table S3). We also identified genes expressed in fibroblasts and epithelial cells encoding cell surface receptors and extracellular molecules, respectively, and reconstructed epithelial-fibroblast (Table S4), fibroblast-fibroblast (Table S5), and epithelial-epithelial interactions (Table S6). To investigate whether the fibroblast-epithelial interactions were applicable to those between fibroblasts and AEC2, we used publicly available transcriptome data of lung mesenchymal subpopulations (Zepp et al., 2017) and analyzed the expression of fibroblast-expressed ligands in mesenchymal subpopulations supporting AEC2. We found that most fibroblast-expressed ligands were highly expressed in lung Axin2+ Pdgfra+ or Pdgfra+ mesenchymal cells (Figure 3C), which support AEC2 and generate alveolospheres.
Figure 3.
Signaling Pathways Upregulated during Alveologenesis and in Mature Lungs
(A and B) Schematic for (A) and summary of (B) fibroblast-epithelial interactome analysis (Table S3). Adam17 is not a direct ligand to Notch2 or Notch3 but was detected in the analysis.
(C and D) Heatmap of fibroblast ligand-encoding genes based on transcriptome data of lung mesenchymal subsets (GSE92699) (C) and our time course transcriptome data (D).
(E–M) Number of SAGE tags (expression after standardization) of the selected genes. Data represent mean ± SEM. *Adjusted p < 0.05; normalized data were examined for statistical significance using TCC package (Sun et al., 2013).
See also Figure S3.
The above-mentioned results suggested that the identified interactions were associated with fibroblast-AEC2 interactions. Moreover, the expression levels of these fibroblast ligands tended to be high during alveolar maturation (P5–P28) and in the mature lung stage (P56) as compared with the saccular stage (E17.5–P2) (Figure 3D), suggesting that the interactions were associated with AEC2 proliferation and differentiation in the former and not the latter periods. Specifically, fibroblast ligands Fgf2, Fgf7 (Figure 3E), Bmp4, Bmp5 (Figure 3G), Tgfb1, and Tgfb3 (Figure 3I) as well as epithelial Fgfr2 (Figure 3F) and Bmpr2 (Figure 3H) were upregulated as the lungs matured, suggesting specific roles for Fgf, Bmp, and Tgf-β signaling in alveologenesis and lung maturation. Fibroblast Fgf10 expression was high throughout the development (Figure 3E), but considering the high expression level of its receptor Fgfr2 during alveolar maturation and in the mature lung stage, its specific role in alveologenesis was also presumed. Although the levels of direct Notch ligands were low in fibroblasts (data not shown), the corresponding receptor Notch2 was highly expressed in epithelial cells in the alveologenesis stage (Figure 3K), suggesting that Notch signaling is involved in alveologenesis (Tsao et al., 2016). Wnt2 and Wnt5a (Figure 3L) levels in fibroblasts as well as epithelial Lrp5 (Figure 3M) and Tgfbr1 (Figure 3J) remained relatively constant throughout lung development. Given the high overall expression level of Lrp5, we presumed that Wnt signaling plays a role in alveologenesis. We also performed a statistical assessment of linear relationships between CLICK-identified fibroblast clusters and epithelial cell clusters (Figure S3A) as well as fibroblast ligands and epithelial receptors (Figure S3B) and found that fibroblast cluster 2 (including Fgf7 and Fgf2) and epithelial cluster 1 (including Fgfr2) were positively correlated (r = 0.49, p = 0.1). Although they were non-significant, we found positive correlations between Fgf7/Fgfr2 (r = 0.22, p = 0.5), Fgf10/Fgfr2 (r = 0.31, p = 0.3), Bmp5/Bmpr2 (r = 0.16, p = 0.6), Tgfb1/Tgfbr1 (r = 0.4, p = 0.2), and Tgfb3/Tgfbr1 (r = 0.41, p = 0.2) (Figure S3B). Thus, the results of the interactome analysis suggest that interactions between fibroblasts and AEC2 in alveologenesis and in mature lungs involve Fgf, Bmp, Tgf-β, Notch, and Wnt signaling.
Fibroblast Ligands and Signaling Pathway Inhibitors Promote Alveolosphere Growth In Vitro
