Abstract
Tissue inhibitor of metalloproteinase 2 (TIMP-2) antitumorigenic, antimetastatic and antiangiogenic activities in vitro and in vivo. Recombinant TIMP-2 is currently undergoing preclinical testing in multiple, murine tumor models. Here we report the development of an inert, injectable peptide hydrogel matrix enabling encapsulation and sustained release of TIMP-2. We studied the TIMP-2 release profile from four β-hairpin peptide gels of varying net electrostatic charge. A negatively charged peptide gel (designated AcVES3) enabling encapsulation of 4 mg/mL of TIMP-2, without effects on rheological properties, facilitated the slow sustained release (0.9%/d) of TIMP-2 over 28 d. Released TIMP-2 is structurally intact and maintains the ability to inhibit MMP activity, as well as suppress lung cancer cell proliferation in vitro. These findings suggest that the AcVES3 hydrogel will be useful as an injectable vehicle for systemic delivery of TIMP-2 in vivo for ongoing preclinical development.
Graphical Abstract
INTRODUCTION
The tumor microenvironment (TME) is recognized for its essential role in regulating cancer progression and metastasis.2–4 The normal tissue microenvironment presents an antitumorigenic barrier in which normal tissue architecture and local homeostatic mechanisms restrict tumor progression.6 The TME is a complex three-dimensional stromal matrix that is composed of similar molecular components as “normal” extracellular matrix (ECM) but are extensively remodeled to support tumor growth and expansion. In addition, the TME contains an infiltrate of stromal cells, such as immune/inflammatory cells, cancer-associated fibroblasts (CAFs), adipocytes, and endothelial cells (both vascular and lymphatic). These infiltrating non-neoplastic cells express a network of cytokines and growth factors that promote tumor growth and modulate immune surveillance. Tumor cells can undergo an epithelial—mesenchymal transition resulting in acquisition of various properties, such as altered adhesion, enhanced migration, and expression of ECM-degrading proteases, that contribute to cancer invasion and metastasis. It is now well established that this process of cancer metastasis is the principal cause of treatment failure and is overwhelmingly associated with the majority of cancer deaths.3,7 The reciprocal, dynamic interactions between cells, both malignant and nonmalignant, and molecular components of the three-dimensional ECM are critical determinants of tissue homeostasis. Disruption of these essential elements underlies the pathogenesis of many chronic disease states, including cancer progression and metastasis.6,8,9 Emerging challenges in the development of new cancer therapies have fostered interest in the development of treatments targeting the TME, including strategies for “normalizing” tissue homeostasis, also referred to as differentiation therapy.4,10,11
The roles of the matrix metalloproteinases (MMPs) in remodeling of the ECM associated with chronic disease states, such as cancer, have been studied extensively.7,12,13 These studies, and the identification of low levels of endogenous MMP inhibitors in tumor tissues, have made MMPs an attractive target for therapeutic intervention. The clinical failure of synthetic MMP inhibitors for cancer therapy was the result of poor study design, lack of efficacy, failure to monitor target MMP activity, and toxicity.12,14 However, novel strategies targeting MMPs for cancer therapy include innovative prodrug designs and targeting based on new structure—function correlates, as well as the use of endogenous MMP inhibitors to normalize the TME.12,15–17
The human genome has four paralogous genes encoding endogenous proteinase inhibitors known collectively as the tissue inhibitors of metalloproteinases (TIMPs). These endogenous inhibitors are well characterized with respect to their inhibitory activities against members of the metzincin superfamily of proteases, which includes the MMPs (also known as the matrixins), the ADAM and ADAMTS, as well as the astacins.18,19 The TIMP family members have similar but distinct protease inhibitory profiles.20–22 TIMPs are multifunctional proteins that, in addition to regulation of protease activity, reportedly modulate cell growth and migration.16 Altered expression of TIMP family members has been associated with a variety of chronic diseases including proliferative diabetic retinopathy, acute kidney injury, neurodegenerative processes, extension of myocardial infarction, and cancer progression, highlighting potential use of TIMPs as biomarkers of disease or as novel therapeutics.23–27
TIMP-2 is an isoform that is abundantly expressed in most normal adult human tissues.16,17 However, decreased TIMP-2 expression is associated with poor survival in human nonsmall cell lung cancer, hepatocellular, breast, and renal cell carcinomas.28–31 TIMP-2 can directly suppress growth-factor-mediated cellular proliferation (fibroblasts and endothelial and tumor cells) in vitro by an MMP-independent mechanism via heterologous receptor inactivation.32–34 TIMP-2 binding to the integrin α3β1 activates the Shp-1 phosphatase that inactivates downstream signaling from receptor tyrosine kinases, e.g., EGFR, FGFR, and VEGFR.35 The combination of MMP inhibitory and direct (MMP-independent) antiproliferative activities makes TIMP-2 an attractive candidate for preclinical therapeutic development. However, successful TIMP-2 therapy requires an innovative approach for the administration of stable, bioactive TIMP-2 protein (or derivatives) in a convenient, long-term manner.
Self-assembling peptide-based hydrogels have been studied as injectable materials for tissue repair, tissue engineering, and vehicles for controlled drug delivery whose physical and biological properties can be regulated by modulating their amino acid compositions.36–46 This allows fine-tuning for a variety of biomedical applications, including vascular anastomosis.47 Schneider et al. have been developing peptide hydrogels for therapeutic delivery using β-hairpin amphiphilic peptides that undergo triggered self-assembly into fibrillar networks that constitute the formation of hydrogel material.48–56 The peptides contain 20 residues and are composed of two β-strands of alternating hydrophilic amino acids and hydrophobic valine residues surrounding a tetrapeptide sequence (VDPPT) which is known to adopt a type II′ β-turn.57 When peptides are initially dissolved in aqueous buffer at low ionic strength and temperature, they are soluble. Self-assembly leading to gelation can be triggered by changes in pH, ionic strength, or temperature. If initially present in the peptide solution, small molecules, proteins, and/or cells can be directly encapsulated during gel formation. Resultant peptide gels exhibit shear-thinning/self-recovery behavior following shear stress.58,59 This allows peptide gels to be injected via syringe to targeted sites, such as a particular tissue. β-Hairpins can be designed to be either cationic or anionic, and in turn, the fibrillar networks formed from their self-assembly are charged. The charge state of a particular gel (either positive or negative) influences the amount and rate of protein released from its fibrillar network.
Gel encapsulated proteins exist as two distinct populations. The first is a freely diffusing mobile population, and the second comprises proteins that have physically adsorbed to the fibril network. The diffusive population is released in an early time regime (days), and the physically absorbed population is released slowly (weeks to months). The amount of protein in a given population is governed by electrostatic interactions made between the gel network and the encapsulated protein.54,58,59 For example, more protein will be adsorbed and thus released more slowly when electrostatic interactions exist between oppositely charged fibrils and protein. Thus, the charge state of the gel and the protein are important determinants in engineering gels for a desired release profile.
In this work, we investigate the utility of four differently charged peptide gels with the aim of developing a sustained delivery system, that allows continuous, long-term (30 day) release of stable, bioactive, recombinant, human TIMP-2. This will provide an alternative strategy to the current method of daily, systemic administration of TIMP-2 during continuing preclinical development.
MATERIALS AND METHODS
Production Purification and Characterization of TIMP-2 6XHis.
