Abstract
Neonatal hippocampal lesions in monkeys impairs normal performance on both relational and working memory tasks, suggesting that the early lesions have impacted the normal development of prefrontal-hippocampal functional interactions necessary for normal performance on these tasks. Given that working memory processes engage distributed neuronal networks associated with the prefrontal cortex, it is critical to explore the integrity of distributed neural networks of dorsolateral prefrontal cortex (dlPFC) following neonatal hippocampal lesions in monkeys. We used resting-state functional MRI to assess functional connectivity of dlPFC networks in monkeys with neonatal neurotoxic hippocampal lesion (Neo-Hibo, n = 4) and sham-operated control animals (Neo-C, n = 4). Significant differences in the patterns of dlPFC functional networks were found between Groups Neo-Hibo and Neo-C. The within-group maps and the between-group comparisons yielded a highly coherent picture showing altered interactions of core regions of the working memory network (medial prefontal cortex and posterior parietal cortex) as well as the dorsal (fundus of superior temporal area and superior temporal cortex) and ventral (V4 and infero-temporal cortex) visual processing areas in animals with Neo-Hibo lesions. Correlations between functional connectivity changes and working memory impairment in the same animals were found only between the dlPFC and visual cortical areas (V4 and infero-temporal cortex). Thus, the impact of the neonatal hippocampal lesions extends to multiple cortical areas interconnected with the dlPFC.
Keywords: resting state fMRI, functional connectivity, rhesus monkey
1. Introduction
Working memory, an ability of temporally holding or manipulating information maintained in memory buffer (Baddeley, 1992), is closely associated with interactive activity in the prefrontal, parietal and medial temporal lobes, especially the hippocampus (Constantinidis et al., 2004; Petrides, 1996). Earlier lesions and electrophysiological studies in monkeys revealed that the dorsolateral prefrontal cortex (areas 8, 46) is reciprocally connected with the dorsal visual stream via the parietal cortex, whereas the ventral visual stream (areas 12, 45) is associated with the inferior temporal cortex (Goldman-Rakic, 1988). This conceptual functional network has been largely confirmed by more recent neuroimaging studies (PET and fMRI) in humans, demonstrating concurrent activation of multiple brain areas when subjects are engaged in working memory tasks (Courtney et al., 1997; Jonides et al., 1993; Owen, 2000; Petrides, 1995a; Ungerleider et al., 1998). In clinical studies, changes in functional networks associated with the dorsolateral prefrontal cortex were reported in patients with mental illnesses, notably with developmental neuropsychiatric disorders such as schizophrenia (Callicott et al., 2000; Kraguljac et al., 2013; Kyriakopoulos et al., 2012; Zhou et al., 2007). Although earlier studies assigned working and episodic memory deficits in schizophrenics to dysfunction of specific neural structures, such as dorsolateral prefrontal cortex and hippocampus, respectively (Kraguljac et al., 2013), more recent fMRI studies have revealed large scale changes in prefrontal cortex functional networks associated with these memory deficits (Baker et al., 2014; Henseler et al., 2010; Kang et al., 2011).
The high similarity of anatomical connections and functional organizations of brain in nonhuman primates, especially the macaque monkeys, and humans makes it possible to mirror human brain pathology with more controllable primate models in which invasive techniques can be applied (Nakahara et al., 2007; Thiebaut de Schotten et al., 2012). On the evidence that neonatal ventral hippocampal lesions in rodents mimic certain aspects of the positive symptoms of schizophrenia, including working and spatial memory deficits (O’Donnell, 2012; Tseng et al., 2009), we investigated memory performance of monkeys that had received neonatal hippocampal lesions in the first two weeks of life. These neonatal lesions yielded severe loss of object recognition memory and episodic-like (relational) memory (Blue et al., 2013; Glavis-Bloom et al., 2013; Zeamer et al., 2013; Zeamer et al., 2010) as well as deficits in working memory related to impaired monitoring processes rather than maintenance processes (Heuer et al., 2011; 2013). These data suggested that neonatal hippocampal lesions impacted the normal development of prefrontal-hippocampal functional associations necessary for normal performance on both episodic and working memory tasks. Given the assumption that working memory processes engage distributed neuronal networks associated with the prefrontal cortex instead of sole brain areas (McIntosh, 1999), it becomes critical to explore the integrity of distributed neural networks of dorsolateral prefrontal cortex following neonatal hippocampal lesions in monkeys. Findings from such studies may provide a greater understanding of the functional alterations in this primate model and provide valuable information for identifying the neural correlates of memory impairments reported in schizophrenic patients as well as in other developmental brain disorders, such as developmental amnesia resulting in hippocampal atrophy from ischemic episode.
