Abstract
Objective
To identify factors associated with pain severity and opioid consumption in the early perioperative period.
Design
Prospective observational cohort study.
Setting
Tertiary academic medical center.
Subjects
Patients with osteoarthritis older than age 45 years undergoing primary total knee replacement at Brigham and Women’s Hospital. A total of 126 patients enrolled.
Methods
Preoperatively, pain questionnaires and quantitative sensory testing were performed on patients to develop a psychosocial and psychophysical profile. Postoperatively, pain scores and opioid consumption were measured as primary end points. Univariate and multiple linear regression analyses were performed to determine the predictive value of these characteristics on perioperative pain scores and opioid consumption.
Results
Regression analysis revealed several predictors of acute postoperative pain scores including temporal summation of pain (TSP; P = 0.001), body mass index (BMI; P = 0.044), number of previous knee surgeries (P = 0.006), and female gender (P = 0.023). Similarly, predictors of opioid utilization included TSP (P = 0.011), BMI (P = 0.02), age (P = <0.001), and tourniquet time (P = 0.003).
Conclusions
The only significant, unique predictors of both pain and opioid consumption were TSP, an index of central pain facilitatory processes, and BMI. Interestingly, psychosocial factors, such as catastrophizing and somatization, although correlated with postoperative pain scores and opioid consumption, generally did not independently explain substantial variance in these measures. This study suggests that BMI and quantitative sensory testing, specifically the temporal summation of pain, may provide value in the preoperative assessment of patients undergoing total knee arthroplasty and other surgeries via predicting their level of risk for adverse pain outcomes.
Keywords: Quantitative Sensory Testing, Total Knee Arthroplasty, predictors of acute pain, Postoperative Pain, Chronic Post-Surgical Pain
Introduction
The problem of pain from knee osteoarthritis has grown substantially over the past 20 years in the United States, a trend likely to continue as the obesity epidemic expands and the population ages [1]. In a parallel fashion, the number of primary total knee arthroplasty (TKA) procedures performed in the United States has increased, more than tripling from 203,600 to 645,100 between 1992 and 2011 [2]. While the majority of patients undergoing primary TKA do well, a significant minority, estimated at 10–34%, report dissatisfaction and continued pain [3].
Importantly, greater acute pain in the immediate postoperative period following TKA has also been associated with an increased likelihood of developing persistent pain [4]. This link between acute and chronic postsurgical pain, although not necessarily causal, has been described for multiple surgical procedures [5] and may allow a window for early preventive intervention.
Previous studies have investigated a variety of demographic and clinical factors for their association with acute pain after TKA. In particular, preexisting pain [6], younger age [7], fatigue [8], and anxiety [9,10] have been associated with greater acute pain after TKA. Pain catastrophizing has been identified as a risk factor for poor pain outcomes after total knee arthroplasty in previous studies [11,12]. Psychosocial differences between patients seem to be closely related to their propensity to experience more severe pain after any injury, including surgery [13]. Similarly, substantial variability exists between individuals in basal pain sensitivity and modulation, as assessed using quantitative sensory testing (QST), whether studied in normal subjects or in patients with chronic pain [14]. Previous studies have also investigated risk factors associated with prolonged opioid use after TKA. In addition to preoperative opioid use, identified risk factors include female gender, age younger than 50 years, anxiety, depression, and low back pain [15]. An elevated preoperative body mass index (BMI) has also been associated with increased postoperative pain and decreased postoperative functional capacity and quality of life in patients undergoing TKA [16].
This prospective study of patients undergoing primary TKA aimed to identify salient, preoperatively assessable factors associated with poorly controlled perioperative pain and opioid consumption during hospital admission in the first two days after surgery. We hypothesized that sources of individual variation in psychosocial and preoperative nociceptive sensitivity, as measured using QST, as well as demographic, surgical, and anesthetic factors, could predict postoperative pain and opioid consumption.
Methods
Subjects
The institutional review board of Brigham and Women’s Hospital approved all study procedures, and written informed consent was obtained from all subjects by study staff during subject enrollment. Subjects in the study met the American College of Rheumatology criteria for knee osteoarthritis and were scheduled to undergo TKA at Brigham & Women’s Hospital (BWH) in Boston, Massachusetts. Other inclusion criteria included age of 45 years or greater and fluency in English. Exclusion criteria were disorders of cognition preventing completion of the study procedures, recent history of a myocardial infarction, presence of an autoimmune disorder, and documented peripheral neuropathy of at least moderate severity.
Participants were recruited by advertising on email, web, and bulletin board announcements. Subjects came for a preoperative study visit, during which they first completed questionnaires and then underwent quantitative sensory testing (QST). This testing usually occurred a few weeks prior to the planned procedure. QST was performed by a single specially trained research associate.
A total of 210 subjects contacted our research coordinator expressing an interest in the study. Of these, 151 subjects met phone screen criteria and scheduled an initial visit. Of those, 140 subjects came to the visit and signed a consent form to enroll in the study. Of those, 126 subjects completed all of the associated preprocedural testing, underwent the arthroplasty, and had complete postoperative pain and opioid data. Patients not included in the final group included four patients who did not pursue the surgical procedure, five patients who pursued their surgical procedures at community hospitals from which we could not obtain complete medical records, and five patients who did not participate for unknown reasons.
