Abstract
Objective
To assess dyskinesia frequency in a population-based cohort of patients with Parkinson disease (PD). Dyskinesia complicates levodopa treatment and affects quality of life.
Methods
Utilizing the 1991–2010 population-based, parkinsonism-incident cohort of Olmsted County, MN (n = 669), accessed via the Rochester Epidemiology Project, we identified patients with PD and abstracted levodopa-related dyskinesia information.
Results
Of 309 patients with PD (46.2% with parkinsonisms), 279 (90.3%) received levodopa. Most (230/279; 82.4%) had been treated by a Mayo Clinic neurologist. Median age of the 309 patients with PD at the time of diagnosis was 74.1 years (range 33.1–97.8 years). Median-age levodopa initiation in this cohort was 75 years (range 37–98 years), and median-duration levodopa treatment was 6 years (range 2 months to 19.8 years). Dyskinesia was documented in 84 of 279 patients (30.1%). Median time from levodopa initiation to dyskinesia onset was 4 years (range 2 months to 20 years); those with dyskinesia (65.5%; 55/84) developed it within 5 years of levodopa initiation (9 within the first year). Dyskinesia was mild in 57/84 (67.9%), moderate in 16/84 (19.1%), and severe in 9/84 (10.7%); severity was not reported in 2 cases. Dyskinesia severity led to levodopa adjustments or amantadine initiation in 60.7% (51/84 of those with dyskinesia), with improvement in 23/51 (45.1%). Thirteen patients with dyskinesia underwent deep brain stimulation, reporting marked improvement. Postmortem examination confirmed Lewy body disease in 7 autopsied cases.
Conclusions
Levodopa-induced dyskinesia affected 30% of the patients with PD in our cohort. Mayo neurologists favoring levodopa dosage optimization treated most patients. Dyskinesia was severe in 3.2% of all levodopa-treated patients with PD (10.7% of all patients with dyskinesia) with marked improvement among those treated with deep brain stimulation.
Levodopa is the gold standard therapy for Parkinson disease (PD), supported by strong evidence and decades of clinical practice.1 Prolonged levodopa administration is associated with motor complications, notably dyskinesia and motor fluctuations.2 Levodopa-induced dyskinesia is defined as involuntary, purposeless, predominantly choreiform movements.3 The incidence of levodopa-induced dyskinesia has been reported to be nearly 40% after 4 to 6 years of treatment.2 Levodopa-induced dyskinesia frequently corresponds to the times of the peak plasma-levodopa concentration and resolves with reduction of the individual levodopa doses. In contrast, medication off-states in PD often manifest with dystonia (e.g., toe-curling, foot-inversion), and these should not be conflated with levodopa-induced dyskinesia. Choreiform dyskinesia may manifest with a biphasic pattern, surfacing at the beginning and end of the plasma-levodopa peak4; however, this is infrequent and will not be addressed further in this report.
Levodopa dyskinesia incidence decreases with age5 but increases with PD duration and, by extension, duration of levodopa treatment. It also is linked to higher levodopa doses.6 These motor complications may influence therapeutic choices7 and quality of life. Strategies have been proposed to prevent levodopa dyskinesia including deferring levodopa, a priori restriction of the levodopa dose, and use of dopamine agonists.8 However, limiting or deferring levodopa treatment tends to compromise parkinsonism control. Oral dopamine agonists (e.g., pramipexole, ropinirole) are not nearly as efficacious as levodopa therapy and have an unfavorable side-effect profile including impulse-control disorders9 and sleep attacks.10 Dopamine agonists also are more likely to provoke hallucinations.11 Of practical relevance, levodopa-induced dyskinesia resolves with reduction of levodopa doses although sometimes at the expense of recurrent parkinsonism.
The aim of this study was to assess the impact of levodopa-induced dyskinesia in our incident cohort of patients with PD. We tabulated the frequency of dyskinesia of any severity among all incident cases of PD in Olmsted County, MN, from 1991 to 2010 and examined potential risk factors for their development and the effects of adjusted medication. For the purpose of this report, we defined levodopa-induced dyskinesia as peak-dose choreiform movements. Levodopa off-state dystonia was not included as a form of levodopa dyskinesia. Biphasic presentations of dyskinesia were not tabulated, since, in our practice, they are infrequent and sometimes overlooked.