Recombinant human TIMP-2 with a C-terminal 6X histidine epitope tag (TIMP-2–6XHis, subsequently referred to as TIMP-2) was prepared and analyzed as previously reported.60 Briefly, HEK-293 F cells in suspension culture were stably transfected with pCDNA 3.1 expression vector bearing the codon optimized TIMP-2 cDNA. Alternatively, HEK-293 F cells previously transduced with the TIMP-2 cDNA and using antibiotic selection were screened for maximal TIMP-2 protein expression levels. These cell lines were used to initiate suspension cultures that underwent sequential expansion of culture volumes (up to 3 L) and then harvested at 7 days after initial seeding. TIMP-2 was purified by an initial Ni2+-IMAC (HisTrap, GE Healthcare Catalog No. 17-5248-02) by stepwise imidazole gradient and then by preparative reverse phase (RP)-HPLC using a POROS R1/10 column (Applied Biosystems, Catalog No. 1-1012-46) in a H2O-acetonitrile gradient with 0.1% trifluoroacetic acid (TFA). HPLC eluents containing purified TIMP-2 were lyophilized, resuspended in Milli-Q H2O, and lyophilized again for final storage at −80 °C. Final characterization and quantitation of the TIMP-2 were performed by SDS-PAGE, western blotting, BCA assay, A280 (using the theoretical (calculated) molar extinction coefficient of TIMP-2), and also TIMP-2 ELISA assay.
Peptide Synthesis and Purification.
Peptides were synthesized on PL-rink resin (Agilent Technologies) using an automated ABI 433A peptide synthesizer (Applied Biosystems). Fmoc-protected amino acids were purchased from Novabiochem. Synthesis was carried out via solid-phase Fmoc-based chemistry with 1H-benzotriazolium-1-[bis(dimethylamino)methylene]-5-chloro-hexafluorophosphate-(1-),3-oxide (HCTU, Peptide International) activation. Resin bound peptide was cleaved and side-chain deprotected using TFA/thioanisole/ethanedithiol/anisole (90:5:3:2) for 2 h under inert gas. Crude peptides were precipitated with cold diethyl ether (Fisher Scientific) after separation of resin by filtration. MAX8 and HLT2 were purified by RP-HPLC using a Vydac C18 Column with solvents consisting of solvent A (0.1% TFA in water) and solvent B (0.1% TFA in 90% acetonitrile). Purified peptide solutions were lyophilized, resulting in pure peptide powders that were utilized in all assays. AcVES3 and IE1 were purified by RP-HPLC using a Phenomenex PolymerX Column with solvents consisting of solvent C (20 mM ammonium bicarbonate in water) and solvent D (20 mM ammonium bicarbonate in 80% acetonitrile). Purified fractions were lyophilized, yielding powder. The ammonium counterions for the glutamates were exchanged with sodium by dissolving pure peptide in solvent C at 1 mg/mL and adding an equal molar equivalence of aqueous NaOH with respect to glutamate content. This solution was lyophilized, yielding a white powder. The purity of all peptides was confirmed by analytical HPLC and electrospray ionization-mass spectrometry, Figure S1.
Oscillatory Shear Rheology.
Rheological assessment was conducted on a Texas Instruments AR-G2 rheometer using a 25 mm stainless steel parallel geometry. A 2 wt % peptide stock solution was prepared in chilled water. This solution was mixed with the chilled HEPES buffer (50 mM HEPES, 300 mM NaCl at pH 7.4) containing 8 mg/mL TIMP2 at a 1:1 ratio, affording a 1 wt % peptide solution containing 4 mg/mL TIMP-2. Then, 300 μL of the peptide solution was transferred to the center of the plate and the upper geometry was lowered to a gap height of 0.5 mm. The temperature of the system was then increased from 5 to 37 °C at 0.5 °C/s. Then, the storage (G′) modulus was monitored for 1 h at a constant angular frequency of 6 rad/s and 0.2% strain at 37 °C. After this, 1000% strain was applied for 30 s to disrupt the material. Subsequently, the ability of the hydrogel to reheal was monitored by measuring the recovery of G′ at 6 rad/s and 0.2% strain for an additional 1 h. Then, dynamic frequency sweeps (0.1–100 rad/s at constant 0.2% strain) and strain sweeps (0.1–1000% strain at constant 6 rad/s) were performed to ensure that time sweep data were collected in the linear viscoelastic regime.
TIMP-2 Release Studies.
For the TIMP-2 release studies, 100 μL of 1 wt % peptide gels containing 4 mg/mL TIMP-2 was prepared in glass vials (12 × 35 mm, Fisher Scientific). After the initiation of gelation, the gels were placed in an incubator at 37 °C for 1 h to ensure complete gelation. After 1 h, 1 mL of HEPES buffer (25 mM HEPES, 150 mM NaCl, pH 7.4) was gently added to the top of each gel. TIMP-2 release was evaluated over the course of 28 days. For each time point, the entire volume of buffer above the gel (1 mL) was removed and replaced with fresh buffer at designated time points: 1 h, 3 h, 6 h, 1 day, 3 days, 7 days, 14 days, 21 days, and 28 days. The concentration of TIMP-2 within the supernatant was determined by absorbance at 280 nm using an Epoch Microplate Spectrophotometer (BioTek Instruments) and was compared to a calibration curve. Release experiments were conducted in triplicate, and the results are presented as the mean ± standard deviation.
Circular Dichroism (CD) Studies.
The AcVES3 gel (1 wt %, 100 μL total volume) was prepared encapsulating 4 mg/mL of TIMP-2 in a glass vial. HEPES buffer (1 mL) was added to the top of the gel. The gel was shaken at 100 rpm at 37 °C for 28 days to allow protein release. The HEPES buffer above the gel was collected and replaced on days 7 and 28, and collected samples were filtered using a 0.22 μm spin filter. Any soluble peptide that may have been released during the course of this study was removed using Amicon ultra centrifugal filters (10K, EMD Millipore). BIS-TRIS propane (BTP) buffer (50 mM BTP, 150 mM NaCl, pH 7.4) was added to the top of the filter to dilute and collect the TIMP-2 protein. The TIMP-2 concentration was then determined by UV at 280 nm using the extinction coefficient (33 180 M−1 cm−1). CD wavelength spectra were measured from 200 to 260 nm at 37 °C using a 1 mm path length quartz cell. The mean residue ellipticity, [θ], was calculated from the equation [θ] = MRW (mean residue weight) × θ/10c × d, where θ is the measured ellipticity (mdeg), c is the concentration (mg/mL), and d is the length of the cell (cm). MRW was calculated from the equation MRW = molecular weight/(N − 1), where N is the number of residues. CD spectra were collected on an AVIV model 420 circular dichroism spectrometer (AVIV Biomedical). Control spectra were also collected using TIMP-2 that had never been encapsulated in gels.
Kinetic Analysis.
