Abstract
Purpose:
To examine the clinical utility of intratumor microRNAs (miRNA) as a biomarker for predicting responses to platinum-based doublet chemotherapy in patients with recurring lung adenocarcinoma (LADC).
Experimental Design:
The expression of miRNAs was examined in LADC tissues surgically resected from patients treated with platinum-based doublet chemotherapy at the time of LADC recurrence. Microarray-based screening of 904 miRNAs followed by quantitative reverse transcription-PCR–based verification in 40 test cohort samples, including 16 (40.0%) responders, was performed to identify miRNAs that are differentially expressed in chemotherapy responders and nonresponders. Differential expression was confirmed in a validation cohort (n = 63 samples), including 18 (28.6%) responders. An miRNA signature that predicted responses to platinum-based doublet chemotherapy was identified and its accuracy was examined by principal component and support vector machine analyses. Genotype data for the TP53-Arg72Pro polymorphism, which is associated with responses to platinum-based doublet chemotherapy, were subsequently incorporated into the prediction analysis.
Results:
A signature comprising three miRNAs (miR1290, miR196b, and miR 135a*) enabled the prediction of a chemotherapeutic response (rather than progression-free and overall survival) with high accuracy in both the test and validation cohorts (82.5% and 77.8%). Examination of the latter was performed using miRNAs extracted from archived formalin-fixed paraffin-embedded tissues. Combining this miRNA signature with the TP53-Arg72 Propolymorphism genotype marginally improved the predictive power.
Conclusion:
The three-miRNA signature in surgically resected primary LADC tissues may by clinically useful for predicting responsiveness to platinum-based doublet chemotherapy in patients with LADC recurrence.
Introduction
Lung adenocarcinoma (LADC) is the most common type of non–small cell lung cancer (NSCLC) and is a leading cause of cancer mortality worldwide (1). Surgical resection is the best curative treatment for NSCLC; however, patients that experience recurrence after surgery and those with advanced disease receive chemotherapy to slow tumor growth and improve survival. LADCs harboring an EGFR mutation or an ALK fusion are primarily treated with specific tyrosine kinase inhibitors (TKI), with response rates of approximately 60% (2, 3). Other oncogene aberrations, such as BRAF, HER2, RET, and ROS1, are also targeted by specific TKIs (4–6), but a major barrier to curative treatment of LADC using TKIs is innate and acquired drug resistance (7, 8). Furthermore, more than 60% of USA/European and more than 30% of Japanese LADC cases do not harbor the oncogene aberrations listed above (9, 10). Such resistant and oncogene-negative LADC cases are treated with chemotherapy. The standard regimens comprise platinum-based doublets, that is, a combination of platinum and another agent; the drugs paired with platinum (cisplatin or carboplatin) include microtubule-targeted agents (paclitaxel, docetaxel, or vinorelbine) and DNA-damaging agents (gemcitabine or irinotecan). A series of trials in unselected patients revealed that the efficacy of each combination is similar, with response rates of 30% to 40% (11–13); thus, identifying biomarkers that can discriminate between patients that will respond to platinum-based doublet chemotherapy and those who may not before treatment will help improving the efficacy of chemotherapy for LADC.
microRNAs (miRNA) are small noncoding RNAs that posttranscriptionally regulate the translation of target genes; these miRNAs show altered expression in a variety of cancers and can modify the malignant properties of cancer cells, including the response to DNA damage (14–16). In fact, functional studies in LADC cell lines have identified a number of miRNAs that modulate sensitivity to platinum-based agents (17–23). Furthermore, miRNAs are stable in formalin-fixed paraffin-embedded (FFPE) tissues, that is, materials used for daily pathologic diagnosis (24, 25). Indeed, intratumor miRNA expression is a promising prognostic marker in patients that have undergone surgical resection(26–31), but few studies have examined the utility of miRNAs as a predictor of chemotherapeutic responses (32). To the best of our knowledge, only two such studies have been reported: one shows that a two-miRNA signature (miR149 and miR375) is associated with responses to platinum-based chemotherapy in NSCLC (n = 38), and the other shows that miR92a-2* expression is associated with chemoresistance in small-cell lung cancer (n = 34; refs. 33, 34).
Here, we investigated the utility of intratumor miRNAs as a biomarker for predicting responses to platinum-based doublet chemotherapy. We examined the expression of miRNAs in 103 surgically resected specimens obtained from patients who received platinum-based chemotherapy upon LADC recurrence to ascertain whether miRNA expression in primary tumors could predict responses to platinum-based doublet chemotherapy in patients who experienced LADC recurrence after surgery. First, a two-step screening process involving 904 miRNAs was performed to identify an miRNA signature with predictive value. The first step was performed using a test cohort comprising 40 frozen tumor samples from which RNAs were isolated, and the second step was performed using a validation cohort comprising 63 cases, for which RNAs from FFPE tissues were available. We identified a three-miRNA signature that predicted responses to platinum based doublet chemotherapy with an accuracy of >75%.