Questionnaires
The Pain Catastrophizing Scale (PCS) was used to assess patient catastrophizing [17]. This questionnaire consists of 13 items asking individuals to reflect on past painful experiences and rate the degree to which they experience negative pain-related thoughts in the content domains of rumination, magnification, and helplessness [18]. The Patient-Reported Outcomes Measurement Information System (PROMIS) brief measures of anxiety and depression were used to assess negative affect. These well-validated multidimensional sets of items assessing cognitive and emotional function use item response theory to provide precise measurement of individual symptom clusters; they have excellent psychometric properties and are widely used [19]. The somatization subscale of the Brief Symptoms Inventory (BSI) was used to assess interindividual variability in the degree of somatic focus. This brief, well-validated six-item scale reflects somatic distress arising from perceptions of bodily dysfunction. Such measures of somatization have proven to be among the strongest psychosocial predictors of the development of chronic pain in longitudinal cohort studies [20]. The Widespread Pain Index (WPI) was used to assess the anatomic extent of pain complaints. This checklist of anatomic regions comprises a critical part of the recently validated update to the diagnostic criteria for fibromyalgia [21]. Patients were also asked to estimate the average number of hours of sleep they get per night.
Quantitative Sensory Testing
Mechanical pain thresholds were assessed using a digital pressure algometer (Somedic) bilaterally at the patella, the trapezius muscle, and the metacarpophalangeal joint of the thumb. Mechanical force was applied using a 0.5-cm2 probe covered with polypropylene pressure-transducing material; pressure was increased at a steady rate of 30 kPA/s until the subject indicated that the pressure was “first perceived as painful.” Reaction to prolonged deep tissue pressure pain was ascertained via cuff pressure algometry. We used a Hokanson rapid cuff inflator; a standard blood pressure cuff was wrapped comfortably around the lower leg over the gastrocnemius muscle, and a computer-controlled air compressor determined the pressure level that was individually tailored for each subject to produce a pain intensity rating of 40/100. Patients were asked to rate the pain experienced from this deep pressure stimulus upon initial inflation, at 60 seconds, and at 120 seconds. Additionally, the patients were asked to rate any ongoing pain at the cuff site 15 seconds following cuff deflation (painful after-sensations). Finally, we assessed conditioned pain modulation (CPM), a noninvasive test of endogenous pain-inhibitory systems using a heterotopic noxious conditioning stimulation paradigm. In brief, participants immersed their dominant hand in a circulating cold water bath maintained at 4°C. Pressure pain threshold (PPTh) was assessed on the contralateral trapezius during immersion, with an increase in PPTh reflecting an engagement of endogenous pain-inhibitory systems. As in prior studies, we calculated a CPM Index that reflected the magnitude of change in PPTh during contralateral hand cold water immersion relative to baseline [22,23]. The CPM Index is calculated using the formula: (PPTh during the cold pressor test/baseline PPTh)*100. Scores over 100 indicate positive/effective CPM (i.e., pain threshold increased during the cold water immersion).
Collection of Primary Outcome Measures
Postoperative pain was measured for each patient using a numeric rating scale (NRS), with 0 being no pain and 10 indicating worst pain. These pain scores reflected the patient’s current pain and could reflect either pain at rest or pain with movement; the medical record did not allow quantification of which percentages were at rest vs which percentages were with activity. Nurses recorded NRS as part of the institutional practice at multiple points throughout the perioperative period and entered this pain score in the electronic record. Each patient had a number of pain scores recorded during different epochs of their hospitalization (post-anesthesia care unit [PACU], postoperative day [POD] 0, POD1, POD2). The overall average postoperative pain score was calculated by taking the average of all pain scores (NRS) listed in the nursing record from arrival in the PACU until discharge from the hospital on POD1 or 2. For the 40 of the 126 patients in this series who remained in the hospital on POD3, NRS scores were recorded until 23:59 on POD2.
Opioid consumption was measured for each patient as the oral morphine milligram equivalent (MME) per day. The MME/d outcome included the time from arrival in the PACU until hospital discharge. For patients in this series who remained in the hospital on POD3, medications administered and time course were recorded until 23:59 on POD2 (the data from day 3 or beyond were not used). Medications administered and time points were available for review for all of the patients via an electronic medical system.
MME was calculated using ratios approved by the hospital’s postoperative pain service. The conversion factors used to calculate oral morphine milligram equivalents from the different opioid formulations were as follows: mg hydromorphone IV × 20, mg hydromorphone PO × 5, mcg fentanyl IV × 3.333, mg morphine IV × 3, mg oxycodone PO × 1.5, mg hydrocodone PO × 1, mg tramadol PO × 0.1. The enteral-to-enteral ratios between different agents were calculated in accordance with published society guidelines [24].
Surgical and Anesthetic Variables
TKA surgeries were performed by eight BWH surgeons, all of whom were experienced and board certified in orthopedic surgery. Peripheral nerve blocks were performed by the regional anesthesia service in a minority of the cases, based on input from the surgeon, the anesthesiologist, and the patient. The decision to use general vs spinal anesthesia was similarly based primarily on the preference of the patient.