Inhibitory activity of recombinant TIMP-2 as well as TIMP-2 released at 37 °C from AcVES3 hydrogels during days 4–7 (referred to as day 7) and days 21–35 (denoted as day 35) postencapsulation were assayed against the recombinant MMP-2 40 kDa catalytic domain, using the MMP-2 Screening Assay Kit (Catalog No. ab139446, Abcam). An additional control tested enzyme inhibition by TIMP-2 incubated for 30 days using similar physiologic buffer conditions (Hank’s balanced salt solution, HBSS) but without prior AcVES3 encapsulation. The MMP-2 40 kDa catalytic domain was assayed at a final concentration of 25 nM (information provided by Abcam Inc. technical support). This assay utilizes MMP cleavage of a thiopeptolide substrate Ac-PLG-thioester-LG-OEt ([S] = 200 μM, non-rate-limiting) coupled assay, as described previously.61,62 Briefly, the MMP-mediated substrate cleavage generates a free sulfhydryl group that coupled with 5,5′-dithiobis(2-nitrobenzoic) acid (DNTB, Ellman’s reagent) leads to formation of 2-nitro-5-thiobenzoic acid, detected by absorbance at 412 nm. A small molecule broad spectrum MMP inhibitor (N-isobutyl-N-(4-methoxyphenylsulfonyl) glycyl hydroxamic acid (NNGH)) was used as a control inhibitor for the assay. Kinetic analyses of freshly prepared TIMP-2 dissolved in HBSS as well as TIMP-2 released from AcVES3 gels (days 7 and 35) or never encapsulated (incubated at 37 °C for 30 days) were conducted at concentrations from 0.5 to 40 nM ([I]:[E] ratios of 0.2–1.6) in triplicate (n = 3). A typical kinetic reaction involves MMP-2 preincubation with TIMP-2 for 1 h, at 37 °C prior to the addition of substrate. The reaction is then monitored (412 nm) at 2 min intervals for a total of 20 min using a Tecan Infinite M1000 Pro plate reader. The initial velocities (vi) and inhibitor constants (Ki) for the TIMP-2 preparations were determined from analysis of these time course experiments. Due to the putative, limited single site interaction of TIMP-2 with the MMP-2 catalytic domain (i.e., lacking C-terminal hemopexin domain interactions), we performed Dixon plots (1/vi vs [IT]) for determination of Ki without correction for tight binding forms.62,63 In addition, end point assays were performed using identical buffer and substrate conditions but with saturating TIMP-2 concentrations (100–200 nM, [I]:[E] ratios ≥4–8 fold) with product optical densities measured at a single time point (20 min, n = 3) before converting to total μM concentrations of 2-nitro-5-thiobenzoic acid produced (mean ± s.d.). Statistical analysis for these end point assays compared (means ± s.d.) the total 2-nitro-5-thiobenzoic acid generated (at indicated TIMP-2 concentrations) with the control (no added TIMP-2 (0 nM)) using a two-tailed student’s t-test (Prism software v 7.0).
A549 Cell Proliferation Assay.
Cell-based assays of TIMP-2 suppression of epidermal growth factor (rhEGF)-induced tumor cell proliferation were conducted as reported previously.32 Briefly, A549 human, lung cancer cells (Catalog No. CCL-185, ATCC) were cultured and maintained in DMEM F-12 media (Lonza, Catalog No. 12–719F) supplemented with 10% FBS and 0.01% PenStrep. Cells (viability >98%) were plated at 20 × 103 cells/well in 96 well plates precoated with 200 μL of 0.1% porcine gelatin solution (Catalog No. ES-006-B, EMD Millipore). Cells were cultured at 37 °C, 5% CO2 in complete growth media (containing 10% FBS) for the first 24 h and then in serum-free media for the next 24 h. Serum starved cells were pretreated with indicated TIMP-2 samples at doses of 0, 5, 10, and 100 nM and incubated at 37 °C for 30 min., prior to stimulation with 100 ng/mL rhEGF. Cell growth following EGF stimulation for 72 h was determined by measuring cell numbers using a standard MTT assay as previously described.32,60 The formazan product was dissolved by adding 100 μL of DMSO and read at 562 nm in a Tecan Infinite M1000 pro plate reader. Mean absorbance readings from the MTT assay were converted to % max EGF proliferation using the formula
Basal Abs560 and EGF Abs560 denote the mean absorbance from wells that received no treatment and those treated with rhEGF alone (with no inhibitor treatment), respectively.
Each assay was performed in triplicate and statistical analysis performed using a student t-test (Prism software, v 7.0).
RESULTS AND DISCUSSION
TIMP-2 Encapsulation and Release of TIMP-2 from Peptide Gels.
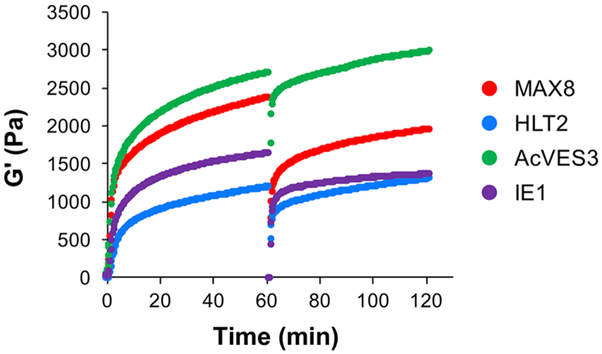
Four different β-hairpin peptide gels were studied for their ability to encapsulate and release TIMP-2 (Table 1, TEM micrographs of peptide fibrils can be found in Figure S2). MAX8 (formal charge of +7) and HLT2 (+5) are cationic, while AcVES3 (−5) and IE1 (−7) are anionic at pH 7.4. Thus, the MAX8 gel is more positively charged compared to the HLT2 gel and the IE1 gel is more negatively charged compared to AcVES3. Recombinant TIMP-2 has a pI of 6.84 (calculated isoelectric point based on protein sequence including the C-terminal His-tag epitope), nearly neutral at pH 7.4. The rheological properties of each gel containing 4 mg/mL TIMP-2 were first investigated to assess their potential for syringe-based delivery. Figure 1 shows the time-sweep oscillatory rheology data measuring the mechanical rigidity (G′) during the onset of gelation as well as the gels’ shear-thin/recovery characteristics. Here, TIMP-2 is codissolved with soluble peptide and gelation triggered at time = 0 and monitored over 60 min. Self-assembly occurs rapidly for each peptide in the presence of TIMP-2 with values of G′ exceeding 200 Pa within minutes that further increase to greater than 1000 Pa over time. After 60 min, the gels are shear-thinned and allowed to recover. Although TIMP-2 slightly impairs the ability of the most positively charged gel (MAX8) and the most negatively charged gel (IE1) to recover after being thinned, it has no effect on either HLT2 or AcVES3 gels. In sum, all four gels can be used to syringe deliver encapsulated TIMP-2, with the HLT2 and AcVES3 gels having slightly better recovery properties.
Table 1.
Sequences of β-Hairpin Peptides and Their Net Charge at pH 7.4
| peptide | sequence | net charge |
|---|---|---|
| MAX8 | VKVKVKVKVDPPTKVEVKVKV−NH2 | +7 |
| HLT2 | VLTKVKTKVDPPTKVEVKVLV−NH2 | +5 |
| AcVES3 | Ac−VEVSVSVEVDPPTEVSVEVEV−NH2 | −5 |
| IE1 | IEIEIEIEVDPPTEIEIEIEI−NH2 | −7 |
Figure 1.
Oscillatory rheology dynamic time sweeps and shear-thin recovery of 1 wt % MAX8, HLT2, AcVES3, and IE1 peptide gels in HEPES buffer (25 mM HEPES, 150 mM NaCl, pH 7.4) with 4 mg/mL encapsulated TIMP-2 monitoring the storage modulus (G′) as a function of time. The first 60 min represents the onset of gelation (strain = 0.2%, frequency = 6 rad/s), after which gels are shear-thinned at 1000% strain for 30 s and allowed to recover by reducing the strain to 0.2%. Recovery is monitored for an additional 60 minutes.