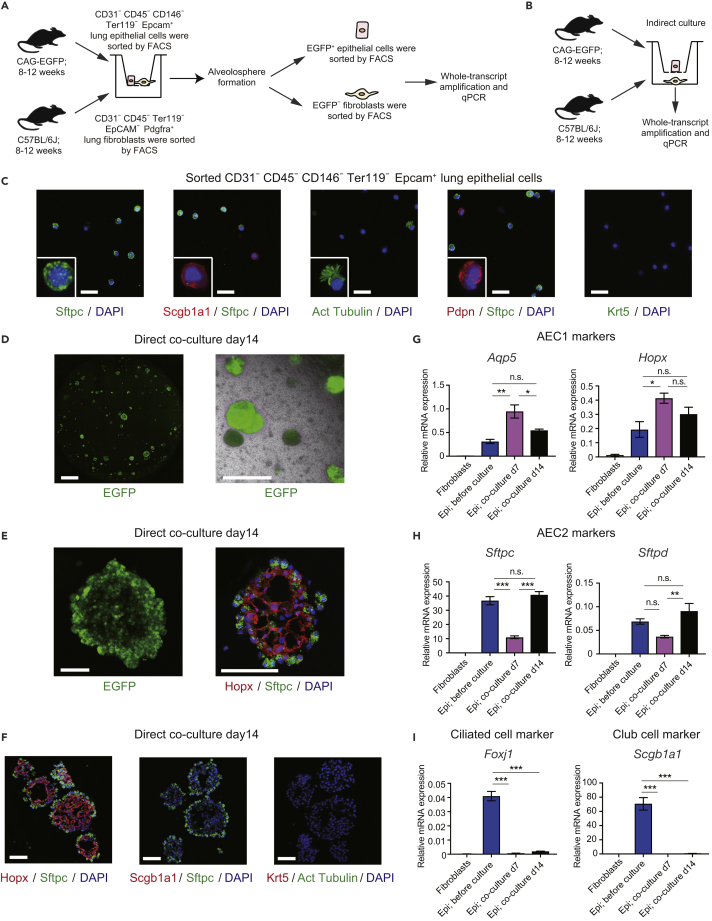
To assess the significance of fibroblast-AEC2 interactions in vitro, we performed an alveolosphere formation assay by directly co-culturing lineage (CD31, CD45, CD146 and Ter119)− Epcam+ EGFP+ epithelial cells from CAG-EGFP mice and fibroblasts from C57BL/6J mice (Figure 4A) (Barkauskas et al., 2013). Epcam+ epithelial cells included AEC2 (89.14% ± 1.34%), club cells (6.85% ± 0.46%), ciliated cells (5.19% ± 0.90%), and AEC1 (0.83% ± 0.49%) (Figure 4C). After alveolosphere generation, EGFP+ epithelial cells and EGFP− fibroblasts were sorted for qPCR analysis. As a control experiment, epithelial cells and fibroblasts were indirectly co-cultured (Figure 4B), but this resulted in no epithelial cell proliferation (data not shown), suggesting that factors expressed higher in direct co-cultures than in indirect co-cultures may promote alveolosphere formation. To understand the mechanism, fibroblasts obtained from the indirect culture were lysed and analyzed by qPCR. Although bulk epithelial cells were sorted for co-culture, the direct culture assay generated mostly Hopx+ Sftpc+ alveolospheres, as determined by immunohistochemistry (Figures 4D–4F) and qPCR (Figures 4G–4I). All 56 immunostained spheres (randomly selected serial sections of 56 spheres fixed after 14 days in culture from three independent experiments) were positive for Sftpc and one Sftpc+ sphere included Sftpc− Scgb1a1+ cells (data not shown) (Lee et al., 2017). We did not detect acetylated tubulin+ ciliated cells or Krt5+ basal cells (Figure 4F). The expression of marker genes of AEC1 (Aqp5 and Hopx; Figure 4G) and AEC2 (Sftpc and Sftpd; Figure 4H) in epithelial cells obtained from the alveolospheres, but not non-alveolar epithelial cell marker genes (Foxj1 and Scgb1a1; Figure 4I), was confirmed by qPCR.
Figure 4.
Alveolospheres Generated from Direct Epithelial-Fibroblast Co-culture
(A) Experimental scheme of alveolosphere formation assay by direct co-culture of 8- to 12-week-old CAG-EGFP epithelial cells and 8- to 12-week-old C57BL/6J fibroblasts.
(B) Experimental scheme of indirect co-culture of epithelial cells and fibroblasts.
(C) Immunocytochemical analysis of sorted Epcam+ cells showing Sftpc+ AEC2, Scgb1a1+ club cells, acetylated tubulin+ ciliated cells, and Pdpn+ AEC1.
(D) Image of a representative well (left; entire Transwell stored in 24-well plates is shown) from direct epithelial-fibroblast co-culture obtained by confocal microscopy and representative merged view obtained by fluorescence microscopy (right).
(E) Enlarged image of the alveolosphere in Figure 4D (left) and alveolosphere labeled with antibodies (right) against Hopx (red) and Sftpc (green).
(F) Collected alveolospheres labeled with antibodies against Hopx/Sftpc (left), Scgb1a1/Sftpc (middle), and Krt5/acetylated tubulin (right).
(G–I) qPCR of AEC1 (G), AEC2 (H), and non-alveolar epithelial (I) markers using cells obtained from direct epithelial-fibroblast co-culture assays (before culture and on days 7 and 14). mRNA levels were normalized to that of Actb. Data represent mean ± SEM (n = 3 wells for cultured cells and n = 3 animals for sorted cells) and are representative of two independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 (one-way ANOVA with Tukey's post hoc test).
Scale bars: 25 μm (C), 1 mm (D), and 100 μm (E and F).
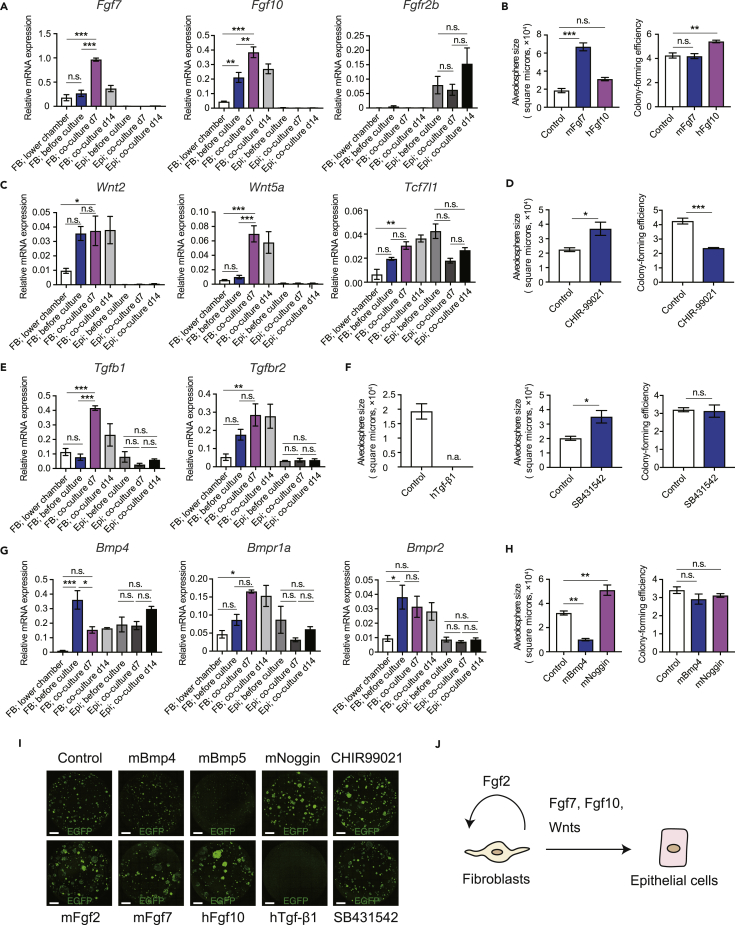
