Abstract
Background
Much new evidence on oral anticoagulation has come to light in recent years. Non-vitamin-K-dependent oral anticoagulants (NOAC) have been developed and have been introduced into clinical practice. In this review, we present the current state of the evidence on anticoagulation for various indications with vitamin K antagonists (VKA) and with NOAC.
Methods
This review is based on pertinent articles retrieved by a selective search in PubMed (search terms: anticoagulation, atrial fibrillation, prosthetic valve, thrombosis, pulmonary embolism) and on specialty society recommendations and relevant guidelines from the years 2000–2018.
Results
The main indications for oral anticoagulation are atrial fibrillation, venous thromboembolism, and status post heart valve replacement. In patients with atrial fibrillation and without valvular heart disease, anticoagulation is recommended for men with a CHA2DS2-VASc score = 1 and for women with a score = 2. NOAC for this indication are associated with a marginally lower rate of stroke than VKA (3.5% vs. 3.8%, number needed to treat [NNT] = 333) as well as a lower rate of major hemorrhage (5.1% vs. 6.2%, NNT = 91). NOAC are contraindicated for patients with mechanical heart valves. Anticoagulation with VKA can be predictably antagonized. Among the various types of NOAC, the anticoagulant effect of dabigatran can be safely antagonized with an antidote; no specific antidote is yet available for apixaban, rivaroxaban, or edoxaban.
Conclusion
The evidence base for anticoagulation over a time frame of several years is inadequate at present, and direct comparative data for the different types of NOAC are not yet available.
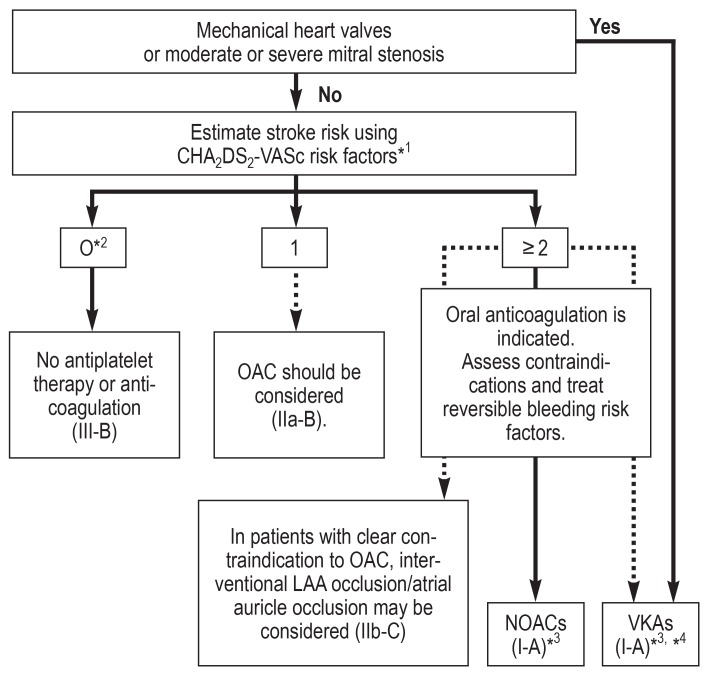
Atrial fibrillation is the most common cardiac arrhythmia, with an estimated prevalence in the adult population of approximately 3% and a significantly higher prevalence among older patients (1) and patients with comorbidities, such as hypertension, heart failure, coronary heart disease, valvular heart disease, diabetes mellitus, or chronic kidney disease (2). Atrial fibrillation is associated with an approximately twofold increase in overall mortality risk among women and a 1.5-fold increase among men; this means, for example, that the life expectancy of a male patient aged 55–64 years with atrial fibrillation is shortened by 5.5 years on average compared to men of the same age without atrial fibrillation (3). Furthermore, atrial fibrillation is associated with an increased rate of heart failure and stroke (4). Current studies have shown that atrial fibrillation was diagnosed in 20 to 30 % of all patients with ischemic stroke before, during or after a stroke event (5, 6). Oral anticoagulation therapy can prevent the majority of ischemic strokes in patients with atrial fibrillation (absolute risk reduction from 6.0% to 2.2%) and extend life (7). Oral anticoagulation is superior to no anticoagulation therapy or aspirin treatment (8). The net benefit applies to almost all patients, except for patients at very low risk of stroke. Consequently, oral anticoagulation is recommended to most patients with atrial fibrillation (figure 1) (2). Despite this strong body of evidence in support of oral anticoagulation therapy, only 46 % of patients with atrial fibrillation receive anticoagulation, according to data from a Swedish registry (1). Severe or less severe hemorrhagic events—especially among older patients—are frequently stated as reasons preventing the use of anticoagulation; thus, here it is crucial to balance risk of stroke and risk of bleeding, using a highly differentiated risk stratification approach. For this end, risk stratification schemes for risk of stroke and bleeding risk were established based on data from various cohorts. The indication for anticoagulation in patients with nonvalvular atrial fibrillation is established using the CHA2DS2VASc score (table 1). The use of the CHA2DS2-VASc score has been recommended in the European guidelines since 2010 and is a class I recommendation for risk stratification in patients with atrial fibrillation (9). Based on the CHA2DS2-VASc score, it is recommended that in the absence of risk factors (CHA2DS2-VASc score of 0 in males or 1 in females) no antiplatelet or anticoagulant therapy should be initiated. With a score of 1 in males or 2 in females, anticoagulation should be considered, weighing the individual bleeding risk against the risk of stroke. In males with a CHA2DS2-VASc score of 2 or females with a score of 3, the benefit of anticoagulation therapy for atrial fibrillation is supported by strong evidence (2).