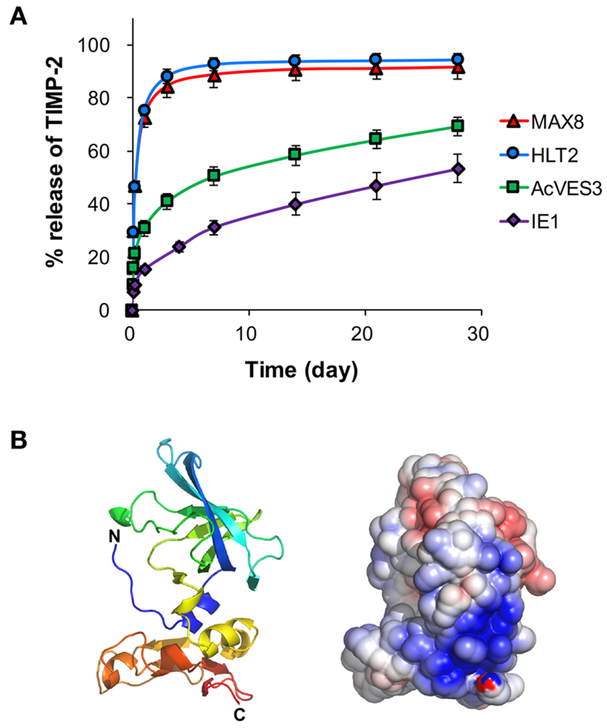
The release of TIMP-2 from 1 wt % MAX8, HLT2, AcVES3, and IE1 gels was monitored in vitro for 28 days (Figure 2A). Over 80% of TIMP-2 was rapidly released within 3 days from the positively charged MAX8 and HLT2 gels. This fast release was somewhat surprising in that earlier model studies investigating the release of neutral model proteins, myoglobin, and IgG from the MAX8 gel showed release profiles consistent with a longer duration reaching plateau levels around day 7.51 However, inspection of TIMP-2’s structure provides insight into this unexpected release behavior. Figure 2B shows a ribbon diagram of TIMP-2 along with a calculated linear Poisson-Boltzmann electrostatic potential surface for the protein rendered in the same pose. The surface map reveals a large dense patch of positively charged solvent-exposed residues near the C-terminus of the protein. It is likely that this positively charged patch is electrostatically repulsed from the positively charged fibrils that comprise the MAX8 and HLT2 gels. In addition, the protein used in our studies contains a C-terminal His-tag (not shown in Figure 2B) that also contributes a modest formal positive charge of +0.23 at pH 7.4. Thus, most of the encapsulated protein exists in the freely diffusing population (vide supra) and is released in an early time regime.
Figure 2.
(A) Cumulative release profiles of TIMP-2 from 1 wt % MAX8, HLT2, AcVES3, and IE1 peptide gels at 37 °C for 28 days. (B) A ribbon diagram of the crystal structure of TIMP2 (1BR9.pdb)(1 that was rendered using PyMOL. The linear Poisson−Boltzmann electrostatic potential was calculated with 0.15 M monovalent ions at 37 °C using the Adaptive Poisson−Boltzmann Solver (APBS) plugin within PyMOL.5 The solvent-accessible electrostatic surface was displayed at ±5 kT levels.
In contrast, the AcVES3 gel released only about 40% of TIMP-2 by day 3 and then exhibited a linear and slow release profile (~0.9%/d; 3.6 μg/d) over the next 25 days. Thus, TIMP-2’s patch of positively charged residues may be interacting with the oppositely charged fibril network, slowing its release. In fact, the more negatively charged IE1 gel released only about 24% of TIMP-2 by day 4 and then exhibited a linear and slow release profile (1.05%/d; 4.2 μg/d). With respect to the AcVES3 gel, the data indicate that less than half of the protein initially encapsulated exists in the freely diffusing population, which is released early. The remainder of the protein is adsorbed to the fibril network and is released more slowly. For the more negatively charged IE1 gel, more of the protein is initially absorbed to the fibril network during encapsulation. Thus, less TIMP-2 exists in the freely diffusing population, which is clearly observed in its release profile where less protein is released in the early time regime as compared to the AcVES3 gel. Irrespective of the gel type, the relative amounts of protein in each population are most likely governed by an equilibrium between bound and free protein as opposed to simply saturating the fibrils’ surfaces with bound protein. This is supported by the fact that similar release profiles are observed from a singular gel, such as AcVES3, when either 2 or 4 mg/mL protein is initially encapsulated (Figure S3). Gratifyingly, the negatively charged peptide gels display the desired release profile for TIMP-2. However, given the superior rheological properties of the AcVES3, it was studied further.
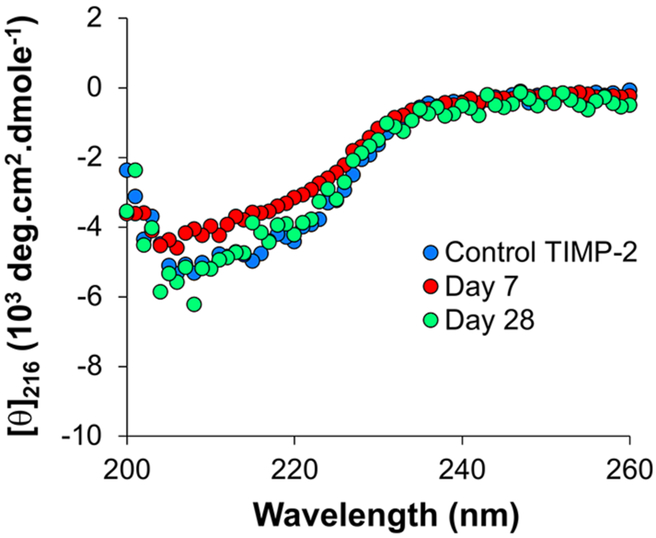
The conformation of TIMP-2 released from the AcVES3 gel was analyzed by CD spectroscopy to ensure that the protein remained folded, Figure 3. The spectra for TIMP-2 released after 7 and 28 days post-encapsulation closely match that of native protein. This suggests that, although the protein electrostatically interacts with the fibril network, that interaction does not result in protein denaturation. Further, released TIMP-2 remained active in ELISA assays, which were used to also follow protein release from the AcVES3 gels (Figure S4). Protein integrity was also assessed by mass spectroscopy (Figure S5), indicating that TIMP-2 is not proteolyzed or chemically modified during its encapsulation or release. Taken together, the data confirm that TIMP-2 is stable while encapsulated for nearly a month (the last time point assessed) at physiologic pH and temperature (37 °C).
Figure 3.
CD spectra of TIMP-2 in BTP buffer (50 mM BTP, 150 mM NaCl, pH 7.4) at 37 °C. Control TIMP-2 that was never encapsulated in a gel (blue). TIMP-2 released from the AcVES3 gel at day 7 (day 1−day 7) and day 28 (day 7−day 28) is in red and green, respectively.
TIMP-2 Retains MMP Inhibitory Activity and Suppresses Tumor Cell Growth In Vitro Following Release from AcVES3 Peptide Gels.