The qPCR analysis revealed upregulation of Fgf7 and Fgf10 in directly but not indirectly co-cultured fibroblasts (Figure 5A). Moreover, upregulation of Fgfr2b, a specific receptor for Fgf7 and Fgf10 (Ornitz and Itoh, 2015), was observed in co-cultured epithelial cells (Figure 5A), suggesting that fibroblasts might upregulate Fgf7 and Fgf10 to interact with AEC2. Given that adding mouse (m)Fgf7 (Zepp et al., 2017) and human (h)Fgf10 (Zacharias et al., 2018) to direct co-cultures increased alveolosphere size and increased colony-forming efficiency, respectively (Figures 5B, 5I, and S4E), it was presumed that Fgf7 and Fgf10 are associated with AEC2 growth. Fgf2 was expressed in fibroblasts and mFgf2 promoted alveolosphere growth (Figures 5I, S4A, S4B, and S4E), but Fgfr1c was expressed only in fibroblasts (Figure S4A), suggesting that Fgf2 is secreted by fibroblasts for self-maintenance. Wnt2 and Wnt5a were upregulated in directly co-cultured fibroblasts (Figure 5C), whereas Tcf7l1, a gene downstream of Wnt signaling (Shy et al., 2013), was upregulated in both co-cultured fibroblasts and epithelial cells (Figure 5C). Since several Wnts can be expressed in lung AEC2 (Nabhan et al., 2018), it was presumed that different Wnts are expressed in and mediate the interaction between fibroblasts and AEC2. The GSK-3β inhibitor CHIR99021, a canonical Wnt pathway agonist (Frank et al., 2016), increased alveolosphere size and decreased colony-forming efficiency (Figures 5D, 5I, and S4E). These findings suggest that Wnt signaling is involved in fibroblast-AEC2 interactions. Regarding Tgf-β/Bmp signaling, Tgfb1, Bmp4, and Bmp5 were more highly expressed in directly as compared with indirectly co-cultured fibroblasts, and their receptors Tgfbr2, Bmpr1a, and Bmpr2 were expressed in both fibroblasts and AEC2 (Figures 5E, 5G, and S4C). Mouse (m)Bmp4 (Zepp et al., 2017) and mBmp5 decreased alveolosphere size (Figures 5H, 5I, S4D, and S4E; no significant change in colony-forming efficiency), and human TGF-β1 interrupted sphere formation (Figures 5F, 5I, and S4E). On the other hand, the TGF-β inhibitor SB431542 (Jain et al., 2015) and Bmp4 inhibitor mNoggin increased alveolosphere size (Figures 5F, 5H, 5I, and S4E; no significant change in colony-forming efficiency), indicating that Bmp/Tgf-β signaling might inhibit AEC2 in the context of fibroblast-epithelial interactions. To assess the number of proliferating cells within the co-culture assays, we used epithelial cells obtained from Fucci mice (Sakaue-Sawano et al., 2008, Tomura et al., 2013) and assessed Fucci-green (mAG)-positive epithelial cells in the wells by flow cytometry (Figures S4F and S4G) and found that the number of actively proliferating (mAG positive) epithelial cells was increased upon addition of SB431542, Fgf7, and Fgf2 (Figures S4F and S4G). These results demonstrate that Fgf7/10, Wnt (Figure 5J), and Bmp/Tgf-β signaling mediate AEC2-fibroblasts interactions and are important regulators of AEC2.
Figure 5.
Fibroblast Ligands and Signaling Pathway Inhibitors Promote Alveolosphere Growth In Vitro
(A, C, E, and G) qPCR of genes related to Fgf (A), Wnt (C), Tgf-β (E), and Bmp (G) signaling using cells obtained from direct and indirect epithelial-fibroblast co-culture assays before culture and day 7 and 14 (n = 3 wells for cultured cells and n = 3 animals for sorted cells). The data are representative of two independent experiments. For “FB; co-culture d7,” data for one sample were not available (n = 2). mRNA levels were normalized to that of Actb.
(B, D, F, and H) Alveolosphere size and colony-forming efficiency after adding mFgf7 or hFgf10 (B), CHIR99021 (D), hTGF-β1 or SB431542 (F), and mBmp4 or mNoggin (H). Sphere size was averaged for alveolospheres in each well. To calculate colony-forming efficiency, all colonies formed in each well were counted and the number of colonies was divided by 5 × 103 (epithelial cells plated). Data are shown for n = 3 wells and are representative of two independent experiments.
(I) Representative wells (epithelial cells; EGFP; entire Transwell stored in 24-well plates is shown) on day 14 after adding fibroblast ligand or signaling pathway inhibitor.
(J) Schematic for Fgf and Wnt signaling in fibroblast-epithelial interactions. Arrowheads indicate presumed activating signals.
Data represent mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 (one-way ANOVA with Tukey's post hoc test or Student's t test). Scale bar: 1 mm. See also Figure S4.