Figure 1.
Recommendation for oral anticoagulation in patients with atrial fibrillation (according to [2])
*1 Chronic heart failure, hypertension, age = 75 years (2 points), diabetes mellitus, stroke/transient ischemic attack/thromboembolism (2 points), preexisting vascular condition, age 65–74 years, female sex
*2 Includes females without other stroke risk factors
*3 IIa-B in females with only 1 additional stroke risk factor
*4 I-B in patients with mechanical heart valve or mitral stenosis
LAA left atrial appendage
NOACs Non-vitamin K antagonist oral anticoagulants
OAC oral anticoagulation
VKAs Vitamin K antagonists
Grades of recommendation and levels of evidence:
Grades of recommendation:
I is recommended/is indicated
IIa should be considered
IIb may be considered
III is not recommended
Evidence level:
A Data from multiple randomized clinical trials or meta-analyses
B Data from 1 randomized clinical trial or multiple large non-randomized trials
C Consensus opinion of experts and/or small studies, retrospective studies or registries
Table 1. Individual thromboembolism risk (CHA2DS2-VASc score) with atrial fibrillation and bleeding risk (HAS-BLED score) (according to [2, 9, 39]).
| CHA2DS2-VASc score |
CHA2DS2-VASc score and risk of stroke per year without anticoagulation |
||
| Risk factor | Points | Score | Percent |
| Heart failure | 1 | 0 | 0% |
|
Hypertension Resting blood pressure >140/90 mm Hg (at least 2 measurements) or current antihypertensive treatment |
1 | 1 | 1.3% |
|
Age 75 years or older |
2 | 2 | 2.2% |
|
Diabetes mellitus Fasting blood glucose >125 mg/dL (7 mmol/L) or treatment with oral antidiabetic drugs and/or insulin |
1 | 3 | 3.2% |
|
Prior stroke, transient ischemic attack or thromboembolic event |
2 | 4 | 4.0% |
|
Vascular disease Prior myocardial infarction, peripheral vascular disease or aortic plaque |
1 | 5 | 6.7% |
|
Age 65–74 years |
1 | 6 | 9.8% |
|
Sex category female |
1 | 7 | 9.6% |
| 8 | 6.7% | ||
| Maximum score | 9 | 9 | 15.2% |
| HAS-BLED score |
HAS-BLED score and bleeding rate per year with anticoagulation |
||
| Risk factor | Points | Score | Percent |
| Hypertension | 1 | 0 | 0.9% |
| Abnormal renal and liver function | 1 or 2 | 1 | 3.4% |
| Stroke | 1 | 2 | 4.1% |
| Bleeding | 1 | 3 | 5.8% |
| Labile INRs | 1 | 4 | 8.9% |
| Age >65 years | 1 | 5 | 9.1% |
| Drugs that predispose bleeding/alcohol | 1 or 2 | 6–9 | insufficient data |
| Maximum score | 9 | ||
However, it should be noted that additional, less established risk factors, such as poor and unstable INR control in patients treated with vitamin K antagonists (VKAs) or a short time in therapeutic range (TTR) are currently not taken into account with this score. The same applies to other factors, including alcohol abuse, chronic renal failure or poor treatment adherence (2).
Currently, the individual bleeding risk is assessed using the HAS-BLED score (table 1) (9). As a general rule, a HAS-BLED score of = 3 indicates an increased bleeding risk; however, this does not mean that these patients should not receive oral anticoagulation for atrial fibrillation. Especially, it is recommended to identify risk factors for bleeding which can be treated and modified.
For patients with atrial flutter, principally the same recommendations for anticoagulation apply (2).
Anticoagulation for first-time atrial fibrillation
Occasionally, new-onset atrial fibrillation is first diagnosed while patients are assessed for other clinical events, such as surgical procedures, a stay in an intensive care unit or hyperthyroidism. These patients should usually receive anticoagulation based on the CHA2DS2-VASc score. However, if the triggering event is resolved (e.g. euthyroidism restored) or if the triggering event can be clearly attributed to another clinical condition/surgery/intervention, discontinuation of anticoagulation could be considered if no other episode of atrial fibrillation has occurred during the follow-up period (10).
