Abstract
The host-adapted human pathogen Neisseria gonorrhoeae is the causative agent of gonorrhea. Consistent with its proposed evolution from an ancestral commensal bacterium, N. gonorrhoeae has retained features that are common in commensals, but it has also developed unique features that are crucial to its pathogenesis. The continued worldwide incidence of gonorrheal infection, coupled with the rising resistance to antimicrobials, and with the difficulties in controlling the disease in developing countries, highlights the need to better understand the molecular basis of N. gonorrhoeae infection. This knowledge will facilitate disease prevention, surveillance and control, improve diagnostics and may help to facilitate the development of effective vaccines or new therapeutics. In this Review, we discuss gender-related symptomatic gonorrheal disease, and provide an overview of the bacterial factors that are important for the different stages of pathogenesis, including transmission, colonization and immune evasion, and discuss the problem of antibiotic resistance.
Subject categories: Biological sciences / Microbiology / Bacteriology [URI /631/326/1320], Biological sciences / Microbiology / Pathogens [URI /631/326/421], Biological sciences / Microbiology / Bacteria / Bacterial pathogenesis [URI /631/326/41/2531], Biological sciences / Microbiology / Bacteria / Bacterial immune evasion [URI /631/326/41/2534], Biological sciences / Microbiology / Bacteria / Bacterial host response [URI /631/326/41/2533], Biological sciences / Microbiology / Bacteria / Bacterial physiology [URI /631/326/41/1969], Biological sciences / Microbiology / Bacteria / Bacterial physiology / Antibacterial drug resistance [URI /631/326/41/1969/2038], Biological sciences / Microbiology / Clinical microbiology [URI /631/326/107], Biological sciences / Ecology / Microbial ecology [URI /631/158/855], Health sciences / Diseases / Infectious diseases / Bacterial infection [URI /692/699/255/1318]
Table of contents blurb
The host-adapted human pathogen Neisseria gonorrhoeae is the causative agent of gonorrhea. In this Review, Quillin and Seifert provide an overview of the bacterial factors that are important for the different stages of pathogenesis, including transmission, colonization and immune evasion, and discuss the problem of antibiotic resistance.
Introduction
Neisseria gonorrhoeae (also known as the gonococcus) is the etiologic agent of gonorrhea, a sexually transmitted infection (STI) that remains a major global public health concern. World Health Organization (WHO) surveillance of clinical strains of N. gonorrhoeae has identified strains that are resistant to most available antibiotics, highlighting the imminent possibility of widespread untreatable gonorrhea infections (Box 1). Treatment recommendations by the WHO are aimed both at elimination of the organism, and to prevent the further spread of antimicrobial-resistant gonorrhea1. With a worldwide incidence of over 78 million cases each year, uncontrolled transmission, and limited treatment options in low-income countries and poorer communities in developed countries, untreatable gonorrhea will result in increases in the incidence and complications from infection1-4.
Box 1: Diagnosis, incidence and epidemiology of Neisseria gonorrhoeae.
Historically, gonorrhea was diagnosed by Gram-stain of the purulent exudate from a patient, showing gram-negative diplococci among polymorphonuclear leukocytes (neutrophils). This method of diagnosis is still used in the developing world and in remote clinics, but in modern facilities, diagnosis is made using a variety of nucleic acid-based assays (for example, nucleic acid amplification tests that identify N. gonorrhoeae-specific nucleic acid signatures or, less commonly, antibody-based assays29. Often, diagnosis is confirmed by culture, growing isolates from clinical exudates on N. gonorrhoeae growth medium and examining growth and bacterial morphology. Primary clinical specimens are isolated on nonselective chocolate agar as well as selective agar containing antimicrobial compounds (vancomycin, colistin, trimethoprim lactate, nystatin, and anisomycin or amphotericin B) that stop the growth of other bacteria and fungi 151. Recently, whole genome sequencing has been employed to study N. gonorrhoeae epidemiology and the spread of resistance, and it has been discovered that genital meningococcal infections are often misdiagnosed as gonococcal infections using nucleic-acid based assays152,153.
In the developing world, where gonorrhea is most prevalent, limited resources for public health surveillance, limited self-reporting of sexually transmitted infection (STI) cases, and barriers to access to complete medical records can prevent accurate assessment of the burden of gonorrhea in the population. Nonetheless, in 2012, the World Health Organization (WHO) reported 78 million cases of gonorrhea occurring worldwide between people ages 15–49, which roughly correspond to 0.6% among men and 0.8% among women. As the number of asymptomatic infections and the number of people who do not seek treatment are unknown, it is likely that the actual number of infections is much higher. The highest prevalence for disease is estimated to occur within the Western Pacific and African regions. The WHO has developed guidelines for effective diagnosis, treatment, and dissemination of infection regarding diagnosis and treatment with the goal of reaching target populations, including: adults, adolescents aged 10–19, people living with HIV, sex workers, men who have sex with men (MSM), and transgendered persons. Untreated gonorrhea patients are at risk for infertility, pelvic inflammatory disease, and, rarely, disseminated gonococcal infection, as well as at risk for transmitting the disease. Gonorrhea is considered a nonulcerative STI, like chlamydia and trichomoniasis, and, along with other nonulcerative STIs, people with gonorrhea have a higher risk of HIV transmission to their partners, due to increased genital shedding of the virus in patients who are co-infected with HIV154,155.
N. gonorrhoeae mainly colonizes the genital mucosa, but it can also colonize the ocular, nasopharyngeal, and anal mucosa5-7. Pathology largely results from damage that is caused by the activation of innate immune responses at the sites of colonization as N. gonorrhoeae does not express potent exotoxins. Complications from untreated, ascending, female genital tract infections can include pelvic inflammatory disease, infertility, and ectopic pregnancy8. Maternal transmission to children during birth can also lead to neonatal blindness9. Untreated N. gonorrhoeae infection can also lead to disseminated gonococcal infection (DGI), potentially giving rise to infectious arthritis and endocarditis10.
N. gonorrhoeae belongs to the genus Neisseria, of which N. gonorrhoeae and Neisseria meningitidis (also known as the meningococcus) are the two pathogenic species, with the latter being a leading cause of bacterial meningitis11. In addition, at least eight non-pathogenic commensal Neisseria species make up a substantial proportion of the human nasal and oropharyngeal flora12. Other Neisseria spp. are able to colonize a range of non-human mammalian and non-mammalian hosts, such as non-human primates, dogs, cats, herbivorous mammals, dolphins, avian species, and insects13. Phylogenetic analyses show that N. gonorrhoeae and N. meningitidis evolved from a common non-pathogenic ancestor, but now represent separate lineages that normally occupy distinct niches, the genital mucosa and nasopharyngeal mucosa, respectively14-17. Although N. meningitidis can withstand dehydration, survive outside the human host for periods of time, and spread via respiratory droplet transmission. N. gonorrhoeae is unviable if dehydrated or exposed to non-physiological temperatures. The events that led to the evolution of two separate organisms that are highly similar in core genome and physiology, and yet cause markedly distinct diseases in different locations of the human body, are not yet understood. As both the commensal and pathogenic Neisseria spp. occupy the same niches, it is often difficult to differentiate colonization factors from virulence factors that are necessary to elicit host damage.
Since N. gonorrhoeae colonizes genital, rectal and oral mucosa, it expresses a repertoire of factors that allow replication and survival in these environmental niches, and factors that modulate and evade the host immune system. Understanding the mechanisms through which N. gonorrhoeae interacts with and evades the host immune system are necessary to facilitate better infection prevention, diagnostic development, surveillance, and the development of vaccines or new treatments. In this Review, we discuss the prevalence of asymptomatic infection in both genders, the main stages of gonococcal pathogenesis from transmission, colonization, adaptation to environmental conditions and immune evasion, and the rise in antimicrobial resistance. Although N. gonorrhoeae and N. meningitidis share many genetic and physiological features, this Review will focus on the host-adaptation and pathogenesis of N. gonorrhoeae.
Symptomatic and asymptomatic infections
Differences in the developmental embryological origins of cells lining the urogenital tracts of men and women have endowed these microenvironments with different surface molecules that act as receptors and co-receptors for N. gonorrhoeae, and lead to differences in the mechanisms by which N. gonorrhoeae survives in the male and female urogenital niches18. In addition, the prevailing dogma in the field is that female genital infections are mostly asymptomatic and male infections are mostly symptomatic18-20. However, there are many studies that show asymptomatic infections are common in both genders21-25. The long-held, highly-repeated supposition that female infections are mostly asymptomatic and males are symptomatic is mainly based on the fact that overt symptoms (that is, inducing immune cell influx and inflammation) in males are easier to diagnose, due to the purulent exudate from the penis and resultant painful urination. Clinical manifestations in women are more likely to go unnoticed, as inflammation does not occur in the same niche as urination and thus is less likely to be painful. Moreover, symptoms of gonorrheal infection in women are more likely to be nonspecific, as the vaginal discharge that is caused by neutrophil influx may be mistaken for bacterial vaginosis, yeast infection, hormonal variation in vaginal secretions, or normal variability in secretions26. Data on the antibody, cytokine, and chemokine composition and general magnitude of the inflammatory response in women is sparse and inconclusive. One clinical study of responses to N. gonorrhoeae in individuals and one study of the response of immortalized vaginal and cervical epithelial cells in culture have resulted in different views. Analyses of the cervical mucus of infected and uninfected women found a lack of strong IgA1 induction, a slight reduction of IgG levels and an absence of proinflammatory IL-1, IL-6, and IL-8 cytokines in infected individuals compared with uninfected individuals27, suggesting there was no fulminant inflammatory response to infection. By contrast, analyses of immortalized vaginal and cervical epithelial cells in vitro showed increased levels of IL-1, IL-6, and IL-8 when infected with N. gonorrhoeae, suggesting that there could be an inflammatory cytokine response during infection28. Due to the widespread prevalence of asymptomatic infections in men and women, it is also plausible that a detectable antibody or cytokine response in genital secretions may not result in detectable physiological symptoms. We suggest that the idea that infections in women are normally asymptomatic more likely reflects differences between the anatomy of the urogenital tract in men and women. To fully understand the epidemiology of gonorrhea, surveillance and diagnostic tests need to be improved for both genders25 to enable faster and less expensive responses to gonorrhea (Box 1).
Transmission
Transmission is often the most understudied stage of infections and this is also true for N. gonorrhoeae infections. A successful pathogen must be able to efficiently transmit to new hosts, and as an obligate human colonizer, N. gonorrhoeae cannot survive outside the host. Transmission between hosts relies on sexual networks to spread the pathogen from the core, high-risk population in which the majority of infections occur, to the fringe, medium-risk group that transmits N. gonorrhoeae back to the core group, and to their partners. High-risk populations include individuals with multiple sexual partners, and individuals who have unprotected sex29. Individuals are also often unaware that they are part of a larger sexual network. N. gonorrhoeae attaches to sperm30,31 and is easily transmitted from men to their partners through the ejaculates, as they contain a high number of bacteria32. However, how the efficiency of transmission from women to their partners is maintained is less apparent. The surface of N. gonorrhoeae must be free of sialic acid to successfully bind and enter urethral epithelial cells of men, and so it is thought that bacterial sialidases, which are secreted by the cervicovaginal microbiota of women, must first desialylate N. gonorrhoeae lipooligosaccharide (LOS) to enable efficient transmission from women to men33.
Establishment of infection
Adherence, colonization and invasion
Following transmission, N. gonorrhoeae establishes contact with the mucosal epithelium to replicate and ultimately transmit to new hosts. N. gonorrhoeae is primarily a mucosal colonizer, attaching to various epithelial surfaces. The primary event establishing infection and the first step in pathogenesis is the bacterial adherence to the epithelium of the mucosa, which is mediated through distinct bacterial surface structures (Figure 1) that include Type IV pili, opacity (Opa) proteins, the LOS, and the major porin, also referred to as PorB. During initial infection, following initial host cell interaction, N. gonorrhoeae attachment and subsequent colonization depends largely on Type IV pili forming microcolonies on the epithelial cell surface34. Type IV pili are outer membrane structures that are crucial for mediating initial cellular adherence, natural transformation competence, twitching motility and immune evasion through antigenic and phase variation35-39. Adherence to the epithelial surface and subsequent pilus retraction bring the gonococci close to the cell surface.