We performed a kinetic analysis of MMP-2 inhibitory activity of the TIMP-2 released from the AcVES3 peptide hydrogels and compared this with freshly prepared control TIMP-2, as well as TIMP-2 incubated under similar physiologic conditions (HBSS buffer at 37 °C), but never encapsulated in hydrogel, for 30 days. Figure 4A shows the Dixon plots of the reciprocal initial velocities versus inhibitor concentrations for TIMP-2 prepared or released from AcVES3 gels under various conditions. These analyses were performed with a range of TIMP-2 to enzyme (MMP-2 catalytic domain) concentration ratios from 0.2 to 1.6 and modeled using Dixon plots for noncompetitive inhibitors to calculate Ki values (Table 2, calculate Ki values obtained by linear regression analysis, R2 = correlation coefficient).64 These calculated Ki values demonstrate that the interaction of TIMP-2 with the catalytic domain of MMP-2 has a low nanomolar affinity (Ki values ranging from 10 to 21 nM) compared with the subnanomolar affinity previously observed for full-length MMP-2.61–63 These differences are attributed to the predicted single, noncompetitive interaction of TIMP-2 with the MMP-2 catalytic site, compared with the multisite interactions previously reported for TIMP-2 inhibition of full-length MMP-2. In addition, there is a slight interassay variability, as demonstrated by two freshly compared TIMP-2 controls (designated TIMP-2 #1 and TIMP-2 #2 prepared on days 1 and 30, respectively, in Figure 4A and B, Table 2) in which the calculated Ki values differ slightly, 12.03 ± 1.30 nM compared with 21.2 ± 0.74 nM, respectively. The Ki values calculated for freshly prepared TIMP-2 solutions (TIMP-2 #1; Ki = 12.03 ± 1.30 nM) and AcVES3 released TIMP-2 on day 7 (day 7; Ki = 10.5 ± 2.05 nM), assayed on the same day, as well as TIMP-2 incubated at 37 °C in physiologic buffer for 30 days (Ki = 14.24 ± 0.55 nM), are in excellent agreement with one another, suggesting no loss of MMP-2 inhibitory activity during 7 days of encapsulation in the AcVES3 gels. Similarly, TIMP-2 incubated at physiologic pH and 37 °C for 30 days (in the absence of the AcVES3 gel) is highly stable and demonstrates no loss of MMP-2 inhibitory activity.
Figure 4.
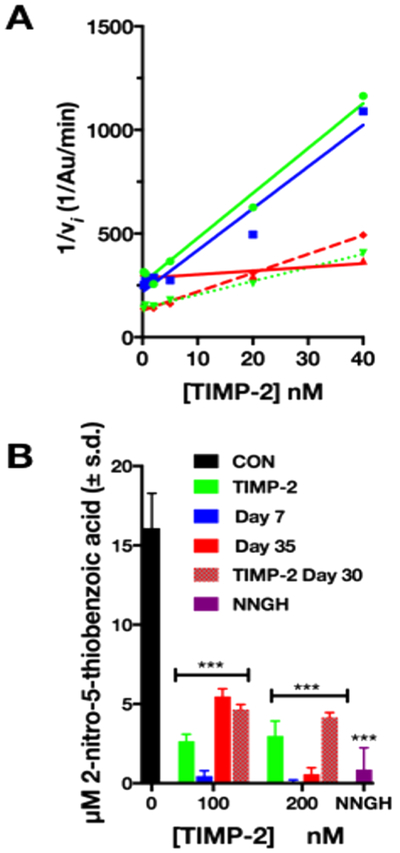
(A) Kinetic analysis of dose dependent inhibition of MMP-2 catalytic activity by TIMP-2. Dixon plots (1/Vi vs [TIMP-2]) for determination of Ki of TIMP-2 inhibition (when [I]:[E] ratios <2) following release from the AcVES3 gel between days 4−7 (day 7; solid blue line with squares) or days 21−35 (day 35, solid red line with triangles), control, freshly prepared solutions (TIMP-2 #1, solid green line with circles; TIMP-2 #2, dotted green line with triangles) or incubated for 30 days at 37 °C (TIMP-2 30 days, dashed red line with diamonds) in HEPES buffer alone. Kinetic analyses measured initial velocities (vi), and Ki values determined from Dixon plots are shown in Table 2. (B) End point analysis of TIMP-2 inhibition of catalytic activity at [I]:[E] ratios >2.5. The μM concentration of the end product, 2-nitro-5-thiobenzoic acid, of the coupled reaction vs [TIMP-2] (nM) from the denoted incubation conditions (***p ≤ 0.001). Con, positive control (0 nM TIMP-2); NNGH, negative control (small molecular MMP inhibitor); TIMP-2, freshly prepared TIMP-2 (never encapsulated); day 7, AcVES3 encapsulated TIMP-2 released between days 4−7; day 35, AcVES3 encapsulated TIMP-2 released between days 21−35; TIMP-2 day 30, TIMP-2 incubated at physiologic pH and 37 °C for 30 days.
Table 2.
KiApp for TIMP-2 Released from AcVES3 and Controls (Days 1−35, R2 = Correlation Coefficient for Linear Regression Analysis)
| TIMP-2 source | Ki | R2 |
|---|---|---|
| TIMP-2 #1 | 12.03 ± 1.30 | 0.98 |
| Day 7 | 10.5 ± 2.05 | 0.95 |
| Day 35 | 160.58 ± 6.78 | 0.65 |
| TIMP-2 #2 | 21.2 ± 0.74 | 0.99 |
| TIMP-2 30 days | 14.24 ± 0.55 | 0.99 |
The TIMP-2 released from AcVES3 gels on day 35 demonstrated an increase of the KiApp value by 1 order of magnitude, 160.58 ± 6.78 nM (compared to 12.03 ± 1.30 nM), suggesting a decrease in TIMP-2 affinity for MMP catalytic domain. However, several important observations are relevant to the potential significance of this observed difference in affinity. First, the variation in this data set is greater as compared to that of the other kinetic assays (R2 = 0.65 versus 0.98 or greater for all others, Table 2). In addition, appendage of a single alanine residue to the N-terminal cysteine reside of the TIMP-2, referred to as the Ala+TIMP-2 analogue, results in complete loss of MMP inhibitor activity against the MMP-2 catalytic domain (Figure S6). A significant reduction in the KiApp, by greater than 5 orders of magnitude (from subnanomolar for TIMP-2 to micromolar), has previously been reported for Ala+TIMP-2 inhibition of full-length MMP-2, although tight binding C-terminal domain interactions between these molecules remain intact.62 By comparison, the decrease in the KiApp observed for AcVES3-released TIMP-2 on day 35 is modest and, as described below, significant loss of MMP inhibitory activity is not observed at higher inhibitor concentrations. TIMP-2 freshly prepared, released from AcVES3 gels on days 7 and 35, or incubated in physiologic buffer at 37 °C for 30 days demonstrate similar, highly significant (0 nM control (CON) vs 100 nM; student t test ***p ≤ 0.001) inhibition of MMP-2 catalytic domain activity in the end point assays (>100 nM concentrations, Figure 4B). This indicates that, although the apparent affinity of AcVES3 released TIMP-2 on day 35 is slightly diminished in the kinetic assay, the MMP-2 inhibitory activity is retained at higher TIMP-2 concentrations despite long-term interaction with the hydrogel. Finally, and most importantly, these findings do not preclude alteration of TIMP-2 cell growth inhibitory activity, that is independent of MMP inhibitory activity (vide infra).27,32,33,35
MMP-2 inhibition by TIMP-2 is reliant on the proper secondary and tertiary structures of the TIMP-2 as well as the co-ordination of the TIMP-2 N-terminal cysteine residue with the catalytic Zn2+ atom in the MMP-2 active site.65 The conserved MMP-2 inhibitory activity, as well as CD spectral analysis, of TIMP-2 released from AcVES3 gels suggests that its tertiary structure remains intact while encapsulated and electrostatically bound to the fibril network of the gel. In addition, the control samples demonstrate that TIMP-2 is stable and retains potency following extended storage at 37 °C in HEPES buffer. The differences in observed KiApp values are modest, suggesting a slight reduction in TIMP-2 binding to the nonphysiologic MMP-2 catalytic site, but such changes are unlikely to affect inhibition of intact, full length MMP-2 in vivo or tumor suppressor activity in vivo, as previously demonstrated for the Ala+TIMP-2 analogue.27,33
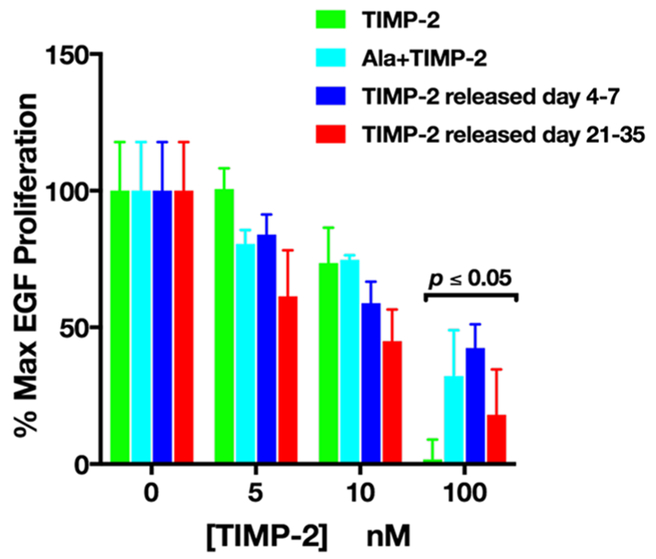
TIMP-2, Ala+TIMP-2, and TIMP-2 released from AcVES3 hydrogels from days 4–7 and days 21–35 all demonstrate statistically significant suppression of rhEGF-induced A549 cell growth at 100 nM concentrations (Figure 5). Prior studies have demonstrated an MMP-independent, growth suppressive effect of TIMP-2 on cancer and endothelial cells in vitro,32,34,35 as well as tumor cells in vivo.27,35 Analysis of TIMP-2’s crystal structure (1BR9) reveals that the B—C loop forms a negatively charged, solvent accessible loop that is present at the base of the N-terminal domain and is reliant on the overall tertiary structure.1 Detailed investigations have identified a stretch of 24 amino acid residues (Ile43—Ala66) within this B—C loop as necessary and sufficient for inhibiting cell proliferation independently of MMP inhibitory activity.35,66 Similar to the retention of MMP inhibitory activity, conservation of the cell inhibitory effects indicates not only that the tertiary structure is maintained but that the encapsulated TIMP-2 also maintains its native surface charge and other modifications that are critical to its overall disposition and interaction with the integrin α3β1 receptor.