Methods
Study population
Utilizing the unique infrastructure of the Rochester Epidemiology Project, we identified 669 incident cases of parkinsonism in Olmsted County, MN, from 1991 to 2010. Details regarding the identification of cases and the clinical diagnostic criteria have been reported elsewhere.12
Standard protocol approvals, registrations, and patient consents
The Mayo Clinic and Olmsted Medical Center institutional review boards approved this study, and participating patients (or their legally authorized representatives) provided written consent to use their medical information.
Case identification
This analysis included patients who fulfilled criteria for the clinical diagnosis of PD and were residents of Olmsted County.12,13 We reviewed the medical records of these patients and abstracted information regarding levodopa treatment, sex, age at onset of parkinsonism, time between initial levodopa administration and onset of dyskinesia, dyskinesia severity, therapy adjustments (levodopa dosage changes or amantadine use), and duration of follow-up. A movement disorders specialist (R.S.) reviewed all cases to ascertain final diagnoses and the presence or absence of dyskinesia.
Dyskinesia was reported as present only if explicitly diagnosed in the clinical records. We graded dyskinesia as mild, moderate, or severe, according to the physician's assessment recorded in the clinical records; then, a movement disorder specialist (R.S.) reviewed all the cases to confirm the presence of dyskinesias and their severity according to the signs reported in the patient medical records. We examined the association between total daily levodopa dose and the odds of developing dyskinesia by separating doses into quintiles; levodopa dose was clearly documented in 247 of 309 patients with PD.
We also reviewed the pathologic reports of dyskinetic patients with PD who underwent autopsy. Standardized neuropathologic criteria were applied by a board-certified neuropathologist (J.E.P.) to establish the definite diagnosis.14,15
Statistical analysis
Wilcoxon rank sum and Fisher exact tests were used to compare characteristics between (1) patients who developed dyskinesia and those who did not, and (2) those with mild to moderate dyskinesia and those with severe dyskinesia. We used logistic regression models to determine the association between levodopa dose and dyskinesia. Model 1 was unadjusted. Model 2 was adjusted for age and sex. Levodopa dose was divided into quintiles. All analyses were completed using Stata version 13.0 (StataCorp LP, College Station, TX).
Data sharing
All the relevant data have been shared and published in this article. Data regarding case ascertainment of parkinsonism and methodology on case identification have been previously published (see Savica et al.12).
Results
Demographics
The median age of the 309 patients with PD at the time of diagnosis was 74.1 years (range 33.1–97.8 years). Of these 309 patients, 279 (90.3%) were treated with levodopa; the median levodopa treatment duration was 6 years (range 2 months to 19.8 years). Median follow-up time from levodopa treatment onset was 9 years (range 2–21 years) among dyskinetic patients and 4 years (range 2 months to 18 years) among nondyskinetic patients (figures 1 and 2). None of the patients were initially treated with a dopamine agonist. Among the 279 patients with PD who were treated with levodopa, 84 (30.1%) developed dyskinesia at any point in time. Of these 84, the median time from levodopa treatment initiation to the onset of dyskinesia was 4 years (range 2 months to 20 years). Forty-eight of 84 dyskinetic patients (57.1%) were male; sex was not associated with the risk of developing dyskinesia (p = 0.39).
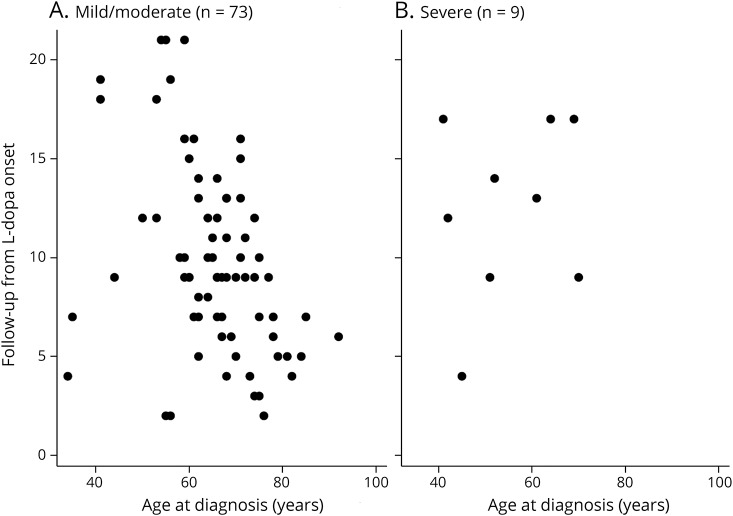
Figure 1. Follow-up from levodopa onset and age at diagnosis of PD as variables for patients with mild/moderate (combined) (A) vs severe (B) dyskinesia.