Anticoagulation after cardiac valve replacement
For patients with mechanical heart valves, life-long oral anticoagulation with a vitamin K antagonist is recommended; however, there is only limited evidence from controlled trials available to support this recommendation (11). The target INR range has to be adapted taking into account the patient’s risk factors and the thrombogenicity of the valve. Here, the position and the type of artificial valve play a key role. Supported by strong evidence, INR patient self-testing is recommended as it helps to continuously achieve the INR target range and maximum TTR. Currently, non-vitamin K antagonist oral anticoagulants (NOACs) are contraindicated in the treatment of these patients (11). In patients with artificial heart valves experiencing a thromboembolic event despite adequate INR control, the additional administration of low-dose aspirin is recommended. After bioprosthetic valve replacement, life-long oral anticoagulation is only recommended in patients with a further indication for anticoagulation, such as atrial fibrillation or pulmonary embolism. After implantation of bioprosthetic valves in mitral or tricuspid position, anticoagulation with a VKA over a period of 3 months is an option to consider. The same applies to the situation after surgical repair of the mitral valve or tricuspid valve (11).
Anticoagulation to prevent venous thromboembolism
Venous thromboembolism (VTE) comprises proximal deep vein thrombosis (DVT) and pulmonary embolism (PE). With an incidence rate of 114 per 100 000 among males and 105 per 100 000 among females, VTE is among the third most common cardiovascular diseases (12). Besides initial acute parenteral treatment (unfractionated heparin [UFH], low-molecular-weight heparin [LMWH] or fondaparinux) during the first 5 to 10 days, oral anticoagulation is required for secondary prophylaxis. For patients with venous thromboembolism due to transient or reversible risk factors (such as surgical procedures, trauma, immobilization, pregnancy, oral contraceptives, or hormone therapy), anticoagulation for a period of 3 months is recommended (13, 14). The parenteral administration of heparin should overlap with the initiation of VKA treatment with a target INR range of 2.0 to 3.0 (evidence level B, reduction of event rate from 20.0% to 6.7%) (13, 15).
For patients with a first episode of unprovoked VTE, oral anticoagulation for at least 3 months is recommended; in this group, prolonged anticoagulation should be considered in patients with low bleeding risk. Further criteria indicating when anticoagulation should rather be prolonged include previously good quality of anticoagulation, increased d-dimer levels after end of treatment, male sex, a long thrombus, proximal thrombus location, existing residual thrombus, severe thrombophilia (e.g. antiphospholipid syndrome), and the patient’s preference to continue anticoagulation therapy (14). After the second episode of unprovoked VTE at the latest, indefinite anticoagulation is recommended (13).
As an alternative to vitamin K antagonists, NOACs can be used. In patients with VTE, however, there are differences in the initial treatment one should be aware of. If it is planned to use dabigatran or edoxaban for maintenance therapy, treatment is continued after the initial administration of UFH, LMWH or fondaparinux without overlapping after day 5 with the oral anticoagulant. If it is planned to treat the patient with apixaban or rivaroxaban, treatment with these medications can be started immediately after the patient has been diagnosed with VTE; yet, an increased initial dose is required for one and three weeks, respectively (13, 16– 18).
Differences between the substances
The choice of the anticoagulant is influenced by numerous factors. Since NOACs have not be been approved for the treatment of patients with valvular atrial fibrillation or artificial heart valve, VKAs continue to be the standard of care. For other indications, NOACs offer the significant advantage of a fixed-dose regimen without the need for routine coagulation monitoring. For the indication of nonvalvular atrial fibrillation, meta-analyses of all approved NOACs found a significantly improved protection against cardioembolic events compared to VKAs (absolute reduction from 3.8% to 3.1%), primarily due to lower intracranial bleeding rates (absolute reduction from 1.5% to 0.7%). Comparisons between various NOACs and VKAs showed significant event rate reductions for dabigatran and apixaban. Furthermore, a minor reduction in all-cause mortality in favor of NOACs was found compared to VKAs (absolute reduction from 7.7 % to 6.9%) while, according to significance, the major bleeding rate was the same (absolute reduction from 6.2% for VKA treatment to 5.1% for NOAC treatment); the single substances apixaban and edoxaban showed a significant advantage over VKA (table 2). In patients receiving NOACs, increased rates of gastrointestinal bleeding have been observed (absolute increase from 2.0% with VKA treatment to 2.6% with NOAC treatment) (19, 20). It is essential to take into account the fact that data from clinical studies must not be uncritically applied in everyday clinical practice where treatment decisions should be based on the specific assessment of the individual patient. In the differential therapy of VKAs versus NOACs for the treatment of atrial fibrillation, the quality of anticoagulation achieved with VKA is of importance since NOACs do not offer an advantage in patients on VKA who achieve good INR control (17). In addition, poor treatment adherence and the lack of an antidote for factor Xa inhibitors in emergency situations are reasons to rather choose VKA therapy.