Figure 5.
Dose dependent inhibition of rhEGF-mediated A549 cell proliferation. Cell proliferation was quantified by determining cell numbers 72 h following rhEGF treatment with and without TIMP-2 pretreatment and normalized to basal cell numbers using a MTT assay (as described). Absorbance values were converted to % max EGF proliferation (mean ± SEM, n = 3). Statistical analysis (t test; comparison of 0 nM vs 100 nM for all conditions; n = 3; p ≤ 0.05) was performed using Prism software (v 7.0).
CONCLUSION
The targeting of matrix metalloproteinase activities for cancer therapy is witnessing a revival of interest following the initial setbacks after failed clinical trials with small molecule MMP inhibitors.67 This is due to the increased understanding of MMP biology, mode of action, and determination of the correlation between the spatiotemporal inhibition of MMP and the pharmacological outcome. Herein, we developed an injectable hydrogel capable of slow sustained release of TIMP-2. In earlier clinical trials, small molecule peptidomimetic MMP inhibitors, such as Batimastat and Marimastat, exhibited low nanomolar IC50 values, but due to the high sequence homologies and structural similarities of the catalytic domains between members of the MMP family, these small molecule inhibitors were relatively nonselective, resulting in off target toxic side effects.12,14,68 Full length endogenous inhibitors, such as TIMP-2, may alleviate the nonspecificity problem through the larger more complex modes of protein—protein interaction that mediate metalloproteinase inhibition, as well as differential binding to cell surface receptors. The composite MMP inhibitory ability and inhibition of cell growth, independent of its MMP inhibitory activity, makes TIMP-2 an attractive candidate for cancer therapy.16,7 Previous studies have demonstrated a significant reduction in overall tumor growth and phenotypic expression of EMT markers in murine xenografts of human A549 lung cancer cells with forced expression of TIMP-2, and the Ala+TIMP-2, the analogue lacking MMP inhibitory activity.27,69 Our data show that a stable, inert encapsulation and delivery system, such as that offered by the AcVES3 gel, should facilitate preclinical development by providing a vehicle for sustained, relatively long-term administration of TIMP-2. This gel complements others reported in the literature of peptide-based hydrogels that are also in development for delivery of proteins.70 Ongoing studies will assess the TIMP-2/AcVES3 delivery platform for in vivo inhibition of tumor growth and metastasis, as well as longterm dose responses, pharmacokinetics, and biodistribution.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge National Institutes of Health, National Cancer Institute Intramural Project support ZIA-SC-009179 and ZIA-Bc-011204 (NCI/CCR Project 8386620) to W.G.S.-S. The authors thank Drs. Sandra Jensen and David Peeney for critical reading of the manuscript and helpful suggestions. We also thank Dr. Scott Walsh for calculation of the electrostatic potential map of TIMP-2 shown in Figure 2B.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.bio-mac.8b00107.
Figure S1, analytical HPLC and ESI-mass spectra of pure peptides; Figure S2, transmission electron microscope (TEM) images of peptide fibrils; Figure S3, release of 2 mg/mL vs 4 mg/mL TIMP-2 from AcVES3 gel; Figure S4, TIMP-2 release measured by ELISA vs absorbance; Figure S5, deconvoluted mass spectra of TIMP-2 released form AcVES3 gel; Figure S6, MMP-2 inhibitory activity of Ala+TIMP-2 (PDF)
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Tuuttila A; Morgunova E; Bergmann U; Lindqvist Y; Maskos K; Fernandez-Catalan C; Bode W; Tryggvason K; Schneider G Three-dimensional structure of human tissue inhibitor of metalloproteinases-2 at 2.1 A resolution. J. Mol. Biol 1998, 284, 1133–1140. [DOI] [PubMed] [Google Scholar]
- (2).Kalluri R The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [DOI] [PubMed] [Google Scholar]
- (3).Lambert AW; Pattabiraman DR; Weinberg RA Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Sounni NE; Noel A Targeting the tumor microenvironment for cancer therapy. Clin. Chem 2013, 59, 85–93. [DOI] [PubMed] [Google Scholar]
- (5).Baker NA; Sept D; Joseph S; Holst MJ; McCammon JA Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl. Acad. Sci. U. S. A 2001, 98, 10037–10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Bissell MJ; Hines WC Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med 2011, 17, 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Liotta LA; Steeg PS; Stetler-Stevenson WG Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 1991, 64, 327–336. [DOI] [PubMed] [Google Scholar]
- (8).Daley WP; Yamada KM ECM-modulated cellular dynamics as a driving force for tissue morphogenesis. Curr. Opin. Genet. Dev 2013, 23, 408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Simian M; Bissell MJ Organoids: A historical perspective of thinking in three dimensions. J. Cell Biol 2017, 216, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Bischof AG; Yuksel D; Mammoto T; Mammoto A; Krause S; Ingber DE Breast cancer normalization induced by embryonic mesenchyme is mediated by extracellular matrix biglycan. Integr Biol. (Camb) 2013, 5, 1045–1056. [DOI] [PubMed] [Google Scholar]
- (11).Martin JD; Fukumura D; Duda DG; Boucher Y; Jain RK Reengineering the Tumor Microenvironment to Alleviate Hypoxia and Overcome Cancer Heterogeneity. Cold Spring Harbor Perspect. Med 2016, 6, a027094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Alaseem A; Alhazzani K; Dondapati P; Alobid S; Bishayee,; Rathinavelu, A. Matrix Metalloproteinases: A challenging paradigm of cancer management. Semin. Cancer Biol [Epub ahead of print, Nov 16, 2017], DOI: 10.1016/j.semcancer.2017.11.008. [DOI] [PubMed] [Google Scholar]
- (13).Kessenbrock K; Plaks V; Werb Z Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Coussens LM; Fingleton B; Matrisian LM Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 2002, 295, 2387–2392. [DOI] [PubMed] [Google Scholar]
- (15).Levin M; Udi Y; Solomonov I; Sagi I Next generation matrix metalloproteinase inhibitors - Novel strategies bring new prospects. Biochim. Biophys. Acta, Mol. Cell Res 2017, 1864, 1927–1939. [DOI] [PubMed] [Google Scholar]