The x-axis = age at Parkinson disease diagnosis and the y-axis = follow-up from levodopa onset.
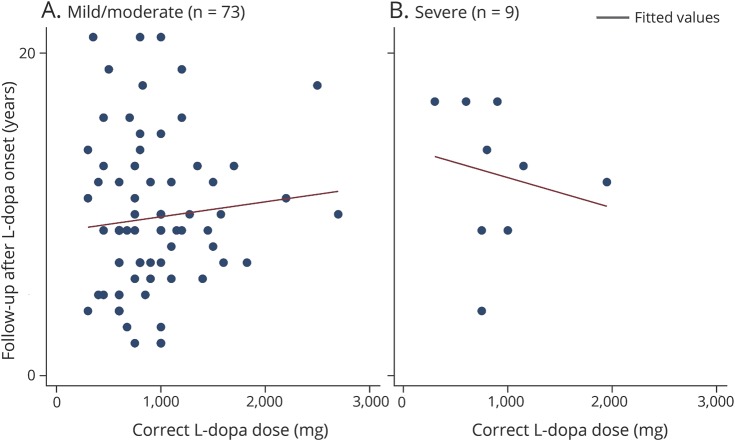
Figure 2. Follow-up from levodopa onset and the levodopa dose-triggering dyskinesias as variables for patients with mild/moderate (combined) (A) vs severe (B) dyskinesia.
The x-axis = the correct levodopa dose that triggered dyskinesias and the y-axis = follow-up from levodopa onset.
Probability of dyskinesia and duration of treatment
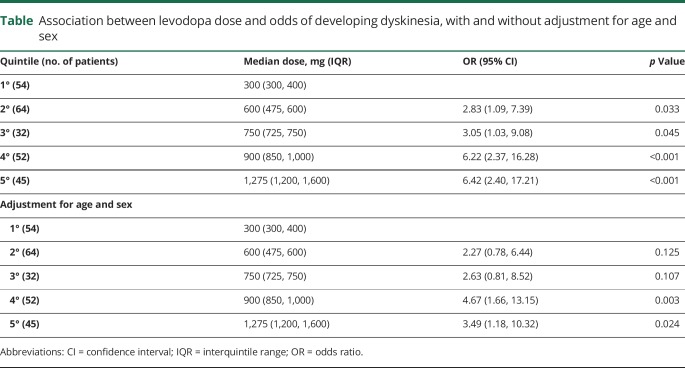
Both the fourth and fifth quintiles of levodopa doses (i.e., higher doses) were associated with increased odds of dyskinesia after adjusting for sex and age (table). Of the 84 dyskinetic patients, 55 (65.5%) developed symptoms within 5 years of levodopa therapy initiation including 9 within the first year. These 9 patients did not differ from the other dyskinetic patients in age at onset of PD (p = 0.54), age at levodopa initiation (p = 0.087), or levodopa dose (p = 0.71).
Table.
Association between levodopa dose and odds of developing dyskinesia, with and without adjustment for age and sex
Severity of dyskinesia, duration of treatment, and age at onset
Among the 84 patients with levodopa dyskinesia, 67.9% were reported as having mild dyskinesia; 19.1%, moderate; and 10.7%, severe. Among the patients who developed severe dyskinesia, the median time between levodopa initiation and onset of dyskinesia was 6 years (range 1–10 years), the median follow-up time after clinical diagnosis was 13 years (range 8–19 years), and the median levodopa treatment duration was 13 years (range 4–17 years). The duration of levodopa treatment did not differ between patients with mild to moderate and those with severe dyskinesia (p = 0.09). Patients with severe dyskinesia were diagnosed with PD at a significantly younger age (median 51.5 years) compared to patients with mild to moderate dyskinesia (median 68.4 years; p = 0.003). There was no difference in the median daily levodopa dose between these 2 groups. Each year of increase in age at onset was associated with reduced odds of developing severe dyskinesia, as compared to development of mild to moderate dyskinesia, in both unadjusted (odds ratio = 0.92, 95% confidence interval 0.87–0.97, p = 0.003) and sex-adjusted (odds ratio = 0.90, 95% confidence interval 0.84–0.96, p = 0.002) models. The Hoehn and Yahr stage at time of onset of severe dyskinesias was unknown for 8 of 9 patients with severe dyskinesia; one patient was stage II.