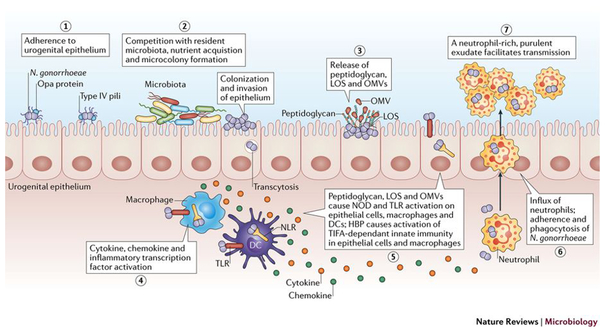
Figure 1: Overview of Neisseria gonorrhoeae infection.
During initial infection, N. gonorrhoeae adheres to host epithelial cells through Type IV pili (step 1), which retract and enable epithelial interactions with other prominent surface structures160,161. After initial adherence, N. gonorrhoeae replicates and forms microcolonies (step 2), and possibly biofilms34,49, and likely competes with the resident microbiota. When colonizing the epithelium, N. gonorrhoeae is capable of invasion and transcytosis. During these initial stages in infection, N. gonorrhoeae releases fragments of peptidoglycan, lipooligosaccharide (LOS), and outer membrane vesicles(OMVs)111,162,163 (step 3) that activate Toll-like receptor (TLR) and nucleotide-binding oligomerization domain-like receptor (NOD) signaling in epithelial cells, macrophages, and dendritic cells (DCs)111,164,165. NOD and TLR signaling from these cells leads to activation of inflammatory transcription factors and the release of cytokines and chemokines (step 4). N. gonorrhoeae also releases heptose-1,7-bisphosphate (HBP) that activates TRAF-interacting protein with forkhead-associated domain (TIFA) immunity115 (step 5). The release of pro-inflammatory cytokines and chemokines by these innate immune signaling pathways creates cytokine and chemokine gradients that recruit large numbers of polymorphonuclear leukocytes (PMNs) neutrophils to the site of infection (step 6), where they interact with and phagocytose N. gonorrhoeae. The influx of neutrophils comprises a purulent exudate that then facilitates tranmission (step 7).
Interactions between Opa proteins with carcinoembryonic antigen-related cell adhesion molecule (CEACAM) receptors and other molecules, like heparin sulfate, are important for adherence, and the Opa-CEACAM interaction may be one of the major adherence interactions. 32,40-42. Opa proteins are abundant outer membrane proteins that mediate adherence after initial contact by Type IV pili, as well as immune evasion by multigene phase variation that result in antigenic variation 43-45. Type IV pili and Opa proteins are expressed during infection of both women and men40,41,46-48, and are considered essential for the colonization of the mucosal epithelium of the genital tract and other sites of infection (Figure 2a). N. gonorrhoeae can form biofilms on abiotic surfaces and epithelial cells in vitro49,50; however, the precise role of biofilms during infection remains to be determined50-52; it is not known whether stable mucosal colonization during infection is mediated by microcolonies, biofilms, or a combination of both.
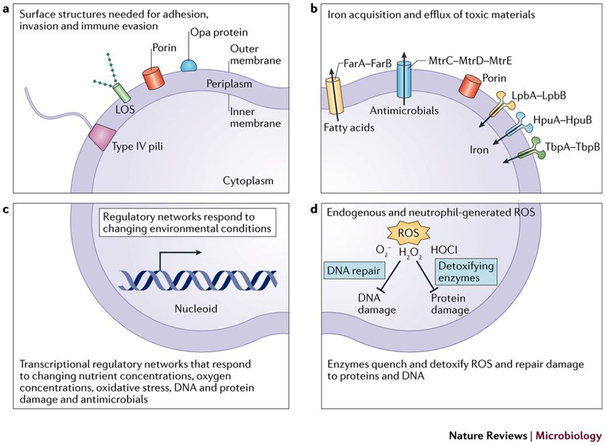
Figure 2: Overview of Neisseria gonorrhoeae pathogenesis factors.
As a host-restricted pathogen, N. gonorrhoeae encodes a relatively small repertoire of pathogenesis and colonization factors compared to other gram-negative bacteria166. a) N. gonorrhoeae uses an array of surface structures to adhere to host cells, occasionally invade host cells, and evade the immune system18,142,143. These surface structures include Type IV pili, LOS, porin, and Opa proteins. b) Efflux pumps protect N. gonorrhoeae from antimicrobials and fatty acid stress, and membrane transporters allow N. gonorrhoeae to coopt nutrients from the surrounding environment66,141,167-169. Pump FarAB controls fatty acid transport, while pump MtrCDE controls antimicrobial peptides. Pumps LpbAB, HpuAB, and TbpAB contribute to iron transport and iron homeostasis. c) A set of transcriptional regulators, discussed in detail in the main text and Figure 3, induce transcriptional programs to adapt and respond to changing environmental conditions during infection82,127,170-173. The regulons that respond to iron levels, oxidative conditions, and oxygen concentration are co-regulated and interconnected. d) Protective enzymes like catalase and MsrAB detoxify reactive oxygen species (ROS), like superoxide anion O2−, hydrogen peroxide H2O2, and hypochlorous acid HOCl, that are generated endogenously and by neutrophils86.
Prominent surface factors porin and LOS also affect colonization. Porin is a nutrient channel and is one of the most abundant gonococcal outer membrane proteins, binds complement factors C4bp and Factor H, and suppresses the neutrophil oxidative burst and neutrophil apoptosis53. LOS is localized to the outer leaflet of the outer membrane and is similar in structure to the ubiquitous bacterial lipopolysaccharide (LPS), although LOS lacks the O-antigen polymer54. LOS is important for adherence and invasion of host cells, variations in LOS affect immune cell recognition, and sialyation of LOS affects serum resistance through complement evasion and host transmission54-56. In addition to colonization of the mucosal epithelium, N. gonorrhoeae can invade epithelial cells. Although less is known about mucosal cell invasion compared to surface colonization, it has been shown that N. gonorrhoeae invades nonciliated cervical epithelial cells and the urethral epithelial cells of men when LOS is desialylated18. It is thought that the interaction between LOS and the asialoglycoprotein receptor promotes epithelial invasion in the urethra of men, whereas CR3 serves as the receptor that mediates invasion in the lower cervical genital tract, and LHr serves as the receptor in the endometrium and fallopian environments. This invasion of the epithelium and the resultant transcytosis of the epithelium could lead to DGI, but the relevance of epithelial cell invasion and transcytosis to uncomplicated infections is less clear. The differences in receptors that mediate epithelial invasion highlights the complex, multifaceted nature of tissues lining genital tract, a central part of the difficulty in establishing appropriate animal and tissue culture models to study a host-restricted pathogen (Box 2).
Box 2: Neisseria gonorrhoeae infection models.
As N. gonorrhoeae has evolved to survive and persist only within the human body, there are limitations to understanding the complexities of the environmental challenges that the bacteria encounter during infection in humans. These limitations may include, but are not limited to, the specific types and local concentrations of specific nutrients (for example, iron, manganese, zinc, glucose, lactate and pyruvate) that are present during infection, the oxygen concentration at different sites during colonization, the heterogeneity of cytokine and chemokine responses between individuals and their immune cell profiles, and the composition of local microbiota that is encountered during infection. Currently, the interaction of N. gonorrhoeae with specific types of host cells (epithelial cells, endothelial cells, neutrophils and macrophages) can be studied ex vivo by using immortalized or primary cell lines. However, immortalized cell lines do not always model human tissues and the heterogeneity of primary human cells can introduce variability into these studies114. The most well-developed mouse model is the estradiol-treated vaginal infection model156 but this model is limited to addressing certain questions, specifically, which factors affect short-term colonization or survival of N. gonorrhoeae in the murine vaginal tract, due to the lack of receptors (CR3, CD46, and CEACAM) that are required for adherence and subsequent colonization to the mouse mucosal epithelium, and other differences in host physiology. The ongoing development of transgenic mouse models that express human receptors will enable the study of N. gonorrhoeae pathogenesis however, these models may not be able to replicate all of the factors that are required for human infection157-159.
Growth and metabolism
Once N. gonorrhoeae adheres to the mucosal epithelium, efficient colonization requires extracellular bacterial replication and nutrient acquisition from the surrounding extracellular milieu. It has not been thoroughly determined which microenvironments are encountered during colonization and thus the exact nutrient composition of each ecological niche that N. gonorrhoeae may inhabit during urogenital, rectal, and orapharyngeal infection is unknown. In laboratory culture, N. gonorrhoeae has complex media requirements. Specifically, bacteria cannot grow in culture without a supplemented source of glucose, glutamine, thiamine, phosphate, iron and carbon dioxide57-59. In order to meet its nutritional requirements, N. gonorrhoeae must interact and possibly compete with resident microbiota for available nutrients60-62 (Figure 1). Indeed, N. gonorrhoeae must acquire nutrients like iron, zinc, and manganese (Figure 3) that are limited by the human host as a defense against bacterial pathogens in a process termed nutritional immunity63,64. As Neisseria spp. lack siderophores, N. gonorrhoeae scavenges iron directly from host-bound complexes, obtaining metals through a series of membrane transport complexes by transporting them into the bacterial cell65-68 (Figure 2). Finally, the influx of neutrophils that occurs during symptomatic colonization (Figure 1), may promote nutrient acquisition by causing leakage of serum components, tissue damage, and exposing N. gonorrhoeae to intracellular nutrient pools following phagocytosis, thus providing nutrients for bacterial growth69-73,74.
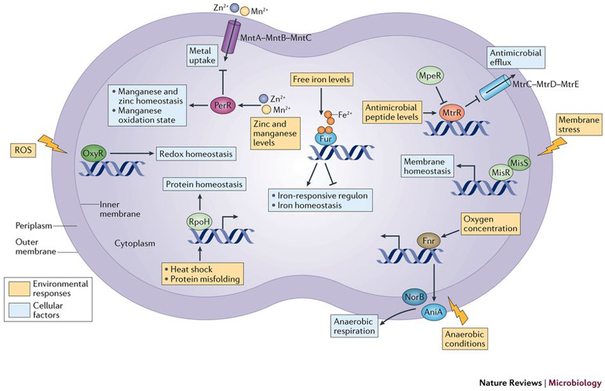
Figure 3: Characterized transcriptional regulatory factors of Neisseria gonorrhoeae.
To adapt to a changing urogenital, rectal, and oropharyngeal environment during infection, N. gonorrhoeae has relatively only a few regulatory networks. N. gonorrhoeae has regulators that specifically respond to metal availability, antimicrobial peptides, oxygen availability, membrane stress, and protein misfolding. These systems are often overlapping, particularly at the level of iron and oxygen availability. Transcriptional regulator MpeR is known to repress the antimicrobial efflux pump operon repressor MtrR; both regulators mediate antimicrobial peptide efflux76,78. Two-component regulatory system composed of sensor histidine kinase and response regulator MisS-MisR respond to membrane perturbations and control membrane homeostasis174. Oxygen-sensor FNR responds to oxygen concentrations and contributes to regulation of genes encoding AniA and NorB, which control a denitrification system required for anaerobic respiration82,171. Iron-response master regulator Fur responds to fluctuating iron levels and controls iron homeostasis under iron-replete and iron-starvation conditions175. PerR responds to fluctuating zinc and manganese levels, controlling metal influx through MntABC and zinc and manganese homeostasis172. RpoH responds to heat shock and protein misfolding and controls a regulon that maintains protein-folding homeostasis 173. OxyR responds to the presence of reactive oxygen species and maintains redox homeostasis176.
Regulatory networks
In order to survive and replicate, N. gonorrhoeae must adapt to changing environmental conditions within the host genital tract, rectum, and oropharynx (Figure 3). The limitations to current N. gonorrhoeae experimental models have prevented gains in specific knowledge of pH, nutrient and oxygen concentrations in the varying ecological niches of the genital mucosa, however, the range of adaptive mechanisms that the bacterium has acquired and maintained over evolutionary time indicate the main contributing environmental changes that may affect survival during infection. These mechanisms regulate global transcriptional changes through transcriptional regulators and two-component systems (Figure 2C, Figure 3), translational regulation through regulatory sRNAs, and clonal variation through phase variation.