Table 2. Analysis of phase III studies comparing all non-vitamin K antagonist oral anticoagulants (NOACs) with warfarin in patients with atrial fibrillation. The table shows risk ratio, absolute risk reduction (ARR) and the number needed to treat (NNT) [according to (19)].
| Study | RE-LY | ROCKET-AF | ARISTOTLE | ENGAGE AF-TIMI 48 | Combined | |||||||
| Outcome |
Dabigatran 150 mg |
Dabigatran 110 mg |
Warfarin |
Rivaroxaban 20 mg |
Warfarin |
Apixaban 5 mg |
Warfarin |
Edoxaban 60 mg |
Edoxaban 30 mg |
Warfarin | NOACs | Warfarin |
| Stroke/SEE | ||||||||||||
| n/N | 134/6076 (2.2%) |
183/6015 (3.0%) |
199/6022 (3.3%) |
269/7081 (3.8%) |
306/7090 (4.3%) |
212/9120 (2.3%) |
265/9081 (2.9%) |
296/7035 (4.2%) |
383/7034 (5.4%) |
337/7036 (4.8%) |
1477/42 361 (3.5%) |
1107/29 229 (3.8%) |
|
Risk ratio [95% CI] |
0.66 [0.53; 0.82] |
0.92 [0.76; 1.12] |
NA | 0.88 [0.75; 1.03] |
NA | 0.80 [0.67; 0.95] |
NA | 0.88 [0.75; 1.02] |
1.14 [0.99; 1.31] |
NA | 0.92 [0.86; 1.00] |
NA |
| ARR (NNT) | 1.1% (91) | 0.3% (382) | NA | 0.5% (194) | NA | 0.6% (169) | NA | 1.2% (172) | −0.6% (NA)*1 | NA | 0.3% (333) | NA |
| p value | 0.0001 | 0.41 | NA | 0.12 | NA | 0.012 | NA | 0.10 | 0.08 | NA | 0.0410 | NA |
| Major bleeding | ||||||||||||
| n/N | 375/6076 (6.2%) |
322/6015 (5.4%) |
397/6022 (6.6%) |
395/7111 (5.6%) |
386/7125 (5.4%) |
327/9088 (3.6%) |
462/9052 (5.1%) |
444/7012 (6.3%) |
292/7002 (4.2%) |
557/7012 (7.9%) |
2155/42 304(5.1%) | 1802/29 211(6.2%) |
|
Risk ratio [95% CI] |
0.94 [0.82; 1.07] |
0.81 [0.70; 0.94] |
NA | 1.03 [0.90; 1.18] |
NA | 0.71 [0.61; 0.81] |
NA | 0.80 [0.71; 0.90] |
0.53 [0.46; 0.60] |
NA | 0.83 [0.79; 0.89] |
NA |
| ARR (NNT) | 0.4% (238) | 1.2% (81) | NA | −0.2% (NA)*2 | NA | 1.5% (67) | NA | 1.6% (63) | 3.7% (27) | NA | 1.1% (94) | NA |
| p value | 0.34 | 0.004 | NA | 0.72 | NA | <0.0001 | NA | 0. 0002 | <0.0001 | NA | <0.0001 | NA |
*1 Number needed to harm = 153; *2 number needed to harm = 729
NA, not available; n/N, number events/total; SEE, systemic embolic event; 95% CI, 95% confidence interval
For the indication of venous thromboembolism, NOACs showed similar efficacy compared to VKAs (recurrent VTEs 2.0% with NOAC treatment, 2.2% with VKA treatment) along with reduced rates of bleeding complications; however, this advantage was small (number needed to treat [NNT]: 149 for major bleeding and 1111 for fatal bleeding) (21, 22).
Vitamin K antagonists
The group of vitamin K antagonists (VKAs) comprises phenprocoumon, acenocoumarol (not approved in Germany) and warfarin. In Germany, phenprocoumon is commonly prescribed and, to a lesser extent, warfarin. While most studies are based on warfarin, a similar effectiveness is assumed for the other substances in this group. VKAs mainly show differences in their half-lives (phenprocoumon 72–270 h, warfarin 36–42 h and acenocoumarol 8–24 h). Numerous drug-drug interactions resulting in an increased effect of VKAs have been described, including interactions with nonsteroidal anti-inflammatory drugs, tetracyclines, erythromycin, sulfonamides (e.g. sulfonylureas), valproate, allopurinol, and levothyroxine; by contrast, the effect of VKAs is reduced by rifampicin, azathioprine, carbamazepine, barbiturates, cholestyramine, and glucocorticoids. In addition, interactions with food have to be taken into account, such as reduction in the effect of VKAs related to the intake of alcohol and vitamin K-rich foods.
A major disadvantage of VKAs is their narrow therapeutic index and the little time patients actually are in the therapeutic INR range (TTR, time in therapeutic range). Even in well-designed studies comparing VKAs with NOACs, the mean TTR was only in the range of 55 to 65% (23– 26). INR self-management can increase TTR and is associated with a lower rate of thromboembolic events among patients receiving VKAs (absolute event reduction from 5.2% to 3.7%) (27).