Medication adjustments
The treating physician adjusted the levodopa dose or prescribed amantadine in 51 of the 84 patients (60.7%) with dyskinesia. Presumably, dyskinesia was not bothersome enough to require medication changes in the others. Marked improvement or resolution of dyskinesia was documented in 23 of 51 patients (45.1%). None of the patients who responded to amantadine required deep brain stimulation (DBS) surgery. Among the 9 severely dyskinetic patients, neither lowering the levodopa dose nor adding amantadine adequately controlled the dyskinesia without substantially increasing parkinsonism.
Deep brain stimulation
Subthalamic nucleus DBS was performed in 13 of 84 dyskinetic patients (15.5%), and all reported marked improvement in dyskinesias after surgery. This included 4 of the 9 severely impaired patients and 3 mildly dyskinetic patients who required DBS surgery to control medically refractory tremor and disabling motor fluctuations along with dyskinesias.
Postmortem examinations
Brain autopsy was available on 7 patients with dyskinesia (median, 4 years from onset of dyskinesia to death; range, 1–9 years). All were classified according to the Consortium to Establish a Registry for Alzheimer's disease, Braak, and National Institute on Aging/Reagan criteria. In all cases, there was greater subcortical than cortical burden of α-synuclein deposits. The pathologic diagnosis was Lewy body disease in all patients. All of these also had pathologic features of Alzheimer neuropathology (showing both neuritic plaques and neurofibrillary tangle deposits in the brain tissues), but only 2 had been clinically diagnosed with cognitive decline. For further details about the pathologic characteristics of our cohort of patients, refer to Turcano et al.16
Discussion
In the last quarter century, PD specialists have typically advocated deferring levodopa and restricting levodopa dosage to reduce the risk of motor complications. The rationale for this strategy was partially based on concerns that more aggressive levodopa treatment put patients at risk for dyskinesia. Of note, the treating clinicians involved were primarily neurologists at Mayo Clinic–Rochester (82.4%) who generally did not subscribe to this levodopa-sparing strategy. None of the patients with PD in this cohort were initially treated with a dopamine-agonist drug. Hence, the dyskinesia outcomes in this cohort are relevant to initial and longer-term treatment of PD.
In this cohort, the overall frequency of levodopa-induced dyskinesia of any severity was only 30%. Although levodopa-induced dyskinesia is seldom problematic (e.g., modestly affecting one limb), it can, nevertheless, be obtrusive and disabling. In this cohort, however, severe dyskinesia was only documented in approximately 10% of patients.
The median age of our PD cohort was 74 years at the time of diagnosis, which likely contributed to the relatively low dyskinesia frequency. Younger age at PD diagnosis is a major risk factor in levodopa-induced dyskinesia.5 For example, PD onset before age 40 years is associated with a dyskinesia frequency of 94% after 5 years of treatment.17 In our cohort, the patients with severe dyskinesia had a significantly younger age at onset than the mild to moderate patients. Our relatively elderly cohort presumably reflects typical PD in the community. It also accounts for the relatively restricted median levodopa treatment duration of 6 years.
These results are consistent with an earlier study in this Olmsted County cohort that documented a 30% 5-year risk of dyskinesia of any severity, increasing to 59% after 10 years of treatment.18 This 30% 5-year risk is also similar to a 36% 4- to 6-year risk in a systematic review of all published reports from the modern levodopa era to 2000.2
It is not surprising that the levodopa dose among the dyskinetic patients was higher than the dose among nondyskinetic patients (p < 0.001; table), since a dose-dependent effect is well recognized.19 Hence, the principal strategy for abolishing dyskinesia is reduction of levodopa dose. In our cohort, 51 of 84 dyskinetic patients required medication adjustment (lowering levodopa dose or initiation of amantadine). Approximately 45% of patients who made these adjustments showed marked improvement in their dyskinesia. Occasionally, patients do not have a sufficiently broad levodopa dose range to treat parkinsonism adequately without exceeding the dyskinesia threshold. In our experience, these patients benefit from DBS, as evidenced by the 13 of our dyskinetic patients who achieved control with DBS.