For specific environmental conditions (for example, metal availability, oxygen concentrations, ROS, protein misfolding, membrane stress, and the presence of antimicrobial peptides (Figure 2b,c,d), N. gonorrhoeae utilizes an array of responsive transcriptional factors to activate and repress small-scale and large-scale adaptive transcriptional programs (Figure 3). During infection, N. gonorrhoeae encounters antimicrobial peptides. Transcriptional regulators MtrR, MtrA, and MpeR contribute to regulation of an antimicrobial efflux pump MtrCDE, which exports antimicrobial peptides75-78. RpoH maintains protein homeostasis and the two-component system MisRS responds to membrane stress79. Portions of the human genital mucosa presents a microaerophilic environment, but different subcellular locations may vary in oxygen concentration. N. gonorrhoeae is capable of either aerobic or anaerobic respiration controlled through a truncated denitrification pathway that is regulated by AniA and NorB80,81. The oxygen-sensing fumarate-nitrate reduction (FNR) regulator is required for activation of this pathway, in addition to the two-component system NarQ-NarP82. Intracellular iron homeostasis is maintained via the Fur regulator, and there is substantial overlap between the iron, anaerobic, and oxidation-responsive regulons, highlighting the fine-tuned and interconnected nature of the relatively small adaptive regulatory networks in N. gonorrhoeae83,84,85,86.
N. gonorrhoeae encodes a specialized repertoire of 34 putative transcriptional regulators, two-component systems, and sRNAs. These systems are relatively small in number, compared to the ~200 transcriptional regulators, two-component systems, and sRNAs found in Escherichia coli. The small number of these regulons in N. gonorrhoeae is likely due to the organism’s long evolutionary history solely colonizing the human host, thereby limiting the diversity of environmental conditions it may encounter and regulons it may require to survive. In addition, a large number of N. gonorrhoeae genes are stochastically modulated by phase variation87, likely reducing the need for transcriptional regulation. During phase variation, Neisseria spp. modulate protein production. Protein production may be altered through changes in transcription efficiency or changes in translation efficiency. Transcription efficiency is altered by varying the numbers of polynucleotide repeat sequences in genes, thus tuning the levels of gene expression. Translation efficiency is altered through changes in gene coding sequence, like the introduction of stop codons or repeat sequences, thus tuning protein production. N. gonorrhoeae is estimated to phase vary over 100 genes that encode a variety of gene products88. Phase variation presumably provides different advantages to the numerous subpopulations that result during colonization. The opa gene family is the most well-characterized phase variable system, both mechanistically and functionally89. Opa phase variation occurs through changes in the number of CTCTT repeats in the leader peptide sequence of each of 11 opa alleles in the chromosome, resulting in altered expression of these genes, affecting host cell adherence and neutrophil stimulation. The ability of N. gonorrhoeae to generate a repertoire of different phenotypes within a clonal lineage promotes long-term adaptation on the bacterial population level. In addition to phase variation of Opa proteins, N. gonorrhoeae also encodes a phase variable methyltransferase, Mod13A that switches between an on and off state of gene expression, regulated changes in polynucleotide repeats that occur during replication. Differences in the amount of Mod13A activity results in altered methylation patterns of promoters and gene expression levels of various genes. This phenomenon is called the Mod13A phasevarion, and influences virulence factor gene expression and biofilm formation90-94. N. gonorrhoeae proteins may also be globally regulated by post-translational modifications, and acetylation has been shown to affect numerous pathways, anaerobic growth, and ability to form biofilms95. Lastly, it is known that N. gonorrhoeae expresses sRNAs. Although many N. gonorrhoeae regulatory sRNAs have not been characterized mechanistically, it is known that the sRNA NrrF is transcribed in response to iron availability and controls a small regulon96 and another sRNA, FnrS, controls a regulon of four genes in response to anaerobic conditions84.
Interactions with the host immune system
Interactions with Complement
The alternative and classical complement pathways are major arms of the innate immune system that converge at the level of protein C3, which can lead to the deposition of opsonin C3b to facilitate bacterial phagocytosis and kill invading pathogens through the formation of membrane attack complexes. The alternative and classical complement pathways are activated by the presence of invading microorganisms, the former by non-specific tissue damage or microorganism binding and the latter through IgG and IgM antibody deposition that leads to clearance by the immune system. Activation of both pathways converges with cleavage and activation of protein C3, which is crucial for maintaining a cascade that results in the assembly of membrane attack complexes, transmembrane pores that form on bacterial surfaces causing lysis and cell death.
The ability of N. gonorrhoeae to evade recognition and attack from the human complement system is a major feature of host adaptation by this species, highlighted by the observation that N. gonorrhoeae resists the action of human complement but is sensitive to animal complement systems97. Patients with complement deficiencies have been found to have a higher risk of systemic N. gonorrhoeae infection98,99. In both the cervical epithelium and human serum, the alternative and classical complement pathways respond to N. gonorrhoeae infection by initiating the complement cascade to opsonize invading bacteria18,100-102. Studies in vitro have shown that N. gonorrhoeae interacts with several complement components103. N. gonorrhoeae evades complement-mediated killing through two general mechanisms: binding to and inactivating complement cascade components and preventing membrane attack complex formation, and presenting as ‘self’ by expressing molecules found in the host on the bacterial surface, and binding to complement regulatory proteins. (Figure 4a).
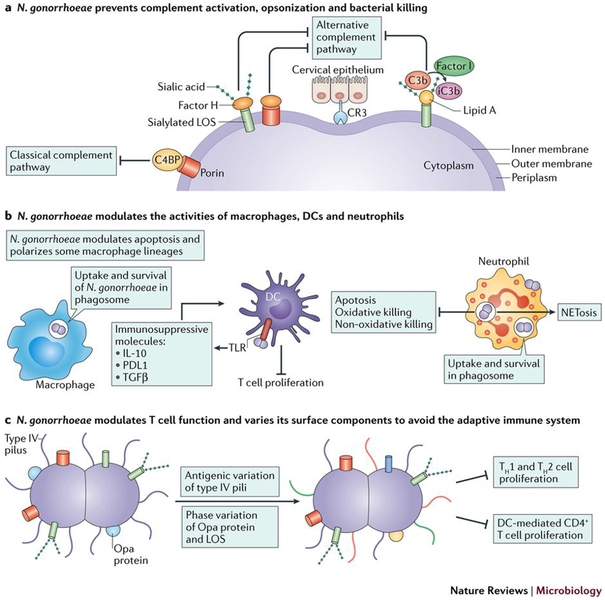
Figure 4: Neisseria gonorrhoeae evades and modulates the innate and adaptive immune system.
a) During infection, both the alternative and classical complement pathways may be activated by N. gonorrhoeae. N. gonorrhoeae binds complement proteins to prevent opsonization and killing by membrane attack complexes18, as well as siaylates its LOS to hide from the complement system177. N. gonorrhoeae binds host Factor H and C4bp, becoming serum resistant by presenting as ‘self’ and shielding itself from complement recognition97,178 N. gonorrhoeae also binds to the alternative complement pathway receptor CR3 and the receptor for iC3b, a process thought to aid in epithelial cell invasion100. N. gonorrhoeae binds C3b through Lipid A on its LOS, rapidly inactivating C3b to iC3b through factor I179. b) N. gonorrhoeae is able to survive in and around macrophages and neutrophils during infection, and modulate the immune activating properties of dendritic cells114,116,122,124,136,164,180. In macrophages, N. gonorrhoeae is able to survive inside the phagosome and modulate apoptosis and production of inflammatory cytokines 114. The bacteria polarize macrophages, resulting in macrophages less capable of T cell activation116, and similarly, dendritic cells exposed to N. gonorrhoeae are less capable of stimulating T cell proliferation136. The interactions of N. gonorrhoeae and neutrophils is complex and is discussed in detail in the text. c) N. gonorrhoeae infection does not generate immunological memory, due to the ability of N. gonorrhoeae to antigenically and phase vary its surface structures including Type IV pili, Opa proteins, and LOS. In addition, N. gonorrhoeae modulates the adaptive immune response by suppressing T helper cell proliferation and subsequent activation through influencing cytokine production134,135,181.
In the first mechanism, N. gonorrhoeae inactivates the complement cascade through factor I. C3b binds to gonococcal LOS through Lipid A, and is rapidly inactivated by factor I-mediated cleavage to iC3b, thus inactivating the complement cascade (Figure 4a)101. In addition, in the cervical epithelium, N. gonorrhoeae binds to the alternative complement pathway receptor CR3 and the receptor for iC3b, which is thought to facilitate epithelial cell invasion100,102.
In the second mechanism, N. gonorrhoeae shields itself from complement recognition, thus subverting complement activation in both the cervical epithelium and in human serum. In the cervical epithelium, N. gonorrhoeae binds alternative complement pathway regulator Factor H through sialylated LOS and porin (Figure 4a). Normally, Factor H acts as an alternative complement pathway regulatory protein that binds sialylated cell structures to protect cells, as host structures bound to Factor H are considered ‘self’ and not targeted for opsonization and lysis104,105. In the serum, N. gonorrhoeae can bind the classical complement pathway regulator C4bp, a molecule which has a similar function as Factor H, to the porin (Figure 4a)106. Moreover, N. gonorrhoeae can bind complement regulatory factor CD46 through the pilus, though the role of this interaction in pathogenesis is not fully defined107. Without the ability to avoid complement recognition and killing, N. gonorrhoeae would not be able to effectively colonize epithelial mucosa and grow, as shown by its sensitivity to killing by animal complement components.
Immune Cell Detection and Signaling
Due to lack of surveillance and difficulty in diagnosing asymptomatic gonorrhea, little is known about how the immune system responds to N. gonorrhoeae during asymptomatic infection, though these infections likely represent a high and likely underreported proportion of infections. Symptomatic infection stimulates the release of pro-inflammatory cytokines and chemokines IL-6, IL-8, IL-1B, IL-17, interferon gamma, and cytokine-expression controlling transcription factor NF-κB, causing an influx of neutrophils to the site of infection, and potentially causing inflammatory damage within the epithelial mucosa74,108-110 (Figure 1). During colonization, the presence of bacterial factors like LOS and peptidoglycan are capable of triggering their cognate innate immune sensors TLR2, TLR4, NOD1, and NOD2, all inducing signaling of the host innate immune system111-113 (Figure 1). In addition, detection by immune sentinel cells like macrophages and dendritic cells55,114 releases a gradient of cytokines and chemokines IL-6, IL-8, IL-1B, IL-17, interferon gamma, and NF-kB, thus resulting in influx of neutrophils. N. gonorrhoeae has also been shown to release heptose-1,7-bisphosphate (HBP), a metabolic intermediate that acts as a pathogen-associated molecular pattern (PAMP) to trigger Traf-interacting protein with forkhead-associated domain (TIFA)-dependent innate immunity115 (Figure 1). Moreover, N. gonorrhoeae can survive within macrophages by modulating apoptosis and cytokine production114, and may polarize macrophages in a way that suppresses T cell proliferation (Figure 4b)116.
As discussed earlier in this Review, N. gonorrhoeae colonization may result in either symptomatic or asymptomatic infection. Symptomatic infection occurs when there is sufficient neutrophil influx into the site of infection to produce a purulent exudate. Although it is known that the presence of a purulent exudate is a result of bacterial innate immune stimulation through cytokine and chemokine signaling neutrophil influx, it is not known whether asymptomatic patients also recruit neutrophils to the site of infection. It is possible that N. gonorrhoeae recruit neutrophils in numbers that are insufficient to produce observable symptoms, but it is also possible that neutrophils are not recruited to the site of infection during asymptomatic colonization. Differences in N. gonorrhoeae Opa variants and their propensity for CEACAM binding and immune stimulation may explain the heterogeneity of symptoms in infected individuals. Opa-CAECAM interactions determine neutrophil adhesion, phagocytosis, and stimulation of the oxidative burst. The N. gonorrhoeae cell surface may lack Opa proteins entirely (Opa-less), express one Opa variant only, or possibly a combination of many Opa alleles. It is known that during human infection there is variability of Opa expression in N. gonorrhoeae, ranging from multiple Opa-expressing to Opa-less strains and Opa expression is correlated with the menstrual cycle47. It is not known whether particular Opa expression patterns predominate during asymptomatic or symptomatic infection. Although CEACAM1, CEACAM3, CEACAM5, and CEACAM6 bind to N. gonorrhoeae Opa variants, only CAECAM1, CEACAM3, and CEACAM5 are expressed on neutrophils117 The Opa-CAECAM3 interaction is the only one known to stimulate a bactericidal neutrophil oxidative burst. Although the underlying mechanism for why some infections elicit observable symptoms and some do not is unknown, it has been proposed that a subset of the bacterial population capable of Opa-CEACAM3 binding induces neutrophil killing of sufficient number of bacteria to prevent massive neutrophil influx and an observable purulent exudate. There is evidence that neutrophils use CEACAM3 as a decoy receptor, as bacterial contact with CEACAM family of receptors enables colonization of other cells types, but on neutrophils enables capture of Opa-expressing variants for subsequent phagocytosis, neutrophil activation, and killing118,119.