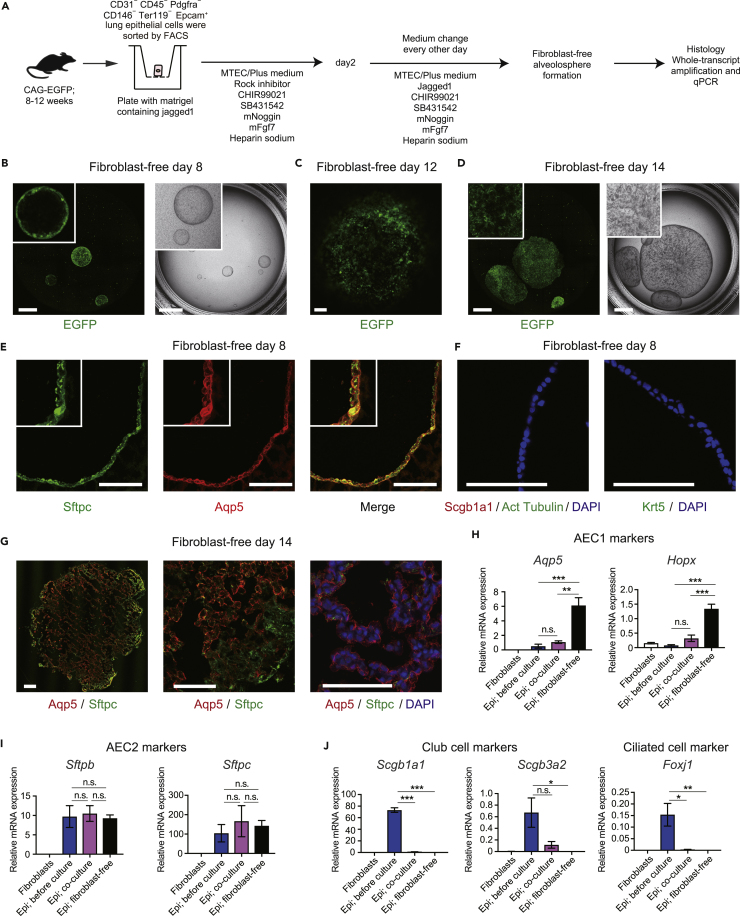
Specific Factors Are Required to Induce Fibroblast-Free Alveolospheres
The results of the co-culture assay suggested that several signaling pathways are critical for AEC2 proliferation. We hypothesized that alveolospheres could be induced to form by adding several fibroblast-derived ligands and signaling inhibitors. To investigate whether fibroblast-free alveolosphere formation was possible, adult epithelial cells from CAG-EGFP mice were sorted and cultured in Matrigel with various combinations of fibroblast-derived ligands and signaling inhibitors. We found that mFgf7, mNoggin, SB431542, CHIR99021, and the Notch ligand Jagged1 (Sato et al., 2009) were necessary to induce the formation of fibroblast-free alveolospheres (Figures 6A–6D and S5A). Jagged1 was added because inactivation of Jagged1 was reported to cause defects in alveologenesis in the developing distal lung (Zhang et al., 2013) and because Notch2 was highly expressed in the adult epithelium (Figure 3K). Single-molecule treatment with mFgf7, mNoggin, SB431542, or CHIR99021 did not induce sphere generation, and their removal from the combination of molecules resulted in no or weak sphere formation (one sphere formed in 2/6 wells in the absence of SB431542 and one sphere formed in 3/6 wells in the absence of mNoggin; Figure S5A). Although a crucial role of Fgf10 was presumed from co-culture assays, substituting mFgf7 with hFgf10 did not yield spheres (Figure S5A). Omitting Jagged1 from the combination of molecules yielded some spheres (up to four spheres per well), but these were fewer in number (spheres in 8/9 wells, with only one sphere in 4/8 wells) than in Jagged1 supplemented cultures (spheres in 9/9 wells, with 5.9 ± 1.1 spheres per well). The observation that the γ-secretase inhibitor DAPT inhibited sphere formation suggests that Notch signaling has positive effects on alveolosphere formation.
Figure 6.
Combination of Fibroblast Ligands and Signaling Pathway Inhibitors Induce the Formation of Fibroblast-Free Alveolospheres
(A) Experimental scheme of the fibroblast-free alveolosphere culture.
(B–D) Representative wells (entire Transwell stored in 24-well plates is shown) from fibroblast-free alveolospheres formation assays on day 8 (B) or 14 (D) and representative fibroblast-free alveolosphere (epithelial cells; EGFP) on day 12 (C). Confocal micrographs (B, left and D, left) and bright-field images (B, right and D, right) are shown.
(E–G) Representative sections of fibroblast-free alveolospheres on day 8 (E and F) and day 14 (G) labeled with antibodies against Aqp5/Sftpc (E and G), Scgb1a1/acetylated tubulin (F left), or Krt5 (F right), showing Sftpc+ Aqp5+ cells on the outside and no cells inside (E) and Sftpc+ AEC2 on the outside and Aqp5+ AEC1 on the inside (G).
(H–J) qPCR analysis of AEC1 (H), AEC2 (I), and non-alveolar epithelial (J) markers using cells obtained from epithelial-fibroblast co-culture (day 14) or fibroblast-free alveolosphere formation assays (day 14). “Fibroblasts” refer to sorted fibroblasts before co-culture and “Epi; before culture” refers to sorted epithelial cells before co-culture. mRNA levels were normalized to that of Actb. Data represent mean ± SEM (n = 3 wells for cultured cells and n = 3 animals for sorted cells) and are representative of two independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 (one-way ANOVA with Tukey's post hoc test).
Scale bars: 1 mm in B and D; 100 μm in C and E–G. See also Figures S5 and S6.