Using Diffusion Tensor Imaging (DTI), we recently revealed significant and enduring alterations of white matter integrity in the hippocampal projection systems after neonatal hippocampal lesions. These white matter changes were observed not only in the fornix and ventromedial prefrontal cortex, but also the temporal stem and optic radiations (Meng et al., 2013; Meng et al., 2014). To further explore the distributed neural networks that have been impacted by these neonatal lesions, resting state functional MRI was used in the present study to assess functional connectivity of dorsolateral prefrontal cortex networks in adult monkeys with neonatal hippocampal lesion and sham-operated control animals. Resting state functional MRI detects the intrinsic functional connectivity between brain areas regardless of stimulation (Ralchle et al., 2007). During rest, blood oxygen level-dependent (BOLD) signal often measured by correlation in low frequency fluctuations (LFF) (<0.1 Hz) reflects the metabolic level of the brain in the absence of extrinsic stimulation in the default mode functional network. Changes of the default mode network provide valuable insights regarding the integrity and functional alteration in normal subjects or patients (Lee et al., 2013), and also in anaesthetized monkeys (Vincent et al., 2007). By calculating the correlation of the time courses of the acquired signal between the dorsolateral prefrontal cortex and other areas, functional connectivity of macaque monkeys in both groups was compared on a voxel-by-voxel basis. More importantly, to explore whether the altered functional networks were associated with the episodic and working memory deficits found in the same animals, correlations were also performed between functional changes in neural networks and working and episodic memory performance.
2. Methods
2.1. Animals
All procedures were carried out and used in full compliance with the Institutional Animal Care and Use Committees of Emory University (IACUC). Four infant macaque monkeys had received sham lesions (Neo-C), and the other four had received neurotoxic lesions of the hippocampus (Neo-Hibo) via bilateral infusion of ibotenic acid (5.0 μl) at the age of about two weeks. Details of the surgical procedures and descriptions of the extent of hippocampal lesions can be found in previous study using the same animals (Heuer and Bachevalier, 2011; Zeamer et al., 2010).
When this MR imaging study was performed, animals were 8–10 years old. They were initially sedated with ketamine (5–10 mg/kg, IM) and then intubated for anesthesia with 1.0–1.5 % isoflurane. An IV catheter was placed for delivering lactated ringers solution (3.5–10 ml/kg/h) during the entire scanning procedure. Animals’ heads were immobilized in a custom-made head holder. During the acquisition of the MRI scans, they were spontaneously breathing under isoflurane at ~1.0% end-tidal inspiratory concentration, mixed with 100% O2. Physiological parameters were maintained in normal ranges (Li et al., 2013) as follows: end-tidal PCO2 = 38–42 mmHg, end-tidal PO2 = 25–35 mmHg, end-tidal O2 saturation = 95–100%, respiration rate = 15 / min, heart rate = 100 / min, body temperature = 37.5 °C maintained by a feedback-regulated circulating warm-water blanket.
2.2. MRI Experiments
All MRI scans were acquired on a Siemens 3T Trio scanner. Resting-state fMRI data were obtained with a Siemens 8-channel phase-array volume coil and a single-shot echo planar imaging (EPI) sequence and following imaging parameters: TE = 25 ms, TR = 2.2 s, data matrix = 64 × 64, voxel size = 1.5 mm × 1.5 mm, slice thickness = 1.5 mm, 34 slices to cover the whole brain, and 300 repetitions done within around 10 minutes. T1-weighted images were acquired by using a 3D MPRage sequence with GRAPPA (R = 2) and following parameters: inversion time = 0.95 s, TE / TR = 3.5 ms / 3 s, voxel size = 1.5 mm × 1.5 mm and slice thickness = 1.5 mm, to build the anatomical macaque template for the image registration. Whole brain field maps were acquired using a gradient echo sequence with TE = 6.24 and 8.7 ms, TR = 500 ms, FOV =96 mm × 96 mm, voxel size = 1.3 mm × 1.3 mm, and slice thickness =1.3 mm.
