Abstract
Since the discovery of CSF1R gene mutations in families with hereditary diffuse leukoencephalopathy with spheroids in 2012, more than 70 different mutations have been identified around the world. Through the analyses of mutation carriers, CSF1R-related leukoencephalopathy has been distinctly characterized clinically, radiologically, and pathologically. Typically, patients present with frontotemporal dementia-like phenotype in their 40s–50s, accompanied by motor symptoms, including pyramidal and extrapyramidal signs. Women tend to develop the clinical symptoms at a younger age than men. On brain imaging, in addition to white matter abnormalities, thinning of the corpus callosum, diffusion-restricted lesions in the white matter, and brain calcifications are hallmarks. Primary axonopathy followed by demyelination was suggested by pathology. Haploinsufficiency of colony-stimulating factor-1 receptor (CSF1R) is evident in a patient with a frameshift mutation, facilitating the establishment of Csf1r haploinsufficient mouse model. These mice develop clinical, radiologic, and pathologic phenotypes consistent with those of human patients with CSF1R mutations. In vitro, perturbation of CSF1R signaling is shown in cultured cells expressing mutant CSF1R. However, the underlying mechanisms by which CSF1R mutations selectively lead to white matter degeneration remains to be elucidated. Given that CSF1R mainly expresses in microglia, CSF1R-related leukoencephalopathy is representative of primary microgliopathies, of which microglia have a pivotal and primary role in pathogenesis. In this review, we address the current knowledge of CSF1R-related leukoencephalopathy and discuss the putative pathophysiology, with a focus on microglia, as well as future research directions.
Owing to advances in molecular genetics, the complexity of hereditary leukoencephalopathies has been gradually unveiled in recent years. The discovery of a causative gene facilitates increasing recognition of the disease and understanding of its clinical, pathologic, and biologic characteristics. CSF1R gene encoding colony-stimulating factor-1 receptor (CSF1R) was identified in 1 of the hereditary leukoencephalopathies, hereditary diffuse leukoencephalopathy with spheroids (HDLS), in 2012.1,2 CSF1R is a transmembrane tyrosine kinase receptor that expresses in mononuclear phagocytic cells. In the brain, it mainly expresses in microglia. Binding of its ligands (CSF1 and interleukin-34) to CSF1R leads to formation of a receptor homodimer on cell surface and subsequent activation of CSF1R through autophosphorylation.3 Activation is necessary to initiate signal transductions, which contribute to development, maintenance, and activation of microglia.4 Therefore, microglia are considered primarily affected in this inherited leukoencephalopathy, referred to as primary microgliopathies,2,5–7 as opposed to secondary microgliopathies, in which microglial activation occurs secondary to other CNS insults.7,8 In line with the recent understanding of this disease concept, we call this disease CSF1R-related leukoencephalopathy9 in this review. We review historical aspects of this unique disease and discuss clinical, radiologic, and pathologic characteristics and putative pathophysiology, with a focus on microglia.
Standard protocol approvals, registrations, and patient consents
We conducted all genetic analyses under approval of the institutional review board (IRB) of Mayo Clinic Florida and Niigata University. Written informed consent was obtained from all research participants. Brain autopsy was performed at the Mayo Clinic Florida with permission obtained from the legal next of kin. The Mayo Clinic IRB Committee exempts autopsy studies from human subject research.
Historical background and nosology
In 1936, pigmentary orthochromatic leukodystrophy (POLD) was reported as a subset of orthochromatic leukodystrophies in a Belgian family by van Bogaert and Nyssen.10 POLD is pathologically characterized by diffuse myelin and axonal loss accompanied by pigmented macrophages. HDLS was first described in a Swedish family in 1984 by Axelsson et al.1 Two neuropathologists, Röyttä11 and Sourander, named HDLS based on its pathologic characteristics: loss of myelin sheaths and axons, widespread white matter degeneration, and numerous neuroaxonal spheroids. Marotti et al.12 discussed the clinical and pathologic similarities between POLD and HDLS.10 Marotti et al.12 reported 3 autopsy-confirmed HDLS cases from a single family, whose brain lesions also contained pigmented macrophages. These authors had a chance to review the original POLD case of van Bogaert and Nyssen and found abundant axonal spheroids in affected white matter reminiscent of HDLS. In the article by Marotti et al.,12 the term adult-onset leukodystrophy with neuroaxonal spheroids and pigmented glia (ALSP) was coined as a comprehensive pathologic term for these conditions. Since then, the similarity between POLD and HDLS has been repeatedly discussed by others and the term “leukoencephalopathy” has been often used alternatively for “leukodystrophy.”13–15 A year after the discovery of the CSF1R gene in HDLS, Nicholson et al.16 identified CSF1R mutations in 2 pathologically diagnosed POLD families (1 mutation had already been found in HDLS2), providing genetic evidence that POLD and HDLS can be categorized as a single disease entity.
There have been several cases in which clinical and pathologic findings were consistent with ALSP, but CSF1R mutations were not identified.17–19 Notably, no CSF1R mutation has been found in the original Swedish family,20 implying that other genes may be associated with these cases. In 2016, homozygous or compound heterozygous mutations in AARS2 gene encoding mitochondrial alanyl-transfer RNA synthetase were identified in 5 patients who were clinically suspected to have ALSP, but were negative for CSF1R mutations.21 Importantly, pathologic findings similar to those of ALSP carrying CSF1R mutation were obtained in 1 of those patients with AARS2 mutation by brain biopsy. However, there are several clinical and radiologic differences between ALSP caused by CSF1R mutation and AARS2-related leukoencephalopathy (AARS2-L).22,23 AARS2-L is not always an adult-onset disease.24 Furthermore, in another case of AARS2-L, no pathologic evidence of ALSP was obtained by brain biopsy.25 Thus, further studies, including detailed analyses of autopsied brains, are required to characterize AARS2-L.
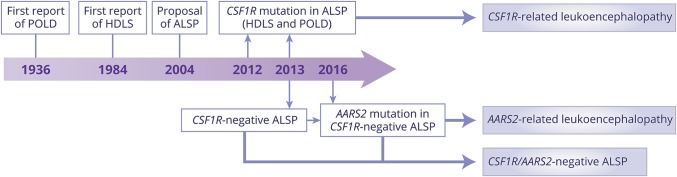
Due to the evolving knowledge of pathology and genetics of this disease entity, its nomenclature is changing (figure 1). In our review, we use the term CSF1R-related leukoencephalopathy9 for descriptive purposes; however, if ALSP is further established as a consistent clinicopathologic entity determined by several genes, a prefix-gene style, as used for genetic movement disorders,26 would be acceptable, such as ALSP-CSF1R. There is controversy which term—leukodystrophy or leukoencephalopathy—is more appropriate.27 Recently, the GLIA Consortium defined leukodystrophies as heritable white matter disorders with neuropathology primarily characterized by involvement of non-neuronal cells.28 According to this definition, ALSP could be categorized under leukodystrophies5 because microglia are considered to be primarily affected in this disease.2
Figure 1. Changing nomenclature with a growing understanding of pathology and genetics.
We currently have at least 3 different leukoencephalopathies from this adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP) axis: colony-stimulating factor-1 receptor (CSF1R)–related leukoencephalopathy, alanyl-transfer RNA synthetase 2 (AARS2)–related leukoencephalopathy, and CSF1R/AARS2-negative ALSP. It is controversial whether AARS2-related leukoencephalopathy can be classified under ALSP. HDLS = hereditary diffuse leukoencephalopathy with spheroids; POLD = pigmentary orthochromatic leukodystrophy.