Non-vitamin K antagonist oral anticoagulants
Non-vitamin K antagonist oral anticoagulants (NOACs) were developed as an alternative to VKAs for oral anticoagulation therapy in patients with nonvalvular atrial fibrillation and venous thromboembolism. The key advantage of NOACs over VKAs is that they allow for a fixed-dose regimen without the need for regular coagulation monitoring. Therefore, treatment with NOACs is especially useful in patients who, despite good compliance, tend to show considerable fluctuation in INRs with VKA treatment, who have drug-drug interactions with VKAs or in whom regular INR monitoring is difficult for logistic reasons. Among the 4 approved NOACs, it can be differentiated between the thrombin (coagulation factor IIa) inhibitor dabigatran and the direct factor Xa inhibitors rivaroxaban, apixaban and edoxaban (table 3) (2, 28, 29). Both patients receiving VKAs and those receiving NOACs should be provided with patient cards and be regularly monitored (30).
Table 3. Overview of the non-vitamin K antagonist oral anticoagulants (NOACs) (according to [2, 28]).
| Dabigatran | Apixaban | Edoxaban | Rivaroxaban | |
| Standard dosing* | 150 mg or 110 mg twice daily |
5 mg twice daily | 60 mg once daily | 20 mg once daily |
| Dose adjustment in patients with chronic kidney disease and for age, weight, co-medication* | No (creatinine clearance <30 ml/min contraindicated) |
2.5 mg twice daily in patients with atrial fibrillation and severe chronic kidney disease (creatinine clearance 15–29 mL/min) or at least 2 of the following criteria: age ≥ 80 years; body weight ≤ 60 kg or serum creatinine ≥ 1.5 mg/dL | 30 mg once daily, in the presence of the following factors: Creatinine clearance 15–49 mL/min, body weight ≤ 60 kg, co-medication with cyclosporine, dronedarone, erythromycin or ketoconazole | 15 mg once daily in patients with atrial fibrillation, if creatinine clearance 15–49 mL/min |
| Bioavailability | 3–7% | 50% | 62% | 66% without food, almost 100% with food |
| Prodrug | Yes | No | No | No |
| Non-renal/renal clearance of the absorbed dose (with normal renal function) | 20% / 80% | 73% / 27% | 50% / 50% | 65% / 35% |
| Hepatic drug metabolism: CYP450 involved | No | Yes (elimination; low CYP3A4 involvement) |
minimal (<10% of elimination) |
Yes (elimination) |
| Absorption with concomitant food intake | No effect | No effect | No effect | +39% more |
| Recommended to take with food? | No | No | No | Compulsory |
| Absorption with H2 blocker/proton pump inhibitor treatment | –12–30% | No effect | No effect | No effect |
| Asian patients | +25% | No effect | No effect | No effect |
| Gastrointestinal tolerability | Dyspepsia 5–10% |
No problems | No problems | No problems |
| Elimination half-life | 12–17 hrs | 12 hrs | 9–11 hrs | 5–9 hrs (younger) 11–13 hrs (older) |
| Specific antidote available | Yes | No | No | No |
* Detailed information of the respective summary of product characteristics should be taken into account.
The anticoagulant effect of dabigatran can be reliably estimated using the ecarin clotting time or, alternatively, using the widely available thrombin time (sensitive across the entire concentration range). The activated partial thromboplastin time (aPTT) increases with dabigatran administration; however, there is no linear relationship between intake and anticoagulant effect. By contrast, reliable monitoring of the effect of factor Xa inhibitors can only be achieved by a costly specific anti-factor Xa activity test. The standard coagulation parameters—Quick one-stage prothrombin time/INR and aPTT—are changed by the administration of factor Xa inhibitors (small or variable increase within the therapeutic range); however, both parameters do not allow reliable quantitative interpretation of the anticoagulant effect.
In patients with renal failure, rivaroxaban, apixaban and edoxaban can normally be prescribed up to a glomerular filtration rate (GFR) of 15 mL/min. In the relevant studies on all 4 NOACs, however, only patients with GFR = 30 mL/min (apixaban = 25 mL/min or creatinine <2.5 mg/dL) were included; consequently, meaningful data are only available for patients with moderately impaired renal function. Moreover, it should be noted that similar to the situation with low-molecular-weight heparins no specific antidote for the factor Xa inhibitors rivaroxaban, apixaban and edoxaban has (yet) become available. For the thrombin inhibitor dabigatran, the antidote idarucizumab is approved which rapidly and safely reverses the anticoagulant effect of the drug (31).
Bridging
Each year, 10% to 20% of all patients on oral anticoagulation require treatment interruption for surgery or interventional procedures (32, 33). Typically, perioperative bridging anticoagulation using unfractionated heparin or low-molecular-weight heparin is undertaken to prevent thromboembolic events during the period without oral anticoagulation. In recent years, several studies have questioned the safety and efficacy of this bridging anticoagulation. For example, the recently published randomized, double-blind BRIDGE study in which patients with moderate risk of thromboembolic events prior to surgical intervention on oral anticoagulation for atrial fibrillation were randomized to receive either dalteparin or placebo found only few thromboembolic events and no significant difference between placebo and dalteparin. However, patients treated with dalteparin showed a higher rate of hemorrhagic events (34). These data suggest that in patients with low to moderate risk anticoagulation should be interrupted and no bridging with low-molecular-weight heparin should be performed.