2.3. Data Processing
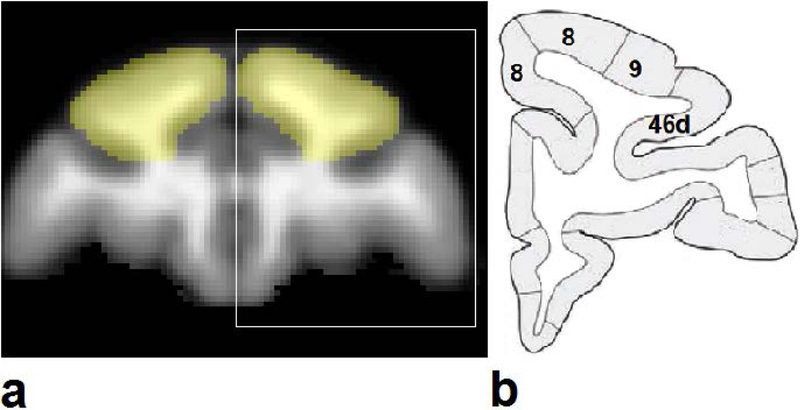
Data were processed with FSL (FMRIB, Oxford) and home-made MATLAB (Mathworks, Natick, MA) scripts. For resting-state fMRI data, image distortion was corrected based on an acquired field map. For each dataset, initial 10 time points were removed to eliminate the instability at the start of scanning, followed by motion correction and slice timing correction for the fact that a functional volume is covered with a series of successively measured 2D slices, and spatial smoothing with FWHM 3 mm. High-pass temporal filtering (Gaussian-weighted least-squares straight line fitting with sigma = 100 s) were applied. Dorsolateral prefrontal cortex (dlPFC), including Brodmann areas 8, 9 and 46d, was selected as the seed in the template derived from the high-spatial resolution T1-weighted anatomical images built from the Neo-C animals (see Fig. 1). The dlPFC areas from the template were registered (12 DOF linear affine transformation) to individual BOLD maps for Neo-C and Neo-Hibo animals. Individual functional connectivity maps were obtained by voxelwise calculation of the correlation coefficients between the time series of the grey matter of each whole brain and the averaged time series of the BOLD signal in the seed dlPFC, followed by a Fisher r-to-z transform to normalize the distribution of the correlation coefficients. The correlation coefficient maps of subjects were then registered to the T1-weighted anatomical template map. Significant activations were determined voxel-by-voxel by applying a two-sample t-test with a threshold of p < 0.05 and corrected with Gaussian random field theory (a kernel size of 3 mm was used for smoothing) and cluster size > 5.
Figure 1.
The seed dorsolateral prefrontal cortex (dlPFC, yellow color) derived from the monkey brain template (T1-weighted image, a), according to the atlas (b, (Saleem et al., 2006)) for the white box region of the template (a).
2.4. Performance on visual memory tasks
As they reached adulthood, animals were tested in a series of recognition and working memory tasks. Visual and spatial recognition memory mediated by the medial temporal lobe structures were assessed with the Visual Paired Comparison tasks (Bachevalier et al., 2008; Nemanic et al., 2002) and working memory mediated by the dorsolateral prefrontal cortex with the object self-ordered (Obj-SO) and serial-order memory (SOMT) tasks (Petrides, 1991; 1995a; b). The VPC is an incidental object recognition (relational) memory task that measures the animals’ ability to look longer at novel stimuli than familiar ones and uses delays of 10–120 sec. The Obj-SO task is a self-order working memory task that requires the monkeys to select one of 3 objects one at a time without choosing an object that they already have selected. The SOMT task is a temporal order task in which animals are presented with a list of 4 objects followed by a probe test presenting only a pair of objects from the list. Animals are rewarded for selecting the object that occurred earlier in the list. Detailed description of the tasks and results have been reported in an earlier publications (Heuer and Bachevalier, 2011). For the animals used in this study, the data indicated severe impairment in all tasks in animals with Neo-Hibo lesions as compared to controls and were used for correlations with changes found in prefrontal functional connectivity. To test the correlations between the areas of the functional network with significant group differences and recognition and working memory scores in Neo-Hibo animals, Pearson’s correlation analyses were conducted using the Fisher Z-scores of connectivity between dlPFC and the other areas, and the memory performance including: the total errors to reach learning criterion in self-order task obtained (Heuer and Bachevalier, 2011), the percent looking at novel pictures at delays of 120 s in visual paired comparison (VPC-delay) task at 4 years of age (Zeamer and Bachevalier, 2013), and the percent looking at the novel re-arranged images in the VPC-object-in-place (VPC-spatial) at 5–6 years of age (Blue et al., 2013). In addition, a one-way ANOVA was applied to assess the statistical significance of the correlations for the three groups with different behavioral measures (self-order, VPC-delay and VPC-spatial).