Initial treatment with dopamine agonists, such as ropinirole7 or pramipexole,20,21 carries a substantially lower risk of motor complications than levodopa. However, agonist monotherapy often does not adequately treat parkinsonism and rarely is sufficient beyond a few years; moreover, these oral dopamine agonists are associated with side effects such as pathologic behaviors,9 daytime sleepiness,7,10,20,21 and hallucinations.7,20,21
Our study has several strengths. First, we utilized the well-defined, population-based incident cohort of parkinsonism patients of the records-linked system of the Rochester Epidemiology Project. The population-based nature of this cohort is less likely to be affected by selection bias than many case series. Although few cases had postmortem neuropathologic diagnostic confirmation, notably, all 7 deceased, dyskinetic patients with PD had evidence of Lewy body disease. Second, the vast majority of patients have been treated and followed by Mayo Clinic neurologists, who have very similar training and practice approaches that favor optimizing levodopa dosage.
This study also has limitations. First, we acknowledge that mild or infrequent incidence of levodopa dyskinesia might not have been reported in the medical records. Moreover, because of the retrospective nature of our study and the relatively small number of diphasic dyskinesias we see in our practice, these kinds of dyskinesias might not have been reported. This may have led to an underestimation of the prevalence of dyskinesia in our cohort. However, we are confident that we detected all the clinically problematic dyskinesias, such as diphasic dyskinesias. Because of the geography of Olmsted County, Mayo Clinic is the only referring center of the area that has a neurology department with movement disorders specialists. Therefore, all patients were seen at least once by a movement disorders specialist. Thus, patients with PD and motor complications are usually likely to be followed by one neurologist in the Movement Disorders Division of the Mayo Clinic.
Second, patients were seen as part of the routine clinical practice without standardization of the recorded data. Third, the shorter follow-up time from levodopa treatment initiation among nondyskinetic patients compared to the dyskinetic ones may have led to an underestimation of the dyskinesia frequency. Fourth, we could not gather data about the severity and progression of PD assessed with the Unified Parkinson's Disease Rating Scale. Fifth, because we had only 9 patients with severe dyskinesia, we did not have sufficient power to examine the percent of severe dyskinesias at a given time after either start of levodopa or time from diagnosis as a function of age at diagnosis with multivariate regression models. However, using scatterplots, we can see that there was not a strong bias of age at PD diagnosis on time from levodopa use to onset of dyskinesias when comparing mild/moderate and severe dyskinesia patients.
In conclusion, our results are relevant to current practice. It is reassuring that early levodopa initiation and appropriately maximal tolerated dose do not appear to predispose to major, disabling dyskinesia.
Acknowledgment
The authors thank Mrs. Lea Dacy for editing and formatting the manuscript.
Glossary
- DBS
deep brain stimulation
- PD
Parkinson disease
Footnotes
CME Course: NPub.org/cmelist
Author contributions
Dr. Turcano wrote the first draft of the manuscript and assisted in data analysis and interpretation of data. Dr. Mielke performed data analysis and critical review of the manuscript for content. Dr. Bower assisted with data collection and critical review of the manuscript for content. Dr. Parisi assisted with data collection and critical review of the manuscript for content. Dr. Cutsforth-Gregory assisted with data collection and critical review of the manuscript for content. Dr. Ahlskog assisted with data collection and critical review of the manuscript for content. Dr. Savica developed the concept for the manuscript, assisted in writing the first draft, provided oversight of data collection and analysis, and performed final critical review for content.
Study funding
The study was supported by the NIH grant R01 AG034676.
Disclosure
P. Turcano reports no disclosures relevant to the manuscript. M. Mielke receives research support from the National Institute on Aging. J. Bower, J. Parisi, and J. Cutsforth-Gregory report no disclosures relevant to the manuscript. J. Ahlskog has authored books on parkinsonism for which he is eligible to receive royalties. R. Savica receives research support from the National Institute on Aging. Go to Neurology.org/N for full disclosures.