Epidemiology
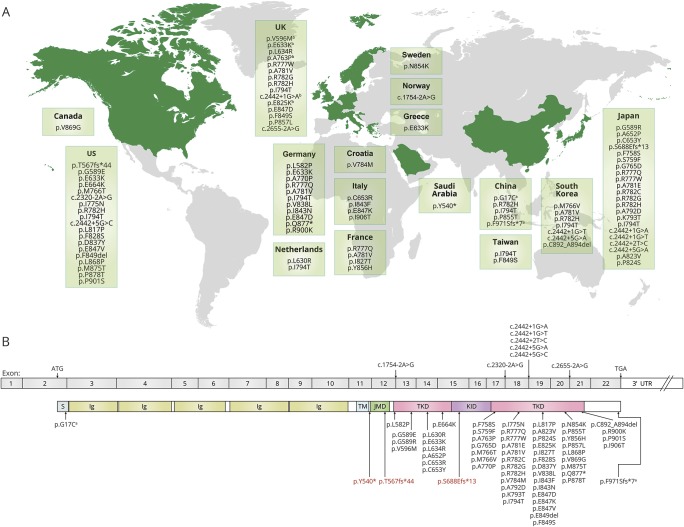
Since diagnosis of CSF1R-related leukoencephalopathy had depended on pathologic findings, the number of definitively diagnosed patients was extremely limited until discovery of the gene mutations. Since 2012, although still rare, genetically diagnosed patients have been increasingly recognized around the world (figure 2A), indicating that this disease has global distribution, and many patients may still be underdiagnosed. One study estimated the frequency of CSF1R-related leukoencephalopathy at 10% (5/48) of adult-onset leukoencephalopathies and implied that it is the most common type,29 whereas in another study, using an MRI pattern-based approach, only 2 of 154 (0.6%) patients with adult-onset leukoencephalopathies were genetically diagnosed.30 Approximately one-third of genetically proven patients have been reported in Japan.23
Figure 2. World distribution of colony-stimulating factor-1 receptor (CSF1R) mutations and CSF1R gene/protein diagram with reported mutations.
(A) World distribution of CSF1R mutations. The countries in which the mutation was reported are shown in green. This world map was created using mapchart (http://mapchart.net/). (B) Diagram of CSF1R gene and protein with reported mutations. All mutations are found within the tyrosine kinase domain (TKD) of CSF1R except for a nonsense mutation, p.Y540*, and 2 frameshift mutations, p.T567fs*44 and p.S688Efs*13, shown in red. These are located in the intervening sequence between transmembrane domain (TM) and juxtamembrane domain (JMD) and in the kinase insert domain (KID). aThese 2 variants, located outside the TKD, have been reported recently in Chinese patients.40 One (p.G17C) is in the signal peptide sequence of CSF1R and the other (p.F971Sfs*7) is at the C-terminal sequence. The former variant does not change the TKD, but might fail to express CSF1R on the cell surface if it influences the signal peptide function, resulting in loss of CSF1R function. The latter induces subtle changes in a couple of the last amino acids of CSF1R, but the TKD remains unchanged. This has been reported as a rare variant in an East Asian population (rs766047383; allele frequency is 0.0004641 based on The Genome Aggregation database, gnomad.broadinstitute.org/). Therefore, the pathogenicity of these variants remains to be determined. bThese 5 mutations were identified in a mixed cohort from the United Kingdom, Greece, and Ireland.29 Ig = immunoglobulin-like domain; UTR = untranslated region.
Clinical features
The mean age at onset of CSF1R-related leukoencephalopathy is 43 years and disease duration is 6.8 years.23 While there is no sex difference in prevalence, women develop disease 7 years earlier than men (40 vs 47 years [95% confidence interval (CI) 3.158–11.177]).23 The cumulative incidence is 10% at age 27 years, 50% at age 43 years, and rises to 95% by age 60 years.23 This disease is clinically characterized by 2 major components: neuropsychiatric and motor symptoms.31 The former represents progressive cognitive decline, depression, apathy, anxiety, irritability, and other behavioral or personality changes, resembling behavioral variant frontotemporal dementia (bvFTD). The latter includes parkinsonian symptoms (i.e., tremor, rigidity, bradykinesia, and postural instability),20 pyramidal signs (e.g., hyperreflexia, spasticity), bulbar signs (e.g., dysarthria, dysphagia), and ataxia. Some patients manifest gait disturbance as a cardinal symptom rather than cognitive and psychiatric symptoms.31 Apraxia, aphasia, and seizures can also develop in one-third of patients.23 Like other leukoencephalopathies, there is no disease-specific clinical picture of CSF1R-related leukoencephalopathy; however, if patients manifest bvFTD at a relatively young age accompanied by motor symptoms and white matter abnormalities on MRI, CSF1R-related leukoencephalopathy should be included in the differential diagnosis. The progression of this illness is rapid. Some rare presentations have been described, such as stroke-like episodes,32 bone cysts,33 and involvement of optic34 and peripheral nerves.35,36 Further investigation is warranted to evaluate significance of these rare findings. Of note, motor symptoms can be more predominant than neuropsychiatric symptoms in young women, who are likely to be misdiagnosed with multiple sclerosis (MS).23 In the literature, we found 24 patients (18 women, 5 men, and 1 whose sex was obscured) diagnosed with MS or demyelinating disorder.2,9,34,36–47 Eleven cases (45.8%) were sporadic. The mean age at onset was 32.1 years, 10 years younger than the overall average. Indeed, 14 (58%) patients initially presented with motor symptoms, including gait disturbance, limb weakness, hemiparesis, dysarthria, and dysphagia, regardless of whether or not they had cognitive impairment. These features may drive MS diagnosis over CSF1R-related leukoencephalopathy.
We recently proposed diagnostic criteria for CSF1R-related leukoencephalopathy.23 The criteria yield high sensitivity (more than 96% of cases fell into probable or possible criteria) and can successfully exclude cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, which is one of the main differential diagnoses and a major cause of adult-onset leukoencephalopathies.30 If a patient fulfils the probable criteria, genetic testing for CSF1R is recommended.
Radiologic features
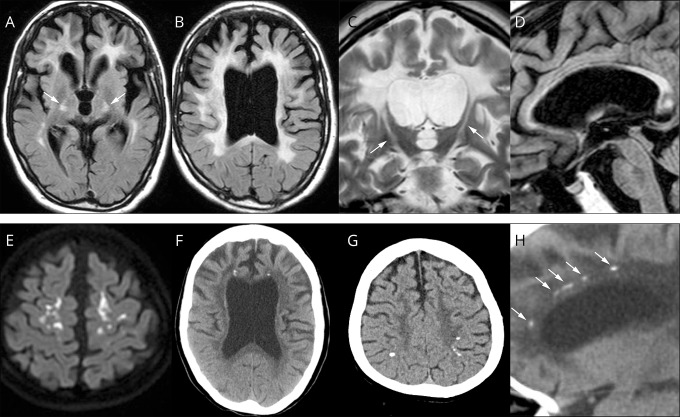
Consistent radiologic findings on brain MRI are white matter abnormalities and enlargement of the lateral ventricles, reflecting the intensive white matter damage (figure 3, A–C). Thinning of the corpus callosum is characteristic and best observed in a sagittal view (figure 3D). These MRI findings develop insidiously48 and are usually seen at the time of symptom onset. White matter changes have been observed 6 years prior to symptom onset.49 White matter lesions are initially patchy, but later confluent and distributed predominantly in the frontal and parietal areas. These are usually bilateral, but not always symmetric. The lesions also involve projection fibers, including pyramidal tracts in the internal capsule and brainstem (figure 3, A and C),23,43 whereas U-fibers are likely spared until the terminal stage of disease. Cortical atrophy over the frontal and parietal lobes becomes evident with disease progression. Diffusion-restricted lesions with reduced apparent diffusion coefficient can be observed in the white matter of some patients (figure 3E). Unlike ischemic lesions, these are persistent for several months or more, presumably reflecting intramyelinic edema.50,51 No contrast enhancement is observed.51,52 Cerebellar dentate nuclei and cerebellar peduncles are spared. An MRI scoring system for assessing severity of white matter lesions and brain atrophy (total severity score ranges from 0 [minimum] to 57 [maximum]) has been proposed.52 Lakshmanan et al.22 were aware of the high incidence (63% of their cohort) of a cavum septum pellucidum and cavum vergae. Consistent with their observation, we found the same findings in our patients.41,48,52 Given that the cavum septum pellucidum and cavum vergae can be seen as normal variants in the general population, comparison studies between CSF1R-related leukoencephalopathy, other neurologic diseases with white matter involvement, and normal controls are required to validate.