- (16).Stetler-Stevenson WG Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci. Signaling 2008, 1, re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Stetler-Stevenson WG; Gavil NV Normalization of the tumor microenvironment: evidence for tissue inhibitor of metal-loproteinase-2 as a cancer therapeutic. Connect. Tissue Res 2014, 55, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Murphy G Riding the metalloproteinase roller coaster. J. Biol. Chem 2017, 292, 7708–7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Apte SS; Parks WC Metalloproteinases: A parade of functions in matrix biology and an outlook for the future. Matrix Biol. 2015. 44–46, 1–6. [DOI] [PubMed] [Google Scholar]
- (20).Brew K; Nagase H The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Actay Mol. Cell Res 2010, 1803, 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Cruz-Munoz W; Khokha R The role of tissue inhibitors of metalloproteinases in tumorigenesis and metastasis. Crit. Rev. Clin. Lab. Sci 2008, 45, 291–338. [DOI] [PubMed] [Google Scholar]
- (22).Arpino V; Brock M; Gill SE The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015, 44–46, 247–254. [DOI] [PubMed] [Google Scholar]
- (23).Abu El-Asrar AM; Ahmad A; Bittoun E; Siddiquei MM; Mohammad G; Mousa A; De Hertogh G; Opdenakker G Differential expression and localization of human tissue inhibitors of metalloproteinases in proliferative diabetic retinopathy. Acta Ophthalmol. 2018, 96, e27–e37. [DOI] [PubMed] [Google Scholar]
- (24).Barlow SC; Doviak H; Jacobs J; Freeburg LA; Perreault PE; Zellars KN; Moreau K; Villacreses CF; Smith S; Khakoo Y; Lee T; Spinale FG Intracoronary delivery of recombinant TIMP-3 after myocardial infarction: effects on myocardial remodeling and function. Am. J. Physiol Heart Crc Physiol 2017, 313, H690–H699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Castellano JM; Mosher KI; Abbey RJ; McBride AA; James ML; Berdnik D; Shen JC; Zou B; Xie XS; Tingle M; Hinkson IV; Angst MS; Wyss-Coray T Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature 2017, 544, 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Meersch M; Schmidt C; Van Aken H; Rossaint J; Gorlich D; Stege D; Malec E; Januszewska K; Zarbock A Validation of cell-cycle arrest biomarkers for acute kidney injury after pediatric cardiac surgery. PLoS One 2014, 9, e110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Bourboulia D; Jensen-Taubman S; Rittler MR; Han HY; Chatterjee T; Wei B; Stetler-Stevenson WG Endogenous angiogenesis inhibitor blocks tumor growth via direct and indirect effects on tumor microenvironment. Am. J. Pathol 2011, 179, 25892600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Beardo P; Truan Cacho D; Izquierdo L; Alcover-Garcia J Alcaraz A; Extramiana J; Mallofre C Cancer-Specific Survival Stratification Derived from Tumor Expression of Tissue Inhibitor of Metalloproteinase-2 in Non-Metastatic Renal Cell Carcinoma. Pathol. Oncol. Res [Epub ahead of print, Nov 4, 2017], DOI: 10.1007/s12253-017-0339-7. [DOI] [PubMed] [Google Scholar]
- (29).Chen X; Zhong SL; Lu P; Wang DD; Zhou SY; Yang SJ; Shen HY; Zhang L; Zhang XH; Zhao JH; Tang JH miR-4443 Participates in the Malignancy of Breast Cancer. PLoS One 2016, 11, e0160780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Kai AK; Chan LK; Lo RC; Lee JM; Wong CC; Wong JC; Ng IO Down-regulation of TIMP2 by HIF-1alpha/miR-210/HIF-3alpha regulatory feedback circuit enhances cancer metastasis in hepatocellular carcinoma. Hepatology 2016, 64, 473–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Zhu L; Yu H; Liu SY; Xiao XS; Dong WH; Chen YN; Xu W; Zhu T Prognostic value of tissue inhibitor of metalloproteinase-2 expression in patients with non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 2015, 10, e0124230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Hoegy SE; Oh HR; Corcoran ML; Stetler-Stevenson WG Tissue inhibitor of metalloproteinases-2 (TIMP-2) suppresses TKR-growth factor signaling independent of metalloproteinase inhibition. J. Biol. Chem 2001, 276, 3203–3214. [DOI] [PubMed] [Google Scholar]
- (33).Seo DW; Li H; Guedez L; Wingfield PT; Diaz T; Salloum R; Wei BY; Stetler-Stevenson WG TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell 2003, 114, 171–180. [DOI] [PubMed] [Google Scholar]
- (34).Fernandez CA; Butterfield C; Jackson G; MOSES MA Structural and functional uncoupling of the enzymatic and angiogenic inhibitory activities of tissue inhibitor of metalloproteinase-2 (TIMP-2): loop 6 is a novel angiogenesis inhibitor. J. Biol. Chem 2003, 278, 40989–40995. [DOI] [PubMed] [Google Scholar]
- (35).Seo DW; Saxinger WC; Guedez L; Cantelmo AR; Albini A; Stetler-Stevenson WG An integrin-binding N-terminal peptide region of TIMP-2 retains potent angio-inhibitory and anti-tumorigenic activity in vivo. Peptides 2011, 32, 1840–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Fukunaga K; Tsutsumi H; Mihara H Self-Assembling Peptide Nanofibers Promoting Cell Adhesion and Differentiation. Biopolymers 2013, 100, 731–737. [DOI] [PubMed] [Google Scholar]
- (37).Joyner K; Taraban MB; Feng Y; Yu YB An Interplay Between Electrostatic and Polar Interactions in Peptide Hydrogels. Biopolymers 2013, 100, 174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Jung JP; Jones JL; Cronier SA; Collier JH Modulating the mechanical properties of self-assembled peptide hydrogels via native chemical ligation. Biomaterials 2008, 29, 2143–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Kumar VA; Shi S; Wang BK; Li IC; Jalan AA; Sarkar, Wickremasinghe NC; Hartgerink JD Drug-Triggered and Cross-Linked Self-Assembling Nanofibrous Hydrogels. J. Am. Chem. Soc 2015, 137, 4823–4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Lin YA; Ou YC; Cheetham AG; Cui HG Rational Design of MMP Degradable Peptide-Based Supramolecular Filaments. Biomacromolecules 2014, 15, 1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Maude S; Ingham E; Aggeli A Biomimetic self-assembling peptides as scaffolds for soft tissue engineering. Nanomedicine 2013, 8, 823–847. [DOI] [PubMed] [Google Scholar]
- (42).Micklitsch CM; Medina SH; Yucel T; Nagy-Smith KJ; Pochan DJ; Schneider JP Influence of Hydrophobic Face Amino Acids on the Hydrogelation of beta-Hairpin Peptide Amphiphiles. Macromolecules 2015, 48, 1281–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Rajagopal K; Lamm MS; Haines-Butterick LA; Pochan DJ; Schneider JP Tuning the pH Responsiveness of beta-Hairpin Peptide Folding, Self-Assembly, and Hydrogel Material Formation. Biomacromolecules 2009, 10, 2619–2625. [DOI] [PubMed] [Google Scholar]