Materials and Methods
Materials
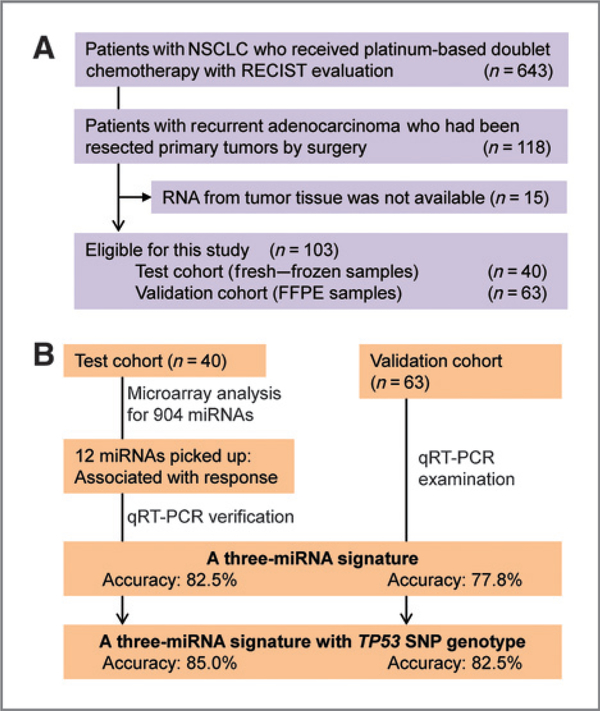
Of note, 103 surgically resected LADC tissues were examined in the present study (Fig. 1A). Briefly, 643 Japanese patients with NSCLC received platinum-based doublet chemotherapy at the National Cancer Center Hospital (NCCH; Tokyo, Japan), between 2000 and 2008, and the therapeutic response was evaluated using the Response Evaluation Criteria In Solid Tumors (RECIST) guidelines (35). None of the patients had received prior treatment with platinum-based chemotherapy. Of the 643 cases, 118 were recurrent cases that had undergone surgical resection at NCCH, and all were pathologically diagnosed with adenocarcinoma. Tumor tissues for RNA extraction were available for 103 of 118 cases; these cases were examined in the present study. Information regarding age, gender, pathologic TNM stage (the 7th classification), smoking habits, postoperative chemotherapy regimens and responses to platinum doublet therapy, and performance status (PS) were retrospectively collected. RNAs isolated from fresh-frozen tissues were available for 40 of 103 cases; these were defined as the test cohort. The RNAs from the test cohort were subjected to miRNA microarray analysis followed by verification by quantitative reverse transcriptase-PCR (qRT-PCR) analysis. RNAs from FFPE tissues were available for the remaining 63 cases; these were defined as the validation cohort. In addition, patients were classified into two categories according to RECIST guidelines: those that responded to platinum-based doublet chemotherapy [complete response (CR) or partial response (PR)] and those that did not [stable disease (SD) or progressive disease (PD)].
Figure 1.
Patients and treatment strategy. A, selection of eligible cases, that is, 103 surgically resected cases that received platinum-based doublet chemotherapy upon LADC recurrence. B, identification and evaluation of a three-miRNA signature for the prediction of responses to chemotherapy.
RNA extraction
RNA was extracted from snap-frozen tissues (test cohort) using TRizol reagent (Thermo Fisher Scientific). The quality and quantity of the RNAs were examined using a Bioanalyzer (Agilent). All RNA samples showed an RNA integrity number RIN >6.0; therefore, they were subjected to microarray analysis. For the validation cohort, RNA was isolated from three unstained FFPE sections (5-mm thick). The area of the carcinoma in the three unstained sections was outlined by referring to a sequential section that was stained with hematoxylin and eosin. Each marked area was macro-dissected using a sterile disposable scalpel and RNA was isolated using the RecoverAll Total Nucleic Acid Isolation Kit (Ambion). Total RNA was quantified using a NanoDrop ND-1000 spectrometer (Thermo Fisher Scientific). The optical density (OD) 260/280 and OD 260/230 ratios were used for quality control.
Microarray experiments
The Human miRNA Microarray Kit release 14 (8 × 15 K; Agilent Technologies), covering 904 miRNAs, was used to screen for miRNAs in the test cohort samples (n = 40). Data were normalized and analyzed using GeneSpring GX software (version 12.5; TOMY Digital Biology). The foldchange in expression was defined as the ratio of expression in responders to that in nonresponders. Normalized and raw expression data were deposited in the Gene Expression Omnibus at the National Center for Biotechnology Information (GSE56264).
Examination of driver oncogene aberrations
All 40 test cohort samples were also screened for oncogene fusions (EML4– and KIF5B–ALK, KIF5B– and CCDC6–RET; and CD74–, EZR–, and SLC34A2–ROS1) by reverse transcription-PCR as previously described (4,36). GenomicDNA was extracted from fresh or frozen samples from all 103 subjects using a QIAamp DNA Mini Kit (QIAGEN) and then analyzed for EGFR, KRAS, BRAF, and HER2 hot spot mutations using the high resolution melting method (37, 38).
qRT-PCR analysis
qRT-PCR of mature miRNA was performed using TaqMan MicroRNA assays (Thermo Fisher Scientific) and the 7900 HT Fast Real-Time PCR System (Thermo Fisher Scientific). cDNA was synthesized using miRNA-specific primers and a TaqMan MicroRNA Reverse Transcription Kit (Thermo Fisher Scientific). RNA (40ng) was reverse transcribed in a 20 μL reaction containing gene-specific RT probes. All assays were performed in triplicate and investigators were blinded to the clinical outcome. All TaqMan probes were purchased from Thermo Fisher Scientific: hsa-miR135a-3p (ID 002232), hsa-miR196b-5p (ID 002215), hsa-miR1181 (Assay ID 241045_mat), hsa-miR31–5p (ID 002279), hsa-miR31–3p (ID 002113), hsa-miR1290 (ID 002863), hsa-miR598 (ID 001988), hsa-miR1 (ID 002222), hsa-miR144–5p (ID 002148), hsa-miR628–5p (ID 002433), hsa-miR449a (ID 001030), and hsa-miR34b-3p (ID 002102). RNU66 (ID 001002) was used as a normalization control. Relative expression of miRNAs was calculated using RQ manager 1.2 (Thermo Fisher Scientific).