Figure 3. Brain MRI/CT findings of colony-stimulating factor-1 receptor (CSF1R)-related leukoencephalopathy.
(A–D, F, H) A 44-year-old woman with CSF1R p.G589R. (E) A 27-year-old woman with CSF1R c.2442+5 G > A. (G) A 31-year-old woman with CSF1R p.A652P. All of these patients have been reported previously.41 (A, B), Bilateral diffuse white matter hyperintensity with pyramidal tract involvement (arrows in A), cortical atrophy, and enlarged lateral ventricles on fluid-attenuated inversion recovery MRI. (C) Longitudinal pyramidal tract involvement (arrows) on coronal T2-weighted image. (D) Thinning of the corpus callosum with hyperintensity on sagittal fluid-attenuated inversion recovery image. (E) Hyperintensity lesions in the subcortical white matter on diffusion-weighted image. (F) Small calcifications located bilaterally near the anterior horns of the lateral ventricles on brain CT image. (G) Calcifications in parietal subcortical white matter. (H) Stepping stone appearance of calcifications (arrows) in the frontal pericallosal region on sagittal CT image.
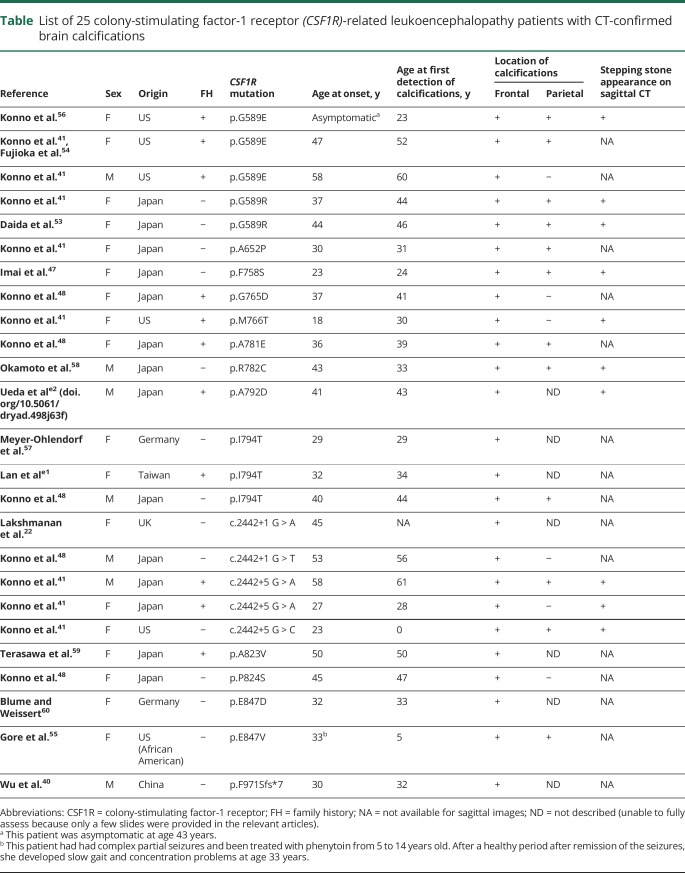
Brain CT scan is also useful for diagnosing CSF1R-related leukoencephalopathy. It can depict brain calcifications in the white matter that are possibly specific to this disease. Based on a review of 25 patients who had CT-confirmed brain calcifications (table)22,40,41,47,48,53–60,e1,e2 (doi.org/10.5061/dryad.498j63f), there were 7 men and 18 women with a mean onset age of 38 years, though significantly younger in women than in men (34.6 vs 46.1 years, respectively, 95% CI 2.580–20.530). The calcifications always distributed in the frontal white matter adjacent to the anterior horns of lateral ventricles (25/25, 100%, bilaterally in most patients) (figure 3F), and to a lesser extent, in the parietal subcortical white matter (12/25, 48%) (figure 3G).41,48 On a sagittal view, a striking pattern of the calcifications can be seen bilaterally in the frontal pericallosal regions, called stepping stone appearance (10/25, 40%) (figure 3H).41 The calcifications can be found at an asymptomatic stage of CSF1R mutation carrier,56,58 even at birth,41 indicating that the calcifications may not be related to clinical symptoms or white matter damages. The size and distribution of the calcifications usually remain stable over time,54,56 but occasionally the calcifications may be reduced in size.41 The calcifications are usually very small, and thus, very easily overlooked; therefore, thin-section (1 mm) CT scan and reconstructed sagittal images are recommended to locate them.41 An exceptional patient has been reported in whom large calcifications were observed.55 In our experience, CSF1R mutations were always found in patients who had these calcifications, suggesting that the brain calcifications if present have extremely high diagnostic value. Of additional interest is that a pericallosal brain calcification distribution pattern is very similar to the distribution of Iba1- and CD68-immunopositive cells in the human fetal brain,e3,e4 raising the possibility of a causal relationship between formation of calcifications and some mutant microglia dysfunction.
Table.
List of 25 colony-stimulating factor-1 receptor (CSF1R)-related leukoencephalopathy patients with CT-confirmed brain calcifications
SPECT demonstrates hypoperfusion in the frontal and parietal cortices37,45,53,59,e5 (doi.org/10.5061/dryad.498j63f). [18F]-fluorodeoxyglucose PET reveals diffuse cortical hypometabolism predominantly in fronto-parietal areas.16,46, e6,e7 These findings are not disease-specific, but may well reflect the affected brain areas. In studies using magnetic resonance spectroscopy, increased choline and decreased N-acetylaspartate levels are constantly observed in white matter lesions,32,34,51,57,e8 indicating demyelination and axonal damage, respectively. The change of metabolite levels can be detected prior to development of symptoms.e8
Pathologic features
A key neuropathologic finding is the combination of widespread white matter degeneration with a loss of myelin and axons, abundant neuroaxonal spheroids, and lipid-laden and pigmented macrophages (figure 4).1 White matter degeneration affects the centrum semiovale, the periventricular white matter, and the corpus callosum, predominantly in the frontal and parietal lobes, with relative sparing of the temporal and occipital lobes. Reactive bizarre astrocytes that appear irregular and hypertrophic are observed in the affected white mattere9 (doi.org/10.5061/dryad.498j63f). While cortical neurons are relatively preserved, ballooned neurons are frequently observed in the overlying cortex. The significance of ballooned neurons remains unknown, but may be associated with impaired axonal transporte10 or dying-back degeneration.15 Interestingly, in contrast to the prominent involvement in the cerebral white matter, other fibers, including arcuate fibers (U-fibers), pencil fibers in the putamen, anterior commissure, fornix, and optic nerves, are generally preserved. Projection fibers, including frontopontine and corticospinal fibers, can be involved. In some patients, pyramidal tract degeneration is observed continuously through the brainstem to the spinal cord.e11
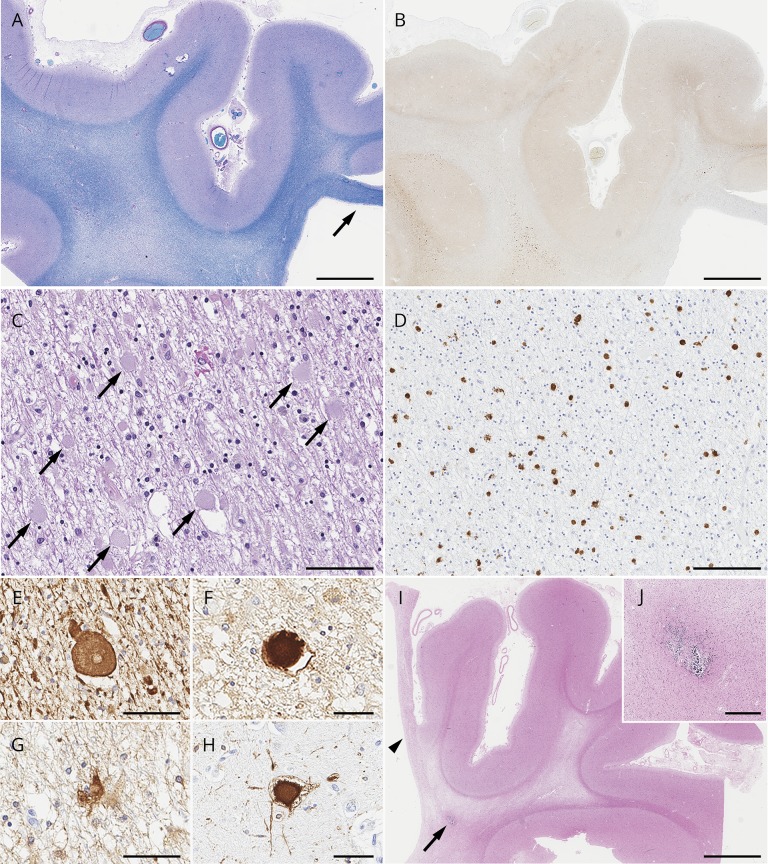
Figure 4. Pathologic findings of colony-stimulating factor-1 receptor (CSF1R)-related leukoencephalopathy.
