Abstract
Objective
To evaluate microstructural characteristics of the corpus callosum using diffusion tensor imaging (DTI) and their relationships to cognitive impairment in Parkinson disease (PD).
Methods
Seventy-five participants with PD and 24 healthy control (HC) participants underwent structural MRI brain scans including DTI sequences and clinical and neuropsychological evaluations. Using Movement Disorder Society criteria, PD participants were classified as having normal cognition (PD-NC, n = 23), mild cognitive impairment (PD-MCI, n = 35), or dementia (PDD, n = 17). Cognitive domain (attention/working memory, executive function, language, memory, visuospatial function) z scores were calculated. DTI scalar values, including fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD), were established for 5 callosal segments on a midsagittal plane, single slice using a topographically derived parcellation method. Scalar values were compared among participant groups. Regression analyses were performed on cognitive domain z scores and DTI metrics.
Results
Participants with PD showed increased AD values in the anterior 3 callosal segments compared to healthy controls. Participants with PDD had significantly increased AD, MD, and RD in the anterior 2 segments compared to participants with PD-NC and most anterior segment compared to participants with PD-MCI. FA values did not differ significantly between participants with PD and participants with HC or among PD cognitive groups. The strongest associations for the DTI metrics and cognitive performance occurred in the most anterior and most posterior callosal segments, and also reflected fronto-striatal and posterior cortical type cognitive deficits, respectively.
Conclusions
Microstructural white matter abnormalities of the corpus callosum, as measured by DTI, may contribute to PD cognitive impairment by disrupting information transfer across interhemispheric and callosal–cortical projections.
Cognitive impairment in Parkinson disease (PD) leads to substantial disability, but its neuroanatomic substrates are incompletely understood.1,2 Gray matter atrophy, especially involving cortico-limbic regions, has been identified on brain MRI in patients with PD with dementia (PDD) and mild cognitive impairment (PD-MCI).3–6 Microstructural white matter alterations in cortical, subcortical–cortical, cingular, and callosal pathways have been reported in PD cognitive impairment using diffusion tensor imaging (DTI), though the presence and location of DTI abnormalities vary across studies.7–14 DTI is an MRI technique that can be used to characterize directional properties of the diffusion of water in different tissues, such as brain white matter. Four commonly evaluated DTI scalar values include fractional anisotropy (FA) (a normalized SD of the eigenvalues), mean diffusivity (MD) (a directionally averaged measure), axial diffusivity (AD) (apparent diffusivity in the direction parallel to the underlying tissue tract), and radial diffusivity (RD) (apparent diffusivity perpendicular to the underlying tissue tract).15
The corpus callosum is a critical structure for interhemispheric information transfer and thereby plays an important role in cognitive function.16 In a prior study, we found macrostructural differences in the corpus callosum associated with PD cognitive impairment with greater volume loss in PDD compared to PD-MCI and cognitively normal (PD-NC) participants.17 Furthermore, regional callosal atrophy predicted performance in certain cognitive domains. Building on this work, we sought to examine the microstructural integrity of the corpus callosum across the PD cognitive spectrum, using a region of interest approach based on the topographic patterns of callosal–cortical projections.18 We investigated 4 DTI scalar values (FA, MD, AD, and RD) in the 5 topographically defined callosal segments in PD-NC, PD-MCI, and PDD participants compared to healthy controls (HCs) and evaluated their relationships to performance measures of different cognitive domains.
Methods
Participants
Participants were part of a prospective study of clinical and neuroimaging markers of PD cognitive impairment, and those having both DTI and structural MRI brain scans were included.17,19,20 Of 107 total MRI scans, 8 were excluded due to motion/field of view issues, with final inclusion of 99 participants comprising 75 patients with PD and 24 HC. PD participants were diagnosed by movement disorders specialists using UK PD Brain Bank Criteria.21 Healthy controls were recruited from the community, had normal cognition and neurologic examinations, and had Mini-Mental State Examination (MMSE) scores ≥28. Detailed inclusion and exclusion criteria for the PD and HC participants have been described previously.17
Standard protocol approvals, registrations, and patient consents
Participants gave informed consent, and the study protocol was approved by the Rush University Institutional Review Board.
Clinical and cognitive evaluations
Clinical evaluations assessed demographics and medications, and disease-related features for participants with PD, including levodopa daily dose equivalents,22 proportion on levodopa or dopamine agonists, Hoehn & Yahr stage,23 proportion with motor fluctuations and dyskinesias, and motor function with the Movement Disorder Society–Unified Parkinson's Disease Rating Scale (MDS-UPDRS)23 in the “on” state. Neuropsychological assessments included the MMSE and a battery of tests grouped into 5 cognitive domains (attention/working memory, executive function, language, memory, and visuospatial domains) according to Movement Disorder Society (MDS) Task Force recommendations and previously described methodology (table 1).19,20,24 Raw scores of neuropsychological tests in each cognitive domain were transformed into z scores based on normative data from HCs at our center.25 z Scores based on local normative samples were utilized in order to be cognizant of local norms and consistent with our prior studies. By transforming the scores into z scores, a normal distribution was necessarily created. Participants with PD were classified into cognitive groups (PD-NC, PD-MCI, PDD) according to MDS Task Force criteria for PD-MCI and PDD and established cognitive classification methodology.26,27 Participants with PD without dementia who did not fulfill MDS PD-MCI criteria were classified as PD-NC.