Statistical Analysis
Analysis of data was performed using SPSS (V 22, Chicago, IL, USA). Data for continuous variables are presented as means and standard deviations, and data for categorical variables are presented as percentages. A temporal summation score was computed by subtracting a patient’s end pressure pain rating from their initial pressure pain rating during the prolonged painful cuff stimulus. Two main outcome variables estimating postoperative pain were collected and calculated as described above: overall average postoperative pain score in postoperative days 0–2 and average daily opioid consumption during the same period. Normality testing using Shapiro-Wilk indicated a normal distribution for individual average pain scores (Statistic = 0.982, df 126, P = 0.100). In order to investigate the inter-relationships between average postoperative pain and average daily opioid use with other variables (demographic, surgical, psychosocial, and psychophysical), Pearson correlation coefficients were calculated, as reported in Tables 2 and 3. In order to assess the predictive potential of these factors on the two measures of postoperative pain, we performed univariate linear regression with average postoperative pain and average daily opioid utilization as dependent variables. In order to assess the independent predictive potential of preoperatively assessed variables, we performed a multiple linear regression analysis, including factors with a significance level of P < 0.05 on the univariate analysis, grouped according to factor type as blocks: Block 1: age, gender, BMI (demographics); Block 2: Brief Pain Inventory average, WPI (previous pain); Block 3: PCS total, BSI, hours sleep (psychosocial); Block 4: TS, painful after-sensations (PAS) (psychophysical); Block 5: tourniquet time, number of previous knee surgeries, intraoperative MME (surgical and anesthetic), as reported in Tables 2 and 3. The adjusted R2 for each block and as individual factor beta coefficients in the multiple linear regression are also reported in Tables 2 and 3. Significance for all tests was set at alpha = 0.05.
Table 2.
Predictors of average pain scores from POD0–2: results of linear regression
| Variable | Pearson Correlation | Univariate Linear Regression |
Multiple Linear Regression |
|||||
|---|---|---|---|---|---|---|---|---|
| Block Model |
||||||||
| R | Adj R2 | P | Beta | P | Adj R2 | P | ||
| Block 1: Demographic | Age | −0.274 | 0.067 | 0.002 | −0.028 | 0.100 | 0.163 | <0.001 |
| Female gender | 0.220 | 0.041 | 0.014 | 0.588 | 0.023 | |||
| BMI | 0.199 | 0.032 | 0.026 | 0.043 | 0.044 | |||
| Block 2: Previous pain | Average pain (BPI) | 0.444 | 0.190 | <0.001 | 0.126 | 0.116 | 0.234 | 0.007 |
| Widespread Pain Index | 0.297 | 0.088 | 0.001 | 0.009 | 0.880 | |||
| Block 3: Psychosocial | Catastrophizing (PCS) | 0.287 | 0.074 | 0.002 | −0.001 | 0.965 | 0.245 | 0.232 |
| Somatization (BSI) | 0.209 | 0.036 | 0.020 | 0.080 | 0.265 | |||
| Sleep hours | −0.300 | 0.082 | 0.001 | −0.097 | 0.388 | |||
| Anxiety | 0.102 | 0.002 | 0.266 | |||||
| Depression | 0.081 | −0.002 | 0.376 | |||||
| Block 4: Psychophysical | Trapezius pressure pain threshold | 0.038 | 0.008 | 0.676 | 0.344 | <0.001 | ||
| Patella pressure pain threshold | −0.040 | −0.008 | 0.685 | |||||
| Conditioned pain modulation | 0.015 | −0.010 | 0.888 | |||||
| Temporal summation of pain | 0.342 | 0.109 | <0.001 | 0.027 | 0.001 | |||
| Painful after-sensations | 0.199 | 0.031 | 0.035 | |||||
| Block 5: Surgical, anesthetic | Number of previous knee surgeries | 0.272 | 0.067 | 0.002 | 0.210 | 0.006 | 0.386 | 0.023 |
| Number of nonorthopedic surgeries | −0.003 | −0.008 | 0.970 | |||||
| Tourniquet time | 0.098 | 0.002 | 0.098 | |||||
| Surgical time | 0.040 | −0.006 | 0.659 | |||||
| Anesthetic type | 0.048 | 0.004 | 0.217 | |||||
| Intraop opioid | 0.194 | 0.030 | 0.030 | 0.001 | 0.795 | |||
BMI = body mass index; BPI = Brief Pain Inventory; BSI = Brief Symptoms Inventory; PCS = Pain Catastrophizing Scale; POD = postoperative day.
Table 3.