(A–H) A 78-year-old man with CSF1R p.M875T who was a member of a previously reported family (VA-27 in reference 2).2,e60 (doi.org/10.5061/dryad.498j63f) At 71 years of age, he developed cognitive impairment followed by personality and behavior change, depression, executive dysfunction, apraxia, parkinsonism, and pyramidal weakness. He died with 7 years disease duration. (A) Luxol fast blue stain shows severe myelinated fiber loss in the superior frontal and cingulate white matter, whereas the U-fibers are relatively spared. Note the thinning of the corpus callosum (arrow). (B) The axonal spheroids in the affected white matter are stained with amyloid precursor protein (APP). (C) Numerous axonal spheroids (arrows) are seen within the frontal white matter (hematoxylin & eosin). (D) CD68-immunopositive macrophages in the frontal white matter. (E, F) An axonal spheroid in the white matter depicted by phosphorylated neurofilament (SMI31) (E) and APP (F). (G) A bizarre astrocyte in the white matter (αB-crystallin). (H) A ballooned neuron in the superior frontal cortex (αB-crystallin). (I, J) A 55-year-old woman with autopsy-confirmed adult-onset leukodystrophy with neuroaxonal spheroids and pigmented glia, but genetic testing was not performed because DNA was unavailable. (I) Note the small calcified lesion (arrow) located in the pericallosal region. An arrowhead indicates the paper-like atrophy of the corpus callosum. (J) An enlarged image of the calcification. Bars in A, B, and I = 5 mm; C and D = 100 μm; E, F, G, and H = 50 μm; and J = 400 μm.
Two pathologic hallmarks, axonal spheroids and pigmented macrophages, are vastly observed in the affected white matter. The spheroids are immunostained with phosphorylated neurofilament, amyloid precursor protein, and ubiquitine9 (doi.org/10.5061/dryad.498j63f). The pigmented macrophages are CD68-positive and contain brown granular pigments that are autofluorescent and stained with periodic acid-Schiff.e9 Electron microscopy reveals heterogeneous appearances of spheroids with combinations of aggregated neurofilaments, mitochondria, vesicles, and dense bodies.1,e9,e12-e17 Thickness of the myelin sheath varies and intramyelinic vacuolations are frequently seen.e14 Macrophages contain ceroid-like ultrastructure with lamellated or fingerprint bodies.e14,e18,e19 The abundance and distribution of spheroids and macrophages seem to be related to the severity of the white matter pathology. According to pathologic staging proposed by Alturkustani et al.,e17 numerous spheroids appear prior to the white matter change. Then the number of spheroids is decreased along with white matter damage. Importantly, any stages can be observed simultaneously in a single patient, implying that lesions may occur multifocally, and thus, only 1 biopsy specimen could fail to detect typical findings.55 This temporal and spatial association between spheroids and white matter lesions supports the idea of primary axonopathy; axonal damage occurs prior to myelin loss.12,15,e5,e17,e17,e20,e21 The pigmented macrophages also become sparse in severely affected white matter.e5,e13,e17 Alturkustani et al.e17 described that CD68-positive macrophages were rarely observed in early stages, whereas others argued that the emergence of these cells precedes axonal injury.e5,e21 In general, there are no pathologic structures immunostained with tau, α-synuclein, and TDP-43.
Tada et al.e22 (doi.org/10.5061/dryad.498j63f) focused on microglia by detailed pathologic analyses. They showed that microglia had relatively small and narrow cytoplasm with thin processes in CSF1R-related leukoencephalopathy (n = 6) compared to controls (n = 6) and other white matter–involved disorders, including Alzheimer disease (n = 1), Nasu-Hakola disease (NHD) (n = 1), adrenoleukodystrophy (n = 1), and Binswanger disease (n = 2).e22 The number of microglia was decreased in brains with CSF1R mutations. Interestingly, distribution of activated microglia (immunopositive for Iba1, P2ry12, and GLUT5) is spatially restricted; more abundant in relatively preserved areas, but sparse in devastated areas, which is consistent with the previous observation by Alturkustani et al.e17, e22 In contrast to the activated microglia, Tada et al.e22 showed that macrophages (immunopositive for Iba1, but negative for P2ry12 and GLUT5) accumulated in the damaged white matter and are involved in phagocytosis. These cells were distinguishable from activated microglia and likely derived from peripheral circulation. They seemed to compensate for the insufficient function of microglia. Ultrastructurally, vesiculation of rough endoplasmic reticulum and disaggregated polyribosomes is observed in microglia.e22 These observations lend support to the concept of primary microgliopathy in which distinct microglial abnormality has a principal role in neurodegeneration. Microglia might lose their normal physiologic function by CSF1R mutation.
Management
Like most other hereditary leukoencephalopathies, there is no cure for CSF1R-related leukoencephalopathy. Although expected benefit is limited, symptomatic therapies should be offered, including antidepressants for depression, muscle relaxants for spasticity, and antiepileptic drugs for epilepsy if tolerable. CSF1R-related leukoencephalopathy may mimic Alzheimer disease in some cases2,46,52,e7,e23,e24 (doi.org/10.5061/dryad.498j63f); however, given that the nucleus basalis of Meynert is usually preserved, cholinesterase inhibitors are unlikely to be beneficial for cognitive impairment. Likewise, for parkinsonian symptoms, levodopa may not be beneficial since dopaminergic neurons in substantia nigra are usually preserved.20 Rehabilitation may be helpful for patients to maintain their physical performance. While mouse disease model showed some evidence of neuroinflammation,e25 no patients have shown positive response to immunotherapies like steroids, interferon β-1a/1b, cyclophosphamide, and plasmapheresis.
As shown for metabolic leukodystrophies, including adrenoleukodystrophy, Krabbe disease, and metachromatic leukodystrophye26 (doi.org/10.5061/dryad.498j63f), hematopoietic stem cell transplantation (HSCT) might be an effective treatment for CSF1R-related leukoencephalopathy. There has been only 1 patient treated with HSCT.e27 The patient did not gain clinical improvement, but no more progression was observed after HSCT. The patient's neurologic condition was described as stable for at least 15 years, suggesting HSCT could be beneficial for this disease.e27 However, how the HSCT works remains to be determined. Unlike the metabolic disorders, CSF1R-related leukoencephalopathy is not associated with an essential enzyme deficiency. In addition, given that microglia are not originally derived from bone marrow and are intrinsically maintained by a self-renewal manner independent of circulating monocytes,e28 it is questioned whether the transplanted bone marrow–derived cells can sufficiently compensate for microglial function and its longevity and self-renewal ability.e29 Although intense investigation is required to validate the utility of HSCT, it is worth noting that HSCT might be an optimal treatment option for this fatal disease.