Moreover, other studies demonstrated that certain surgical procedure with low bleeding risk can be performed without interruption of VKA anticoagulation. These data refer to pacemaker or defibrillator implantations (35), extractions of teeth (36) and cataract surgery (37). Furthermore, NOAK anticoagulation is received by a large proportion of the patient in whom bridging of the interrupted treatment with parenteral anticoagulation appears to be unnecessary.
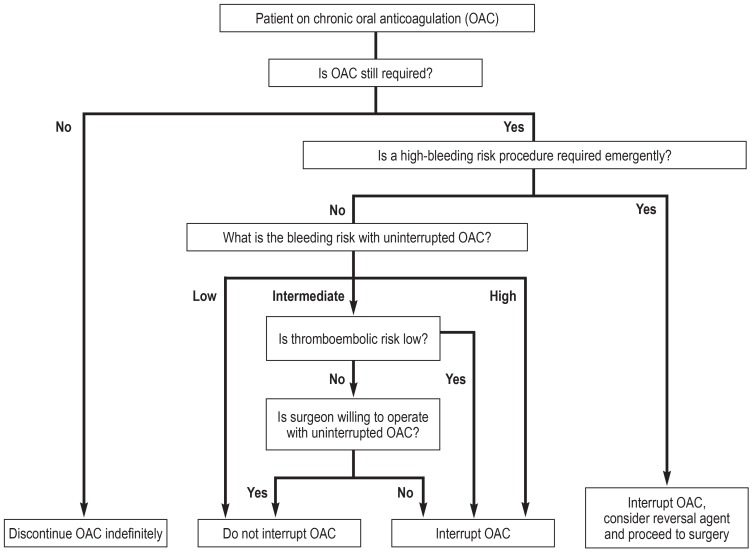
In the light of these data, risk stratification for perioperative management is recommended and should be adapted to the procedure and the individual risk (32). Decision-making for or against bridging should take into account the individual risk of thromboembolism, the individual bleeding risk and the bleeding risk associated with the surgery/procedure type (Figure 2) (32).
Figure 2A.
Decision tree for interruption of anticoagulation (adapted from [32])
For patients treated with NOACs, the following recommendations apply: No bridging with unfractionated heparin or low-molecular-weight heparin when NOAC treatment is interrupted; this also applies to patients with high thromboembolic risk. Preoperatively, it should be clearly defined for how many days treatment will be interrupted; for this decision, the factors renal function, age, individual bleeding risk, bleeding risk of surgery, and concomitant treatments, for example with aspirin, are to be considered (table 4). For procedures where complete and immediate hemostasis is achieved, it is recommended to restart treatment with NOACs after a period of 6 to 8 hours. For procedures associated with a high bleeding risk, it is recommended to restart NOAC treatment after a period of 48 to 72 hours; in this case, the aim is to provide venous thromboembolism prophylaxis, e.g. with low-molecular-weight heparin, 6 to 8 hours after surgery. Currently, no data on the effect of NOAC dose reduction are available. VKA therapy should not be bridged using NOACs (38).
Table 4. Management of last intake of non-vitamin K antagonist oral anticoagulants (NOACs) before elective procedures taking into consideration ?bleeding risk and renal function (adapted from [38]).