On day 8 of culture, fibroblast-free alveolospheres became larger than day 14 co-cultured alveolospheres (Figure S5B) and were globular with an empty inner part (Figure 6B). Around day 12, the inner part began to fill with cells in a lattice pattern (Figures 6C and 6D). Immunohistochemical analysis revealed that on day 8, the spheres contained Aqp5+ Sftpc+ cells (Figure 6E). We did not detect any Scgb1a1+, acetylated tubulin+, or Krt5+ cells (Figure 6F). On day 14, Aqp5+ AEC1 accumulated inside the spheres (Figure 6G), and qPCR analysis confirmed the expression of AEC1 (Figure 6H) and AEC2 (Figure 6I) markers but not non-alveolar epithelial markers (Figure 6J). In addition, the alveolospheres did not express fibroblast or fibroblast activation marker genes (Tsukui et al., 2013) (Figures S5C and S5D), suggesting an absence of epithelial-mesenchymal transition or fibroblast contamination. Since airway cells were included in the Epcam+ cell population that we used for fibroblast-free culture (Figures 4C and 6A), we sorted the (CD31/CD45/Pdgfra/CD146/Ter119)− (lineage-negative) CD24− (airway cell-negative) (Chen et al., 2012) CD34−/Sca1− (Scgb1a1+/Sftpc+ cell-negative) (Kim et al., 2005) MHCII+/Epcam+ (Hasegawa et al., 2017) population that was enriched in AEC2 (98.45% ± 0.65%; Figures S6A–S6C) and confirmed that it generates alveolospheres under fibroblast-free conditions (Figures S6D–S6G).
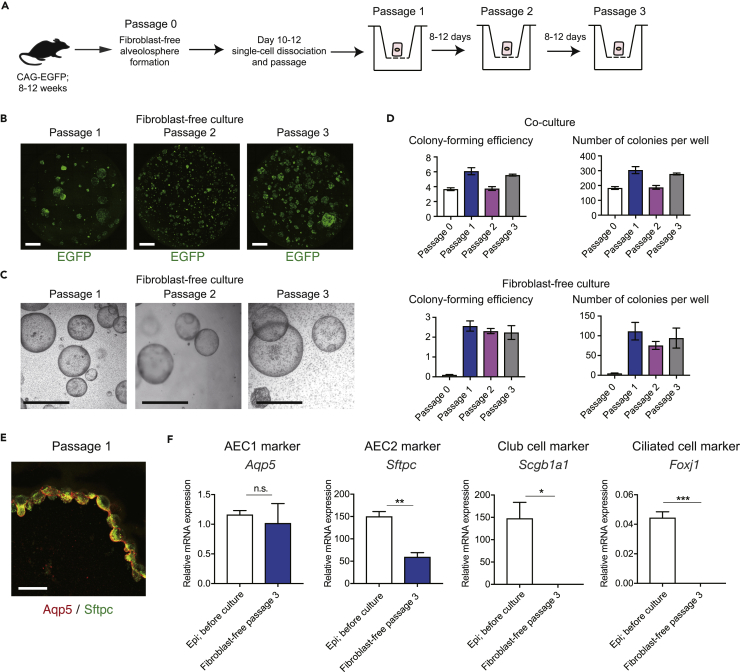
The alveolospheres could be passaged even as single-cell suspensions (Figures 7A–7C). Although the colony-forming efficiency of passaged fibroblast-free alveolospheres was less than that of co-cultured alveolospheres, the efficiency of passaged fibroblast-free alveolospheres was stable for at least three passages (P1, 2.57% ± 0.26%; P2, 2.31% ± 0.13%; and P3, 2.24% ± 0.35%) (Figure 7D). We confirmed Sftpc and Aqp5 expression in the passaged alveolospheres and did not detect any non-alveolar markers (Figures 7E and 7F). These results indicate that a specific molecule and inhibitor combination promotes the clonal proliferation of AEC2 and is thus critical for AEC2.
Figure 7.
Passage of Fibroblast-Free Alveolospheres as Single-Cell Suspensions
(A) Experimental scheme of fibroblast-free alveolosphere passaging. Colonies were dissociated and 2 × 103–5 × 103 cells were plated.
(B) Confocal micrographs of representative wells of passaged spheres (epithelial cells; EGFP; entire Transwell stored in 24-well plates is shown) after 8–12 days.
(C) Bright-field images of representative wells of passaged spheres.
(D) Colony-forming efficiency of fibroblast-free and co-cultured alveolospheres. To calculate colony-forming efficiency, all colonies formed in each well were counted and the number of colonies was divided by 5 × 103 (for primary cultures and for passaging of co-cultured alveolospheres) or 2 × 103–5 × 103 (for passaging of fibroblast-free alveolospheres). Colony-forming efficiency of primary fibroblast-free alveolosphere culture (P0) was 0.1% ± 0.02 (5.0 ± 0.9 spheres per well). Fibroblast-free alveolospheres were passaged at least three times without loss of colony-forming efficiency. For P0, n = 12 wells for fibroblast-free culture and n = 9 wells for co-culture pooled from at least two independent cell sorting; for P1, P2, or P3, n = 3 or 4 wells for fibroblast-free culture and n = 6 wells for co-culture pooled from at least two independent passaging procedures.
(E) Representative section of passaged fibroblast-free alveolosphere (passage 1) on day 8 labeled with antibodies against Aqp5/Sftpc.
(F) qPCR analysis of AEC1, AEC2, and non-alveolar epithelial markers using cells obtained from passaged spheres (passage 3, day 8). Data represent mean ± SEM (n = 3 wells for cultured cells and n = 3 animals for sorted cells) and are representative of two independent experiments. mRNA levels were normalized to that of Actb. *p < 0.05, **p < 0.01, ***p < 0.001 (unpaired Student's t test [two-tailed]).
Scale bar: 1 mm (B and C), 25 μm (E).
Discussion
To clarify fibroblast-AEC2 cell interactions, we performed a time course SAGE-seq analysis and identified time-specific transcriptional features of epithelial cells and fibroblasts that we used to generate a list of possible fibroblast-AEC2 interactions. We also showed that alveolospheres can be generated under mesenchymal cell-free conditions using a specific set of factors that promote alveolosphere growth in epithelial-fibroblast co-cultures and may thus be critical regulators of AEC2 proliferation.