Pathophysiology
Genetic perspective
As of January 2018, 71 different CSF1R mutations (56 missense mutations, 8 splice-site mutations, 3 frameshift mutations, 2 nonsense mutations, and 2 small deletions) were found in more than 100 cases around the world (figure 2B). Apparently, there is no phenotype–genotype correlation. While this disease is an autosomal dominant trait, sporadic cases have often been reported. This may be partially explained by incomplete penetrancee30 (doi.org/10.5061/dryad.498j63f) and genetic mosaicisme27 . In addition, de novo mutations have been identified in sporadic cases, including a monozygotic twin pair.2,42, e30-e32 Almost all mutations were located in the tyrosine kinase domain (TKD) of CSF1R.23 While mutations have been found in any exons encoding the TKD, so far, they have been most frequently identified in exons 18 and 19. In vitro, ligand-induced autophosphorylation of CSF1R was not detected in cells expressing mutant CSF1R, indicating loss of CSF1R signaling is relevant to the disease.2,16,47,48,e33 Splice-site mutations produce aberrant splice variants lacking a part of TKD, supporting the loss of function hypothesis.2,41,44,48,e34,e35 Flow cytometry analysis showed that cell surface expression of mutant CSF1R was decreased compared to that of wild-type CSF1R in transfected cells,e33 but another study yielded sustained expression of mutant CSF1R.e36 Our cell surface biotinylation assay revealed that mutant CSF1R (p.I794T and an aberrant splice variant produced by c.2442+1 G > T) expressed on the cell surface comparable to the wild-type (Konno et al.,48). Mutant CSF1R does not inhibit autophosphorylation of wild-type CSF1R when the mutant CSF1R is transfected in cells stably expressing the wild-type CSF1R, suggesting that dominant-negative mechanism is unlikely.
Haploinsufficiency of CSF1R
Haploinsufficiency of CSF1R was evident in a patient with a frameshift variant (p.S688Efs*13) not located within the TKD, but within the kinase insert domain.48 Theoretically, the mutant mRNA was supposed to be degenerated by nonsense-mediated mRNA decaye37 (doi.org/10.5061/dryad.498j63f). To support this, mRNA and protein expression levels of CSF1R were indeed reduced in the patient's brain.48 Accordingly, frameshift or nonsense mutations that induce premature stop codon have been found outside the TKD.37, e38 Reduced expression of CSF1R was also observed in patients' brains with missense and splice-site mutations by immunohistochemistry,35,48,57,e5,e22 indicating that any type of CSF1R mutation may cause quantitative and functional loss of CSF1R, which underlies the pathogenesis of CSF1R-related leukoencephalopathy. A heterozygous truncating CSF1R mutation (p.Y540*) was identified in first-cousin parents from a study of consanguineous families.e38 Two of their children had lethal phenotype showing generalized osteopetrosis, Dandy-Walker malformation with agenesis of the corpus callosum, and extensive subependymal and periventricular calcifications. Homozygosity was not confirmed in these children due to unavailable samples; however, their phenotypes were similar to those of Csf1r-knockout mouse (Csf1r −/−).e39, e40 This report further supports the concept that the quantitative loss of CSF1R plays a crucial role in human brain integrity; the smaller the amount of CSF1R, the more severe the phenotype becomes.
Mouse model
A Csf1r haploinsufficient mouse model (Csf1r +/−) has been establishede25 (doi.org/10.5061/dryad.498j63f). In adulthood, these mice develop similar clinical findings observed in patients with CSF1R mutations, including cognitive decline, behavioral changes, and motor symptoms. By 12 months of age, white matter abnormalities, enlargement of the lateral ventricles, and thinning of the corpus callosum become evident on MRI. In addition, dysmyelination and axonal spheroids are revealed by electron microscopy. This mouse model provides strong evidence that CSF1R haploinsufficiency is enough to cause white matter degeneration.48,e25 However, there are several differences between human disease and the mouse model. Olfactory dysfunction, hypermyelination in the presymptomatic stage, and increased oligodendrocyte precursors in the cortex observed in the mouse modele25 have not been described or fully investigated in patients with CSF1R mutation. Interestingly, microglial density was increased in this Csf1r haploinsufficient mouse, inconsistent with findings in human patients.e22 An increased microglia in Csf1r +/− mice might induce microglial-mediated inflammation.4, e25 To our knowledge, no clear evidence of neuroinflammation has been shown in the brains of patients with CSF1R-related leukoencephalopathy. Therefore, this should be assessed further in human autopsied brains.
Putative mechanism by CSF1R mutations
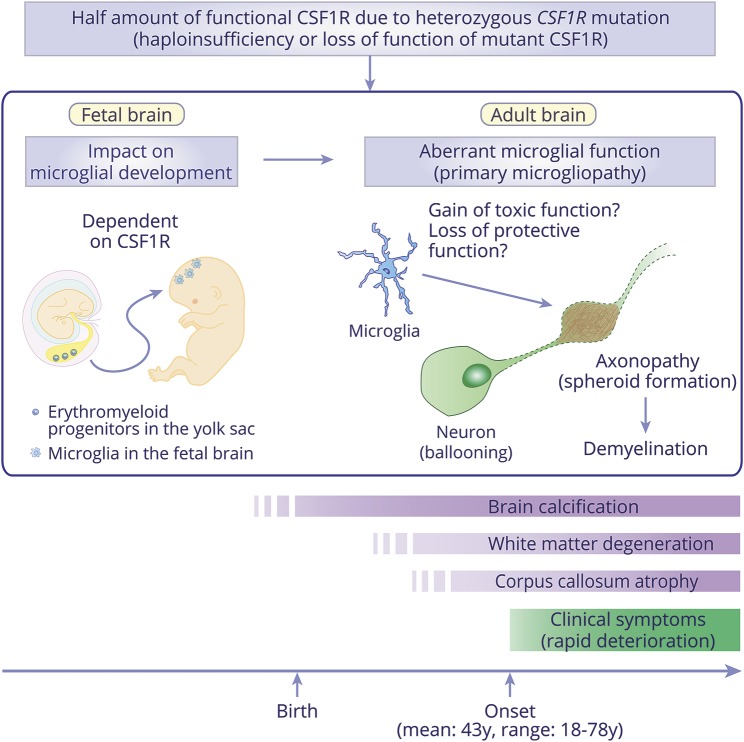
In mice, microglia originate from CSF1R-expressing erythromyeloid progenitors that appear in the yolk sac and migrate into the fetal brain during early embryogenesise41, e42 (doi.org/10.5061/dryad.498j63f). A recent study showed that a somatic mutation in erythromyeloid progenitors causes postnatal neurodegeneration through the activation of mutant microglia,e43 providing evidence that microgliopathy could stem from an early event in the erythromyeloid progenitors. CSF1R is an essential factor for development and maintenance of microglia.4,7 Csf1r-knockout mice showed nearly total loss of microglia, but peripheral monocytes were preserved, suggesting that microglial development is dependent on CSF1R.e39, e42 Taking these facts into account, we assume that microglial development and maturation in the fetal brain comprise quantitative and functional loss of CSF1R due to CSF1R mutations (figure 5). Given the heterozygous state of mutation carriers, a half amount of functional microglia may be sufficient to grow the carriers to adulthood; however, certain damages are presumed to insidiously accumulate in the brain white matter, or perhaps, only aging may affect the physiologic conditions of the brain with aberrant microglia. Once the threshold is exceeded, clinical symptoms develop in the 40s–50s and progress rapidly. Aberrant microglia that acquire toxic function or lose protective function may play a pivotal role in white matter degeneration (figure 5).