Arguments as to whether neutrophil infiltration to sites of infection primarily benefits the host or pathogen
Similarly, although it is known that N. gonorrhoeae is able to both evade and modulate host immune responses, there is disagreement whether neutrophil recruitment ultimately serves to benefit the host or the pathogen or both. One hypothesis is that N. gonorrhoeae aim to remain undetected by the immune system because immune stimulation ultimately benefits the host. This hypothesis is supported by the observation of prevalent asymptomatic infections in humans, the observation that asymptomatic partners are infectious, and data showing bacterial shedding does not correlate with neutrophil infiltration in mice120. An alternative hypothesis is that neutrophil inflammation at sites of infection serves primarily to benefit the pathogen, largely through facilitating transmission. In this scenario, the neutrophil exudate carries live, replicating bacteria, by analogy to a Trojan horse74. Data supporting the argument that neutrophil inflammation benefits the pathogen shows that, although the presence of N. gonorrhoeae recruits neutrophils to the site of infection, most bacteria, (except those expressing Opa variants that engage CEACAM3 on neutrophils) are able to survive and replicate inside and outside of neutrophils86,121-127. In this scenario, it is possible that the transfer of infected neutrophils between partners through sexual contact with purulent exudate is a mechanism that the bacterium exploits for efficient transmission from women to their partners74. Several components of seminal plasma, like lactoferrin77, are chemoattractants for neutrophils128. However, testing any transmission hypothesis is difficult since human transmission studies are unethical and there is no animal model that accurately models sexual transmission. The ambiguity of the role of neutrophils in disease pathogenesis stems from the heterogeneous nature of interactions between N. gonorrhoeae and neutrophils, lack of knowledge regarding what causes some infections to be symptomatic whereas others show symptoms, and limitations to experimental models. Box 2 addresses the usefulness and limitations to current models that are used to study mechanisms of N. gonorrhoeae pathogenesis. As this is a system in equilibrium, it is probable that specific host-pathogen interactions sway the equilibrium in either direction, ultimately determining whether neutrophil influx benefits the host or the pathogen for each individual infection.
Adaptive Immunity and Issues with Vaccine Development
It is known that individuals that have been treated for gonorrhea can be repeatedly infected, with no development of immunological memory. An experimental gonococcal infection model study in men showed that initial infection failed to provide protection against repeated infection with the identical strain within 21 days of initial infection129. N. gonorrhoeae evades the adaptive arm of the human immune system by several mechanisms. N. gonorrhoeae undergoes antigenic and phase variation of the surface-exposed Type IV pili, Opa proteins, and LOS to escape immunity45,48,130 (Figure 4c). The carbohydrate structures of LOS also have a role in immune evasion by mimicking host molecules. Many N. gonorrhoeae lipooligosaccharides show cross reactivity with antibodies that recognize human glycosphingolipid surface antigens, particularly on human erythrocytes, mimicking human surface antigens and contributing to the difficulty of vaccine development131-133. In addition, N. gonorrhoeae has been shown to actively suppress the adaptive immune response by modulating IL-10 production from mouse iliac lymph node cells, CD4+ T cells and genital tract explants by modulating TGF-β cytokine production in BALB/c mouse vaginal cells, and type 1 regulatory T cell activity from CD4+ T cells, thus preventing T helper 1 and T helper 2 cell development134,135. Moreover, dendritic cells that have been exposed to N. gonorrhoeae are no longer capable of inducing CD4+ T cell proliferation136. Whereas N. gonorrhoeae stimulates a large innate immune response from the human host and suppresses the adaptive immune response, these interactions of N. gonorrhoeae on both arms of the immune system can both extend infections and allow repeated infections to the high-risk group of the population137.
Strategies that have searched for protective antigens by comparing infected individuals have been unsuccessful for N. gonorrhoeae vaccine development since natural protection is uncommon if it exists at all. The ability of N. gonorrhoeae to antigenically and phase vary multiple surface proteins has reduced the number of viable vaccine antigen candidates. Vaccine development has also been hampered by the lack of a global systematic vaccine antigen analysis where many antigen candidates are consistently tested in a high-throughput manner, as compared to the few than have been tested in disparate experiments. Purified pili and killed whole cells have been tested, but neither has resulted in a viable vaccine138. The lack of viable vaccine antigen candidates, combined with limitations to the current animal models, have also impeded progress. A recently published study from New Zealand, wherein young adults were inoculated with a group B outer membrane vesicle meningococcal vaccine, showed reduced rates of gonorrhea in that population139. This is the first time a vaccine has shown reduced rates of gonorrhea in those inoculated, and follow-up studies are needed to determine whether the vaccine confers true protection or if the reduced rates are not actually in response to the vaccination. If a viable vaccine were generated, producing and distributing a substantial amount of vaccine to reduce worldwide gonorrhea prevalence would be a large economic challenge and unlikely to be undertaken by the pharmaceutical industry. The necessary follow-up studies, production, and distribution of a viable vaccine will require nonprofit and government support for funding.
Antimicrobial resistance
Without an effective vaccine, antibiotics have been the only effective method for controlling gonorrhea, but the efficacy of antibiotics is now in question. The main molecular mechanisms that are used by bacteria to develop antimicrobial resistance are: protective alteration of antibiotic targets, decreased influx of antibiotics into the cell through transport proteins, increased efflux out of the cell via multi-drug efflux pumps, and expression of antibiotic degrading enzymes. Different strains of N. gonorrhoeae have evolved numerous resistance determinants using all these mechanisms to inhibit killing by all major classes of antibiotics (Figure 5a). There has been substantial research into β-lactam resistance mechanisms of N. gonorrhoeae. Transpeptidase penicillin binding protein 2 (PBP2), encoded by the penA gene is a periplasmic transpeptidase and the main lethal target of cephalosporins; most resistant isolates contain mosaic mutations in penA140. Pump MtrCDE and its repressor MtrR contribute to N. gonorrhoeae resistance through antimicrobial efflux141. Variants of the major porin protein, encoded by porB, contribute to resistance to β-lactams, but resistance requires a concomitant mutation in mtrR142. Interestingly, when all known resistance determinants are transformed into a susceptible recipient strain from a resistant strain, the transformants do not reconstitute the full level of resistance of the donor strain, suggesting the presence of one or more undiscovered factors that cannot be transferred to a recipient strain through transformation143. Moreover, expression levels of the Mtr efflux pump, controlled by MtrR, affects bacterial fitness during vaginal infection of female mice through an unknown mechanism144. This is an important example of how the development of antibiotic resistance can influence fitness, as historically the field has treated bacterial pathogenesis as a separate area of study from antimicrobial resistance. In addition, a greater understanding of N. gonorrhoeae antibiotic resistance determinants, especially in the context of increased fitness by enhancement of colonization or pathogenesis, is important due to the high potential for horizontal gene transfer between pathogenic and commensal Neisseria spp. within the human host, indicating a potential reservoir for antimicrobial resistant genes.
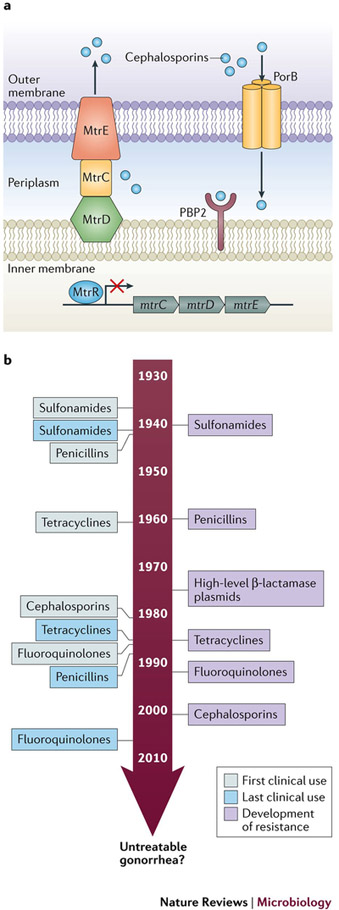
Figure 5: Antibiotic resistance in Neisseria gonorrhoeae a. Main resistance determinants of N. gonorrhoeae.
Transpeptidase penicillin binding protein 2 (PBP2), encoded by penA is a periplasmic transpeptidase and the main lethal target of cephalosporins; most resistant isolates contain mosaic mutations in penA. The efflux pump, MtrCDE, composed of subunits MtrE, MtrC, and MtrD, and its repressor MtrR contribute to N. gonorrhoeae resistance through antimicrobial efflux. The major porin protein, PorB, encoded by penB, is also a main resistance determinant that cannot manifest independently, but requires concomitant mutation in mtrR. b. Timeline of antibiotic resistance development. Since the treatment of N. gonorrhoeae with sulfonamides in the 1930s, N. gonorrhoeae have acquired genetic resistance determinants that prevent killing by all major classes of antibiotics that are used as first line methods of treatment for gonorrhea infection143,145,147,182. As shown in the timeline, each new class of antibiotics that served as first line treatment for N. gonorrhoeae have been stopped as all strains gained resistance. Recently, resistance was observed for the last available first line treatment for N. gonorrhoeae infection, the extended-spectrum cephalosporins143. The ability of N. gonorrhoeae to evolve resistance has lead the World Health Organization and Center for Disease Control, United States to term it a ‘superbug’, and speculate that, if new therapies are not developed soon, we may face an era of untreatable antimicrobial resistant gonorrhea3.
The human history of antibiotic development is matched by the history of N. gonorrhoeae developing and retaining resistance to all new effective antibiotics (Figure 5b). These antimicrobials include sulfonamides, penicillins, tetracyclines, macrolides, and fluoroquinolones143,145. Recent treatment failures using cefixime and ceftriaxone, and the extended-spectrum cephalosporins, β-lactam antibiotics that are used as the last available first line treatment for gonorrhea, have highlighted the potential for untreatable gonorrhea to become a widespread public health epidemic146. Due to lack of quality metadata on individuals that have been infected with N. gonorrhoeae, their sexual networks, and phenotypic and genotypic characteristics of the gonococcal population, we can only speculate on the reasons for antimicrobial resistance emergence and spread. Antimicrobial resistance has likely been facilitated by unrestricted access to and over-prescription of antimicrobials, particularly in the WHO’s West Pacific Region147. In addition, N. gonorrhoeae is naturally competent for transformation, thus it is able to take up gonococcal DNA, and to a lesser extent other bacterial DNA, from the environment, and recombine it efficiently with homologous sequences in the gonococcal genome148,149. The high propensity for N. gonorrhoeae to take up DNA from the environment adds to the likelihood that N. gonorrhoeae genes encoding antibiotic resistance determinants will mutate and become resistant. Transformation can produce mosaic alleles in genes that represent antimicrobial resistance determinants, wherein two orthologous or paralogous genes combine to form a mosaic, resistant, variant gene150. In addition, mutations can arise within a gene, conferring antibiotic resistance to that gene product. Based on previous observations it is reasonable to predict the spread of N. gonorrhoeae that is resistant to cephalosporins will continue to increase. Suspected multi-drug resistant strains are genotyped by multilocus sequence typing (MLST) and N. gonorrhoeae multi-antigen sequence typing (NG-MAST), but it important to continue hypothesis-driven molecular research to understand the molecular mechanisms of action for N. gonorrhoeae antibiotic resistance determinants. Indeed, this will enable the development of novel therapeutics and heighten our understanding of how resistance persists in a population and spreads between strains.
Conclusions
In this Review, we have summarized our knowledge of the course of N. gonorrhoeae pathogenesis, from transmission, adherence, colonization and invasion, to evasion of the innate and adaptive immunity. We have emphasized the difficulty in studying a host-adapted human pathogen. Due to the multifaceted nature of the urogenital tract, rectum, and oropharynx, composed of many different types of epithelial tissue, with innate immune cell composition varying by individual and unknown concentrations of oxygen and nutrients, substantial challenges exist to develop tissue culture and animal models to study the obligate human pathogen N. gonorrhoeae. Advances in standardizing primary tissue culture cell techniques and transgenic mouse models may help to ameliorate these challenges. In addition, we have argued that asymptomatic infections are common in men and women. Though the prevailing dogma currently holds that infections in women are mainly asymptomatic whereas infections in men are not, many studies show asymptomatic infections are prevalent in both sexes. We argue that the prevailing hypothesis more likely stems from physiological and anatomical differences in the urogenital tract between genders, making neutrophil influx in males much more obvious and easier to diagnose compared with gonorrhea in women.