Statistical analysis
Differences in miRNA expression levels between responders and nonresponders were tested by the Mann–Whitney U test using Graphpad Prism v5.0 (Graphpad Software Inc). Spearman correlation analysis was used to examine the correlation between microarray and qRT-PCR data (Graph-pad Prism v5.0). Linear discriminant analysis was performed for each cohort to distinguish responders from nonresponders (JMP 10 software; SAS Institute) based on miRNA expression (i.e., the miRNA signature). Continuous expression values for a single miRNA or for plural miRNAs, that is, ΔCt values obtained by qRT-PCR against RNU66, were included as variables in the analysis. Receiver operating characteristic (ROC) curves were generated to evaluate response sensitivity and the are a under the curve(AUC)was calculated (JMP 10). Principal component analysis (PCA) of the expression of three miRNAs (miR1290, miR196b, and miR135a*) was performed using JMP 10.
Results
Sample selection
The aim of this study was to identify biomarkers in patients with metastatic LADC who relapsed following potential curative surgical resection. Therefore, surgically resected primary tumor tissues from 103 LADC patients who were treated with platinum-based doublet chemotherapy up on recurrence were selected form Irna profiling (Fig. 1A). The cases were assigned to a test cohort (n = 40; RNAs from frozen tissue available) or a validation cohort (n = 63; RNAs from FFPE tissues available) according to the availability of tumor tissue samples. Patients in both cohorts were classified as responders (CR and PR) or nonresponders (SD and PD) to platinum-based doublet chemotherapy according to the RECIST criteria (Materials and Methods; Supplementary Table S1). In this study, platinum-based doublet chemotherapy includes several different regimens. The cohorts were similar in terms of clinicopathologic characteristics such as age, gender, smoking habits, pathologic stage, representative oncogene mutations, therapeutic regimen, and therapeutic response (Table 1). The samples were subjected to a two-step screening procedure to identify miRNAs whose expression is associated with responses to platinum-based doublet chemotherapy (Fig. 1B).
Table 1.
Clinicopathologic characteristics on surgically resected primary lung ADC
| Test cohort (n = 40) | Validation cohort (n = 63) | P | |
|---|---|---|---|
| Age, y | 0.20 | ||
| Mean (range) | 59 (47–67) | 59 (32–72) | |
| Gender number (%) | |||
| Male | 23 (58) | 28 (44) | 0.23 |
| Female | 17 (42) | 35 (56) | |
| Smoking (pack year) number (%) | |||
| Never smoker | 16 (40) | 33 (52) | 0.09 |
| <20 | 3 (8) | 10 (16) | |
| ≥20 | 21 (52) | 20 (32) | |
| TP53 genotype number (%) | 0.49 | ||
| Arg/Arg | 20 (50) | 24 (38) | |
| Arg/Pro | 16 (40) | 33 (52) | |
| Pro/Pro | 4 (10) | 6 (10) | |
| Driver oncogene mutation number (%) | |||
| Negative | 14 (35) | 28 (44) | 0.34 |
| Positive | 26 (65) | 35 (56) | |
| EGFR mutation | 19 | 28 | |
| KRAS mutation | 3 | 7 | |
| HER2 mutation | 3 | 0 | |
| BRAF mutation | 1 | 0 | |
| Driver oncogene fusion number (%) | |||
| Negative | 36 (90) | NE | |
| Positive | 4 (10) | NE | |
| ALK fusion | 2 | NE | |
| RET fusion | 0 | NE | |
| ROS1 fusion | 2 | NE | |
| TNM stage at initial diagnosis number (%) | 0.83 | ||
| IA | 6 (15) | 5 (8) | |
| IB | 5 (13) | 12 (19) | |
| IIA | 4 (10) | 10 (16) | |
| IIB | 2 (5) | 2 (3) | |
| IIIA | 18 (45) | 26 (41) | |
| IIIB | 2 (5) | 0 | |
| IV | 3 (8) | 8 (13) | |
| TNM stage at recurrence number (%) | 0.32 | ||
| IA | 0 | 0 | |
| IB | 0 | 0 | |
| IIA | 0 | 0 | |
| IIB | 0 | 0 | |
| IIIA | 0 | 0 | |
| IIIB | 0 | 0 | |
| IV | 0 | 0 | |
| Recurrent portion | 1 | ||
| Local/regional | 2 (5) | 3 (5) | |
| Metastasis | 38 (95) | 60 (95) | |
| M1a | 22 | 28 | |
| M1b | 16 | 32 | |
| Platinum-based regimens number (%) | 0.44 | ||
| Platinum + paclitaxel | 30 (75) | 42 (67) | |
| Platinum + gemcitabine | 9 (23) | 14 (22) | |
| Platinum + docetaxel | 0 | 4 (6) | |
| Platinum + pemetrexed | 0 | 2 (3) | |
| Platinum + vinorelbine | 1 (2) | 1 (2) | |
| Tumor response number (%) | |||
| Responder | 16 (40) | 18 (29) | 0.31 |
| CR | 1 | 0 | |
| PR | 15 | 18 | |
| Nonresponder | 24 (60) | 45 (71) | |
| SD | 21 | 35 | |
| PD | 3 | 10 |
NOTE: The P value from the Fisher's exact test.