Predictors of daily opioid consumption from POD0–2, results of linear regression
| Variable | Pearson Correlation | Univariate Linear Regression |
Multiple Linear Regression |
|||||
|---|---|---|---|---|---|---|---|---|
| Block Model |
||||||||
| R | Adj R2 | P | Beta | P | Adj R2 | P | ||
| Block 1: Demographic | Age | −0.425 | 0.174 | <0.001 | −1.76 | <0.001 | 0.247 | <0.001 |
| Female gender | −0.009 | −0.008 | 0.921 | |||||
| BMI | 0.217 | 0.040 | 0.015 | 1.46 | 0.020 | |||
| Block 2: Previous pain | Average pain (BPI) | 0.310 | 0.088 | 0.001 | 0.767 | 0.732 | 0.299 | 0.013 |
| Widespread Pain Index | 0.305 | 0.085 | 0.001 | 2.46 | 0.159 | |||
| Block 3: Psychosocial | Catastrophizing (PCS) | 0.021 | −0.008 | 0.227 | 0.698 | 0.087 | 0.322 | 0.032 |
| Somatization (BSI) | 0.211 | 0.037 | 0.018 | −1.62 | 0.424 | |||
| Sleep hours | −0.145 | 0.012 | 0.126 | |||||
| Anxiety | 0.069 | −0.004 | 0.453 | |||||
| Depression | 0.094 | 0.001 | 0.305 | |||||
| Block 4: Psychophysical | Trapezius pressure pain threshold | 0.076 | −0.004 | 0.431 | 0.381 | 0.003 | ||
| Patella pressure pain threshold | 0.103 | 0.001 | 0.295 | |||||
| Conditioned pain modulation | 0.045 | −0.009 | 0.664 | |||||
| Temporal summation of pain | 0.322 | 0.095 | <0.001 | 0.605 | 0.011 | |||
| Painful after-sensations | 0.061 | −0.005 | 0.521 | |||||
| Block 5: Surgical, anesthetic | Number of previous knee surgeries | 0.129 | 0.008 | 0.154 | 0.460 | 0.002 | ||
| Number of nonorthopedic surgeries | −0.004 | −0.008 | 0.968 | |||||
| Tourniquet time | 0.331 | 0.091 | <0.001 | 0.454 | 0.003 | |||
| Surgical time | 0.034 | −0.007 | 0.706 | |||||
| Anesthetic type | 0.131 | 0.009 | 0.153 | |||||
| PACU average pain | 0.378 | 0.136 | 0.001 | 3.53 | 0.025 | |||
BMI = body mass index; BPI = Brief Pain Inventory; BSI = Brief Symptoms Inventory; PCS = Pain Catastrophizing Scale; POD = postoperative day.
Results
A demographic summary of our cohort of 126 patients undergoing primary unilateral TKA for osteoarthritis is listed in Table 1. These procedures took place from March 2011 to December 2015.
Table 1.
Patient demographics and surgical variables
| Mean ± SD (Range) or No. (%) | |
|---|---|
| Age, y | 65 ± 7.8 (49–87) |
| BMI, kg/m2 | 30.5 ± 6.1 (19–50) |
| Female | 73/126 (58) |
| Race | |
| Non-Hispanic white | 114 (91) |
| African American | 6 (4.5) |
| Asian | 2 (1.5) |
| Other/not available | 4 (3) |
| ASA class | |
| 1 | 2(1.5) |
| 2 | 82(65) |
| 3 | 42 (33.5) |
| Taking opioids prior to TKA | 6 (4.5) |
| Anesthesia type | |
| Neuraxial | 94 (74.6) |
| General | 29 (23) |
| Neuraxial converted to general | 3 (2.4) |
ASA = american society of anesthesiologists; BMI = body mass index; TKA = total knee arthroplasty.
All pain scores recorded from 126 patients are depicted in Figure 1. The NRS mean and standard deviation were as follows: PACU 2.08 ± 2.84, POD0 (post-PACU) 3.43 ± 2.67, POD1 3.78 ± 2.47, POD2 3.91 ± 2.60, POD3 2.96 ± 2.5. The minimum number of patient assessments a patient received was 12, the mean and median were 26, and the maximum was 53. The range for all periods was 0 to 10, with the preponderance of NRS scores of 0 in the PACU corresponding to patients who had a spinal anesthetic.
Figure 1.
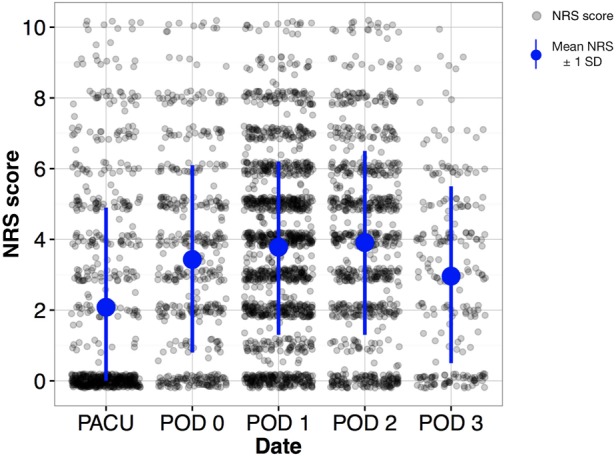
All recorded pain numerical rating scores (NRS) across the entire postoperative course. Standard deviation is depicted by the vertical line within each epoch, with mean NRS depicted by the circle along each standard deviation line. PACU = post-anesthesia care unit; POD = postoperative day.