Our transcriptome analysis revealed stage-specific transcriptional signatures during lung development. E13.5 and E15.5 epithelial cells clustered separately from other epithelial cells by unsupervised hierarchical clustering, which is in accordance with a recent proposal to classify lung development into two distinct processes—branching morphogenesis (E9.5–E16.5) and alveolar epithelial differentiation (E16.5–P30) (Chao et al., 2016). Furthermore, our analysis revealed distinct DEG clusters at each developmental stage, including the saccular (E17.5–P5) and alveolar maturation (P5–P28) stages, that differed in terms of GO and KEGG pathway enrichment, implying that each process is governed by distinct molecular mechanisms. For example, the Shh signaling pathway was specifically enriched in fibroblast C4 by KEGG pathway enrichment analysis, and the expression of Shh-related genes was accordingly upregulated at P2, suggesting that this pathway is activated at the saccular stage. Our time course transcriptome data provide a resource for future studies on the mechanisms of lung development.
The results of our epithelial-fibroblast co-culture alveolosphere formation assay showed that Fgf/Wnt signaling stimulated, whereas Tgf-β/Bmp signaling suppressed, alveolosphere growth. Fgf signaling has been shown to play a critical role in lung development (Ornitz and Itoh, 2015); AEC2 obtained from Sftpc-CreERT2:R26R-EYFP mice revealed that Fgf7 promoted alveolosphere growth (Zepp et al., 2017), which is in accordance with our findings that this effect was induced by Fgf2 and Fgf10. Fgf2 was expressed in fibroblasts but not in epithelial cells and presumably acted on fibroblasts themselves. Experiments using Fgf2 knockout mice demonstrated that Fgf2 was required for epithelial repair and maintenance of epithelial integrity following bleomycin injury (Guzy et al., 2015). Our data suggest that this may be due to the suppression of fibroblast activation and proliferation required for alveolar damage repair. Although very important, the reasons accounting for why direct contact of epithelial cells with fibroblasts is required for alveolosphere formation and why direct co-culture drives upregulation of fibroblast ligands, such as Fgf7 and Fgf10, are unclear. Because Tcf7l1 was upregulated in both co-cultured fibroblasts and epithelial cells, it was presumed that different Wnts are expressed in both fibroblasts and epithelial cells and mediate the interaction between fibroblasts and AEC2. Wnts are signals with a typical range of 1–2 cells (Farin et al., 2016), and therefore, the mutual Wnt interactions between epithelial cells and fibroblasts that exist only in direct culture could be one of the reasons why direct co-culture generates alveolospheres and upregulates Fgf7 and Fgf10 in fibroblasts.
Fibroblast-free alveolospheres were generated by adding Tgf-β, Bmp4, and GSK-3β inhibitors and Fgf7 and Notch ligands to the culture medium, indicating that both positive and negative regulation in the form of Fgf/Wnt/Notch and Tgf-β/Bmp signaling, respectively, are required for AEC2 proliferation. Since receptors of both Bmp (Bmpr1a/2) and Tgf-β (Tgfbr1/2) are expressed in epithelial cells and fibroblasts, the results obtained from the co-culture assay are not applicable to the fibroblast-AEC2 interaction. However, we directly demonstrated with the fibroblast-free alveolosphere formation assay that Tgf-β/Bmp4 inhibition was necessary for AEC2 proliferation. Tgf-β maintains bone marrow hematopoietic stem cells in a state of hibernation (Yamazaki et al., 2011), and we presume that Tgf-β family members also act on AEC2 to maintain their quiescence in tissue homeostasis. However, the potential roles of autocrinological and paracrinological effects of Tgf-β/Bmp signaling in the context of the alveolar stem cell niche in vivo remain to be determined. Wnt signaling has been reported to be a critical pathway for AEC2 (Frank et al., 2016, Nabhan et al., 2018, Zacharias et al., 2018). Because GSK-3β inhibition was required for alveolosphere formation, our results suggest that upregulation of Wnt/β-catenin downstream target genes are important for AEC2. Wnt5a has been reported to both activate and repress Wnt/β-catenin signaling (van Amerongen et al., 2012); therefore, further studies are required to clarify the specific Wnt as well as downstream signaling mechanisms that are critical for AEC2. Notch2 signaling in AEC2 was shown to be important for alveologenesis using Notch2cNull mice (Tsao et al., 2016), and we confirmed the importance of Notch signaling in the generation of alveolospheres. Interestingly, Jagged1 is also required for intestinal organoid formation (Sato et al., 2009); thus, Notch signaling may be conserved among endoderm-derived epithelial cells.
To examine AEC2, it is essential to culture them in vitro. However, there has been no report describing the establishment of passaged cell lines that exhibit morphologic and molecular characteristics of AEC2 (Gonzalez and Dobbs, 2013). Epithelial cell-fibroblast co-cultured alveolospheres are passageable (Barkauskas et al., 2017), but the results obtained from co-culture assays cannot be generalized to AEC2 itself. We established a protocol for passageable fibroblast-free alveolosphere generation that will allow researchers to culture AEC2 for further analysis in the future.
In conclusion, the time course transcriptome analysis of epithelial cells and fibroblasts during alveologenesis and in mature lungs revealed dynamic transcriptional profiles. We identified putative regulators of these processes and generated passageable fibroblast-free alveolospheres using a specific combination of fibroblast ligands and signaling pathway inhibitors. Our results provide a resource for future studies on the molecular mechanisms of lung development.