Abbreviations: NE, not examined; Platinum, cisplatin or carboplatin.
Differential expression of miRNAs between responders and nonresponders
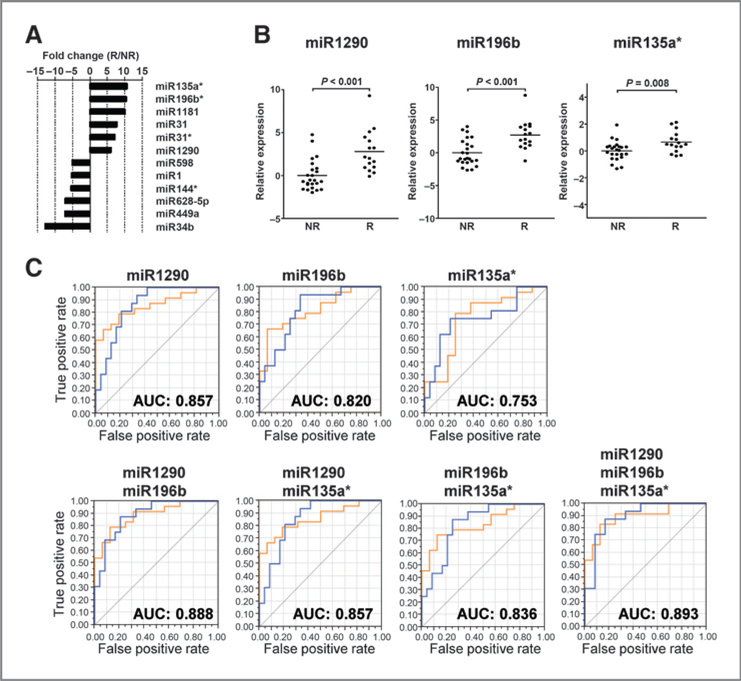
First, we used microarray analysis to identify miRNAs in test cohort samples that were differentially expressed in LADC tissues from responders and nonresponders to platinum-based doublet chemotherapy. Fifty-nine miRNAs were identified (P < 0.05; Welch t test; Supplementary Table S2). Of these, 28 were upregulated in responders and 31 were downregulated.
Next, to identify the limited number of miRNAs that can be used to deduce responsiveness in the clinic, we searched for miRNAs showing highly differential expression between responders and nonresponders. Twelve miRNAs (miR135a*, miR196b, miR1181, miR31, miR31, miR1290, miR598, miR1, miR144, miR628–5p, miR449a, and miR34b) showing a >5-fold change in expression were identified as potential candidates and investigated further (Fig. 2A;Supplementary Table S2). The expression of these 12 miRNAs was reanalyzed by qRT-PCR using the same RNA samples used in the microarray experiments. A good agreement between the qRT-PCR (ΔCt value) and microarray (log2 signal) data (as indicated by the Pearson correlation coefficient) was observed for 10 of the 12 miRNAs (excluding miR1181 and miR598; Supplementary Fig. S1). qRT-PCR identified three miRNAs (miR1290, miR196b, and miR135a*) as differentially expressed in responders and non-responders (P<0.001, P<0.001, and P<0.008, respectively; Fig. 2B; Supplementary Table S3). The fold changes observed in the qRT-PCR experiment were lower than those observed in the microarray experiment. This may be due to the higher sensitivity of the former. Several samples that gave no significant signal (calculated as zero) in the microarray experiment yielded ΔCt values in the qRT-PCR experiment (Supplementary Fig. S1), leading to a reduction in the fold change values (Supplementary Table S3).
Figure 2.
Selection of the three miRNAs whose expression was associated with responses to platinum-based doublet chemotherapy upon LADC recurrence. A, twelve miRNAs differentially expressed in responders (R) and nonresponders (NR) to platinumbased doublet chemotherapy in the test cohort. The diagram depicts miRNAs showing a >5-fold change in expression and with a P value of <0.05. The fold change is represented by the ratio of R to NR derived from the microarray data. B, expression of miRNAs in NR and R in the test cohort, as measured by qRT-PCR. Dot plots, miRNA relative threshold cycle values. Expression was normalized to that of RNU66. Threshold cycle values relative to the mean value in NR are shown on a log2 scale. Horizontal bars, the mean expression value; P values (Mann–Whitney test) are indicated. C, ROC analysis was performed for miR1290, miR196b, and miR135a* in the test cohort. The AUC value is shown. The blue line, the results for responders and the orange line represents the results for NR
We next used linear discriminant analysis to examine these three miRNAs for their potential to discriminate responders from nonresponders. For this, continuous expression values for a single miRNA or a combination of two or three miRNAs, that is, ΔCt values obtained by qRT-PCR, were included as variables in the analysis. ROC curves were plotted to examine both sensitivity and specificity. The results showed that a combination of all three miRNAs provided the best discrimination, with an AUC of 0.893 (Fig. 2C). This combination of three miRNAs is, henceforth, referred to as the “three-miRNA signature.”