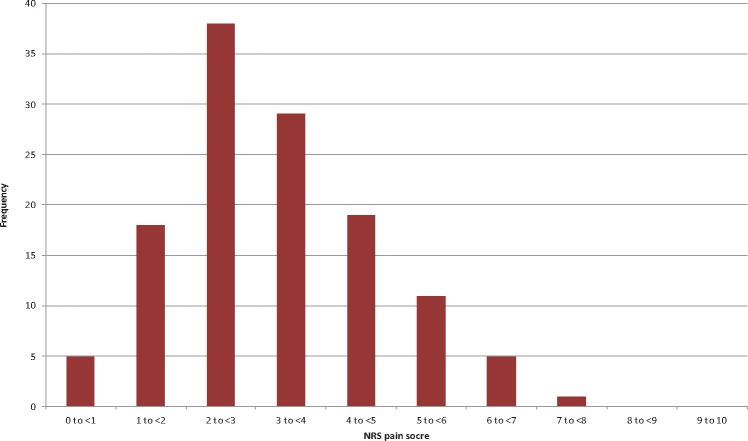
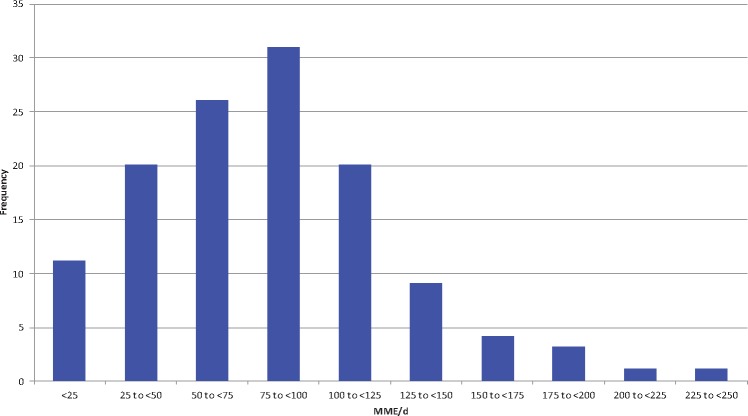
The distribution of patients’ average pain scores and daily opioid utilization (morphine mg equivalents) are shown in Figures 2 and 3, respectively. Individual patients’ average pain scores ranged from 0.71 to 7.9, with a mean of 3.3 and an SD of 1.44. The opioid utilization/d ranged from 3.8 to 244.1 mg/d, with a mean of 81.6 mg/d and an SD of 45.1 mg/d. The average pain NRS scores and MME per day were significantly correlated, with those reporting higher pain scores also requiring higher total daily opioid (Pearson R = 0.389 on PACU/POD0, R = 0.555 on POD1, and R = 0.471 on POD2, all P < 0.001), as depicted in Figure 4.
Figure 2.
Distribution of average numerical rating scale (NRS) pain score by patient. For each patient, the summation of all recorded NRS pain scores was divided by the number of observations. Frequency denotes the number of patients in each category.
Figure 3.
Distribution of average oral morphine milligram equivalent (MME) per day over a two-day postoperative period. For each patient, the summation of all MME doses was divided by the total number of hospital days. Frequency denotes the number of patients in each category.
Figure 4.
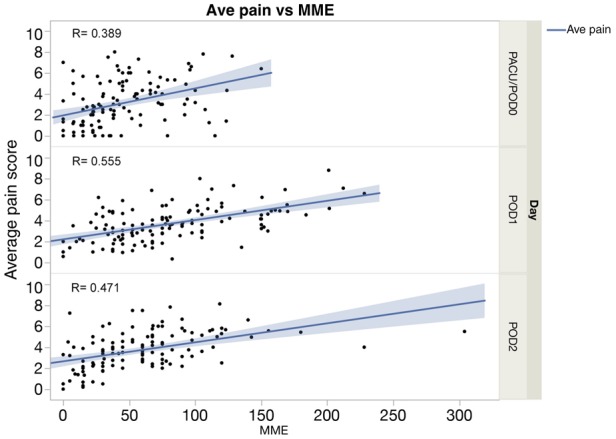
Values are overlaid with lines of best fit. MME = morphine milligram equivalent; PACU = post-anesthesia care unit; POD = postoperative day.
The correlations of average pain score and opioid utilization with other factors, including demographic, psychosocial, anesthetic, and surgical factors, are listed in Tables 2 and 3. Many factors were significantly correlated with pain, including age, female gender, BMI, preoperative pain levels, catastrophizing, somatization, average hours of sleep, temporal summation of pain, painful after-sensations, and number of previous knee surgeries. Similar factors were associated with average daily opioid utilization, with the notable exceptions of female gender, catastrophizing, and sleep disturbance, as well as painful after-sensations and number of previous knee surgeries. Longer tourniquet times were also associated with greater opioid utilization.
Prediction of Postoperative Pain
Univariate linear regression was then performed to assess the predictive potential for these factors, revealing a similar pattern as the correlational analysis (younger age, preexisting pain severity, pain catastrophizing, average hours of sleep, temporal summation of pain, painful after-sensations, and number of previous knee surgeries), as described in Table 2. Similar factors were predictive of opioid requirement on univariate analysis, as described in Table 3. However, unlike for pain scores, sleep and painful after-sensations were not significant predictors, while tourniquet time was a significant predictor.
Because many of the measured factors are interrelated, we performed multiple linear regression analysis in order to assess the independent contribution of factors to the prediction of postoperative pain, including factors with a significance level of P < 0.05 in the univariate analysis. Table 2 (right columns) lists the proportion of variance in average pain scores explained with the addition of each block of factors (adjusted R2), with these factors explaining 38.6% of the variance in pain scores. Demographic, previous pain, psychophysical profile, and surgical-anesthetic blocks all significantly contributed to explaining some portion of the variance in postoperative pain scores. Individual factor contributions are also listed in Table 2. Factors that were independent predictors included gender, BMI, temporal summation of pain, and number of previous knee surgeries.