Limitations of the Study
One of the limitations of our study is that bulk epithelial cells were used for co-culture alveolosphere formation assays. Although nearly 90% of Epcam+ epithelial cells were found to be Sftpc+, the Epcam+ population includes airway cells. For this reason, results obtained using bulk Epcam+ epithelial cells may not be generalizable to AEC2. We sought to overcome this limitation by using purified AEC2 (MHCII-positive) for fibroblast-free alveolosphere assays. Further studies using genetically modified mice that allow direct isolation of Sftpc+ AEC2 are needed to clarify this point. In addition, SAGE-seq included few samples for each time point; therefore, the statistical power might have been low for some of the statistical analyses. Specifically, correlation analysis requires more samples for more biologically meaningful analyses. Use of AEC1 markers Aqp5 and Hopx in qPCR analyses might be another limitation. Although the AEC1 markers have long been used to distinguish AEC1 immunohistochemically (Flodby et al., 2010), in some single cell RNA-sequencing studies, weak mRNA expression of AEC1 markers in AEC2 has been reported (Treutlein et al., 2014).
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Shun-ichi Fujita and Shin Aoki for technical assistance, Yutaka Inagaki for providing Col1a2-GFP mice, Atsushi Miyawaki for providing Fucci mice, and the University of Tokyo Graduate Program for Leaders in Life Innovation for their assistance with Ion Proton Sequencing. This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas 17H06392; Grants-in-Aid for Scientific Research (B) 16H05203 and (C) 16K08730; and a Grant-in-Aid for Challenging Research (Exploratory) 17K19546.
Author Contributions
K.S. conceived and performed experiments and wrote the manuscript. S.S. conceived and performed SAGE-seq analysis. T.N. performed cell culture. S.H., S.U., S.Y., and K.M. provided expertise and feedback.
Declaration of Interests
The authors declare no competing interests.
Published: January 25, 2019
Footnotes
Supplemental Information includes Transparent Methods, six figures, and eight tables and can be found with this article online at https://doi.org/10.1016/j.isci.2018.12.022.
Data and Software Availability
Raw data from RNA sequencing have been deposited in the GEO. The GEO accession number for the RNA sequencing data of epithelial cells and fibroblasts from Col1a2-GFP mice reported in this paper is GSE113160 and RNA sequencing data of E13.5, E15.5, P14, and P56 epithelial cells from C57BL/6J mice is GSE109847.
Supplemental Information
References
- Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkauskas C.E., Chung M.-I., Fioret B., Gao X., Katsura H., Hogan B.L.M. Lung organoids: current uses and future promise. Development. 2017;144:986–997. doi: 10.1242/dev.140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkauskas C.E., Cronce M.J., Rackley C.R., Bowie E.J., Keene D.R., Stripp B.R., Randell S.H., Noble P.W., Hogan B.L.M. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao C.-M., Moiseenko A., Zimmer K.-P., Bellusci S. Alveologenesis: key cellular players and fibroblast growth factor 10 signaling. Mol. Cell. Pediatr. 2016;3:17. doi: 10.1186/s40348-016-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Matsumoto K., Brockway B.L., Rackley C.R., Liang J., Lee J.-H., Jiang D., Noble P.W., Randell S.H., Kim C.F. Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells. 2012;30:1948–1960. doi: 10.1002/stem.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Val S., Ponticos M., Antoniv T.T., Wells D.J., Abraham D., Partridge T., Bou-Gharios G. Identification of the key regions within the mouse pro-alpha 2(I) collagen gene far-upstream enhancer. J. Biol. Chem. 2002;277:9286–9292. doi: 10.1074/jbc.M111040200. [DOI] [PubMed] [Google Scholar]
- Farin H.F., Jordens I., Mosa M.H., Basak O., Korving J., Tauriello D.V.F., de Punder K., Angers S., Peters P.J., Maurice M.M. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature. 2016;530:340–343. doi: 10.1038/nature16937. [DOI] [PubMed] [Google Scholar]
- Flodby P., Borok Z., Banfalvi A., Zhou B., Gao D., Minoo P., Ann D.K., Morrisey E.E., Crandall E.D. Directed expression of Cre in alveolar epithelial type 1 cells. Am. J. Respir. Cell Mol. Biol. 2010;43:173–178. doi: 10.1165/rcmb.2009-0226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D.B., Peng T., Zepp J.A., Snitow M., Vincent T.L., Penkala I.J., Cui Z., Herriges M.J., Morley M.P., Zhou S. Emergence of a wave of Wnt signaling that regulates lung alveologenesis by controlling epithelial self-renewal and differentiation. Cell Rep. 2016;17:2312–2325. doi: 10.1016/j.celrep.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R.F., Dobbs L.G. Isolation and culture of alveolar epithelial Type I and Type II cells from rat lungs. Methods Mol. Biol. 2013;945:145–159. doi: 10.1007/978-1-62703-125-7_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzy R.D., Stoilov I., Elton T.J., Mecham R.P., Ornitz D.M. Fibroblast growth factor 2 is required for epithelial recovery, but not for pulmonary fibrosis, in response to bleomycin. Am. J. Respir. Cell Mol. Biol. 2015;52:116–128. doi: 10.1165/rcmb.2014-0184OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K., Sato A., Tanimura K., Uemasu K., Hamakawa Y., Fuseya Y., Sato S., Muro S., Hirai T. Fraction of MHCII and EpCAM expression characterizes distal lung epithelial cells for alveolar type 2 cell isolation. Respir. Res. 2017;18:150. doi: 10.1186/s12931-017-0635-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriges M., Morrisey E.E. Lung development: orchestrating the generation and regeneration of a complex organ. Development. 2014;141:502–513. doi: 10.1242/dev.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B.L.M., Barkauskas C.E., Chapman H.A., Epstein J.A., Jain R., Hsia C.C.W., Niklason L., Calle E., Le A., Randell S.H. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R., Barkauskas C.E., Takeda N., Bowie E.J., Aghajanian H., Wang Q., Padmanabhan A., Manderfield L.J., Gupta M., Li D. Plasticity of Hopx(+) type I alveolar cells to regenerate type II cells in the lung. Nat. Commun. 