Figure 5. Microglia-oriented hypothesis for colony-stimulating factor-1 receptor (CSF1R)-related leukoencephalopathy.
Based on the haploinsufficiency or loss of function of mutant CSF1R, it is thought that microglia possess a half amount of functional CSF1R in patients with CSF1R-related leukoencephalopathy. This may influence not only microglial physiologic function in adult brains, but also microglial development in fetal brains since embryonic microglial development and maturation are dependent on CSF1R. Therefore, the character of microglia in patients may be somewhat different from individuals with a full amount of functional CSF1R, even at the time of birth. In adult brains, aberrant microglia may have a pivotal role in the white matter degeneration, which is characterized by primary axonopathy (spheroid formation shown in brown) and following demyelination (shown as dot lines surrounding the neuronal axon). Ballooned neurons are frequently found in the overlying cortex. Given that patients develop clinical symptom in adult, a half amount of functional CSF1R is considered to be sufficient for surviving to adulthood; however, white matter degeneration and corpus callosum atrophy could precede symptom onset. Importantly, brain calcification can be observed at birth, implying that the calcifications are independent of white matter degeneration or clinical symptoms.
Other cell contribution
Not as much as microglia, the CSF1R also expresses in other brain cells. It has been reported that CSF1R is expressed in a small number of neurons in the hippocampus and cortex, and its expression level is increased by brain injury. The upregulated CSF1R in neurons exerts a neuroprotective effecte44 (doi.org/10.5061/dryad.498j63f). Mutant CSF1R in neurons might fail to induce this neuroprotective effect and eventually exaggerate neurodegeneration. Nandi et al.e45 showed that during early postnatal development, CSF1R expressed in neuronal progenitor cells and contributed to regulation, proliferation, and neuronal differentiation of these cells. It is of interest whether CSF1R mutations affect neurogenesis and neuronal survival even at the beginning of life.4 Considering that the subventricular zone, where neurogenesis occurs, is close to the place where the brain calcifications are observed in CSF1R-related leukoencephalopathy,e45,e46 there might be a pathophysiologic link between neuronal progenitor cells and calcifications. Csf1r haploinsufficient mouse model would provide further insights regarding this point.
Other microgliopathies in humans
In recent years, the relatively new concept of microgliopathies6 has attracted attention since the role of microglia-related genes in neurologic disorders has been elucidated7,e47 (doi.org/10.5061/dryad.498j63f). In addition to CSF1R-related leukoencephalopathy, there are at least 2 human disorders in which microglia are likely to be affected primarily: NHD caused by homozygous or compound heterozygous mutations of triggering receptor expressed on myeloid cells 2 (TREM2) or TYRO protein tyrosine kinase-binding protein (TYROBP)e48,e49 and pseudo–toxoplasmosis, other agents, rubella, cytomegalovirus, and herpes simplex (TORCH) syndrome caused by homozygous or compound heterozygous mutation of ubiquitin-specific protease 18 (USP18).e50
TREM2 is a cell-surface receptor that expresses in myeloid cells and interacts with a transmembrane adaptor protein TYROBP. In the brain, the TREM2–TYROBP complex exclusively expresses in microgliae51 (doi.org/10.5061/dryad.498j63f). In NHD, loss-of-function mutations in TREM2/TYROBP are considered to result in malfunction of microglia and osteoclasts, leading to brain pathology and bone manifestations, respectively.e52-e54 USP18 negatively regulates type I interferon signaling.e55 In the mouse brain, USP18 abundantly expresses in microglia in the white matter and has a role in microglial quiescence.e56 In pseudo-TORCH, loss of function of USP18 is thought to induce white matter abnormality through type I interferon-mediated neuroinflammation following microglial activation.e56,e57 Both diseases, as well as CSF1R-related leukoencephalopathy, are clinically heterogeneous, but can be characterized by distinct white matter abnormalities, indicating that primary microglial malfunction may directly involve white matter changes. Moreover, brain calcifications are commonly seen in these diseases. In NHD, brain calcifications are usually observed in the basal ganglia, but occasionally found in the fronto-parietal subcortical white matter, reminiscent of those of CSF1R-related leukoencephalopathy.e58,e59 The subcortical and periventricular white matter are favorite sites of calcifications in pseudo-TORCH. These calcifications may occur secondary to brain damage, so-called dystrophic calcifications. However, given that calcifications in CSF1R-related leukoencephalopathy can be detected before clinical manifestation even early in life, it would be worth investigating whether mutant microglia are also primarily associated with the formation of calcified lesions independent of white matter change.
Discussion
Since the discovery of CSF1R mutations, CSF1R-related leukoencephalopathy has been increasingly recognized as a unique disease among adult-onset hereditary leukoencephalopathies. Moreover, it has attracted attention in terms of primary microgliopathy.5,7 Clinical, radiologic, and pathologic characteristics have since been elucidated; however, there are still many questions to be answered. Why do clinical symptoms of this disease only manifest in adults? What is the determinant yielding clinical difference between the sexes? Are there any biochemical or imaging biomarkers for predicting disease onset or progression? Are brain calcifications observed in the mouse model? How is the spheroid formation initiated? Are microglia truly dysfunctional? How do microglia and other cells contribute to pathogenesis? Are there any other possible treatments?
There are only a few studies focusing on molecular mechanism of this disease. Given the rarity of this illness, international collaboration should be encouraged to solve the questions and find an effective treatment. If the concept of microgliopathy is established without doubt for CSF1R-related leukoencephalopathy, development of microglia-targeted therapy will be an ideal and attractive approach that might be applicable in other microgliopathies. In addition, preventive interventions are desirable because asymptomatic mutation carriers can be found, even occasionally, by head CT scans showing the specific brain calcifications. In line with this, a longitudinal follow-up study of asymptomatic carriers is required not only to clarify the natural course of CSF1R-related leukoencephalopathy, but also to find biochemical or imaging biomarkers related to disease progression. Finally, there is no doubt that other genes are associated with CSF1R/AARS2-negative ALSP. A causative gene is still missing in the first Swedish family. Identifying these genes will open a new research field and facilitate understanding of adult-onset hereditary leukoencephalopathies.
Acknowledgment
The authors thank the patients and their families for their cooperation; Drs. Daita Kaneda (Kosaiin Fuzoku Hospital, Osaka, Japan), Yuichi Tashiro (National Hospital Organization Mito Medical Center, Ibaraki, Japan), and Takayoshi Tokutake (Brain Research Institute, Niigata University, Japan) for providing brain imaging data; Linda Rousseau and Virginia Phillips (Mayo Clinic Florida, Jacksonville) for histologic support; Monica Castanedes-Casey (Mayo Clinic Florida, Jacksonville) for immunohistochemistry support; and Audrey Strongosky (Mayo Clinic Florida, Jacksonville) for her assistance in recruitment of patients, maintaining the databases, and enrolling patients into the deeded Mayo Clinic Florida autopsy program.
Glossary
- AARS2-L
AARS2-related leukoencephalopathy
- ALSP
adult-onset leukodystrophy with neuroaxonal spheroids and pigmented glia
- bvFTD
behavioral variant frontotemporal dementia
- CI
confidence interval
- CSF1R
CSF1R gene encoding colony-stimulating factor-1 receptor
- HDLS
hereditary diffuse leukoencephalopathy with spheroids
- HSCT
hematopoietic stem cell transplantation
- IRB
institutional review board
- MS
multiple sclerosis
- NHD
Nasu-Hakola disease
- POLD
pigmentary orthochromatic leukodystrophy
- TKD
tyrosine kinase domain
- TORCH
toxoplasmosis, other agents, rubella, cytomegalovirus, and herpes simplex
- TREM2
triggering receptor expressed on myeloid cells 2
- TYROBP
TYRO protein tyrosine kinase-binding protein
- USP18
ubiquitin-specific protease 18
Author contributions
T. Konno: drafting the manuscript, design and conceptualization of the review, acquisition, analysis, and interpretation of data, and final approval of the manuscript. K. Kasanuki: reviewing the manuscript, acquisition and interpretation of data, creating a pathologic figure, and final approval of the manuscript. T. Ikeuchi: reviewing the manuscript and final approval of the manuscript. D.W. Dickson: reviewing the manuscript, creating a pathologic figure, and final approval of the manuscript. Z.K. Wszolek: reviewing the manuscript, design and conceptualization of the review, interpretation of the literature, and final approval of the manuscript.