Multiple linear regression was also performed for postoperative opioid utilization, including factors with a significance level of P < 0.05 in the univariate analysis. Table 3 (right columns) lists the proportion of variance in average daily opioid utilization explained with the addition of each block of factors (adjusted R2), showing that these factors accounted for 44.6% of the variance among individuals in the study. There was a significant contribution from all blocks (demographics, previous pain, psychosocial, psychophysical testing, and surgical), as described in Table 3. However, factors that were independent predictors of daily opioid utilization included age, BMI, temporal summation of pain, tourniquet time, and PACU pain scores.
Discussion
Pain, whether chronic or acute, is a complex phenomenon, governed by a myriad of influences from distinct domains. This prospective study identified predictive factors for both pain and opioid requirement from several domains, including younger age, high preexisting pain, pain catastrophizing, temporal summation of pain, and number of previous knee surgeries. Interestingly, preoperatively measured temporal summation of pain and BMI were the independent predictors of both higher postoperative pain and opioid requirements.
Quantitative Sensory Testing
QST uses standardized measurement of responses to calibrated stimuli and represents an extension and refinement of the bedside clinical examination of the sensory system. A number of recent, large studies have applied QST to patients with a variety of pain syndromes (often neuropathic pain conditions) in order to examine sensory profiles or subgroups [25–28]. The present findings highlight the role detection of variable sensitivity may play in predicting pain and analgesic medication requirement in the early postoperative period.
The application of QST to a preoperative patient population, most of whom do not have a preexisting neuropathic pain condition, represents a relatively novel use of this modality to quantify the variability in baseline nociceptive processing that is observed in the larger population. Previous studies have also suggested that patients with more severe acute and chronic postoperative pain have lower pain thresholds, a greater propensity to experience temporal summation, painful after-sensations, and other responses consistent with enhanced pain facilitation both retrospectively and prospectively [23,29–33]. A previous cohort analysis has also illustrated that lower forearm pressure pain thresholds, representing heightened widespread pain sensitivity, are associated with greater pain severity 12 months after arthroplasty [34]. A few prior reports have noted associations between preoperative QST measures and postoperative opioid requirements [35], although none of these prior studies assessed temporal summation.
The finding that TSP was an independent predictor of both pain scores and daily opioid utilization in the early postoperative period after TKA is notable. It suggests that patient-specific differences in nociceptive processing could account, at least in part, for the variations in pain and opioid requirements in the perioperative period. Although no causal inference can be drawn, these differences were at least detectable by differences observed in a QST such as TSP.
The present study adds to the evidence that substantial interpatient variability in postsurgical pain intensity exists. In addition, the severity of acute postoperative pain is itself an important unique predictor of persistent postsurgical pain [4,5]. As such, detection of the sources of variability in acute pain may help guide development of predictive algorithms and tailored management protocols to prevent both acute and chronic postsurgical pain, particularly if uncontrolled acute postsurgical pain in fact contributes to the development of persistent pain. It is notable that a recent prospective study indicated that patients reporting severe persistent pain after TKA had enhanced preoperative temporal summation compared with patients without chronic pain or with mild chronic postoperative pain [36]. Our findings support the idea that temporal summation may predict acute postoperative pain and opioid requirement as well. Patients at high risk of postoperative pain could potentially be identified during preoperative assessment, based on their demographic information and limited brief psychophysical and psychosocial testing, triggering the application of preventive therapies or consultation with a pain management specialist in the perioperative period. However, larger studies in multiple surgical types are needed to validate the use of these factors as screening tools for poor clinical outcomes, and studies enriched with these high-risk patients may help define the most useful tools that may help prevent acute and persistent postoperative pain.
The present work did not identify catastrophizing as an independent predictor of acute postoperative pain or opioid requirements, perhaps as a result of this patient sample being notably free of other preexisting psychiatric illness and chronic pain syndromes.
Body Mass Index
The present finding that an elevated BMI is an independent predictor of both pain scores and opioid consumption is in line with the existing literature’s observation that obesity is associated with increased postoperative disability and other complications [37]. It is conceivable that increased force on the surgical site may contribute to increased pain. Fortunately, obesity is an easily identifiable preoperative parameter, potentially lending itself to be used as part of algorithm used to flag patients in need of perioperative pain specialists or for whom the risks and benefits of the surgery should be reassessed.
Strengths of Study
One strength of this study is its prospective design. Patients who were essentially free of significant preexisting psychiatric illness, chronic pain syndromes, or regular opioid use were densely and systematically phenotyped from a psychosocial and psychophysical standpoint before their surgery. This phenotyping captures the natural variability in pain processing between people in a cohort that would not appear to most surgeons to be high risk for developing chronic pain after knee surgery. Identifying patients as “high risk” for increased acute pain and opioid use from within this “average-risk” pool, which is likely representative of the majority of patients considered for TKA, may prove particularly useful for surgical patient selection in the future. Another strength was the relatively comprehensive capture of pain score data in the postoperative period, not relying on a single reported pain score, but rather harnessing the collective set of data points that are used by in-hospital providers to guide postoperative care. All analgesics administered, whether by the patient from a patient-controlled analgesic (PCA) delivery system or by a nurse, were recorded in an electronic medical record system. Pain NRS scores were abundant, recorded at a minimum of every four hours but often, as required by hospital guidelines, much more frequently when analgesics were administered. Perhaps as a result of this relatively rich data set, there was a normal distribution of pain NRS. There was also a concordance of NRS and opioid analgesic administration, suggesting nonrandom administration of analgesics by care providers, but also implying that opioid utilization may serve more as a proxy than a confounder for pain scores.