Ability of the three-miRNA signature to predict responses to chemotherapy
To validate the findings in the test cohort, we next examined the expression of the three-miRNA signature in the validation cohort (Supplementary Fig. S2 and Supplementary Table S3). We then performed a linear discriminant analysis to evaluate the potential of the three-miRNA signature as a biomarker. The mean expression levels of the three miRNAs were higher in responders than in nonresponders in the validation cohort, although the difference in the expression of miR135a* did not reach a statistical significance. However, a combination of all three miRNAs again was better able to distinguish responders from nonresponders (AUC = 0.837) than a single miRNA or a combination of two miRNAs (Supplementary Fig. S3).
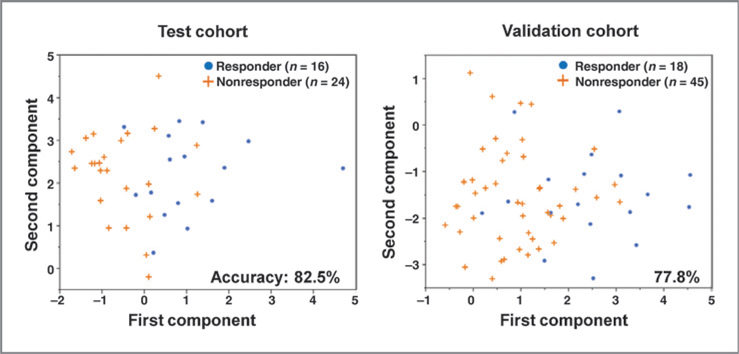
PCA and support vector machine (SVM) analysis showed a predictive response of 37.5% for the test cohort containing 40% responders and a predictive response of 31.7% for the validation cohort containing 28.6% responders. The signature predicts responders and nonresponders in the test and validation cohorts with an accuracy of 82.5% and 77.8%, respectively (Fig. 3). The sensitivity, specificity, and positive and negative predictive values were similar for both cohorts (Supplementary Table S4). There was no significant difference in the clinical characteristics between true responders/nonresponders and predicted responders/nonresponders (Supplementary Table S1). Taken together, these results show that the predictive ability of the three-miRNA signature was confirmed in the independent validation cohort, and that the signature is still predictive even if archive FFPE tissues are used for analysis. Specificity and negative predictive values greaterthan sensitivity and positive predictive values suggest that the three-miRNA signature predicts nonresponders better than responders (Supplementary Table S4).
Figure 3.
PCA. A PCA–SVM strategy using three miRNAs (miR1290, miR196b, and miR135a*) was used to construct a classifier, which could distinguish responders from nonresponders. The blue dots represent the responders and the orange crosses represent the nonresponders in the test (left) and validation cohorts (right). The classifier had a predictive accuracy of 82.5% for the test cohort and an accuracy of 77.8% for the validation cohort.
Combining the three-miRNA signature with the TP53-Arg72Pro polymorphism genotype
We previously showed in the same study population (i.e., the 640 cases shown in Fig. 1A) that the Arg72Pro polymorphism in the TP53 gene in noncancerous (germline) DNA is associated with responses to platinum-based doublet chemotherapy: The response rate is higher in those harboring the TP53–72Pro polymorphism (35). Therefore, we combined the three-miRNA signature with the TP53Arg72Pro genotype data to ascertain whether the predictive power of the miRNA signature was enhanced. We dichotomized the study cohorts into two subgroups (patients with theTP53–72 Pro allele and those without) and examined the predictive accuracy of the three-miRNA signature. We found that the predictive accuracy marginally improved in both the test (85.0%) and validation (82.5%) cohorts (Fig. 1B).
The three-miRNA signature predicts responses to chemotherapy irrespective of driver oncogene aberrations and clinical characteristics
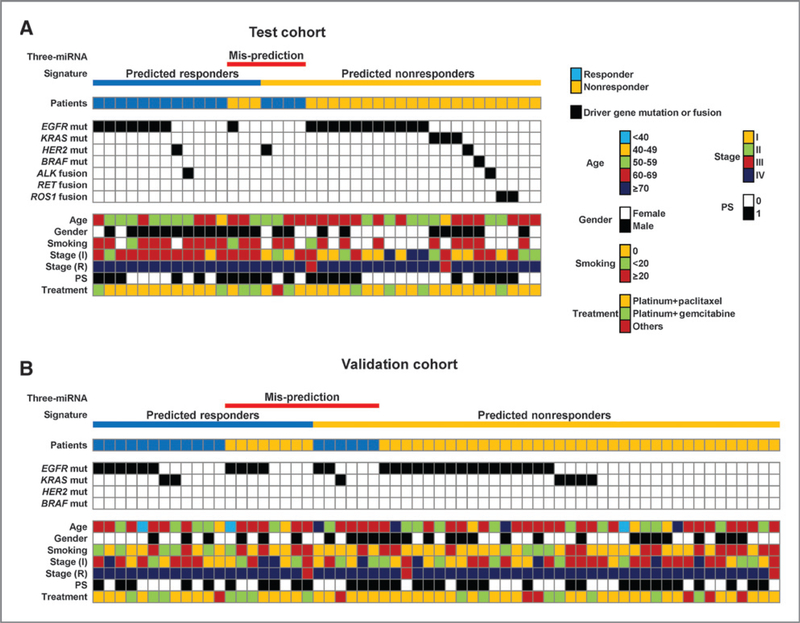
Driver oncogene aberrations in LADC are a critical factor that determines the therapeutic strategy for each patient. In addition, such aberrations are associated with clinical characteristics, as represented by the predominance of EGFR mutation in females and never-smokers. Therefore, we next addressed whether the ability of the three-miRNA signature to predict responses to chemotherapy was affected by driver oncogene alterations or clinical characteristics. The three miRNA signature failed to predict responses in 21 of 103 cases (21%). The test and validation cohorts contained seven (one with an EGFR mutation, one with a HER2 mutation, and five aberration-negative cases) and 14 (six with EGFR mutations, one with a KRAS mutation, and seven aberration-negative cases) nonpredicted cases, respectively (Fig. 4). The nonprediction rate was 15% (7/47 cases) for patients harboring the EGFR mutation, 10% (1/10) for those harboring the KRAS mutation, 33% (1/3) for those harboring the HER2 mutation, and 32% (12/38) for aberration-negative cases; therefore, there was no significant correlation between driver gene status and nonprediction. Similarly, there was no significant association between clinical factors and nonprediction; thus, the three-miRNA signature may be a useful biomarker for predicting the responses of patients with LADC to chemotherapy, irrespective of driver oncogene aberrations and clinical characteristics.