Study funding
No targeted funding reported.
Disclosure
T. Konno receives research support from JSPS Overseas Research Fellowships and is partially supported by a gift from Carl Edward Bolch, Jr. and Susan Bass Bolch. K. Kasanuki and T. Ikeuchi report no disclosures relevant to the manuscript. D. Dickson is supported by the NIH/NINDS P50 NS072187, P50 AG16574. Z. Wszolek is partially supported by the NIH/NINDS P50 NS072187, NIH/NIA (primary) and NIH/NINDS (secondary) 1U01AG045390-01A1, Mayo Clinic Center for Regenerative Medicine, Mayo Clinic Neuroscience Focused Research Team (Cecilia and Dan Carmichael Family Foundation, and the James C. and Sarah K. Kennedy Fund for Neurodegenerative Disease Research at Mayo Clinic in Florida), a gift from The Sol Goldman Charitable Trust, and Donald G. and Jodi P. Heeringa. Go to Neurology.org/N for full disclosures.
Publication history
Received by Neurology February 26, 2018. Accepted in final form July 27, 2018.
References
- 1.Axelsson R, Röyttä M, Sourander P, Akesson HO, Andersen O. Hereditary diffuse leucoencephalopathy with spheroids. Acta Psychiatr Scand Suppl 1984;314:1–65. [PubMed] [Google Scholar]
- 2.Rademakers R, Baker M, Nicholson AM, et al. Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat Genet 2011;44:200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pixley FJ, Stanley ER. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol 2004;14:628–638. [DOI] [PubMed] [Google Scholar]
- 4.Chitu V, Gokhan Ş, Nandi S, Mehler MF, Stanley ER. Emerging roles for CSF-1 receptor and its ligands in the nervous system. Trends Neurosci 2016;39:378–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Knaap MS, Bugiani M. Leukodystrophies: a proposed classification system based on pathological changes and pathogenetic mechanisms. Acta Neuropathol 2017;134:351–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianchin MM, Martin KC, de Souza AC, de Oliveira MA, Rieder CR. Nasu-Hakola disease and primary microglial dysfunction. Nat Rev Neurol 2010;6:2. [DOI] [PubMed] [Google Scholar]
- 7.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 2014;15:300–312. [DOI] [PubMed] [Google Scholar]
- 8.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol 2010;6:193–201. [DOI] [PubMed] [Google Scholar]
- 9.Prieto-Morin C, Ayrignac X, Ellie E, Tournier-Lasserve E, Labauge P. CSF1R-related leukoencephalopathy mimicking primary progressive multiple sclerosis. J Neurol 2016;263:1864–1865. [DOI] [PubMed] [Google Scholar]
- 10.Van Bogaert L, Nyssen R. Le type tardif de la leukodystrophie progressive familiale. Rev Neurol 1936;65:21–45. [Google Scholar]
- 11.Röyttä M. The first neuropathological studies on HDLS. J Neuropathol Exp Neurol 2015;74:587. [DOI] [PubMed] [Google Scholar]
- 12.Marotti JD, Tobias S, Fratkin JD, Powers JM, Rhodes CH. Adult onset leukodystrophy with neuroaxonal spheroids and pigmented glia: report of a family, historical perspective, and review of the literature. Acta Neuropathol 2004;107:481–488. [DOI] [PubMed] [Google Scholar]
- 13.Wider C, Van Gerpen JA, DeArmond S, Shuster EA, Dickson DW, Wszolek ZK. Leukoencephalopathy with spheroids (HDLS) and pigmentary leukodystrophy (POLD): a single entity? Neurology 2009;72:1953–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali ZS, Van Der Voorn JP, Powers JM. A comparative morphologic analysis of adult onset leukodystrophy with neuroaxonal spheroids and pigmented glia: a role for oxidative damage. J Neuropathol Exp Neurol 2007;66:660–672. [DOI] [PubMed] [Google Scholar]
- 15.Itoh K, Shiga K, Shimizu K, Muranishi M, Nakagawa M, Fushiki S. Autosomal dominant leukodystrophy with axonal spheroids and pigmented glia: clinical and neuropathological characteristics. Acta Neuropathol 2006;111:39–45. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson AM, Baker MC, Finch NA, et al. CSF1R mutations link POLD and HDLS as a single disease entity. Neurology 2013;80:1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez-Vega I, Perez de Heredia-Goni K, Santos-Juanes J, et al. Sporadic adult-onset leucodystrophy with axonal spheroids and pigmented glia with no mutations in the known targeted genes. Histopathology 2016;68:308–312. [DOI] [PubMed] [Google Scholar]
- 18.Oboudiyat C, Bigio EH, Bonakdarpour B, et al. Diffuse leukoencephalopathy with spheroids presenting as primary progressive aphasia. Neurology 2015;85:652–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura T, Ishizawa K, Mitsufuji T, et al. A clinicopathological and genetic study of sporadic diffuse leukoencephalopathy with spheroids: a report of two cases. Neuropathol Appl Neurobiol 2013;39:837–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sundal C, Fujioka S, Van Gerpen JA, et al. Parkinsonian features in hereditary diffuse leukoencephalopathy with spheroids (HDLS) and CSF1R mutations. Parkinsonism Relat Disord 2013;19:869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynch DS, Zhang WJ, Lakshmanan R, et al. Analysis of mutations in AARS2 in a series of CSF1R-negative patients with adult-onset leukoencephalopathy with axonal spheroids and pigmented glia. JAMA Neurol 2016;73:1433–1439. [DOI] [PubMed] [Google Scholar]
- 22.Lakshmanan R, Adams ME, Lynch DS, et al. Redefining the phenotype of ALSP and AARS2 mutation-related leukodystrophy. Neurol Genet 2017;3:e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konno T, Yoshida K, Mizuno T, et al. Clinical and genetic characterization of adult-onset leukoencephalopathy with axonal spheroids and pigmented glia associated with CSF1R mutation. Eur J Neurol 2017;24:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dallabona C, Diodato D, Kevelam SH, et al. Novel (ovario) leukodystrophy related to AARS2 mutations. Neurology 2014;82:2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szpisjak L, Zsindely N, Engelhardt JI, Vecsei L, Kovacs GG, Klivenyi P. Novel AARS2 gene mutation producing leukodystrophy: a case report. J Hum Genet 2017;62:329–333. [DOI] [PubMed] [Google Scholar]
- 26.Marras C, Lang A, van de Warrenburg BP, et al. Nomenclature of genetic movement disorders: recommendations of the international Parkinson and movement disorder society task force. Mov Disord 2016;31:436–457. [DOI] [PubMed] [Google Scholar]
- 27.Salsano E. Leukodystrophy or genetic leukoencephalopathy? Nature does not make leaps. Mol Genet Metab 2015;114:491–493. [DOI] [PubMed] [Google Scholar]
- 28.Vanderver A, Prust M, Tonduti D, et al. Case definition and classification of leukodystrophies and leukoencephalopathies. Mol Genet Metab 2015;114:494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lynch DS, Jaunmuktane Z, Sheerin UM, et al. Hereditary leukoencephalopathy with axonal spheroids: a spectrum of phenotypes from CNS vasculitis to parkinsonism in an adult onset leukodystrophy series. J Neurol Neurosurg Psychiatry 2016;87:512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayrignac X, Carra-Dalliere C, Menjot de Champfleur N, et al. Adult-onset genetic leukoencephalopathies: a MRI pattern-based approach in a comprehensive study of 154 patients. Brain 2015;138:284–292. [DOI] [PubMed] [Google Scholar]