Limitations
There are a number of limitations to this study. First, it did not include a measure of functional capacity, such as the ability to participate in physical therapy during the postoperative period, which was in part due to lack of sufficient detail in postoperative physical therapy notes. Future studies would benefit from explicitly engaging in-hospital physical therapy teams in assessment and measurement of functional impairment due to pain. Second, the enrollment of patients was voluntary and as such subject to potential participation bias. Third, the study group was predominantly Caucasian, potentially limiting its generalizability to other patient populations Fourth, as a real-world clinical study, the postoperative management of these patients employed several different postoperative pathways for pain management, which varied as individual surgeons altered these over time, and included various combinations of long-acting opioids, gabapentinoids, and local anesthetics including pericapsular injection, likely contributing somewhat to the variability in pain scores and opioid utilization seen between individuals. This may account in part for the fact that there was a significant amount of variance that was unaccounted for in predicting the NRS and MME. Given this added variability, it seems all the more remarkable that preoperatively assessed psychosocial and psychophysical variables did in fact significantly correlate with postoperative opioid consumption. Last, the number of patients in this study was prohibitively small for any meaningful examination of the contribution of each of the many individual analgesic management factors, and it was not designed in a way to systematically assess the importance of these differences in analgesic regimens. Future studies with larger subject numbers and more strictly defined analgesic pathways may answer the actual contribution of each of these analgesic variables to acute postoperative pain.
Conclusions
Of the many assessed psychosocial and psychophysical factors in this study (pain catastrophizing, sleep disturbance, somatization, painful after-sensations), the only independent predictors of both pain and opioid utilization were BMI and preoperatively assessed temporal summation of pain, indicating that testing for QST and BMI may be useful to predict patient trajectory after surgery. Importantly, the adaptation of TSP testing to a portable clinic-friendly bedside format has yet to be tested for similar predictive potential.
Patients at high risk of postoperative pain could then potentially be identified during preoperative assessment, based on their demographic information and limited brief psychophysical and psychosocial testing, triggering the application of preventive therapies or consultation with a pain management specialist in the perioperative period. However, larger studies in multiple surgical types are needed to validate the use of these factors as screening tools for poor clinical outcomes, and studies enriched with these high-risk patients may help define the most useful tools to help prevent acute and persistent postoperative pain.
Acknowledgments
The authors would like to acknowledge Chuan-Chin Huang of Brigham and Women’s Hospital for his statistical consultation and the subjects for their time and effort in participating in the study.
Funding sources: This study was funded by National Institutes of Health grant R01 AG034982 (R. R. Edwards, coauthor).
Conflicts of interest: None for all authors.
References
- 1. Nguyen US, Zhang Y, Zhu Y, et al. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: Survey and cohort data. Ann Intern Med 2011;155(11):725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthritis and Related Conditions, Chapter 4. Available at: http://www.boneandjointburden.org/2013-report/iv-arthritis/iv (accessed February 2016).
- 3. Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P.. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systemic review of prospective studies in unselected patients. BMJ Open 2012;2(1):e000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Puolakka PA, Rorarius MG, Roviola M.. Persistent pain following knee arthroplasty. Eur J Anaesthesiol 2010;27(5):455–60. [DOI] [PubMed] [Google Scholar]
- 5. Kehlet H, Jensen TS, Woolf CJ.. Persistent postsurgical pain: Risk factors and prevention. Lancet 2006;367(9522):1618–25. [DOI] [PubMed] [Google Scholar]
- 6. Rakel BA, Blodgett NP, Bridget Zimmerman M, et al. Predictors of postoperative movement and resting pain following total knee replacement. Pain 2012;153(11):2192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roth ML, Tripp DA, Harrison MH, Sullivan M, Carson P.. Demographic and psychosocial predictors of acute perioperative pain for total knee arthroplasty. Pain Res Manag 2007;12(3):185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lindberg MF, Miaskowski C, Rustøen T, et al. Preoperative pain, symptoms, and psychological factors related to higher acute pain trajectories during hospitalization for total knee arthroplasty. PLoS One 2016;11(9):e0161681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pinto PR, McIntyre T, Ferrero R, Almeida A, Araújo-Soares V.. Predictors of acute postsurgical pain and anxiety following primary total hip and knee arthroplasty. J Pain 2013;14(5):502–15. [DOI] [PubMed] [Google Scholar]
- 10. Lunn TH, Gaarn-Larsen L, Kehlet H.. Prediction of postoperative pain by preoperative pain response to heat stimulation in total knee arthroplasty. Pain 2013;154(9):1878–85. [DOI] [PubMed] [Google Scholar]