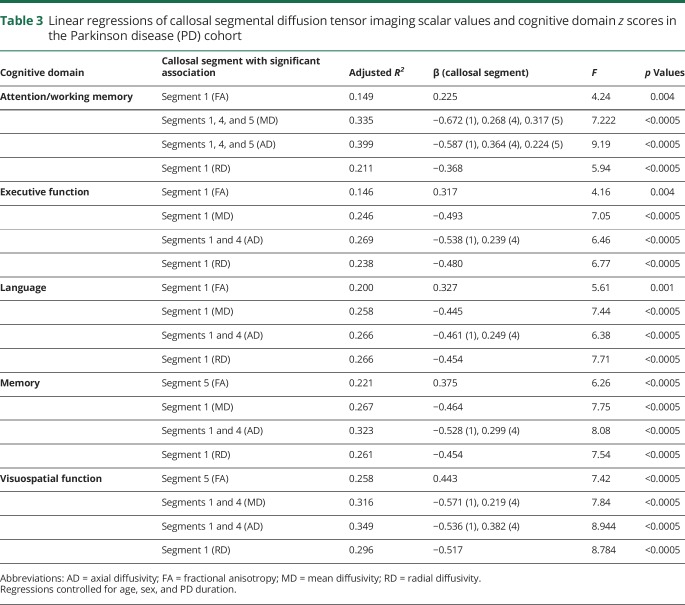
Table 1.
Cognitive domains and neuropsychological tests
MRI acquisition and processing
Participants underwent MRI brain scans at Rush University Medical Center using a 1.5T General Electric (Fairfield, CT) Signa scanner including DTI and T1-weighted sequences. T1-weighted fast spoiled gradient echo sequence or magnetization-prepared rapid gradient-echo sequences were obtained with previously described parameters.17 DTI sequences included diffusion-weighted, single shot, echoplanar imaging with the following parameters: 38 slices, repetition time 12,100 ms, echo time 97 ms, field of view 25 cm, matrix 128 × 128, slice thickness 3 mm, zero gap. DTI sequences were acquired with 6 repetitions in the anterior–posterior commissural plane and using 2 degrees of diffusion weighting (b values = 0 s/mm2 and 800 s/mm2). Diffusion weights were applied in 26 noncollinear directions with 2 repetitions of b = 0 and 2 repetitions of each diffusion-weighted image. Raw diffusion-weighted images were aligned to the corresponding nonweighted image (B0) and corrected for eddy currents and motion artifacts using the FSL software package (fmrib.ox.ac.uk/fsl).28 A diffusion tensor model was fit for each diffusion-weighted image. FA, MD, AD, and RD values were obtained from each DTI image with FSL. Using FMRIB’s nonlinear image registration tool (FNIRT)29 within FSL, all FA, MD, AD, and RD images were nonlinearly registered to the mean FA template derived from the Illinois Institute of Technology (IIT) Human Brain Atlas, a high-quality standardized atlas in 256 × 256 × 256 space.30
Masks for region of interest (ROI)–based analyses were created from the IIT Human Brain Atlas. The principal diffusion direction volume was derived from the IIT Human Brain Atlas mean tensor volume using the DTI-TK software package (dti-tk.sourceforge.net). ROI masks were drawn overlying the principal diffusion direction volume using ITK-SNAP (itksnap.org). The corpus callosum was identified on a midsagittal plane, single slice based on the principal direction of diffusion. Only voxels with a transverse principal direction of diffusion were included in the mask in order to minimize inclusion of noncallosal regions within the ROI.
Five segments of the corpus callosum (figure 1) were established according to the callosal–cortical topographical parcellation scheme of Hofer and Frahm,18 which was used because of its derivation of callosal boundaries from human studies. In this parcellation scheme, the cortical projections of the 5 segments as delineated by Hofer and Frahm18 using DTI-based tractography are as follows: segment 1 (most anterior), prefrontal cortex; segment 2, premotor and supplementary motor cortex; segment 3, motor cortex; segment 4, sensory cortex; segment 5 (most posterior), parietal, temporal, and occipital cortex. Figure 1 depicts the geometric boundaries of each segment. Because boundaries of the callosal segments as outlined by Hofer and Frahm are defined only at the midsagittal plane, we focused our analyses to this region. Separate ROI masks were drawn as follows: (1) the inferior axis of the corpus callosum was established by drawing a line at the corpus callosum base connecting the most anterior–inferior and posterior–inferior callosal points; (2) lines orthogonal to the inferior axis were established to divide the corpus callosum into 5 segments defined by Hofer and Frahm; and (3) separate ROI masks were established for each of the 5 segments (figure 1). A visual inspection of ROI masks overlaid against normalized FA, MD, AD, and RD images for each scan was performed blinded to diagnosis and confirmed good fit within the corpus callosum for all scans. Mean FA, MD, AD, and RD values for each callosal segment were calculated using FSL for each participant.
Figure 1. Hofer and Frahm corpus callosal segmentation scheme and corpus callosum segment region of interest (ROI) masks.
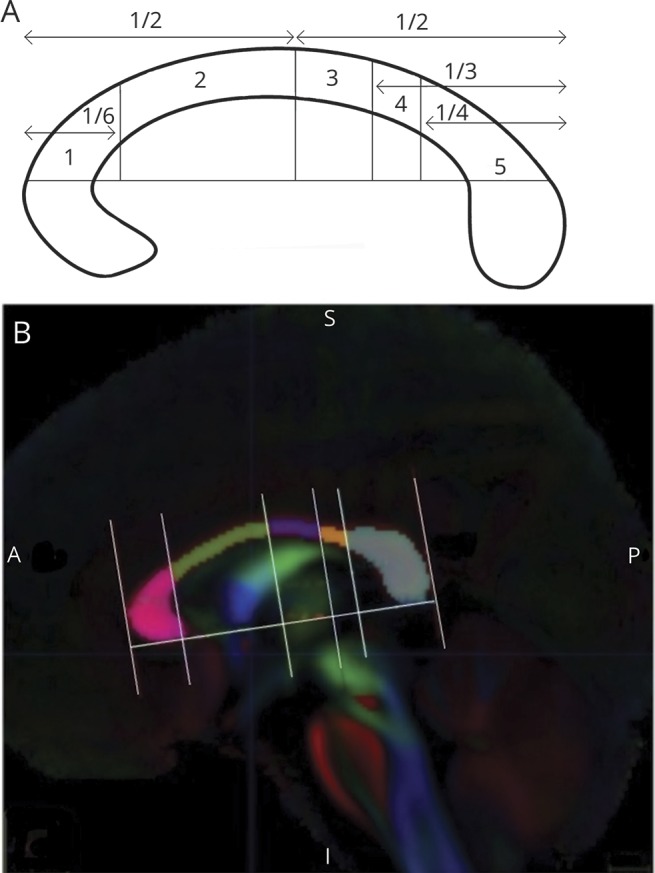
(A) Hofer and Frahm corpus callosal segmentation scheme. (B) Corpus callosum segment ROI masks.