2015;6:6727. doi: 10.1038/ncomms7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M., Furumichi M., Tanabe M., Sato Y., Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.F.B., Jackson E.L., Woolfenden A.E., Lawrence S., Babar I., Vogel S., Crowley D., Bronson R.T., Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Lee J.-H., Tammela T., Hofree M., Choi J., Marjanovic N.D., Han S., Canner D., Wu K., Paschini M., Bhang D.H. Anatomically and functionally distinct lung mesenchymal populations marked by Lgr5 and Lgr6. Cell. 2017;170:1149–1163.e12. doi: 10.1016/j.cell.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulley D., Wienhold M., Sun X. The pulmonary mesenchyme directs lung development. Curr. Opin. Genet. Dev. 2015;32:98–105. doi: 10.1016/j.gde.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan S.E., Harvey C.S., Jackson S.K. Retinoids, retinoic acid receptors, and cytoplasmic retinoid binding proteins in perinatal rat lung fibroblasts. Am. J. Physiol. 1995;269:L463–L472. doi: 10.1152/ajplung.1995.269.4.L463. [DOI] [PubMed] [Google Scholar]
- McQualter J.L., Bertoncello I. Concise review: Deconstructing the lung to reveal its regenerative potential. Stem Cells. 2012;30:811–816. doi: 10.1002/stem.1055. [DOI] [PubMed] [Google Scholar]
- Nabhan A., Brownfield D.G., Harbury P.B., Krasnow M.A., Desai T.J. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science. 2018;137:eaam6603. doi: 10.1126/science.aam6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz D.M., Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015;4:215–266. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng T., Frank D.B., Kadzik R.S., Morley M.P., Rathi K.S., Wang T., Zhou S., Cheng L., Lu M.M., Morrisey E.E. Hedgehog actively maintains adult lung quiescence and regulates repair and regeneration. Nature. 2015;526:578–582. doi: 10.1038/nature14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov A., Zehorai E., Procaccia S., Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta. 2011;1813:1619–1633. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Sakaue-Sawano A., Kurokawa H., Morimura T., Hanyu A., Hama H., Osawa H., Kashiwagi S., Fukami K., Miyata T., Miyoshi H. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 2008;132:487–498. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]
- Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Schultz C.J., Torres E., Londos C., Torday J.S. Role of adipocyte differentiation-related protein in surfactant phospholipid synthesis by type II cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;283:L288–L296. doi: 10.1152/ajplung.00204.2001. [DOI] [PubMed] [Google Scholar]
- Sharan R., Maron-Katz A., Shamir R. CLICK and EXPANDER: a system for clustering and visualizing gene expression data. Bioinformatics. 2003;19:1787–1799. doi: 10.1093/bioinformatics/btg232. [DOI] [PubMed] [Google Scholar]
- Shy B.R., Wu C.-I., Khramtsova G.F., Zhang J.Y., Olopade O.I., Goss K.H., Merrill B.J. Regulation of Tcf7l1 DNA binding and protein stability as principal mechanisms of Wnt/β-catenin signaling. Cell Rep. 2013;4:1–9. doi: 10.1016/j.celrep.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Nishiyama T., Shimizu K., Kadota K. TCC: an R package for comparing tag count data with robust normalization strategies. BMC Bioinformatics. 2013;14:219. doi: 10.1186/1471-2105-14-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomura M., Sakaue-Sawano A., Mori Y., Takase-Utsugi M., Hata A., Ohtawa K., Kanagawa O., Miyawaki A. Contrasting quiescent G0 phase with mitotic cell cycling in the mouse immune system. PLoS One. 2013;8:e73801. doi: 10.1371/journal.pone.0073801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein B., Brownfield D.G., Wu A.R., Neff N.F., Mantalas G.L., Espinoza F.H., Desai T.J., Krasnow M.A., Quake S.R. Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature. 2014;509:371–375. doi: 10.1038/nature13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao P.-N., Matsuoka C., Wei S.-C., Sato A., Sato S., Hasegawa K., Chen H.-K., Ling T.-Y., Mori M., Cardoso W.V. Epithelial Notch signaling regulates lung alveolar morphogenesis and airway epithelial integrity. Proc. Natl. Acad. Sci. U S A. 2016;113:8242–8247. doi: 10.1073/pnas.1511236113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukui T., Ueha S., Abe J., Hashimoto S.-I., Shichino S., Shimaoka T., Shand F.H.W., Arakawa Y., Oshima K., Hattori M. Qualitative rather than quantitative changes are hallmarks of fibroblasts in bleomycin-induced pulmonary fibrosis. Am. J. Pathol. 2013;183:758–773. doi: 10.1016/j.ajpath.2013.06.005. [DOI] [PubMed] [Google Scholar]
- van Amerongen R., Fuerer C., Mizutani M., Nusse R. Wnt5a can both activate and repress Wnt/β-catenin signaling during mouse embryonic development. Dev. Biol. 2012;369:101–114. doi: 10.1016/j.ydbio.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert T., De Langhe S.P. Wnt and FGF mediated epithelial-mesenchymal crosstalk during lung development. Dev. Dyn. 2015;244:342–366. doi: 10.1002/dvdy.24234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S., Ema H., Karlsson G., Yamaguchi T., Miyoshi H., Shioda S., Taketo M.M., Karlsson S., Iwama A., Nakauchi H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147:1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- Zacharias W.J., Frank D.B., Zepp J.A., Morley M.P., Alkhaleel F.A., Kong J., Zhou S., Cantu E., Morrisey E.E. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;143:3639. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepp J.A., Zacharias W.J., Frank D.B., Cavanaugh C.A., Zhou S., Morley M.P., Morrisey E.E. Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell. 2017;170:1134–1148.e10. doi: 10.1016/j.cell.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Loch A.J., Radtke F., Egan S.E., Xu K. Jagged1 is the major regulator of Notch-dependent cell fate in proximal airways. Dev. Dyn. 2013;242:678–686. doi: 10.1002/dvdy.23965. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.