| Dabigatran | Apixaban – Edoxaban – Rivaroxaban | |||
| Creatinine clearance | Low bleeding risk | High bleeding risk | Low bleeding risk | High bleeding risk |
| ≥ 80 mL/min | ≥ 24 h | ≥ 48 h | ≥ 24 h | ≥ 48 h |
| 50–80 mL/min | ≥ 36 h | ≥ 72 h | ≥ 24 h | ≥ 48 h |
| 30–50 mL/min | ≥ 48 h | ≥ 96 h | ≥ 24 h | ≥ 48 h |
| 15–30 mL/min | Not indicated | Not indicated | ≥ 36 h | ≥ 48 h |
| <15 ml/min | No approved NOAC indication | |||
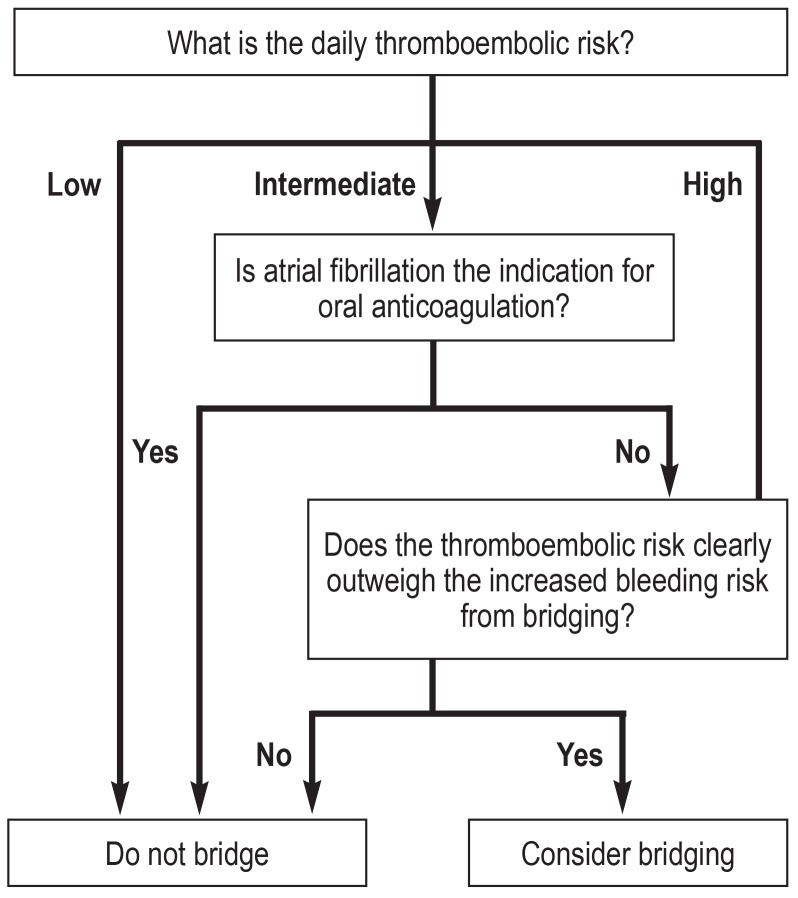
With bridging decision-making, the bleeding risk is frequently underestimated compared to the thromboembolic risk. Bridging therapy is indicated in patients with high thromboembolic risk. In patients with moderate thromboembolic risk, benefits should be carefully weighed against risks; patients with low thromboembolic risk treated with NOACs should not receive bridging anticoagulation.
Figure 2b.
Decision tree for bridging anticoagulation (adapted from [32])
Key Messages.
Atrial fibrillation, venous thromboembolism and cardiac valve replacement are the main indications for oral anticoagulation.
For atrial fibrillation, the use of the CHA2DS2-VASc score is recommended; anticoagulation is indicated if score = 1 for males and = 2 for females.
In patients with mechanical heart valves, life-long oral anticoagulation with vitamin K antagonists (VKAs) is recommended.
Non-vitamin K antagonist oral anticoagulants (NOACs) are considered an alternative to VKAs in patients with nonvalvular atrial fibrillation or venous thromboembolism.
Elective surgery or interventional procedures require an individual risk-benefit assessment to determine whether or not and, if yes, what type of bridging anticoagulation is indicated.
Acknowledgments
Translated from the original German by Ralf Thoene, MD
Footnotes
Conflict of interest
Dr. Altiok received reimbursement of meeting participation fees, travel expenses and accommodation expenses as well as lecture fees from Daiichi Sankyo. Fees for conducting clinical contract research studies from Bayer were passed on to the University Hospital RWTH Aachen.
Prof. Marx provided lecture and consulting services for Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, and Daiichi Sankyo. All of these fees were received by the University Hospital RWTH Aachen. Furthermore, he was responsible for third-party funding received by the University Hospital RWTH Aachen from Boehringer Ingelheim, Daiichi Sankyo and Pfizer.