Statistical analyses
Clinical characteristics
Clinical features were compared across groups (PD-NC, PD-MCI, PDD, HC) using analyses of variance for years of education, MMSE scores, and cognitive domain z scores, and among PD groups, for years of disease, levodopa daily dose equivalents, and MDS-UPDRS motor scores; post hoc comparisons were adjusted for multiple comparisons using Scheffé corrections to permit comparisons of each group with all other groups. For all statistical analyses in the study, the nominal significance level was set at p < 0.05. Nonparametric tests were used for comparisons across all groups for sex (χ2) and among PD groups for the proportions of patients on dopamine agonists, with dyskinesias, or with motor fluctuations (χ2) and Hoehn & Yahr staging (Kruskal-Wallis, with post hoc tests using Mann-Whitney U controlling for multiple comparisons).
Callosal DTI metrics
Mean FA, MD, AD, and RD values for each of the 5 callosal segments were compared between the entire PD cohort and HC participants using multivariate analyses of covariance (MANCOVA) covarying for age and sex. If a main effect of group was present, post hoc univariate analyses of covariance (ANCOVA) were used to evaluate between-group differences, covarying for age and sex. Mean FA, MD, AD, and RD values for each of the 5 callosal segments also were compared between PD-NC and HC participants using MANCOVA and covarying for age and sex, in order to evaluate whether differences in scalar values related to factors other than cognition. If a main effect of group was present, post hoc univariate ANCOVA were used to evaluate between-group differences, covarying for age and sex. Finally, mean FA, MD, AD, and RD values for the 5 callosal segments were compared across the PD cognitive groups using MANCOVA and covarying for age, sex, and PD duration. If a main effect of group occurred, post hoc ANCOVA were used for groupwise comparisons, covarying for age, sex, and PD duration, and with Bonferroni corrections for post hoc comparisons.
Relationships between callosal DTI metrics and cognitive domains in PD
Linear regression analyses were performed to investigate relationships between cognitive domain z scores and callosal segment DTI values. Separate linear regression models were completed for each cognitive domain z score (attention/working memory, executive function, language, memory, and visuospatial function) with each DTI metric (FA, MD, AD, RD). For each regression model, the dependent variable was the cognitive domain z score with age, sex, and PD duration as force-entered covariates in the first block and DTI measures (mean FA, MD, AD, or RD values, each run independently) of the 5 callosal segments as stepwise-entered independent variables in the second block.
Data availability
Anonymized data will be shared at the request of qualified investigators.
Results
Participant characteristics
Seventy-five participants with PD (PD-NC, n = 23; PD-MCI, n = 35; PDD, n = 17) and 24 HCs were included (table 2). There were no significant differences in age or sex. The overall model comparing education years among the 4 groups was significant (F3,95 = 3.070, p = 0.032), but the post hoc pairwise comparisons were not significant. Comparison of education years between the HC and all participants with PD also was not significant (t[97] = 1.54, p = 0.13). Disease duration significantly differed among PD groups (F2,71 = 5.305, p = 0.007) with longer durations in PDD compared to PD-NC and PD-MCI participants (post hoc comparison: p = 0.034 and 0.01, respectively). Hoehn & Yahr staging and MDS-UPDRS motor scores significantly differed across groups, with worse performance in PDD compared to PD-MCI and PD-NC participants, and in PD-MCI compared to PD-NC participants. There were no differences among PD groups in the presence of motor fluctuations, dyskinesias, or treatment with levodopa. While a smaller percentage of PDD and PD-MCI participants were on dopamine agonists compared to PD-NC participants, this difference was not statistically significant. As expected, MMSE scores were significantly different among groups (F3,95 = 43.87, p < 0.0005), with worse scores in those with PDD compared to PD-MCI, PD-NC, and HC participants (all p < 0.0005) and in those with PD-MCI compared to HC (p = 0.016); there were no significant differences between PD-NC and HC participants (p = 0.073). Cognitive domain z scores differed significantly across all groups (p < 0.0005) with greatest impairment in PDD compared to all other groups (p < 0.0005). PD-MCI participants performed worse cognitively compared to PD-NC (p < 0.01) and HC (all p < 0.0005 except visuospatial domain [p = 0.004]). There were no significant differences in cognitive domain z scores between PD-NC and HC participants.
Table 2.