- 31.Ikeuchi T, Mezaki N, Miura T. Cognitive dysfunction and symptoms of movement disorders in adult-onset leukoencephalopathy with axonal spheroids and pigmented glia. Parkinsonism Relat Disord 2018;46(suppl 1):S39–S41. [DOI] [PubMed] [Google Scholar]
- 32.Battisti C, Di Donato I, Bianchi S, et al. Hereditary diffuse leukoencephalopathy with axonal spheroids: three patients with stroke-like presentation carrying new mutations in the CSF1R gene. J Neurol 2014;261:768–772. [DOI] [PubMed] [Google Scholar]
- 33.La Piana R, Webber A, Guiot MC, Del Pilar Cortes M, Brais B. A novel mutation in the CSF1R gene causes a variable leukoencephalopathy with spheroids. Neurogenetics 2014;15:289–294. [DOI] [PubMed] [Google Scholar]
- 34.Shu Y, Long L, Liao S, et al. Involvement of the optic nerve in mutated CSF1R-induced hereditary diffuse leukoencephalopathy with axonal spheroids. BMC Neurol 2016;16:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann S, Murrell J, Harms L, et al. Enlarging the nosological spectrum of hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS). Brain Pathol 2014;24:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Donato I, Stabile C, Bianchi S, et al. A novel CSF1R mutation in a patient with clinical and neuroradiological features of hereditary diffuse leukoencephalopathy with axonal spheroids. J Alzheimers Dis 2015;47:319–322. [DOI] [PubMed] [Google Scholar]
- 37.Guerreiro R, Kara E, Le Ber I, et al. Genetic analysis of inherited leukodystrophies: genotype-phenotype correlations in the CSF1R gene. JAMA Neurol 2013;70:875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inui T, Kawarai T, Fujita K, et al. A new CSF1R mutation presenting with an extensive white matter lesion mimicking primary progressive multiple sclerosis. J Neurol Sci 2013;334:192–195. [DOI] [PubMed] [Google Scholar]
- 39.Sundal C, Baker M, Karrenbauer V, et al. Hereditary diffuse leukoencephalopathy with spheroids with phenotype of primary progressive multiple sclerosis. Eur J Neurol 2015;22:328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu L, Liu J, Sha L, et al. Sporadic cases with novel mutations and pedigree in hereditary leukoencephalopathy with axonal spheroids. J Alzheimers Dis 2017;56:893–898. [DOI] [PubMed] [Google Scholar]
- 41.Konno T, Broderick DF, Mezaki N, et al. Diagnostic value of brain calcifications in adult-onset leukoencephalopathy with axonal spheroids and pigmented glia. AJNR Am J Neuroradiol 2017;38:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saitoh BY, Yamasaki R, Hayashi S, et al. A case of hereditary diffuse leukoencephalopathy with axonal spheroids caused by a de novo mutation in CSF1R masquerading as primary progressive multiple sclerosis. Mult Scler 2013;19:1367–1370. [DOI] [PubMed] [Google Scholar]
- 43.Kleinfeld K, Mobley B, Hedera P, Wegner A, Sriram S, Pawate S. Adult-onset leukoencephalopathy with neuroaxonal spheroids and pigmented glia: report of five cases and a new mutation. J Neurol 2013;260:558–571. [DOI] [PubMed] [Google Scholar]
- 44.Saitoh B-Y, Yoshida K, Hayashi S, et al. Sporadic hereditary diffuse leukoencephalopathy with axonal spheroids showing numerous lesions with restricted diffusivity caused by a novel splice site mutation in the CSF1R gene. Clin Exp Neuroimmunol 2013;4:76–81. [Google Scholar]
- 45.Kitani-Morii F, Kasai T, Tomonaga K, et al. Hereditary diffuse leukoencephalopathy with spheroids characterized by spastic hemiplegia preceding mental impairment. Intern Med 2014;53:1377–1380. [DOI] [PubMed] [Google Scholar]
- 46.Kim EJ, Shin JH, Lee JH, et al. Adult-onset leukoencephalopathy with axonal spheroids and pigmented glia linked CSF1R mutation: report of four Korean cases. J Neurol Sci 2015;349:232–238. [DOI] [PubMed] [Google Scholar]
- 47.Imai T, Kato B, Ohshima J, Hasegawa Y. Hereditary diffuse leukoencephalopathy with spheroids (HDLS) carrying a novel CSF1R mutation (p.Phe758Ser) with characteristic brain calcifications: a case report. Neurol Med 2017;87:670–675. [Google Scholar]
- 48.Konno T, Tada M, Tada M, et al. Haploinsufficiency of CSF-1R and clinicopathologic characterization in patients with HDLS. Neurology 2014;82:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Gerpen JA, Wider C, Broderick DF, Dickson DW, Brown LA, Wszolek ZK. Insights into the dynamics of hereditary diffuse leukoencephalopathy with axonal spheroids. Neurology 2008;71:925–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mateen FJ, Keegan BM, Krecke K, Parisi JE, Trenerry MR, Pittock SJ. Sporadic leucodystrophy with neuroaxonal spheroids: persistence of DWI changes and neurocognitive profiles: a case study. J Neurol Neurosurg Psychiatry 2010;81:619–622. [DOI] [PubMed] [Google Scholar]
- 51.Bender B, Klose U, Lindig T, et al. Imaging features in conventional MRI, spectroscopy and diffusion weighted images of hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS). J Neurol 2014;261:2351–2359. [DOI] [PubMed] [Google Scholar]
- 52.Sundal C, Van Gerpen JA, Nicholson AM, et al. MRI characteristics and scoring in HDLS due to CSF1R gene mutations. Neurology 2012;79:566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daida K, Nishioka K, Li Y, Nakajima S, Tanaka R, Hattori N. CSF1R mutation p.G589R and the distribution pattern of brain calcification. Intern Med 2017;56:2507–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fujioka S, Broderick DF, Sundal C, Baker MC, Rademakers R, Wszolek ZK. An adult-onset leukoencephalopathy with axonal spheroids and pigmented glia accompanied by brain calcifications: a case report and a literature review of brain calcifications disorders. J Neurol 2013;260:2665–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gore E, Manley A, Dees D, Appleby BS, Lerner AJ. A young-onset frontal dementia with dramatic calcifications due to a novel CSF1R mutation. Neurocase 2016;22:257–262. [DOI] [PubMed] [Google Scholar]
- 56.Konno T, Broderick DF, Wszolek ZK. Brain calcification in a CSF1R mutation carrier precedes white matter degeneration. Mov Disord 2017;32:1493–1495. [DOI] [PubMed] [Google Scholar]
- 57.Meyer-Ohlendorf M, Braczynski A, Al-Qaisi O, et al. Comprehensive diagnostics in a case of hereditary diffuse leukodystrophy with spheroids. BMC Neurol 2015;15:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okamoto M, Takeshita J, Takahashi K, Tanaka A, Yoshida K, Kuriyama M. Adult-onset leukoencephalopathy with axonal spheroids and pigmented glia: a case presented brain calcification and corpus callosum atrophy from over 10 years before the onset of dementia. Rinsho Shinkeigaku 2017;57:521–526. [DOI] [PubMed] [Google Scholar]
- 59.Terasawa Y, Osaki Y, Kawarai T, et al. Increasing and persistent DWI changes in a patient with hereditary diffuse leukoencephalopathy with spheroids. J Neurol Sci 2013;335:213–215. [DOI] [PubMed] [Google Scholar]
- 60.Blume J, Weissert R. Suspected perinatal depression revealed to be hereditary diffuse leukoencephalopathy with spheroids. J Mov Disord 2017;10:59–61. [DOI] [PMC free article] [PubMed] [Google Scholar]