Due to the host-restricted life cycle of N. gonorrhoeae and the limitations of existing tissue culture and animal model systems, many niches of the microenvironments the gonococci inhabits and replicates within are yet unknown. Particularly, the nutrient and oxygen availability, the magnitude of innate immune responses, and the microbiota composition that is specific to the different sites within the male and female genital tracts may be markedly different, requiring N. gonorrhoeae to adopt distinct adaptive programs in response to local conditions as infection progresses. Deep sequencing of multiple clinical strains and further characterization of common intersecting gene regulons between strains can help elucidate which gene networks are important for colonization and pathogenesis. In addition, the continued development of tissue culture systems that are composed of different cell types, for example, modeling the different tissues that line the ascending vaginal, cervical, and fallopian tube epithelia, and transgenic mouse models with humanized epithelial surface receptors can help to generate more complex model microenvironments that are relevant to human infection. Very little data exists on the host and bacterial factors that contribute to infections that do not display overt symptoms, and more sensitive surveillance, screening and diagnostics are needed to characterize bacterial strains and host cell factors that contribute to asymptomatic colonization.
Despite the massive amount of data from sequencing projects showing the vast diversity of microbial species, scientists and policy makers still tend to think of all major groups of bacteria as being alike. N. gonorrhoeae is an example of a host-restricted organism that has been on a singular evolutionary path, existing often currently, and likely throughout its’ evolution, as a commensal-like organism in equilibrium with the host, but retaining the ability to elicit inflammation. Efficient horizontal gene transfer mechanisms have contributed to the rise in antimicrobial resistance. The stochastic alteration of gene expression and antigenic properties through phase variation and antigenic variation has hindered the development of viable vaccine candidates. Understanding the underlying mechanisms by which N. gonorrhoeae evades immune detection and develops antimicrobial resistance factors will aid in the development of new therapeutics for gonorrhea.
Key points.
The urogenital tract is a complex environment composed of many different types of epithelial tissues and innate immune cells that sample the surrounding milieu. As a host-adapted organism, Neisseria gonorrhoeae can only interact with human forms of many molecules. Moreover, due to environmental heterogeneities, as well as unknown concentrations of oxygen and nutrients in this niche, substantial challenges exist for developing tissue culture and animal models. Advances in standardizing primary tissue culture cell techniques and transgenic mouse models may help to ameliorate these challenges.
Although the prevailing view is that female infections are mainly asymptomatic whereas male infections are not, many studies show asymptomatic infections are prevalent in both sexes. The observations underlying the current dogma likely come from physiological and anatomical differences in the male and female urogenital tracts, making neutrophil influx in men much more noticeable and easier to diagnose than infections in women.
N. gonorrhoeae is an obligate human pathogen with the ability to evade and modulate both the innate and adaptive immune systems to benefit its replication and survival. The host-restricted pathogen has subsequently evolved a relatively small but effective set of regulatory mechanisms to quickly adapt to changing oxygen and nutrient concentrations.
As N. gonorrhoeae progresses through the stages of disease pathogenesis: transmission, adherence, colonization and invasion, and immune evasion, the bacterium expresses many virulence factors to promote survival and replication while remaining minimally invasive and discoverable by immune cells. It has yet to be settled in the filed whether the vast neutrophil influx following symptomatic infection benefits the host or the pathogen.
Due to its natural competence, propensity for horizontal gene transfer, efficient transformation and relatively dynamic variable genome, N. gonorrhoeae has rapidly developing resistance to every major class of antibiotic. With worldwide antimicrobial resistance on the rise, it is necessary to understand the mechanisms by which resistance determinants confer resistance and develop novel therapies to avoid an era of untreatable gonorrhea infection.
The Neisseria genus is composed of many commensals and one other pathogen, Neisseria meningiditis that are closely related to N. gonorrhoeae genetically but are phenotypically distinct and occupy different niches. Due to this similarity, it is often difficult to determine which factors are specific virulence factors for N. gonorrhoeae, and which facilitate colonization. More research is needed to determine which factors confer pathogenicity and differentiate these two pathogens.
Acknowledgements
Work in the Seifert laboratory was supported by the NIH grant R37-AI033493. S.Q. was partially supported by NIH T32-AI0007476.
Glossary
- Exotoxins
are bacterial secreted proteins that damage host cells.
- Pelvic inflammatory disease (PID)
is a clinical syndrome where infected fallopian tube tissues are damaged by the host inflammatory response to bacteria.
- Ectopic pregnancy
is one sequelae of PID occurs when a fertilized egg implants anywhere other than the uterine lining, such as in the fallopian tube, risking organ damage and blood loss.
- Biofilms
are structured formations of bacterial cells within an extracellular matrix that stick to one another and together on a surface.
- Microcolonies
are collections of bacterial cells that exist as discrete formations.
- Neutrophil oxidative burst
refers to the release of reactive oxygen species (ROS) H2O2, O2- and HOCl from the neutrophil through NADPH oxidase and subsequent processing by myeloperoxidase.
- Transcytosis
is the transit of the cellular epithelium by a bacterium.
- Siderophores
are low molecular weight iron-binding chemical compounds secreted by bacteria to chelate iron for subsequent uptake into the bacterial cell.
- Microaerophillic
environments are those where oxygen is limited, but not zero.
- Nutritional Immunity
is a host’s ability to sequester important nutrients during infection.
- Phase variation
is stochastic form of genetic change that varies gene expression ON/OFF or UP/DOWN.
- Complement system
is an innate immune defense that recognizes and kills microorganisms through opsonization and formation of membrane attack complexes.
- Alternative complement pathway
is one arm of the complement system triggered by C3b binding to a microbe or other surface, damaged tissues, and foreign materials.
- Classical complement pathway
is one arm of the complement system triggered by antigen-antibody complexes with IgG and IgM antibodies.
- Purulent Exudate
is the hallmark symptom of gonorrhea infection, a liquid genital secretion composed of neutrophils and N. gonorrhoeae.
- C3
is an innate immune protein at the center of the alternative and classical complement pathways.
- Membrane attack complexes
are groups of proteins, formed of complement components C8 and C9 that form pores in the membranes of microbes.
- Opsonization
refers to the process by which host molecules bind to the surface of a microorganism to enhance phagocytosis.
- Factor H
is a control protein of the complement alternative pathway that binds C3b, displacing Factor Bb and enabling cleavage and subsequent inactivation of C3b to C3bi by Factor I.
- C4bp
is a classical pathway complement regulatory protein, akin to Factor H in the alternative pathway that regulates complement activation on host cells.
- Outer membrane vesicles
are secreted from the bacterial envelope and can contain a variety of cellular material.
- Oxidative burst
is an antimicrobial response through the release of reactive oxygen species from host cells.
- Granule fusion
refers to the event in which neutrophil granules containing antimicrobial compounds fuse with the phagosome or cell membrane.
- Neutrophil extracellular traps (NETs)
are released from stimulated neutrophils and are composed mainly of DNA and antimicrobial host proteins.
- Phagosome
is a membrane-bound compartment within a phagocyte that is derived from the cell membrane during phagocytosis.
- Antigenic variation
is a reversible process by which a microorganism provides many different versions of a gene product at a frequency higher than the normal mutation rate.
- Mosaic Alleles
refer to gene allele produced by recombination of different gene sequences.
- Multilocus sequence typing
is a system to define strains of a species by defining DNA sequence alleles of a defined series of housekeeping genes.
Author biographies
Sarah Jane Quillin is a PhD, MPH candidate in microbiology in the Driskill Graduate Program at Northwestern University in Dr. Hank Seifert’s laboratory. She has a BS in Biochemistry and a BA in Chemistry from the University of Chicago.
H Steven Seifert (Hank) is the John Edward Porter Professor of Biomedical Science in the Department of Microbiology-Immunology at the Northwestern University Feinberg School of Medicine in Chicago Illinois. Hank received his B.S. degree in Chemistry from Beloit College, Wisconsin; his Ph.D. degree in Molecular Biology from the Pennsylvania State University, Pennsylvania, and did his postdoctoral work at the Research Institute of Scripps Clinic in California. His laboratory group has developed genetic tools for the Neisseria, has elucidated the molecular mechanisms allowing the recombination-based pilin antigenic variation system of the Neisseria, the assembly and function of the Type IV pilus, and the interactions of Neisseria with host cells.
Footnotes
Competing interests statement
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Microbiology thanks M. A. Apicella, S. Gray-Owen and M. W. Russell for their contribution to the peer review of this work.
Uncategorized References
- 1.in WHO Guidelines for the Treatment of Neisseria gonorrhoeae WHO Guidelines Approved by the Guidelines Review Committee (2016). [Google Scholar]
- 2.Carmona-Gutierrez D, Kainz K & Madeo F Sexually transmitted infections: old foes on the rise. Microbial cell 3, 361–362, doi: 10.15698/mic2016.09.522 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unemo M et al. The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: phenotypic, genetic and reference genome characterization. J Antimicrob Chemother 71, 3096–3108, doi: 10.1093/jac/dkw288 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman L et al. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS One 10, e0143304, doi: 10.1371/journal.pone.0143304 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JS, Choi HY, Lee JE, Lee SH & Oum BS Gonococcal keratoconjunctivitis in adults. Eye 16, 646–649, doi: 10.1038/sj.eye.6700112 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Noble RC, Cooper RM & Miller BR Pharyngeal colonisation by Neisseria gonorrhoeae and Neisseria meningitidis in black and white patients attending a venereal disease clinic. British Journal of Venereal Diseases 55, 14–19 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danby CS et al. Patterns of Extragenital Chlamydia and Gonorrhea in Women and Men Who Have Sex With Men Reporting a History of Receptive Anal Intercourse. Sex Transm Dis 43, 105–109, doi: 10.1097/OLQ.0000000000000384 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Little JW Gonorrhea: update. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics 101, 137–143, doi: 10.1016/j.tripleo.2005.05.077 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Sandstr:om, I. Etiology and diagnosis of neonatal conjunctivitis. Acta Paediatrica Scandinavica 76, 221–227 (1987). [DOI] [PubMed] [Google Scholar]
- 10.Masi AT & Eisenstein BI Disseminated gonococcal infection (DGI) and gonococcal arthritis (GCA): II. Clinical manifestations, diagnosis, complications, treatment, and prevention. Semin Arthritis Rheum 10, 173–197 (1981). [DOI] [PubMed] [Google Scholar]
- 11.Hoffman O & Weber RJ Pathophysiology and treatment of bacterial meningitis. Therapeutic advances in neurological disorders 2, 1–7, doi: 10.1177/1756285609337975 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marri PR et al. Genome sequencing reveals widespread virulence gene exchange among human Neisseria species. PLoS One 5, e11835, doi: 10.1371/journal.pone.0011835 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu G, Tang CM & Exley RM Non-pathogenic Neisseria: members of an abundant, multi-habitat, diverse genus. Microbiology 161, 1297–1312, doi: 10.1099/mic.0.000086 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Maiden MC & Harrison OB Population and Functional Genomics of Neisseria Revealed with Gene-by-Gene Approaches. J Clin Microbiol 54, 1949–1955, doi: 10.1128/JCM.00301-16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bratcher HB, Corton C, Jolley KA, Parkhill J & Maiden MC A gene-by-gene population genomics platform: de novo assembly, annotation and genealogical analysis of 108 representative Neisseria meningitidis genomes. BMC Genomics 15, 1138, doi: 10.1186/1471-2164-15-1138 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joseph B et al. Virulence evolution of the human pathogen Neisseria meningitidis by recombination in the core and accessory genome. PLoS One 6, e18441, doi: 10.1371/journal.pone.0018441 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maiden MC Population genomics: diversity and virulence in the Neisseria. Curr Opin Microbiol 11, 467–471 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards JL & Apicella MA The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin Microbiol Rev 17, 965–981, table of contents (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sparling PF Biology of Neisseria gonorrhoeae. 3rd edn, (McGraw-Hill, 1999). [Google Scholar]
- 20.Walker CK & Sweet RL Gonorrhea infection in women: prevalence, effects, screening, and management. International journal of women's health 3, 197–206, doi: 10.2147/IJWH.S13427 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan SJ, Schwebke JR, Aaron KJ, Van Der Pol B & Hook EW 3rd. Meatal Swabs Contain Less Cellular Material and Are Associated with a Decrease in Gram Stain Smear Quality Compared to Urethral Swabs in Men. J Clin Microbiol 55, 2249–2254, doi: 10.1128/JCM.00423-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muzny CA et al. Sexually Transmitted Infection Risk among Women Is Not Fully Explained by Partner Numbers. South Med J 110, 161–167, doi: 10.14423/SMJ.0000000000000621 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Grimley DM et al. Sexually transmitted infections among urban shelter clients. Sex Transm Dis 33, 666–669, doi: 10.1097/01.olq.0000223285.18331.4d (2006). [DOI] [PubMed] [Google Scholar]
- 24.Geisler WM, Yu S & Hook EW 3rd. Chlamydial and gonococcal infection in men without polymorphonuclear leukocytes on gram stain: implications for diagnostic approach and management. Sex Transm Dis 32, 630–634 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Xiong M et al. Analysis of the sex ratio of reported gonorrhoea incidence in Shenzhen, China. BMJ open 6, e009629, doi: 10.1136/bmjopen-2015-009629 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This epidemiological study of 1106 male and 1420 female participants in Shenzen, China, shows that undiagnosed, unreported gonorrhea infections were common in both men and women, and the reported incidence sex ratio was overestimated by a factor of 7.9.