Figure 4.
Response prediction by the three-miRNA signature according to clinicopathologic factors in the test (A) and validation cohorts (B). Driver gene mutations and clinical features are shown: patients (blue, responder; orange, nonresponder); driver gene (black, EGFR, KRAS, HER2, BRAF mutation- or ALK, RET, or ROS1 fusion-positive; white, negative); age (blue, <40; orange, 40–49; green, 50–59; red, 60–69; and navy blue, 70); gender (white, female; black, male); smoking (orange, pack years = 0; green, <20; and red, 20); tumor stage at initial diagnosis (I) and at recurrence (R; orange, I; green, II; red, III; and navy blue, VI); PS (white, 0; black, 1); treatment (orange, platinum + paclitaxel; green, platinum + gemcitabine; and red, other).
Discussion
Here, we performed miRNA expression profiling of patients who initially underwent surgical resection for primary LADC and were then treated with platinum-based doublet chemotherapy upon recurrence. We identified a three-miRNA signature (miR1290, miR196b, and miR135a*) that predicts whether patients with recurring LADC respond to and, therefore, will benefit from platinum-based doublet chemotherapy. Even patients with LADC harboring druggable on cogene aberrations (that are resistant to treatment with TKIs) may be treated with platinum-based doublet chemotherapy; therefore, platinum-based doublet chemotherapy is a major therapeutic strategy for almost all patients with LADC(39–41). Personalized therapy, in which a drug with the greatest chance of eliciting a response (i.e., tumor shrinkage) is chosen specifically for each patient, is the first critical step toward improved prognosis for patients with LADC with advanced and recurrent disease; indeed, clinical trials examining the effect of new drugs on NSCLC have set improved response rates as their primary endpoint (10); thus, response to treatment according to the RECIST criteria ratherthan survival was the outcome measure selected for the present study. The three-miRNA signature will facilitate personalized therapy for LADC and will include platinum-based doublet therapy as an option.
Here, we examined the three-miRNA signature of primary tumors to predict the responsiveness of recurrent tumors. Itis noteworthy that the biologic characteristics of recurrent tumors are not the same as those of primary tumors due to tumor cell heterogeneity in the primary lesions and the accumulation of additional genetic/epigenetic changes during progression. Thus, at present our findings are applicable to the treatment of recurrent tumors for which corresponding primary tumor tissue samples are available. The finding that the three-miRNA signature is predictive in archived primary tumor tissues is an advantage; patients are spared the additional burden of further tissue sampling for genetic analysis. However, it is also worth analyzing recurrent tumors and inoperable advanced tumors to find out whether the three miRNA signature is applicable to patients for whom archived surgical tissues are not available. The finding that the signature can be identified in archived FFPE tissues is also an advantage and will facilitate translation to the clinic.
We also examined the combination of the three-miRNA signature with the TP53-Arg72Pro polymorphism genotype to see whether this provided greater predictive accuracy, as blood cells used for genotyping polymorphisms are easily obtained from patients. However, the improvement was only marginal. Therefore, more polymorphisms associated with responses to platinum-based doublet therapy must be identified if we are to achieve any marked improvement over the three-miRNA signature alone. In addition, we used a fold change >5 as a criterion for identifying candidate miRNAs that are differentially expressed between responders and nonresponders. Using less stringent or other statistical criteria may lead to the identification of more miRNAs that are useful for prediction.
The three-miRNA signature predicted responses irrespective of the presence of driver oncogene aberrations. This is important when we consider that LADCs that have acquired resistance to specific TKIs are treated with platinum-based doublet chemotherapy. However, unfortunately, the present study cohort did not include samples from patients with EGFR- or ALK-positive LADC that received TKIs before platinum-based doublet chemotherapy. Such cases should be examined to address this issue.