Demographic features of the study cohort
DTI metrics: Comparisons between PD vs HC, PD-NC vs HC, and across PD cognitive groups
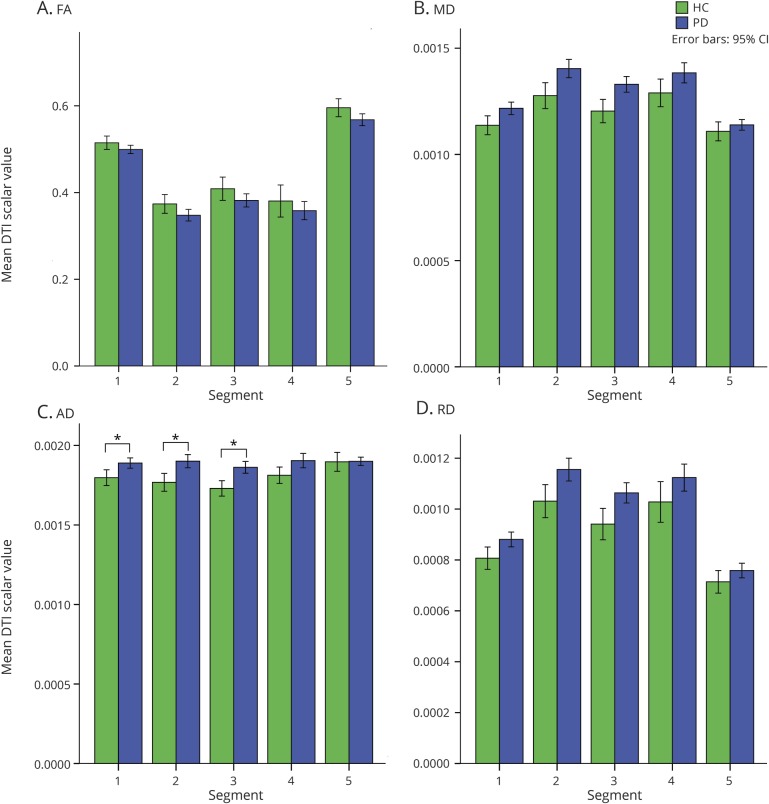
In the MANCOVA comparing FA, MD, AD, and RD values of the callosal segments between the entire PD cohort and HC, covarying for age and sex, only AD was significantly different in multivariate tests (F5,91 = 2.45, p = 0.039) (figure 2). Post hoc univariate tests revealed significant differences in AD values in callosal segment 1 (F3,95 = 12.73, p < 0.0005), segment 2 (F3,95 = 6.25, p = 0.001), and segment 3 (F3,95 = 7.93, p < 0.0005). Group pairwise comparisons demonstrated increased AD values in PD compared to HC (segment 1, p = 0.033; segment 2, p = 0.006; segment 3, p = 0.002). There were no significant differences between PD and HC in mean FA, MD, or RD values for the callosal segments.
Figure 2. Comparison of diffusion tensor imaging (DTI) scalar values between Parkinson disease (PD) (all cognitive groups combined) and healthy control (HC) participants.
(A) Fractional anisotropy (FA). (B) Mean diffusivity (MD). (C) Axial diffusivity (AD). (D) Radial diffusivity (RD). CI = confidence interval.
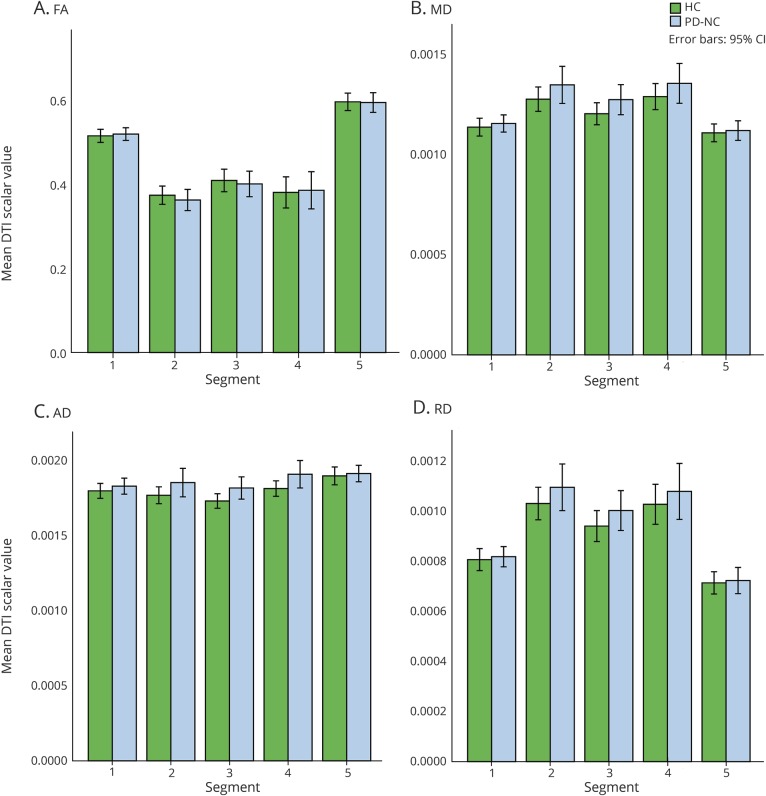
In MANCOVA comparing mean FA, MD, AD, and RD values of the callosal segments between PD-NC and HC, controlling for age and sex, multivariate tests demonstrated no significant differences in any of the scalar values: FA (F5,39 = 0.17, p = 0.97), MD (F5,39 = 0.83, p = 0.54), AD (F5,39 = 1.65, p = 0.17), and RD (F5,39 = 0.51, p = 0.77) (figure 3).
Figure 3. Comparison of diffusion tensor imaging (DTI) scalar values between Parkinson disease normal cognition (PD-NC) and healthy control participants.
(A) Fractional anisotropy (FA). (B) Mean diffusivity (MD). (C) Axial diffusivity (AD). (D) Radial diffusivity (RD). CI = confidence interval.