- 26.Hook EW 3rd. Gender differences in risk for sexually transmitted diseases. The American journal of the medical sciences 343, 10–11, doi: 10.1097/MAJ.0b013e31823ea276 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Hedges SR, Mayo MS, Mestecky J, Hook EW 3rd & Russell MW Limited local and systemic antibody responses to Neisseria gonorrhoeae during uncomplicated genital infections. Infect Immun 67, 3937–3946 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fichorova RN, Desai PJ, Gibson FC 3rd & Genco CA Distinct proinflammatory host responses to Neisseria gonorrhoeae infection in immortalized human cervical and vaginal epithelial cells. Infect Immun 69, 5840–5848 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease, C. & Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports 63, 1–19 (2014). [PMC free article] [PubMed] [Google Scholar]
- 30.James-Holmquest AN, Swanson J, Buchanan TM, Wende RD & Williams RP Differential attachment by piliated and nonpiliated Neisseria gonorrhoeae to human sperm. Infection & Immunity 9, 897–902 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harvey HA et al. Gonococcal lipooligosaccharide is a ligand for the asialoglycoprotein receptor on human sperm. Mol Microbiol 36, 1059–1070 (2000). [DOI] [PubMed] [Google Scholar]; This study shows that gonococcal LOS binds to the ASGP-R receptor on human sperm, possibly contributing to male-to-female transmission.
- 32.Cohen MS et al. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. Journal of Infectious Diseases 169, 532–537 (1994). [DOI] [PubMed] [Google Scholar]
- 33.Ketterer MR et al. Desialylation of Neisseria gonorrhoeae Lipooligosaccharide by Cervicovaginal Microbiome Sialidases: The Potential for Enhancing Infectivity in Men. J Infect Dis 214, 1621–1628, doi: 10.1093/infdis/jiw329 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higashi DL et al. Dynamics of Neisseria gonorrhoeae attachment: microcolony development, cortical plaque formation, and cytoprotection. Infect Immun 75, 4743–4753, doi: 10.1128/IAI.00687-07 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craig L, Pique ME & Tainer JA Type IV pilus structure and bacterial pathogenicity. Nat Rev Microbiol 2, 363–378, doi: 10.1038/nrmicro885 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Obergfell KP & Seifert HS The Pilin N-terminal Domain Maintains Neisseria gonorrhoeae Transformation Competence during Pilus Phase Variation. PLoS Genet 12, e1006069, doi: 10.1371/journal.pgen.1006069 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berry J-L & Pelicic V Exceptionally widespread nanomachines composed of type IV pilins: the prokaryotic Swiss Army knives. Vol. 39 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cahoon LA & Seifert HS Transcription of a cis-acting, noncoding, small RNA is required for pilin antigenic variation in Neisseria gonorrhoeae. PLoS Pathog 9, e1003074, doi: 10.1371/journal.ppat.1003074 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that transcription of a small, cis-acting, noncoding RNA initiates within theguanine quartet (G4) coding sequence to allow the formation of the G4 structure required for pilin antigenic variation.
- 39.Dietrich M et al. Activation of NF-kappaB by Neisseria gonorrhoeae is associated with microcolony formation and type IV pilus retraction. Cell Microbiol 13, 1168–1182, doi: 10.1111/j.1462-5822.2011.01607.x (2011). [DOI] [PubMed] [Google Scholar]
- 40.Swanson J, Barrera O, Sola J & Boslego J Expression of outer membrane protein II by gonococci in experimental gonorrhea. J Exp Med 168, 2121–2129 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jerse AE et al. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J Exp Med 179, 911–920 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that when Opa-negative variants of N. gonorrhoeae strain FA1090 were inoculated into human male volunteers thata majority of bacteria cultured from the infected subjects were Opa positive expressing a variety of different Opa variants.
- 42.Virji M, Makepeace K, Ferguson DJ & Watt SM Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol Microbiol 22, 941–950 (1996). [DOI] [PubMed] [Google Scholar]
- 43.Simms AN & Jerse AE In vivo selection for Neisseria gonorrhoeae opacity protein expression in the absence of human carcinoembryonic antigen cell adhesion molecules. Infect Immun 74, 2965–2974, doi: 10.1128/IAI.74.5.2965-2974.2006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lambden PR, Heckels JE, James LT & Watt PJ Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. Journal of General Microbiology 114, 305–312 (1979). [DOI] [PubMed] [Google Scholar]
- 45.Stern A, Brown M, Nickel P & Meyer TF Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell 47, 61–71 (1986). [DOI] [PubMed] [Google Scholar]
- 46.Swanson J et al. Gonococcal pilin variants in experimental gonorrhea. J Exp Med 165, 1344–1357 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.James JF & Swanson J Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun 19, 332–340 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seifert HS, Wright CJ, Jerse AE, Cohen MS & Cannon JG Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J Clin Invest 93, 2744–2749, doi: 10.1172/JCI117290 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson MT, Byerly L, Apicella MA & Seifert HS Seminal plasma promotes Neisseria gonorrhoeae aggregation and biofilm formation. J Bacteriol, doi: 10.1128/JB.00165-16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steichen CT, Cho C, Shao JQ & Apicella MA The Neisseria gonorrhoeae biofilm matrix contains DNA, and an endogenous nuclease controls its incorporation. Infect Immun 79, 1504–1511, doi: 10.1128/iai.01162-10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greiner LL et al. Biofilm Formation by Neisseria gonorrhoeae. Infect Immun 73, 1964–1970, doi: 10.1128/IAI.73.4.1964-1970.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steichen CT, Shao JQ, Ketterer MR & Apicella MA Gonococcal cervicitis: a role for biofilm in pathogenesis. J Infect Dis 198, 1856–1861, doi: 10.1086/593336 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wetzler LM, Blake MS, Barry K & Gotschlich EC Gonococcal porin vaccine evaluation: comparison of Por proteosomes, liposomes, and blebs isolated from rmp deletion mutants. Journal of Infectious Diseases 166, 551–555 (1992). [DOI] [PubMed] [Google Scholar]
- 54.Song W, Ma L, Chen R & Stein DC Role of lipooligosaccharide in Opa-independent invasion of Neisseria gonorrhoeae into human epithelial cells. J Exp Med 191, 949–960 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Vliet SJ et al. Variation of Neisseria gonorrhoeae lipooligosaccharide directs dendritic cell-induced T helper responses. PLoS Pathog 5, e1000625, doi: 10.1371/journal.ppat.1000625 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wetzler LM, Barry K, Blake MS & Gotschlich EC Gonococcal lipooligosaccharide sialylation prevents complement- dependent killing by immune sera. Infection & Immunity 60, 39–43 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]; A study showing that sialylation of gonococcal LOS prevents opsonophagocytosis by immune sera, leading to later confirmation that sialylation of LOS prevents complement activation and killing.
- 57.Kellogg DS Jr., Peacock WL, Deacon WE, Brown L & Pirkle CI Neisseria gonorrhoeae. I. Virulence genetically linked to clonial variation. Journal of Bacteriology 85, 1274–1279 (1963). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spence JM, Wright L & Clark VL Laboratory maintenance of Neisseria gonorrhoeae. Curr Protoc Microbiol Chapter 4, Unit 4A 1, doi: 10.1002/9780471729259.mc04a01s8 (2008). [DOI] [PubMed] [Google Scholar]; This study compared selectively passaged piliated N. gonorrhoeae capable of infecting human volunteers and nonselectively passaged nonpiliated clonal variants that became noninfectious, allowing researchers to realize infectivity can be phenotypically followed by following piliated and nonpiliated colony morphology.
- 59.Platt DJ Carbon dioxide requirement of Neisseria gonorrhoeae growing on a solid medium. Journal of Clinical Microbiology 4, 129–132 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.St Amant DC, Valentin-Bon IE & Jerse AE Inhibition of Neisseria gonorrhoeae by Lactobacillus species that are commonly isolated from the female genital tract. Infect Immun 70, 7169–7171 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spurbeck RR & Arvidson CG Inhibition of Neisseria gonorrhoeae epithelial cell interactions by vaginal Lactobacillus species. Infect Immun 76, 3124–3130 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spurbeck RR & Arvidson CG Lactobacillus jensenii surface-associated proteins inhibit Neisseria gonorrhoeae adherence to epithelial cells. Infect Immun 78, 3103–3111, doi: 10.1128/IAI.01200-09 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cassat JE & Skaar EP Iron in infection and immunity. Cell host & microbe 13, 509–519, doi: 10.1016/j.chom.2013.04.010 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doherty CP Host-pathogen interactions: the role of iron. The Journal of nutrition 137, 1341–1344 (2007). [DOI] [PubMed] [Google Scholar]
- 65.Bonnah RA & Schryvers AB Preparation and characterization of Neisseria meningitidis mutants deficient in production of the human lactoferrin-binding proteins LbpA and LbpB. Journal of Bacteriology 180, 3080–3090 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Noinaj N, Buchanan SK & Cornelissen CN The transferrin-iron import system from pathogenic Neisseria species. Mol Microbiol 86, 246–257, doi: 10.1111/mmi.12002 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evans RW & Oakhill JS Transferrin-mediated iron acquisition by pathogenic Neisseria. Biochem Soc Trans 30, 705–707 (2002). [DOI] [PubMed] [Google Scholar]
- 68.Kehl-Fie TE & Skaar EP Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol 14, 218–224, doi: 10.1016/j.cbpa.2009.11.008 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ovcinnikov NM & Delektorskij VV Electron microscope studies of gonococci in the urethral secretions of patients with gonorrhoea. British Journal of Venereal Diseases 47, 419–439 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farzadegan H & Roth IL Scanning electron microscopy and freeze-etching of gonorrhoeal urethral exudate. British Journal of Venereal Diseases 51, 83–91 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Evans BA Ultrastructural study of cervical gonorrhea. Journal of Infectious Diseases 136, 248–255 (1977). [DOI] [PubMed] [Google Scholar]
- 72.King G, James JF & Swanson J Studies on gonococcus infection. XI. Comparison of in vivo and vitro association of Neisseria gonorrhoeae with human neutrophils. Journal of Infectious Diseases 137, 38–43 (1978). [DOI] [PubMed] [Google Scholar]
- 73.Apicella MA et al. The pathogenesis of gonococcal urethritis in men: confocal and immunoelectron microscopic analysis of urethral exudates from men infected with Neisseria gonorrhoeae. Journal.of.Infectious.Diseases. 173, 636–646 (1996). [DOI] [PubMed] [Google Scholar]
- 74.Criss AK & Seifert HS A bacterial siren song: intimate interactions between Neisseria and neutrophils. Nat Rev Microbiol 10, 178–190, doi: 10.1038/nrmicro2713 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lucas CE, Hagman KE, Levin JC, Stein DC & Shafer WM Importance of lipooligosaccharide structure in determining gonococcal resistance to hydrophobic antimicrobial agents resulting from the mtr efflux system. Molecular Microbiology 16, 1001–1009 (1995). [DOI] [PubMed] [Google Scholar]
- 76.Zalucki YM, Dhulipala V & Shafer WM Dueling regulatory properties of a transcriptional activator (MtrA) and repressor (MtrR) that control efflux pump gene expression in Neisseria gonorrhoeae. mBio 3, e00446–00412, doi: 10.1128/mBio.00446-12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study compares the binding affinities and regulatory competition between MtrCDE efflux pump operon activator MtrA and repressor MtrR, building on previous data characterizing this important antimicrobial resistance pump and its transcriptional regulation.