- (44).Szkolar L; Guilbaud JB; Miller AF; Gough JE; Saiani A Enzymatically triggered peptide hydrogels for 3D cell encapsulation and culture. J. Pept. Sci 2014, 20, 578–584. [DOI] [PubMed] [Google Scholar]
- (45).Rodriguez AL; Bruggeman KF; Wang Y; Wang TY; Williams RJ; Parish CL; Nisbet DR Using minimalist selfassembling peptides as hierarchical scaffolds to stabilise growth factors and promote stem cell integration in the injured brain. J. Tissue Eng. Regener. Med 2017, DOI: 10.1002/term.2582. [DOI] [PubMed] [Google Scholar]
- (46).Rodriguez AL; Wang TY; Bruggeman KF; Williams RJ; Parish CL; Nisbet DR Tailoring minimalist self-assembling peptides for localized viral vector gene delivery. Nano Res. 2016, 9, 674–684. [Google Scholar]
- (47).Smith DJ; Brat GA; Medina SH; Tong DD; Huang Y; Grahammer J; Furtmuller GJ; Oh BC; Nagy-Smith KJ; Walczak P; Brandacher G; Schneider JP A multiphase transitioning peptide hydrogel for suturing ultrasmall vessels. Nat. Nanotechnol 2016, 11, 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Schneider JP; Pochan DJ; Ozbas B; Rajagopal K; Pakstis L; Kretsinger J Responsive hydrogels from the intramolecular folding and self-assembly of a designed peptide. J. Am. Chem. Soc 2002, 124, 15030–15037. [DOI] [PubMed] [Google Scholar]
- (49).Haines-Butterick L; Rajagopal K; Branco M; Salick D; Rughani R; Pilarz M; Lamm MS; Pochan DJ; Schneider JP Controlling hydrogelation kinetics by peptide design for threedimensional encapsulation and injectable delivery of cells. Proc. Natl. Acad. Sci. U. S. A 2007, 104, 7791–7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Branco MC; Pochan DJ; Wagner NJ; Schneider JP Macromolecular diffusion and release from self-assembled beta-hairpin peptide hydrogels. Biomaterials 2009, 30, 1339–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Branco MC; Pochan DJ; Wagner NJ; Schneider JP The effect ofprotein structure on their controlled release from an injectable peptide hydrogel. Biomaterials 2010, 31, 9527–9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Altunbas A; Lee SJ; Rajasekaran SA; Schneider JP; Pochan DJ Encapsulation of curcumin in self-assembling peptide hydrogels as injectable drug delivery vehicles. Biomaterials 2011, 32, 5906–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Medina SH; Li S; Howard OZ; Dunlap M; Trivett A; Schneider JP; Oppenheim JJ Enhanced immunostimulatory effects of DNA-encapsulated peptide hydrogels. Biomaterials 2015, 53, 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Nagy-Smith K; Yamada Y; Schneider JP Protein release from highly charged peptide hydrogel networks. J. Mater. Chem. B 4, 1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Sun JE; Stewart B; Litan A; Lee SJ; Schneider JP; Langhans SA; Pochan DJ Sustained release of active chemo-therapeutics from injectable-solid beta-hairpin peptide hydrogel. Biomater. Sci 2016, 4, 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Sinthuvanich C; Nagy-Smith KJ; Walsh STR; Schneider JP Triggered Formation of Anionic Hydrogels from Self-Assembling Acidic Peptide Amphiphiles. Macromolecules 2017, 50, 5643–5651. [Google Scholar]
- (57).Nagy-Smith K; Moore E; Schneider J; Tycko R Molecular structure of monomorphic peptide fibrils within a kinetically trapped hydrogel network. Proc. Natl. Acad. Sci. U. S. A 2015, 112, 9816–9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Yan C; Altunbas A; Yucel T; Nagarkar RP; Schneider JP; Pochan DJ Injectable solid hydrogel: mechanism of shear-thinning and immediate recovery of injectable beta-hairpin peptide hydrogels. SoftMatter 2010, 6, 5143–5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Yan C; Mackay ME; Czymmek K; Nagarkar RP; Schneider JP; Pochan DJ Injectable Solid Peptide Hydrogel as a Cell Carrier: Effects of Shear Flow on Hydrogels and Cell Payload. Langmuir 2012, 28, 6076–6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Chowdhury A; Brinson R; Wei B; Stetler-Stevenson WG Tissue Inhibitor of Metalloprotease-2 (TIMP-2): Bioprocess Development, Physicochemical, Biochemical, and Biological Characterization of Highly Expressed Recombinant Protein. Biochemistry 2017, 56, 6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Ye QZ; Johnson LL; Yu AE; Hupe D Reconstructed 19 kDa catalytic domain of gelatinase A is an active proteinase. Biochemistry 1995, 34, 4702–4708. [DOI] [PubMed] [Google Scholar]
- (62).Wingfield PTP; Sax JKJ; Stahl SJS; Kaufman JJ; Palmer II; Chung VV; Corcoran MLM; Kleiner DED; Stetler-Stevenson WGW Biophysical and functional characterization of full-length, recombinant human tissue inhibitor of metalloproteinases-2 (TIMP-2) produced in Escherichia coli. Comparison of wild type and amino-terminal alanine appended variant with implications for the mechanism of TIMP functions. J. Biol. Chem 1999, 274, 21362–21368. [DOI] [PubMed] [Google Scholar]
- (63).Kleiner DE Jr.; Unsworth EJ; Krutzsch HC; Stetler-Stevenson WG Higher-order complex formation between the 72-kilodalton type IV collagenase and tissue inhibitor of metal-loproteinases-2. Biochemistry 1992, 31, 1665–1672. [DOI] [PubMed] [Google Scholar]
- (64).Segel IH Enzyme Kinetics; Wiley-Interscience: London/Sydney/Toronto, 1975. [Google Scholar]
- (65).Gomis-Ruth FX; Maskos K; Betz M; Bergner A; Huber R; Suzuki K; Yoshida N; Nagase H; Brew K; Bourenkov GP; Bartunik H; Bode W Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 byTIMP-1. Nature 1997, 389, 77–81. [DOI] [PubMed] [Google Scholar]
- (66).Kim HJ; Cho YR; Kim SH; Seo DW TIMP-2-derived mer peptide inhibits endothelial cell proliferation and migration through cAMP/PKA-dependent mechanism. Cancer Lett. 2014, 343, 210–216. [DOI] [PubMed] [Google Scholar]
- (67).Vandenbroucke RE; Libert C Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Rev. Drug Discovery 2014, 13, 904–927. [DOI] [PubMed] [Google Scholar]
- (68).Hidalgo M; Eckhardt SG Development of matrix metalloproteinase inhibitors in cancer therapy. J. Natl. Cancer Inst 2001, 93, 178–193. [DOI] [PubMed] [Google Scholar]
- (69).Bourboulia D; Han H; Jensen-Taubman S; Gavil N; Isaac,; Wei B; Neckers L; Stetler-Stevenson WG TIMP-2 modulates cancer cell transcriptional profile and enhances E-cadherin/beta-catenin complex expression in A549 lung cancer cells. Oncotarget 2013, 4, 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Li Y; Wang F; Cui H Peptide-Based Supramolecular Hydrogels for Delivery of Biologics. Bioeng Transl Med 2016, 1, 306–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.