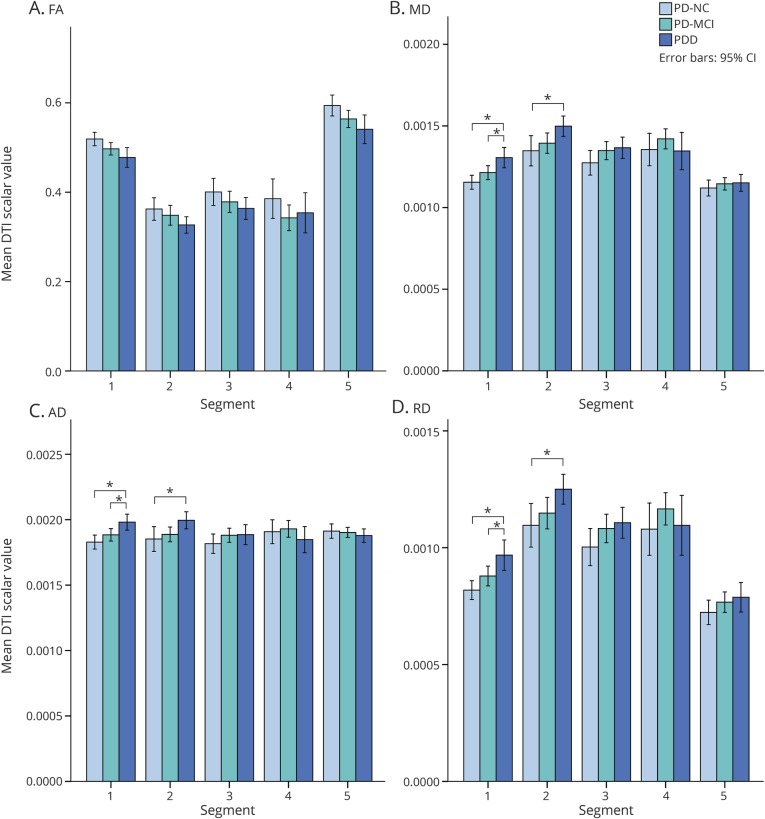
In the MANCOVA comparing mean FA, MD, AD, and RD values of the callosal segments across the PD cognitive groups (i.e., PD-NC, PD-MCI, and PDD), controlling for age, sex, and PD duration, multivariate tests demonstrated significant differences in the values of diffusivity measures: MD (F10,128 = 3.39, p = 0.001), AD (F10,128 = 3.66, p < 0.0005), and RD (F10,128 = 2.70, p = 0.005), but not in FA (figure 4). Post hoc ANCOVA revealed significant group differences in MD, AD, and RD values for segment 1 (F5,69 = 9.67, p < 0.0005; F5,69 = 8.03, p < 0.0005; and F5,69 = 9.36, p < 0.0005, respectively) and segment 2 (F5,69 = 3.73, p = 0.005; F5,69 = 3.05, p = 0.015; and F5,69 = 3.89, p = 0.004, respectively). Group pairwise comparisons showed increased MD, AD, and RD values for PDD compared to PD-NC participants in segment 1 (all p < 0.0005) and segment 2 (p = 0.030, p = 0.033, and p = 0.035, respectively) and increased MD, AD, and RD values for PDD compared to PD-MCI participants in segment 1 (p = 0.014, p = 0.018, and p = 0.020, respectively).
Figure 4. Comparison of diffusion tensor imaging (DTI) scalar values among Parkinson disease cognitive groups.
(A) Fractional anisotropy (FA). (B) Mean diffusivity (MD). (C) Axial diffusivity (AD). (D) Radial diffusivity (RD). CI = confidence interval; PD-MCI = Parkinson disease with mild cognitive impairment; PD-NC = Parkinson disease, cognitively normal; PDD = Parkinson disease with dementia.
Relationships between callosal DTI metrics and cognitive domains in PD
For the PD cognitive groups, regression models, controlling for age, sex, and PD duration as forced-entry variables, showed significant associations among callosal segment FA, MD, AD, and RD values and cognitive domain z scores (table 3). FA values for the callosal segments were significantly associated with attention/working memory, executive function, and language domain z scores in segment 1 (p < 0.0005 for all, except for attention/working memory, p = 0.004), and with memory and visuospatial function domain z scores in segment 5 (p < 0.0005). MD values were significantly related to attention/working memory domain z scores in callosal segments 1, 4, and 5; memory, executive function, and language domain z scores in segment 1; and visuospatial function domain z scores in segments 1 and 4 (all p < 0.0005). AD values were significantly associated with attention/working memory domain z scores in callosal segments 1, 4, and 5, and memory, executive function, language, and visuospatial function domain z scores in segments 1 and 4 (all p < 0.0005). RD values were significantly associated with all cognitive domain z scores in segment 1 only (p < 0.0005).
Table 3.
Linear regressions of callosal segmental diffusion tensor imaging scalar values and cognitive domain z scores in the Parkinson disease (PD) cohort
Discussion
Our study revealed increased measures of diffusivity, particularly in the anterior callosal segments, with (1) greater AD values in PD compared to HC and (2) increased AD, MD, and RD in the most cognitively impaired PD group (i.e., PDD), compared to PD-NC and to PD-MCI participants, but (3) no differences in DTI scalar values in those PD with normal cognition compared to HC. These findings suggest that there are abnormalities in the callosal white matter that may be specifically linked to the presence of cognitive impairment in PD. Furthermore, we found significant associations between DTI measures and performance in the different cognitive domains in PD. Our findings uphold classic brain–behavior relationships including the anterior–posterior dissociations that are particularly relevant in understanding cognitive deficits in PD.31,32 These are supported by our findings of a predominance of anteriorly located DTI abnormalities associated with deficits in attention/working memory and executive function cognitive domains and of posteriorly located DTI abnormalities with memory, visuospatial, and executive function cognitive domains.