- 77.Thaler CJ, Vanderpuye OA, McIntyre JA & Faulk WP Lactoferrin binding molecules in human seminal plasma. Biol Reprod 43, 712–717 (1990). [DOI] [PubMed] [Google Scholar]
- 78.Mercante AD et al. MpeR regulates the mtr efflux locus in Neisseria gonorrhoeae and modulates antimicrobial resistance by an iron-responsive mechanism. Antimicrob Agents Chemother 56, 1491–1501, doi: 10.1128/AAC.06112-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laskos L, Ryan CS, Fyfe JA & Davies JK The RpoH-mediated stress response in Neisseria gonorrhoeae is regulated at the level of activity. J Bacteriol 186, 8443–8452 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Householder TC, Belli WA, Lissenden S, Cole JA, and Clark VL cis- and trans- acting elements involved in the regulation of aniA, the gene encoding the major anaerobically induced outer membrane protein in Nesseria gonorrhoeae. Journal of Bacteriology 181, 5411–5551 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mellies J, Rudel T and Meyer TF Transcriptional regulation of pilC2 in Neisseria gonorrhoeae: response to oxygen availability and evidence for growth-phase regulation in Escherichia coli. Molecular and General Genetics 255, 285–293 (1997). [DOI] [PubMed] [Google Scholar]
- 82.Whitehead RN et al. The small FNR regulon of Neisseria gonorrhoeae: comparison with the larger Escherichia coli FNR regulon and interaction with the NarQ-NarP regulon. BMC Genomics 8, 35 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berish SA, Subbarao S, Chen CY, Trees DL & Morse SA Identification and cloning of a fur homolog from Neisseria gonorrhoeae. Infection & Immunity 61, 4599–4606 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies and initially characterizes the major Fur iron-regulatory protein in N. gonorrhoeae.
- 84.Isabella VM & Clark VL Deep sequencing-based analysis of the anaerobic stimulon in Neisseria gonorrhoeae. BMC Genomics 12, 51, doi: 10.1186/1471-2164-12-51 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies a wide array of genes that are differentially expressed under aerobic and anaerobic conditions in microaerophile N. gonorrhoeae, highlighting the large overlap between genes that are differentially regulated in response to low oxygen, changes in iron levels, and the presence of reactive oxygen species.
- 85.Ducey TF, Carson MB, Joshua O, Stintzi AP & D.W., D. Identification of the iron-responsive genes of Neisseria gonorrhoaea by microarray analysis in defined medium. Journal of Bacteriology 187, 4865–4874 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stohl EA, Criss AK & Seifert HS The transcriptome response of Neisseria gonorrhoeae to hydrogen peroxide reveals genes with previously uncharacterized roles in oxidative damage protection. Mol Microbiol 58, 520–532, doi: 10.1111/j.1365-2958.2005.04839.x (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Makino S, van Putten JP & Meyer TF Phase variation of the opacity outer membrane protein controls invasion by Neisseria gonorrhoeae into human epithelial cells. Embo Journal 10, 1307–1315 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows a positive correlation between the expression of Opa proteins and the binding and invasion into Chang conjunctiva human epithelial cells.
- 88.Snyder LA, Butcher SA & Saunders NJ Comparative whole-genome analyses reveal over 100 putative phase-variable genes in the pathogenic Neisseria spp. Microbiology 147, 2321–2332 (2001). [DOI] [PubMed] [Google Scholar]
- 89.Jordan PW, Snyder LA & Saunders NJ Strain-specific differences in Neisseria gonorrhoeae associated with the phase variable gene repertoire. BMC Microbiol 5, 21, doi: 10.1186/1471-2180-5-21 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Srikhanta YN et al. Phasevarions mediate random switching of gene expression in pathogenic Neisseria. PLoS Pathog 5, e1000400, doi: 10.1371/journal.ppat.1000400 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study characterizes phase-variable DNA methyltransferase activity in N. gonorrhoeae, showing it affects gene expression of virulence-related genes, antimicrobial resistance, human epithelial cervical cell interactions, and biofilm formation.
- 91.Gawthorne JA, Beatson SA, Srikhanta YN, Fox KL & Jennings MP Origin of the diversity in DNA recognition domains in phasevarion associated modA genes of pathogenic Neisseria and Haemophilus influenzae. PLoS One 7, e32337, doi: 10.1371/journal.pone.0032337 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jen FE, Seib KL & Jennings MP Phasevarions mediate epigenetic regulation of antimicrobial susceptibility in Neisseria meningitidis. Antimicrob Agents Chemother 58, 4219–4221, doi: 10.1128/AAC.00004-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Post DMB et al. Identification and characterization of AckA-dependent protein acetylation in Neisseria gonorrhoeae. PLoS One 12, e0179621, doi: 10.1371/journal.pone.0179621 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seib KL, Jen FE, Scott AL, Tan A & Jennings MP Phase variation of DNA methyltransferases and the regulation of virulence and immune evasion in the pathogenic Neisseria. Pathog Dis 75, doi: 10.1093/femspd/ftx080 (2017). [DOI] [PubMed] [Google Scholar]
- 95.Gibson FP, Leach DR & Lloyd RG Identification of sbcD mutations as cosuppressors of recBC that allow propagation of DNA palindromes in Escherichia coli K-12. Journal of Bacteriology 174, 1222–1228 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jackson LA, Pan JC, Day MW & Dyer DW Control of RNA stability by NrrF, an iron-regulated small RNA in Neisseria gonorrhoeae. J Bacteriol, doi: 10.1128/JB.00839-13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ngampasutadol J et al. Human factor H interacts selectively with Neisseria gonorrhoeae and results in species-specific complement evasion. J Immunol 180, 3426–3435, doi:180/5/3426 [pii] (2008). [DOI] [PubMed] [Google Scholar]; This study demonstrates how sialyated LOS binds human Factor H and prevents complement-mediated killing of N. gonorrhoeae.
- 98.Densen P Interaction of complement with Neisseria meningitidis and Neisseria gonorrhoeae. [Review]. Clinical Microbiology Reviews 2 Suppl, S11–S17 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Petersen BH, Graham JA & Brooks GF Human deficiency of the eighth component of complement. The requirement of C8 for serum Neisseria gonorrhoeae bactericidal activity. Journal of Clinical Investigation 57, 283–290 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Edwards JL, Brown EJ, Ault KA & Apicella MA The role of complement receptor 3 (CR3) in Neisseria gonorrhoeae infection of human cervical epithelia. Cell Microbiol 3, 611–622 (2001). [DOI] [PubMed] [Google Scholar]
- 101.Edwards JL & Apicella MA The role of lipooligosaccharide in Neisseria gonorrhoeae pathogenesis of cervical epithelia: lipid A serves as a C3 acceptor molecule. Cell Microbiol 4, 585–598 (2002). [DOI] [PubMed] [Google Scholar]
- 102.Edwards JL et al. A co-operative interaction between Neisseria gonorrhoeae and complement receptor 3 mediates infection of primary cervical epithelial cells. Cell Microbiol 4, 571–584 (2002). [DOI] [PubMed] [Google Scholar]
- 103.Schweinle JE et al. Interaction of Neisseria gonorrhoeae with classical complement components, C1-inhibitor, and a monoclonal antibody directed against the Neisserial H.8 antigen. Journal of Clinical Investigation 83, 397–403 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ram S et al. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. Journal of Experimental Medicine 187, 743–752 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ram S et al. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. Journal of Experimental Medicine 188, 671–680 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ram S et al. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae. J Exp Med 193, 281–295 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gill DB & Atkinson JP CD46 in Neisseria pathogenesis. Trends Mol Med 10, 459–465 (2004). [DOI] [PubMed] [Google Scholar]
- 108.Feinen B & Russell MW Contrasting Roles of IL-22 and IL-17 in Murine Genital Tract Infection by Neisseria gonorrhoeae. Frontiers in immunology 3, 11, doi: 10.3389/fimmu.2012.00011 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Edwards JL & Butler EK The Pathobiology of Neisseria gonorrhoeae Lower Female Genital Tract Infection. Front Microbiol 2, 102, doi: 10.3389/fmicb.2011.00102 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Melly MA, McGee ZA & Rosenthal RS Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. Journal of Infectious Diseases 149, 378–386 (1984). [DOI] [PubMed] [Google Scholar]; This study demonstrates the ability of different N. gonorrhoeae peptidoglycan monomers to damage human fallopian tube mucosal cells in tissue culture.
- 111.Mavrogiorgos N, Mekasha S, Yang Y, Kelliher MA & Ingalls RR Activation of NOD receptors by Neisseria gonorrhoeae modulates the innate immune response. Innate Immun 20, 377–389, doi: 10.1177/1753425913493453 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fisette PL, Ram S, Andersen JM, Guo W & Ingalls RR The Lip lipoprotein from Neisseria gonorrhoeae stimulates cytokine release and NF-kappaB activation in epithelial cells in a Toll-like receptor 2-dependent manner. J Biol Chem 278, 46252–46260, doi: 10.1074/jbc.M306587200M306587200 [pii] (2003). [DOI] [PubMed] [Google Scholar]
- 113.Massari P et al. Cutting edge: Immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J Immunol 168, 1533–1537 (2002). [DOI] [PubMed] [Google Scholar]
- 114.Chateau A & Seifert HS Neisseria gonorrhoeae survives within and modulates apoptosis and inflammatory cytokine production of human macrophages. Cell Microbiol 18, 546–560, doi: 10.1111/cmi.12529 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gaudet RG et al. INNATE IMMUNITY. Cytosolic detection of the bacterial metabolite HBP activates TIFA-dependent innate immunity. Science 348, 1251–1255, doi: 10.1126/science.aaa4921 (2015). [DOI] [PubMed] [Google Scholar]
- 116.Ortiz MC et al. Neisseria gonorrhoeae Modulates Immunity by Polarizing Human Macrophages to a M2 Profile. PLoS One 10, e0130713, doi: 10.1371/journal.pone.0130713 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sadarangani M, Pollard AJ & Gray-Owen SD Opa proteins and CEACAMs: pathways of immune engagement for pathogenic Neisseria. FEMS Microbiol Rev 35, 498–514, doi: 10.1111/j.1574-6976.2010.00260.x (2011). [DOI] [PubMed] [Google Scholar]
- 118.Schmitter T, Agerer F, Peterson L, Munzner P & Hauck CR Granulocyte CEACAM3 is a phagocytic receptor of the innate immune system that mediates recognition and elimination of human-specific pathogens. J Exp Med 199, 35–46, doi: 10.1084/jem.20030204 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sarantis H & Gray-Owen SD The specific innate immune receptor CEACAM3 triggers neutrophil bactericidal activities via a Syk kinase-dependent pathway. Cell Microbiol 9, 2167–2180, doi:CMI947 [pii] 10.1111/j.1462-5822.2007.00947.x (2007). [DOI] [PubMed] [Google Scholar]
- 120.Packiam M, Veit SJ, Anderson DJ, Ingalls RR & Jerse AE Mouse strain-dependent differences in susceptibility to Neisseria gonorrhoeae infection and induction of innate immune responses. Infect Immun 78, 433–440, doi:IAI.00711–09 [pii] 10.1128/IAI.00711-09 [doi] (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dilworth JA, Hendley JO & Mandell GL Attachment and ingestion of gonococci human neutrophils. Infection & Immunity 11, 512–516 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]; This early study shows adherence and ingestion of two different gonococci strains by polymorphonuclear leukocyte neutrophils (PMNs)
- 122.Criss AK & Seifert HS Neisseria gonorrhoeae suppresses the oxidative burst of human polymorphonuclear leukocytes. Cell Microbiol 10, 2257–2270, doi: 10.1111/j.1462-5822.2008.01205.x (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates how different types of Opa-expressing or Opaless N. gonorrhoeae grown under different conditions differ in their ability to elicit a PMN oxidative burst, as well as the ability of some strains to inhibit the PMN oxidative burst.