- 11. Riddle DL, Wade JB, Jiranek WA, Kong K.. Preoperative pain catastrophizing predicts pain outcome after knee arthroplasty. Clin Orthop Relat Res 2010;468(3):798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Theunissen M, Peters ML, Bruce J, Gramke HF, Marcus MA.. Preoperative anxiety and catastrophizing: A systematic review and meta-analysis of association with chronic postsurgical pain. Clin J Pain 2012;28(9):819–41. [DOI] [PubMed] [Google Scholar]
- 13. Edwards RR, Dworkin RH, Sullivan MD, Turk D, Wasan A.. The role of psychosocial processes in the development and maintenance of chronic pain. J Pain 2016;17(9):T70–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cruz-Almeida Y, Fillingim RB.. Can quantitative sensory testing move us closer to mechanism-based pain management? Pain Med 2014;15(1):61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bedard NA, Pugely AJ, Westermann RW, et al. Opioid use after total knee arthroplasty. J Arthroplasty 2017;32(8):2390–4. [DOI] [PubMed] [Google Scholar]
- 16. Rodriguez-Merchan EC. The influence of obesity on the outcome of TKR: Can the impact of obesity be justified from the viewpoint of the overall health care system? HSS J 2014;10(2):167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sullivan MJ, Bishop SR, Pivik J.. The pain catastrophizing scale: Development and validation. Psychol Assess 1995;7(4):524–32. [Google Scholar]
- 18. Edwards RR, Cahalan C, Calahan C, et al. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol 2011;7(4):216–24. [DOI] [PubMed] [Google Scholar]
- 19. Cella D, Riley W, Stone A.. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol 2010;63(11):1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fillingim RB, Ohrbach R, Greenspan JD, et al. Psychological factors associated with development of TMD: The OPPERA prospective cohort study. J Pain 2013;14(12):T75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Häuser W, Jung E, Erbslöh-Möller B, et al. Validation of the Fibromyalgia Survey Questionnaire within a cross-sectional survey. PLoS One 2012;7(5):e37504.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Edwards RR, Dolman AJ, Martel MO, et al. Variability in conditioned pain modulation predicts response to NSAID treatment in patients with knee osteoarthritis. BMC Musculoskelet Disord 2016;17(1):284.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Edwards RR, Mensing G, Cahalan C, et al. Alteration in pain modulation in women with persistent pain after lumpectomy: Influence of catastrophizing. J Pain Symptom Manage 2013;46(1):30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Washington State Agency Medical Directors’ Group (AMDG). 2015 Interagency Guideline on Prescribing Opioids for Pain. Available at: http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf (accessed May 2016).
- 25. Edwards RR, Dworkin RH, Turk DC, et al. Patient phenotyping in clinical trial of chronic pain treatment: IMPACTT recommendations. Pain 2015;157(9):1851–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Freeman R, Baron R, Bouhassira D, Cabrera J, Emir B.. Sensory profiles of patients with neuropathic pain based on the neuropathic pain symptoms and signs. Pain 2014;155(2):367–76. [DOI] [PubMed] [Google Scholar]
- 27. Gierthmuhlen J, Maier C, Baron R, et al. Sensory signs in complex regional pain syndrome and peripheral nerve injury. Pain 2012;153:765–74. [DOI] [PubMed] [Google Scholar]
- 28. Maier C, Baron R, Tolle TR, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain 2010;150:439–50. [DOI] [PubMed] [Google Scholar]
- 29. Schreiber KL, Martel MO, Shnol H, et al. Persistent pain in postmastectomy patients: Comparison of psychophysical, medical, surgical, and psychosocial characteristics between patients with and without pain. Pain 2013;154(5):660–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Skou ST, Graven-Nielsen T, Rasmussen S, et al. Widespread sensitization in patients with chronic pain after revision total knee arthroplasty. Pain 2013;154(9):1588–94. [DOI] [PubMed] [Google Scholar]
- 31. Skou ST, Graven-Nielsen T, Rasmussen S, et al. Facilitation of pain sensitization in knee osteoarthritis and persistent post-operative pain: A cross-sectional study. Eur J Pain 2014;18(7):1024–31. [DOI] [PubMed] [Google Scholar]
- 32. Wylde V, Palmer S, Learmonth ID, Dieppe P.. The association between pre-operative pain sensitisation and chronic pain after knee replacement: An exploratory study. Osteoarthritis Cartilage 2013;21(9):1253–6. [DOI] [PubMed] [Google Scholar]
- 33. Wright A, Moss P, Sloan K, et al. Abnormal sensory quantitative sensory testing is associated with persistent pain one year after TKA. Clin Orthop Relat Res 2015;473(1):246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wylde V, Sayers A, Lenguerrand E, et al. Preoperative widespread pain sensitization and chronic pain after hip and knee replacement: A cohort analysis. Pain 2015;156(1):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coghill R, Houle TT, Seid MH, et al. Multifactorial preoperative predictors for postcesarean section pain and analgesic requirement. Anesthesiology 2006;104(3):417–25. [DOI] [PubMed] [Google Scholar]
- 36. Petersen KK, Arendt-Nielsen L, Simonsen O, Wilder-Smith O, Laursen MB.. Presurgical assessment of temporal summation of pain predicts the development of chronic postoperative pain 12 months after total knee replacement. Pain 2015;156(1):55–61. [DOI] [PubMed] [Google Scholar]
- 37. Sun K, Li H.. Body mass index as a predictor of outcome in total knee replacement: A systemic review and meta-analysis. Knee 2017;24(5):917–24. [DOI] [PubMed] [Google Scholar]