Increased AD within the corpus callosum has been demonstrated in other neurodegenerative diseases, including Alzheimer disease,33 Huntington disease (HD),34 and amyotrophic lateral sclerosis35 and in areas of white matter atrophy in HD34 and Friedreich ataxia.36 In patients with PD, areas of increased AD have been demonstrated in the substantia nigra37 and right hemispheric frontal white matter projections in patients with PD with cognitive impairment.38 One longitudinal study showed a faster rate of increase in AD metrics in several brain regions in PD compared to HC.39 On the other hand, areas of reduced AD were noted in the body and splenium of the corpus callosum in a smaller cohort (n = 22) of participants with PD in a tract-based spatial statistics (TBSS)40 study specifically excluding participants with PD with notable cognitive impairment.41 Reduced AD also has been associated with white matter axonal injury in animal models,15,42 but the biophysical substrates underlying these alterations remain unresolved as other human disease studies report both increases and decreases.34,36 Some studies suggest that atrophy may lead to increased extra-axonal space and smaller axonal caliber, potentially mediating greater water diffusion parallel to axons, resulting in increased AD.34,36 Of interest, the callosal regions revealing increased AD in the current study overlap with the callosal areas in which we previously found macrostructural alterations, with participants with PD demonstrating volume loss in the anterior 2/5th–3/5th of the corpus callosum.17 Callosal atrophy may contribute to altered AD, though actual microstructural tissue pathology cannot be inferred from imaging differences alone.43
Among the PD cognitive groups, we found not only increased AD, but also increased values in other diffusivity measures (i.e., RD, MD) in the anterior corpus callosum as well as in relation to the severity of PD cognitive impairment. Increased diffusivity occurred to a greater extent in those with PDD compared to those with PD-NC in the anterior half of the corpus callosum, but also in those with PDD compared to those with PD-MCI in the anterior 1/6th of the corpus callosum. These DTI differences potentially may reflect abnormalities in myelination. For example, demyelination increased RD in a mouse model, potentially indicating less restricted water movement in directions perpendicular to underlying fiber tracts with myelin sheath breakdown.44 However, RD may also be affected by axonal loss43 or differences in axonal packing density or diameter.45 MD is considered to be inversely related to membrane density and sensitive to cellularity, edema, or necrosis.15 Thin, unmyelinated axons appear to be selectively vulnerable to developing abnormal proteinaceous aggregations characteristic of PD46 and interestingly, the anterior corpus callosum has the highest concentration of small, unmyelinated axons in the structure.47 The anterior-predominant DTI differences occurring in PD may potentially reflect this preferential distribution of thin, unmyelinated axons in the corpus callosum.
We found relationships between DTI scalar values and cognitive domain performance in several callosal segments in participants with PD. Moreover, these relationships support proposed brain–behavior hypotheses of anterior–posterior dissociations and PD cognitive dysfunction.31 The most anterior callosal region (segment 1) demonstrated the strongest association with DTI values and cognitive domains: AD, RD, and MD values in this region were associated with all 5 cognitive domain scores, while FA values in segment 1 were associated with attention/working memory, executive function, and language performance. Given anterior callosal–prefrontal cortical connections, these alterations in microstructural integrity may affect information transfer to prefrontal regions and thereby play a role in the “frontal–striatal” cognitive deficits of PD. In addition, we found significant associations between FA values in the posterior callosal region (segment 5) and memory and visuospatial cognitive domains. Given connections of this most posterior callosal region to temporal, parietal, and occipital cortices, altered FA in callosal segment 5 may contribute to the “posterior cortical type” deficits recognized in PD cognitive impairment. The finding of significant relationships among DTI scalar values and cognitive domains in callosal areas in which groupwise differences were not detected highlights the multifaceted and complex nature of these relationships and need for studies with larger sample sizes of cognitive groups.
While DTI studies that include the corpus callosum have been conducted, our study differs in several methodologic aspects from others reported in the literature. One notable difference is our use of a topographically defined parcellation scheme and ROI-based approach to focus solely on the corpus callosum, examining callosal segments and their neuroanatomically defined projections.18 Other prior PD studies have documented DTI abnormalities in the corpus callosum amidst other white matter regions using whole brain TBSS approaches, and many have examined FA or MD only,7–10 though others included evaluations of AD and RD.38,41 Additionally examining AD and RD helps expand our understanding of diffusion tensor models including comparisons of the role of diffusivity rates in different directions (MD, AD, RD) vs the directional preference of diffusion (FA). One study examined FA, MD, AD, and RD in the corpus callosum in a parkinsonian cohort (n = 18), but the study was small, with mixed parkinsonian diagnoses, and did not evaluate cognitive status.13 Some studies have identified significant differences in FA and MD between PD-MCI and PD-NC participants, but we did not.7,8,10,11 We did find lower average FA and higher average MD values (nonsignificant) in PD-MCI compared to PD-NC participants in all callosal segments, but significant increases in MD were seen only in the most cognitively impaired participants with PD (PDD) in the anterior callosal segments. Methodologic differences such as our focus on the corpus callosum and its segments rather than all white matter tracts, an ROI approach rather than whole brain assessment, application of different definitions of PD cognitive impairment, and differing participant selection may explain different study results. Our finding of increased AD in the corpus callosum in participants with PD, in distinction from the finding of decreased callosal AD values of Georgiopoulos et al.,41 may reflect both differences in methodology and cognitive status of the PD cohorts, as the latter study specifically excluded patients with PD with MMSE scores <25. Our finding of no difference in DTI scalar values between PD-NC and HC further suggests that the differences in DTI scalar values seen in our study are specific for cognitive impairment in the context of PD, as these differences were notably most pronounced in the PD group with greatest cognitive impairment even when controlling for potential confounding variables such as age and disease duration.
Strengths of our study include a large cohort of patients with PD diagnosed by movement disorders experts, representing a breadth of cognitive abilities, and established using well-defined cognitive criteria. Evaluation of the corpus callosum in the midsagittal plane offers the advantage of being one of the few areas in the brain with less concern regarding effects of crossing or divergent white matter fibers on DTI measures, given a relatively homogeneous and uniformly aligned population of white matter fibers.15 We also utilized an ROI-based imaging evaluation, which can offer greater regional specificity. In this method, we specifically constructed ROI masks based on the principal diffusion direction and included only areas with clear left–right orientation of underlying fibers so as to minimize the effects of partial volume averaging, particularly at borders of tissue types. We acknowledge potential study limitations such that the midsagittal plane provides one view of the corpus callosum, and findings may differ in callosal areas outside this most midline, central part. Currently, these neuroimaging analyses represent research tools, though they may gain greater clinical application in the future. Future histopathologic studies of the corpus callosum may help elucidate the underlying microstructural nature of the neuroimaging DTI differences, and tractography imaging analyses may offer insights regarding callosal–cortical connectivity. Another critical evaluation for future studies is how abnormalities in the corpus callosum fit into the broader context of white matter alterations in PD cognitive impairment, including whether its changes occur relatively earlier or later compared to alterations in other white matter structures and whether the corpus callosum is preferentially affected. Longitudinal studies tracking DTI changes in the corpus callosum over time in the same individuals will allow greater understanding of the role of white matter microstructure in PD cognitive impairment.