References
- 1.Bjorck S, Palaszewski B, Friberg L, Bergfeldt L. Atrial fibrillation, stroke risk, and warfarin therapy revisited: a population-based study. Stroke. 2013;44:3103–3108. doi: 10.1161/STROKEAHA.113.002329. [DOI] [PubMed] [Google Scholar]
- 2.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Wolf PA, D‘Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 4.Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 5.Grond M, Jauss M, Hamann G, et al. Improved detection of silent atrial fibrillation using 72-hour Holter ECG in patients with ischemic stroke: a prospective multicenter cohort study. Stroke. 2013;44:3357–3364. doi: 10.1161/STROKEAHA.113.001884. [DOI] [PubMed] [Google Scholar]
- 6.Kishore A, Vail A, Majid A, et al. Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Stroke. 2014;45:520–526. doi: 10.1161/STROKEAHA.113.003433. [DOI] [PubMed] [Google Scholar]
- 7.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 8.Hart RG, Pearce LA, Aguilar MI. Adjusted-dose warfarin versus aspirin for preventing stroke in patients with atrial fibrillation. Ann Intern Med. 2007;147:590–592. doi: 10.7326/0003-4819-147-8-200710160-00018. [DOI] [PubMed] [Google Scholar]
- 9.Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 10.Diener HC, Aisenberg J, Ansell J, et al. Choosing a particular oral anticoagulant and dose for stroke prevention in individual patients with non-valvular atrial fibrillation: part 1. Eur Heart J. 2017;38:852–859. doi: 10.1093/eurheartj/ehv643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 12.Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28:370–372. doi: 10.1161/ATVBAHA.108.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–3069. doi: 10.1093/eurheartj/ehu283. 3069a-k. [DOI] [PubMed] [Google Scholar]
- 14.Gerlach H, Hach-Wunderle V, Konstantinides S, et al. S2-Leitlinie: Diagnostik und Therapie der Venenthrombose und der Lungenembolie. AWMF Leitlinien-Register: AWMF. 2015 [Google Scholar]
- 15.Brandjes DP, Heijboer H, Buller HR, de Rijk M, Jagt H, ten Cate JW. Acenocoumarol and heparin compared with acenocoumarol alone in the initial treatment of proximal-vein thrombosis. N Engl J Med. 1992;327:1485–1489. doi: 10.1056/NEJM199211193272103. [DOI] [PubMed] [Google Scholar]
- 16.Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 17.Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378:615–624. doi: 10.1056/NEJMoa1711948. [DOI] [PubMed] [Google Scholar]
- 18.Joung S, Robinson B. Venous thromboembolism in cancer patients in Christchurch, 1995-1999. N Z Med J. 2002;115:257–260. [PubMed] [Google Scholar]
- 19.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 20.López-López JA, Sterne JAC, Thom HHZ, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ. 2017;359 doi: 10.1136/bmj.j5058. j5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA, Huisman MV. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2014;12:320–328. doi: 10.1111/jth.12485. [DOI] [PubMed] [Google Scholar]
- 22.Robertson L, Kesteven P, McCaslin JE. Oral direct thrombin inhibitors or oral factor Xa inhibitors for the treatment of deep vein thrombosis. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD010956.pub2. CD010956. [DOI] [PubMed] [Google Scholar]
- 23.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 24.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 25.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 26.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 27.Heneghan C, Ward A, Perera R, et al. Self-monitoring of oral anticoagulation: systematic review and meta-analysis of individual patient data. Lancet. 2012;379:322–334. doi: 10.1016/S0140-6736(11)61294-4. [DOI] [PubMed] [Google Scholar]
- 28.Heidbuchel H, Verhamme P, Alings M, et al. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2013;15:625–651. doi: 10.1093/europace/eut083. [DOI] [PubMed] [Google Scholar]
- 29.Salazar CA, del Aguila D, Cordova EG. Direct thrombin inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in people with non-valvular atrial fibrillation. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD009893.pub2. CD009893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arzneimittelkommission der deutschen Ärzteschaft (AkdÄ) Leitfaden „Orale Antikoagulation bei nicht valvulärem Vorhofflimmern“. Empfehlungen zum Einsatz der direkten oralen Antikoagulanzien Dabigatran (Pradaxa®), Apixaban (Eliquis®), Edoxaban (Lixiana®) und Rivaroxaban (Xarelto®), 2. überarbeitete Auflage September 2016. www.akdae.de/Arzneimitteltherapie/LF/PDF/OAKVHF.pdf (last accessed on 12 October 2018) [Google Scholar]
- 31.Pollack CV Jr., Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal—full cohort analysis. N Engl J Med. 2017;377:431–441. doi: 10.1056/NEJMoa1707278. [DOI] [PubMed] [Google Scholar]
- 32.Rechenmacher SJ, Fang JC. Bridging anticoagulation: primum non nocere. J Am Coll Cardiol. 2015;66:1392–1403. doi: 10.1016/j.jacc.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Lange CM, Fichtlscherer S, Miesbach W, Zeuzem S, Albert J. The periprocedural management of anticoagulation and platelet aggregation inhibitors in endoscopic interventions. Dtsch Arztebl Int. 2016;113:129–135. doi: 10.3238/arztebl.2016.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823–833. doi: 10.1056/NEJMoa1501035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birnie DH, Healey JS, Wells GA, et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med. 2013;368:2084–2093. doi: 10.1056/NEJMoa1302946. [DOI] [PubMed] [Google Scholar]
- 36.Bajkin BV, Popovic SL, Selakovic SD. Randomized, prospective trial comparing bridging therapy using low-molecular-weight heparin with maintenance of oral anticoagulation during extraction of teeth. J Oral Maxillofac Surg. 2009;67:990–995. doi: 10.1016/j.joms.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 37.Schlitt A, Jámbor C, Spannagl M, Gogarten W, Schilling T, Zwißler B. The perioperative management of treatment with anticoagulants and platelet aggregation inhibitors. Dtsch Arztebl Int. 2013;110:525–532. doi: 10.3238/arztebl.2013.0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association practical guide on the use of non-vitamin-K antagonist anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J. 2017;38:2137–2149. doi: 10.1093/eurheartj/ehw058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly, Drugs/alcohol concomitantly) score. J Am Coll Cardiol. 2011;57:173–180. doi: 10.1016/j.jacc.2010.09.024. [DOI] [PubMed] [Google Scholar]