This study has several limitations. First, it was retrospective in nature, so the ability of the three-miRNA signature to predict responses needs to be further validated using more samples. Here, different types of tumor tissue for the test and validation sets (bulk frozen and micro dissected FFPE tissues, respectively), which contained patients that had undergone several different chemotherapeutic regimens, were subjected to analysis; therefore, we may have over- or underestimated predictive value. Thus, further studies that use a larger number of samples obtained according to a defined experimental procedure and take factors such as previous treatment regimen, disease stage, and PS into account are required. In addition, prospective studies, for example, studies using samples from patients treated with a single therapeutic regimen, and the analysis of primary and recurrent tumors and inoperable advanced tumors, should be conducted to confirm the utility of the three-microRNA signature. Second, although the three-miRNA signature was significantly associated with response to chemotherapy, differences in progression-free survival were only suggestive (Supplementary Fig. S4). We chose the response as the primary endpoint of efficacy to identify subgroups for which chemotherapy does work (35). However, treatment is continued after the failure of platinum-based doublet chemotherapy; therefore, clinical response alone would not be enough to improve the outcome. Third, the functional relevance of miR1290, miR196b, and miR135a* to the chemosensitivity of LADC remains unclear. Interestingly, a recent study shows that the expression of miR196b is upregulated in patients with rectal adenocarcinoma that respond to neoadjuvant chemoradiotherapy (capecitabine or 5-fluorouracil), which supports the findings of the present study (42). However, preliminary experiments examining the exogenous expression of the three miRNAs in LADC cell lines did not show increased sensitivity to a platinum agent, cisplatin (CDDP). Therefore, the direct or indirect effects of miRNAs on chemosensitivity should be further investigated.
Supplementary Material
Translational Relevance.
The use of biomarkers to identify patients that will respond to platinum-based doublet chemotherapy before treatment is a critical strategy for improving the efficacy of chemotherapy for lung adenocarcinoma (LADC). Here, we report that the expression profile of three miRNAs in surgically resected primary tissues is clinically useful for predicting responsiveness to platinum-based doublet chemotherapy in patients with LADC recurring after initial surgical resection. This three-miRNA signature may be useful for the clinical management of LADC.
Acknowledgments
The authors thank Yoko Shimada for technical assistance.
Grant Support
This work was supported in part by the Advanced Research for Medical Products Mining Program of the National Institute of Biomedical Innovation (NIBIO), Grants-in-Aid from the Ministry of Health, Labor, and Welfare for the Third Term Comprehensive 10 year Strategy for Cancer Control, and Grant-in-Aid from Japan Society for the Promotion of Science (JSPS) for Scientific Research (B). Grant-in-Aid for Young Scientists (B; 24790340) and Third-Term Comprehensive Control Research for Cancer (H25-Young Scientists-009).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–57. [DOI] [PubMed] [Google Scholar]
- 3.Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385–94. [DOI] [PubMed] [Google Scholar]
- 4.Kohno T, Ichikawa H, Totoki Y, Yasuda K, Hiramoto M, Nammo T, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med 2012;18:375–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipson D, Capelletti M, Yelensky R, Otto G, Parker A, Jarosz M, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med 2012;18:382–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeuchi K, Soda M, Togashi Y, Suzuki R, Sakata S, Hatano S, et al. RET, ROS1, and ALK fusions in lung cancer. Nat Med 2012;18:378–81. [DOI] [PubMed] [Google Scholar]
- 7.Awad MM, Engelman JA, Shaw AT. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N Engl J Med 2013;369:1173. [DOI] [PubMed] [Google Scholar]
- 8.Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, et al. EML4–ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 2010;363:1734–9. [DOI] [PubMed] [Google Scholar]
- 9.Oxnard GR, Binder A, Janne PA. New targetable oncogenes in non– small cell lung cancer. J Clin Oncol 2013;31:1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohno T, Tsuta K, Tsuchihara K, Nakaoku T, Yoh K, Goto K. RET fusion gene: translation to personalized lung cancer therapy. Cancer Sci 2013;104:1396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohe Y, Ohashi Y, Kubota K, Tamura T, Nakagawa K, Negoro S, et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non–small cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol 2007;18:317–23. [DOI] [PubMed] [Google Scholar]
- 12.Scagliotti GV, De Marinis F, Rinaldi M, Crino L, Gridelli C, Ricci S, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non–small cell lung cancer. J Clin Oncol 2002;20:4285–91. [DOI] [PubMed] [Google Scholar]
- 13.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non– small cell lung cancer. N Engl J Med 2002;346:92–8. [DOI] [PubMed] [Google Scholar]
- 14.Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet 2011;12:99–110. [DOI] [PubMed] [Google Scholar]
- 15.Kasinski AL, Slack FJ. Epigenetics and genetics. microRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer 2011;11:849–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer 2006;6:259–69. [DOI] [PubMed] [Google Scholar]
- 17.Bian HB, Pan X, Yang JS, Wang ZX, De W. Upregulation of microRNA451 increases cisplatin sensitivity of non–small cell lung cancer cell line (A549). J Exp Clin Cancer Res 2011;30:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong Z, Zhong Z, Yang L, Wang S, Gong Z. microRNA-31 inhibits cisplatin-induced apoptosis in non–small cell lung cancer cells by regulating the drug transporter ABCB9. Cancer Lett 2014;343:249–57. [DOI] [PubMed] [Google Scholar]