- 123.Gunderson CW & Seifert HS Neisseria gonorrhoeae elicits extracellular traps in primary neutrophil culture while suppressing the oxidative burst. mBio 6, doi: 10.1128/mBio.02452-14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Criss AK, Katz BZ & Seifert HS Resistance of Neisseria gonorrhoeae to non-oxidative killing by adherent human polymorphonuclear leucocytes. Cell Microbiol 11, 1074–1087, doi: 10.1111/j.1462-5822.2009.01308.x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Johnson MB & Criss AK Resistance of Neisseria gonorrhoeae to neutrophils. Front Microbiol 2, 77, doi: 10.3389/fmicb.2011.00077 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Soler-Garcia AA & Jerse AE A Neisseria gonorrhoeae catalase mutant is more sensitive to hydrogen peroxide and paraquat, an inducer of toxic oxygen radicals. Microb Pathog 37, 55–63 (2004). [DOI] [PubMed] [Google Scholar]
- 127.Gunesekere IC et al. Ecf, an alternative sigma factor from Neisseria gonorrhoeae, controls expression of msrAB, which encodes methionine sulfoxide reductase. J Bacteriol 188, 3463–3469 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pilch B & Mann M Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome biology 7, R40, doi: 10.1186/gb-2006-7-5-r40 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schmidt KA et al. Experimental gonococcal urethritis and reinfection with homologous gonococci in male volunteers. Sex Transm Dis 28, 555–564 (2001). [DOI] [PubMed] [Google Scholar]
- 130.Cahoon LA & Seifert HS Focusing homologous recombination: pilin antigenic variation in the pathogenic Neisseria. Mol Microbiol 81, 1136–1143, doi: 10.1111/j.1365-2958.2011.07773.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mandrell RE, Griffiss JM & Macher BA Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes [published erratum appears in J Exp Med 1988 Oct 1;168(4):1517]. Journal of Experimental Medicine 168, 107–126 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mandrell RE Further antigenic similarities of Neisseria gonorrhoeae lipooligosaccharides and human glycosphingolipids. Infection & Immunity 60, 3017–3020 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gulati S, McQuillen DP, Mandrell RE, Jani DB & Rice PA Immunogenicity of Neisseria gonorrhoeae lipooligosaccharide epitope 2C7, widely expressed in vivo with no immunochemical similarity to human glycosphingolipids. J Infect Dis 174, 1223–1237 (1996). [DOI] [PubMed] [Google Scholar]
- 134.Liu Y, Islam E, Jarvis G, Gray-Owen S & Russell M Neisseria gonorrhoeae selectively suppresses the development of Th1 and Th2 cells, and enhances Th17 cell responses, through TGF-α-dependent mechanisms. Mucosal Immunol (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu Y, Liu W & Russell MW Suppression of host adaptive immune responses by Neisseria gonorrhoeae: role of interleukin 10 and type 1 regulatory T cells. Mucosal Immunol 7, 165–176, doi: 10.1038/mi.2013.36 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu W et al. Neisseria gonorrhoeae suppresses dendritic cell-induced, antigen-dependent CD4 T cell proliferation. PLoS One 7, e41260, doi: 10.1371/journal.pone.0041260 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu Y, Feinen B & Russell MW New concepts in immunity to Neisseria gonorrhoeae: innate responses and suppression of adaptive immunity favor the pathogen, not the host. Front Microbiol 2, 52, doi: 10.3389/fmicb.2011.00052 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jerse AE, Bash MC & Russell MW Vaccines against gonorrhea: current status and future challenges. Vaccine 32, 1579–1587, doi: 10.1016/j.vaccine.2013.08.067 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Petousis-Harris H et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet 390, 1603–1610, doi: 10.1016/S0140-6736(17)31449-6 (2017). [DOI] [PubMed] [Google Scholar]
- 140.Zhao S et al. Genetics of chromosomally mediated intermediate resistance to ceftriaxone and cefixime in Neisseria gonorrhoeae. Antimicrob Agents Chemother 53, 3744–3751, doi: 10.1128/AAC.00304-09 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hagman KE et al. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 141, 611–622 (1995). [DOI] [PubMed] [Google Scholar]
- 142.Zhao S, Tobiason DM, Hu M, Seifert HS & Nicholas RA The penC mutation conferring antibiotic resistance in Neisseria gonorrhoeae arises from a mutation in the PilQ secretin that interferes with multimer stability. Mol Microbiol 57, 1238–1251 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Unemo M & Shafer WM Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 27, 587–613, doi: 10.1128/CMR.00010-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jerse AE et al. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect Immun 71, 5576–5582 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Unemo M & Shafer WM Antibiotic resistance in Neisseria gonorrhoeae: origin, evolution, and lessons learned for the future. Annals of the New York Academy of Sciences 1230, E19–E28, doi: 10.1111/j.1749-6632.2011.06215.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Centers for Disease C & Prevention. CDC Grand Rounds: the growing threat of multidrug-resistant gonorrhea. MMWR. Morbidity and mortality weekly report 62, 103–106 (2013). [PMC free article] [PubMed] [Google Scholar]
- 147.Unemo M & Nicholas RA Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future microbiology 7, 1401–1422, doi: 10.2217/fmb.12.117 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Aas FE, Lovold C & Koomey M An inhibitor of DNA binding and uptake events dictates the proficiency of genetic transformation in Neisseria gonorrhoeae: mechanism of action and links to Type IV pilus expression. Mol Microbiol 46, 1441–1450 (2002). [DOI] [PubMed] [Google Scholar]
- 149.Hamilton HL & Dillard JP Natural transformation of Neisseria gonorrhoeae: from DNA donation to homologous recombination. Mol Microbiol 59, 376–385 (2006). [DOI] [PubMed] [Google Scholar]
- 150.Bowler LD, Zhang QY, Riou JY & Spratt BG Interspecies recombination between the penA genes of Neisseria meningitidis and commensal Neisseria species during the emergence of penicillin resistance in N. meningitidis: natural events and laboratory simulation. Journal of Bacteriology 176, 333–337 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ng LK & Martin IE The laboratory diagnosis of Neisseria gonorrhoeae. Can J Infect Dis Med Microbiol 16, 15–25 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.De Silva D et al. Whole-genome sequencing to determine transmission of Neisseria gonorrhoeae: an observational study. Lancet Infect Dis 16, 1295–1303, doi: 10.1016/S1473-3099(16)30157-8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Harrison OB et al. Genomic analysis of urogenital and rectal Neisseria meningitidis isolates reveals encapsulated hyperinvasive meningococci and coincident multidrug-resistant gonococci. Sex Transm Infect, doi: 10.1136/sextrans-2016-052781 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Johnson LF & Lewis DA The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex Transm Dis 35, 946–959, doi: 10.1097/OLQ.0b013e3181812d15 (2008). [DOI] [PubMed] [Google Scholar]
- 155.Kalichman SC, Pellowski J & Turner C Prevalence of sexually transmitted co-infections in people living with HIV/AIDS: systematic review with implications for using HIV treatments for prevention. Sex Transm Infect 87, 183–190, doi: 10.1136/sti.2010.047514 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jerse AE et al. Estradiol-Treated Female Mice as Surrogate Hosts for Neisseria gonorrhoeae Genital Tract Infections. Front Microbiol 2, 107, doi: 10.3389/fmicb.2011.00107 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zarantonelli ML et al. Transgenic mice expressing human transferrin as a model for meningococcal infection. Infect Immun 75, 5609–5614, doi: 10.1128/IAI.00781-07 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gu A, Zhang Z, Zhang N, Tsark W & Shively JE Generation of human CEACAM1 transgenic mice and binding of Neisseria Opa protein to their neutrophils. PLoS One 5, e10067, doi: 10.1371/journal.pone.0010067 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li G et al. Establishment of a human CEACAM1 transgenic mouse model for the study of gonococcal infections. Journal of microbiological methods 87, 350–354, doi: 10.1016/j.mimet.2011.09.012 (2011). [DOI] [PubMed] [Google Scholar]; This study presents and characterizes a transgenic mouse model for gonorrhea infection, wherein the mouse has been created expressing a humanized CEACAM receptor molecule important for adherence and colonization, allowing N. gonorrhoeae to intravaginally colonize the mouse.
- 160.Winther-Larsen HC et al. in 13th International Pathogenic Neisseria Conference. [Google Scholar]
- 161.Pearce WA & Buchanan TM Attachment role of gonococcal pili. Optimum conditions and quantitation of adherence of isolated pili to human cells in vitro. Journal of Clinical Investigation 61, 931–943 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kaparakis M et al. Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cell Microbiol 12, 372–385, doi: 10.1111/j.1462-5822.2009.01404.x CMI1404 [pii] (2010). [DOI] [PubMed] [Google Scholar]
- 163.Zhou X et al. Hexa-acylated lipid A is required for host inflammatory response to Neisseria gonorrhoeae in experimental gonorrhea. Infect Immun 82, 184–192, doi: 10.1128/IAI.00890-13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Singleton TE, Massari P & Wetzler LM Neisserial porin-induced dendritic cell activation is MyD88 and TLR2 dependent. J Immunol 174, 3545–3550, doi:174/6/3545 [pii] (2005). [DOI] [PubMed] [Google Scholar]
- 165.Liu X et al. Gonococcal lipooligosaccharide suppresses HIV infection in human primary macrophages through induction of innate immunity. J Infect Dis 194, 751–759 (2006). [DOI] [PubMed] [Google Scholar]
- 166.Remmele CW et al. Transcriptional landscape and essential genes of Neisseria gonorrhoeae. Nucleic Acids Res 42, 10579–10595, doi: 10.1093/nar/gku762 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lee EH & Shafer WM The farAB-encoded efflux pump mediates resistance of gonococci to long-chained antibacterial fatty acids. Mol Microbiol 33, 839–845, doi:mmi1530 [pii] (1999). [DOI] [PubMed] [Google Scholar]
- 168.Lee EH, Rouquette-Loughlin C, Folster JP & Shafer WM FarR regulates the farAB-encoded efflux pump of Neisseria gonorrhoeae via an MtrR regulatory mechanism. J Bacteriol 185, 7145–7152 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Warner DM, Folster JP, Shafer WM & Jerse AE Regulation of the MtrC-MtrD-MtrE efflux-pump system modulates the in vivo fitness of Neisseria gonorrhoeae. J Infect Dis 196, 1804–1812 (2007). [DOI] [PubMed] [Google Scholar]
- 170.Seib KL et al. Characterization of the OxyR regulon of Neisseria gonorrhoeae. Mol Microbiol 63, 54–68 (2007). [DOI] [PubMed] [Google Scholar]
- 171.Overton TW et al. Coordinated regulation of the Neisseria gonorrhoeae-truncated denitrification pathway by the nitric oxide-sensitive repressor, NsrR, and nitrite-insensitive NarQ-NarP. J Biol Chem 281, 33115–33126 (2006). [DOI] [PubMed] [Google Scholar]
- 172.Wu HJ et al. PerR controls Mn-dependent resistance to oxidative stress in Neisseria gonorrhoeae. Mol Microbiol 60, 401–416 (2006). [DOI] [PubMed] [Google Scholar]
- 173.Gunesekere IC et al. Comparison of the RpoH-dependent regulon and general stress response in Neisseria gonorrhoeae. J Bacteriol 188, 4769–4776 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gangaiah D et al. Both MisR (CpxR) and MisS (CpxA) Are Required for Neisseria gonorrhoeae Infection in a Murine Model of Lower Genital Tract Infection. Infect Immun 85, doi: 10.1128/IAI.00307-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yu C, McClure R, Nudel K, Daou N & Genco CA Characterization of the Neisseria gonorrhoeae Iron and Fur Regulatory Network. J Bacteriol 198, 2180–2191, doi: 10.1128/JB.00166-16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tseng HJ, McEwan AG, Apicella MA & Jennings MP OxyR acts as a repressor of catalase expression in Neisseria gonorrhoeae. Infect Immun 71, 550–556 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kim JJ, Zhou D, Mandrell RE & Griffiss JM Effect of exogenous sialylation of the lipooligosaccharide of Neisseria gonorrhoeae on opsonophagocytosis. Infection & Immunity 60, 4439–4442 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Blom AM & Ram S Contribution of interactions between complement inhibitor C4b-binding protein and pathogens to their ability to establish infection with particular emphasis on Neisseria gonorrhoeae. Vaccine 26 Suppl 8, I49–55 (2008). [DOI] [PubMed] [Google Scholar]
- 179.Jarvis GA Analysis of C3 deposition and degradation on Neisseria meningitidis and Neisseria gonorrhoeae. Infection & Immunity 62, 1755–1760 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yu Q et al. Association of Neisseria gonorrhoeae Opa(CEA) with dendritic cells suppresses their ability to elicit an HIV-1-specific T cell memory response. PLoS One 8, e56705, doi: 10.1371/journal.pone.0056705 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liu Y, Liu W & Russell MW Suppression of host adaptive immune responses by Neisseria gonorrhoeae: role of interleukin 10 and type 1 regulatory T cells. Mucosal Immunol, doi: 10.1038/mi.2013.36 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ison CA, Deal C & Unemo M Current and future treatment options for gonorrhoea. Sex Transm Infect 89 Suppl 4, iv52–56, doi: 10.1136/sextrans-2012-050913 (2013). [DOI] [PubMed] [Google Scholar]