Glossary
- AD
axial diffusivity
- ANCOVA
analyses of covariance
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- HC
healthy control
- HD
Huntington disease
- IIT
Illinois Institute of Technology
- MANCOVA
multivariate analyses of covariance
- MD
mean diffusivity
- MDS
Movement Disorder Society
- MDS-UPDRS
Movement Disorder Society–Unified Parkinson's Disease Rating Scale
- MMSE
Mini-Mental State Examination
- PD
Parkinson disease
- PD-MCI
Parkinson disease with mild cognitive impairment
- PD-NC
Parkinson disease with normal cognition
- PDD
Parkinson disease with dementia
- RD
radial diffusivity
- ROI
region of interest
- TBSS
tract-based spatial statistics
Footnotes
CME Course: NPub.org/cmelist
Author contributions
I.O. Bledsoe: design/conceptualization of the study, analysis/interpretation of the data, drafting/revising the manuscript for intellectual content. G.T. Stebbins: design/conceptualization of the study, analysis/interpretation of the data, drafting/revising the manuscript for intellectual content. D. Merkitch: analysis/interpretation of the data, drafting/revising the manuscript for intellectual content. J.G. Goldman: design/conceptualization of the study, analysis/interpretation of the data, drafting/revising the manuscript for intellectual content.
Study funding
Dr. Goldman and this research were supported by NIH K23NS060949 and the Parkinson's Disease Foundation. The Rush University Section of Parkinson Disease and Movement Disorders is supported by a center grant from the Parkinson's Disease Foundation.
Disclosure
I. Bledsoe reports no disclosures relevant to the manuscript. G. Stebbins has received grant/research support from NIH, Michael J. Fox Foundation, Dystonia Coalition, CHDI, and International Parkinson and Movement Disorder Society; consulting fees from Acadia Pharmaceuticals, Adamas Pharmaceuticals, Inc., Biogen, Ceregene, Inc., CHDI Management, Inc., Ingenix Pharmaceutical Services (i3 Research), Neurocrine Biosciences, Inc., Pfizer, Inc., and Tool4Patients; and honoraria from the International Parkinson and Movement Disorder Society, American Academy of Neurology, and Michael J. Fox Foundation. D. Merkitch reports no disclosures relevant to the manuscript. J. Goldman has received grant/research support from NIH, Michael J. Fox Foundation, Parkinson Foundation, CHDI, Rush University, Acadia, and Biotie/Accorda (site-PI); consulting fees from Acadia and Aptinyx; and honoraria from the International Parkinson and Movement Disorder Society and American Academy of Neurology. Go to Neurology.org/N for full disclosures.
Publication history
Received by Neurology April 2, 2018. Accepted in final form August 23, 2018.
References
- 1.Leroi I, McDonald K, Pantula H, Harbishettar V. Cognitive impairment in Parkinson disease: impact on quality of life, disability, and caregiver burden. J Geriatr Psychiatry Neurol 2012;25:208–214. [DOI] [PubMed] [Google Scholar]
- 2.Aarsland D, Creese B, Politis M, et al. Cognitive decline in Parkinson disease. Nat Rev Neurol 2017;13:217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duncan GW, Firbank MJ, O'Brien JT, Burn DJ. Magnetic resonance imaging: a biomarker for cognitive impairment in Parkinson's disease? Mov Disord 2013;28:425–438. [DOI] [PubMed] [Google Scholar]
- 4.Pan PL, Shi HC, Zhong JG, et al. Gray matter atrophy in Parkinson's disease with dementia: evidence from meta-analysis of voxel-based morphometry studies. Neurol Sci 2013;34:613–619. [DOI] [PubMed] [Google Scholar]
- 5.Melzer TR, Watts R, MacAskill MR, et al. Grey matter atrophy in cognitively impaired Parkinson's disease. J Neurol Neurosurg Psychiatry 2012;83:188–194. [DOI] [PubMed] [Google Scholar]
- 6.Duncan GW, Firbank MJ, Yarnall AJ, et al. Gray and white matter imaging: a biomarker for cognitive impairment in early Parkinson's disease? Mov Disord 2016;31:103–110. [DOI] [PubMed] [Google Scholar]
- 7.Melzer TR, Watts R, MacAskill MR, et al. White matter microstructure deteriorates across cognitive stages in Parkinson disease. Neurology 2013;80:1841–1849. [DOI] [PubMed] [Google Scholar]
- 8.Agosta F, Canu E, Stefanova E, et al. Mild cognitive impairment in Parkinson's disease is associated with a distributed pattern of brain white matter damage. Hum Brain Mapp 2014;35:1921–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hattori T, Orimo S, Aoki S, et al. Cognitive status correlates with white matter alteration in Parkinson's disease. Hum Brain Mapp 2012;33:727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Z, Shemmassian S, Wijekoon C, Kim W, Bookheimer SY, Pouratian N. DTI correlates of distinct cognitive impairments in Parkinson's disease. Hum Brain Mapp 2014;35:1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiltshire K, Concha L, Gee M, Bouchard T, Beaulieu C, Camicioli R. Corpus callosum and cingulum tractography in Parkinson's disease. Can J Neurol Sci 2010;37:595–600. [DOI] [PubMed] [Google Scholar]
- 12.Chen B, Fan GG, Liu H, Wang S. Changes in anatomical and functional connectivity of Parkinson's disease patients according to cognitive status. Eur J Radiol 2015;84:1318–1324. [DOI] [PubMed] [Google Scholar]
- 13.Worker A, Blain C, Jarosz J, et al. Diffusion tensor imaging of Parkinson's disease, multiple system atrophy and progressive supranuclear palsy: a tract-based spatial statistics study. PLoS One 2014;9:e112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamagata K, Motoi Y, Tomiyama H, et al. Relationship between cognitive impairment and white-matter alteration in Parkinson's disease with dementia: tract-based spatial statistics and tract-specific analysis. Eur Radiol 2013;23:1946–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexander AL, Hurley SA, Samsonov AA, et al. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connect 2011;1:423–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doron KW, Gazzaniga MS. Neuroimaging techniques offer new perspectives on callosal transfer and interhemispheric communication. Cortex 2008;44:1023–1029. [DOI] [PubMed] [Google Scholar]