- 19.Galluzzi L, Morselli E, Vitale I, Kepp O, Senovilla L, Criollo A, et al. miR181a and miR-630 regulate cisplatin-induced cancer cell death. Cancer Res 2010;70:1793–803. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Li L, Guan Y, Liu X, Meng Q, Guo Q. miR-92b regulates the cell growth, cisplatin chemosensitivity of A549 non–small cell lung cancer cell line and target PTEN. Biochem Biophys Res Commun 2013;440: 604–10. [DOI] [PubMed] [Google Scholar]
- 21.Pouliot LM, Shen DW, Suzuki T, Hall MD, Gottesman MM. Contributions of microRNA dysregulation to cisplatin resistance in adenocarcinoma cells. Exp Cell Res 2013;319:566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song L, Li Y, Li W, Wu S, Li Z. miR-495 enhances the sensitivity of non– small cell lung cancer cells to platinum by modulation of copper transporting P-type adenosine triphosphatase A (ATP7A). J Cell Biochem 2014;115:1234–42. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Zhong M, Liu W, Li J, Huang J, Zheng L. Alterations of microRNAs in cisplatin-resistant human non–small cell lung cancer cells (A549/DDP). Exp Lung Res 2011;37:427–34. [DOI] [PubMed] [Google Scholar]
- 24.Voortman J, Goto A, Mendiboure J, Sohn JJ, Schetter AJ, Saito M, et al. MicroRNA expression and clinical outcomes in patients treated with adjuvant chemotherapy after complete resection of non–small cell lung carcinoma. Cancer Res 2010;70:8288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oue N, Anami K, Schetter AJ, Moehler M, Okayama H, Khan MA, et al. High miR-21 expression from FFPE tissues is associated with poor survival and response to adjuvant chemotherapy in colon cancer. Int J Cancer 2014;134:1926–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito M, Schetter AJ, Mollerup S, Kohno T, Skaug V, Bowman ED, et al. The association of microRNA expression with prognosis and progression in early-stage, non–small cell lung adenocarcinoma: a retrospective analysis of three cohorts. Clin Cancer Res 2011;17:1875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akagi I, Okayama H, Schetter AJ, Robles AI, Kohno T, Bowman ED, et al. Combination of protein coding and noncoding gene expression as a robust prognostic classifier in stage I lung adenocarcinoma. Cancer Res 2013;73:3821–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schetter AJ, Leung SY, Sohn JJ, Zanetti KA, Bowman ED, Yanaihara N, et al. microRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA 2008;299:425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathe EA, Nguyen GH, Bowman ED, Zhao Y, Budhu A, Schetter AJ, et al. microRNA expression in squamous cell carcinoma and adenocarcinoma of the esophagus: associations with survival. Clin Cancer Res 2009;15:6192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen GH, Schetter AJ, Chou DB, Bowman ED, Zhao R, Hawkes JE, et al. Inflammatory and microRNA gene expression as prognostic classifier of Barrett’s-associated esophageal adenocarcinoma. Clin Cancer Res 2010;16:5824–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Y, Schetter AJ, Yang GB, Nguyen G, Mathe EA, Li P, et al. microRNA and inflammatory gene expression as prognostic marker for overall survival in esophageal squamous cell carcinoma. Int J Cancer 2013;132:2901–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hummel R, Hussey DJ, Haier J. microRNAs: predictors and modifiers of chemo- and radiotherapy in different tumour types. Eur J Cancer 2010;46:298–311. [DOI] [PubMed] [Google Scholar]
- 33.Berghmans T, Ameye L, Willems L, Paesmans M, Mascaux C, Lafitte JJ, et al. Identification of microRNA-based signatures for response and survival for non–small cell lung cancer treated with cisplatin-vinorelbine A ELCWP prospective study. Lung Cancer 2013;82:340–5. [DOI] [PubMed] [Google Scholar]
- 34.Ranade AR, Cherba D, Sridhar S, Richardson P, Webb C, Paripati A, et al. MicroRNA 92a-2: a biomarker predictive for chemoresistance and prognostic for survival in patients with small cell lung cancer. J Thorac Oncol 2010;5:1273–8. [DOI] [PubMed] [Google Scholar]
- 35.Shiraishi K, Kohno T, Tanai C, Goto Y, Kuchiba A, Yamamoto S, et al. Association of DNA repair gene polymorphisms with response to platinum-based doublet chemotherapy in patients with non–small cell lung cancer. J Clin Oncol 2010;28:4945–52. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida A, Kohno T, Tsuta K, Wakai S, Arai Y, Shimada Y, et al. ROS1rearranged lung cancer: a clinicopathologic and molecular study of 15 surgical cases. Am J Surg Pathol 2013;37:554–62. [DOI] [PubMed] [Google Scholar]
- 37.Okayama H, Kohno T, Ishii Y, Shimada Y, Shiraishi K, Iwakawa R, et al. Identification of genes upregulated in ALK-positive and EGFR/ KRAS/ALK-negative lung adenocarcinomas. Cancer Res 2012;72: 100–11. [DOI] [PubMed] [Google Scholar]
- 38.Kinno T, Tsuta K, Shiraishi K, Mizukami T, Suzuki M, Yoshida A, et al. Clinicopathological features of non–small cell lung carcinomas with BRAF mutations. Ann Oncol 2014;25:138–42. [DOI] [PubMed] [Google Scholar]
- 39.Gerber DE, Minna JD. ALK inhibition for non–small cell lung cancer: from discovery to therapy in record time. Cancer Cell 2010;18:548–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drilon A, Wang L, Hasanovic A, Suehara Y, Lipson D, Stephens P, et al. Response to Cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov 2013;3:630–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davies KD, Le AT, Theodoro MF, Skokan MC, Aisner DL, Berge EM, et al. Identifying and targeting ROS1 gene fusions in non–small cell lung cancer. Clin Cancer Res 2012;18:4570–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svoboda M, Sana J, Fabian P, Kocakova I, Gombosova J, Nekvindova J, et al. MicroRNA expression profile associated with response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer patients. Radiat Oncol 2012;7:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.