References
- 1.Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA 2014;311:1670–1683. [DOI] [PubMed] [Google Scholar]
- 2.Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 2001;16:448–458. [DOI] [PubMed] [Google Scholar]
- 3.Thanvi B, Lo N, Robinson T. Levodopa-induced dyskinesia in Parkinson's disease: clinical features, pathogenesis, prevention and treatment. Postgrad Med J 2007;83:384–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muenter MD, Sharpless NS, Tyce GM, Darley FL. Patterns of dystonia (“I-D-I” and “D-I-D-”) in response to L-dopa therapy for Parkinson's disease. Mayo Clin Proc 1977;52:163–174. [PubMed] [Google Scholar]
- 5.Kumar N, Van Gerpen JA, Bower JH, Ahlskog JE. Levodopa-dyskinesia incidence by age of Parkinson's disease onset. Mov Disord 2005;20:342–344. [DOI] [PubMed] [Google Scholar]
- 6.Schrag A, Quinn N. Dyskinesias and motor fluctuations in Parkinson's disease: a community-based study. Brain 2000;123(pt 11):2297–2305. [DOI] [PubMed] [Google Scholar]
- 7.Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE. A five-year study of the incidence of dyskinesia in patients with early Parkinson's disease who were treated with ropinirole or levodopa. N Engl J Med 2000;342:1484–1491. [DOI] [PubMed] [Google Scholar]
- 8.Barone P. Clinical strategies to prevent and delay motor complications. Neurology 2003;61:S12–S16. [DOI] [PubMed] [Google Scholar]
- 9.Hassan A, Bower JH, Kumar N, et al. Dopamine agonist-triggered pathological behaviors: surveillance in the PD clinic reveals high frequencies. Parkinsonism Relat Disord 2011;17:260–264. [DOI] [PubMed] [Google Scholar]
- 10.Frucht S, Rogers JD, Greene PE, Gordon MF, Fahn S. Falling asleep at the wheel: motor vehicle mishaps in persons taking pramipexole and ropinirole. Neurology 1999;52:1908–1910. [DOI] [PubMed] [Google Scholar]
- 11.Baker WL, Silver D, White CM, et al. Dopamine agonists in the treatment of early Parkinson's disease: a meta-analysis. Parkinsonism Relat Disord 2009;15:287–294. [DOI] [PubMed] [Google Scholar]
- 12.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Incidence and pathology of synucleinopathies and tauopathies related to parkinsonism. JAMA Neurol 2013;70:859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999;56:33–39. [DOI] [PubMed] [Google Scholar]
- 14.Forno LS. Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol 1996;55:259–272. [DOI] [PubMed] [Google Scholar]
- 15.McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis 2006;9:417–423. [DOI] [PubMed] [Google Scholar]
- 16.Turcano P, Mielke MM, Josephs KA, et al. Clinicopathologic discrepancies in a population-based incidence study of parkinsonism in Olmsted County: 1991–2010. Mov Disord 2017;32:1439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinn N, Critchley P, Marsden CD. Young onset Parkinson's disease. Mov Disord 1987;2:73–91. [DOI] [PubMed] [Google Scholar]
- 18.Van Gerpen JA, Kumar N, Bower JH, Weigand S, Ahlskog JE. Levodopa-associated dyskinesia risk among Parkinson disease patients in Olmsted County, Minnesota, 1976–1990. Arch Neurol 2006;63:205–209. [DOI] [PubMed] [Google Scholar]
- 19.Warren Olanow C, Kieburtz K, Rascol O, et al. Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson's disease. Mov Disord 2013;28:1064–1071. [DOI] [PubMed] [Google Scholar]
- 20.Parkinson Study Group. Pramipexole vs levodopa as initial treatment for Parkinson disease: a randomized controlled trial. JAMA 2000;284:1931–1938. [DOI] [PubMed] [Google Scholar]
- 21.Parkinson Study Group CALM Cohort Investigators. Long-term effect of initiating pramipexole vs levodopa in early Parkinson disease. Arch Neurol 2009;66:563–570. [DOI] [PubMed] [Google Scholar]