- 17.Goldman JG, Bledsoe IO, Merkitch D, Dinh V, Bernard B, Stebbins GT. Corpus callosal atrophy and associations with cognitive impairment in Parkinson disease. Neurology 2017;88:1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofer S, Frahm J. Topography of the human corpus callosum revisited: comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 2006;32:989–994. [DOI] [PubMed] [Google Scholar]
- 19.Goldman JG, Stebbins GT, Dinh V, et al. Visuoperceptive region atrophy independent of cognitive status in patients with Parkinson's disease with hallucinations. Brain 2014;137:849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldman JG, Holden S, Bernard B, Ouyang B, Goetz CG, Stebbins GT. Defining optimal cutoff scores for cognitive impairment using Movement Disorder Society Task Force criteria for mild cognitive impairment in Parkinson's disease. Mov Disord 2013;28:1972–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson's disease. Arch Neurol 1993;50:140–148. [DOI] [PubMed] [Google Scholar]
- 22.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–2653. [DOI] [PubMed] [Google Scholar]
- 23.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society–sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 24.Goldman JG, Holden S, Ouyang B, Bernard B, Goetz CG, Stebbins GT. Diagnosing PD-MCI by MDS Task Force criteria: how many and which neuropsychological tests? Mov Disord 2015;30:402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology 2005;25:163–175. [DOI] [PubMed] [Google Scholar]
- 26.Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson's disease dementia: recommendations from the Movement Disorder Society Task Force. Mov Disord 2007;22:2314–2324. [DOI] [PubMed] [Google Scholar]
- 27.Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord 2012;27:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage 2012;62:782–790. [DOI] [PubMed] [Google Scholar]
- 29.Andersson JLR, Jenkinson M, Smith S. Non-Linear Registration Aka Spatial Normalisation: FMRIB Technical Report TR07JA2. Oxford: FMRIB Centre; 2007. [Google Scholar]
- 30.Zhang S, Arfanakis K. Role of standardized and study-specific human brain diffusion tensor templates in inter-subject spatial normalization. J Magn Reson Imaging 2013;37:372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams-Gray CH, Mason SL, Evans JR, et al. The CamPaIGN study of Parkinson's disease: 10-year outlook in an incident population-based cohort. J Neurol Neurosurg Psychiatry 2013;84:1258–1264. [DOI] [PubMed] [Google Scholar]
- 32.Kulisevsky J, Pagonabarraga J. Cognitive impairment in Parkinson's disease: tools for diagnosis and assessment. Mov Disord 2009;24:1103–1110. [DOI] [PubMed] [Google Scholar]
- 33.Salat DH, Tuch DS, van der Kouwe AJ, et al. White matter pathology isolates the hippocampal formation in Alzheimer's disease. Neurobiol Aging 2010;31:244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosas HD, Lee SY, Bender AC, et al. Altered white matter microstructure in the corpus callosum in Huntington's disease: implications for cortical “disconnection”. NeuroImage 2010;49:2995–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metwalli NS, Benatar M, Nair G, Usher S, Hu X, Carew JD. Utility of axial and radial diffusivity from diffusion tensor MRI as markers of neurodegeneration in amyotrophic lateral sclerosis. Brain Res 2010;1348:156–164. [DOI] [PubMed] [Google Scholar]
- 36.Della Nave R, Ginestroni A, Diciotti S, Salvatore E, Soricelli A, Mascalchi M. Axial diffusivity is increased in the degenerating superior cerebellar peduncles of Friedreich's ataxia. Neuroradiology 2011;53:367–372. [DOI] [PubMed] [Google Scholar]
- 37.Schuff N, Wu IW, Buckley S, et al. Diffusion imaging of nigral alterations in early Parkinson's disease with dopaminergic deficits. Mov Disord 2015;30:1885–1892. [DOI] [PubMed] [Google Scholar]
- 38.Theilmann RJ, Reed JD, Song DD, et al. White-matter changes correlate with cognitive functioning in Parkinson's disease. Front Neurol 2013;4:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Wu IW, Tosun D, Foster E, Schuff N, Parkinson's Progression Markers I. Progression of regional microstructural degeneration in Parkinson's disease: a multicenter diffusion tensor imaging study. PLoS One 2016;11:e0165540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006;31:1487–1505. [DOI] [PubMed] [Google Scholar]
- 41.Georgiopoulos C, Warntjes M, Dizdar N, et al. Olfactory impairment in Parkinson's disease studied with diffusion tensor and magnetization transfer imaging. J Parkinsons Dis 2017;7:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 2003;20:1714–1722. [DOI] [PubMed] [Google Scholar]
- 43.Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage 2013;73:239–254. [DOI] [PubMed] [Google Scholar]
- 44.Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 2005;26:132–140. [DOI] [PubMed] [Google Scholar]
- 45.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics 2007;4:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braak H, Rüb U, Schultz C, Del Tredici K. Vulnerability of cortical neurons to Alzheimer's and Parkinson's diseases. J Alzheimers Dis 2006;9:35–44. [DOI] [PubMed] [Google Scholar]
- 47.Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res 1992;598:143–153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared at the request of qualified investigators.