3. Results
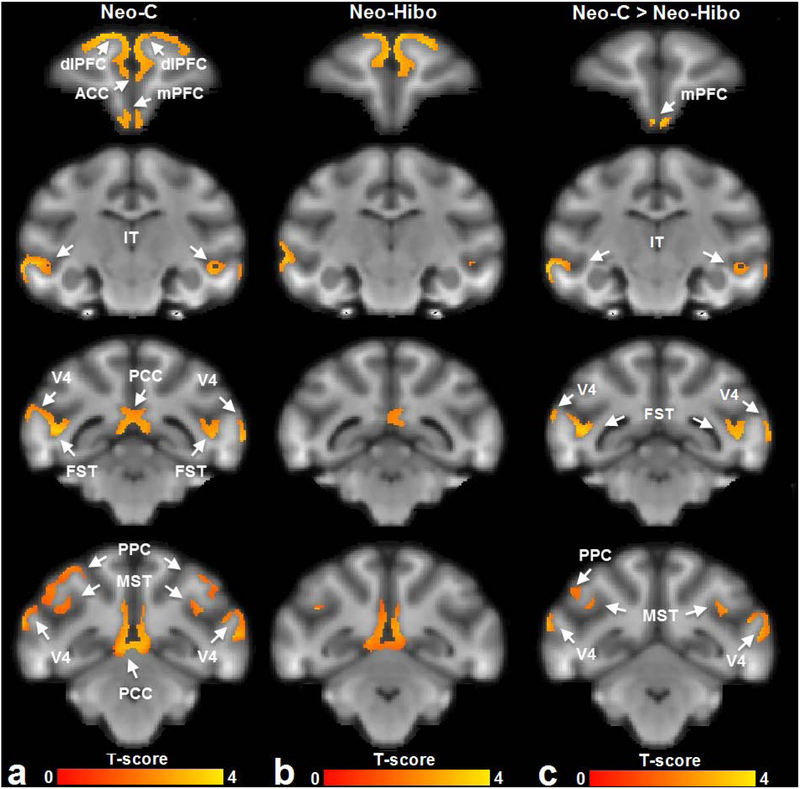
As shown in Figure 2a, the functional connectivity with dlPFC in the sham-operated controls involved the medial prefontal cortex (mPFC), inferotemporal cortex (IT), posterior cingulate cortex (PCC), posterior parietal cortex (PPC), fundus of superior temporal sulcus (FST and MST) and visual cortex (V4) in Neo-C group. In contrast, the functional connectivity with dlPFC in Neo-Hibo group was restricted to only PCC, IT and MST (Fig, 2b). Voxel-by-voxel analysis showed that for Group Neo-Hibo there was significant reduction in functional connectivity of dlPFC with mPFC, IT, MST, FST, PPC, V4 (Fig. 2c).
Figure 2.
The functional connectivity networks of dorsolateral prefrontal cortex derived from (a) animals with sham-operations Neo-C and (b) animals with Neo-Hibo lesions, by a one-sample t-test. A two-sample t-test (c) further showed that for the Neo-Hibo group functional connectivity decreased between dorsolateral prefrontal cortex (dlPFC) and medial prefontal cortex (mPFC), infero-temporal cortex (IT), fundus of superior temporal area (FST), posterior parietal cortex (PPC), medial superior temporal cortex (MST) and visual cortical area (V4). Other abbreviations: ACC: anterior cingulate cortex; PCC: posterior cingulate cortex. The brain template was generated with the T1-weighted images of Neo-C animals.
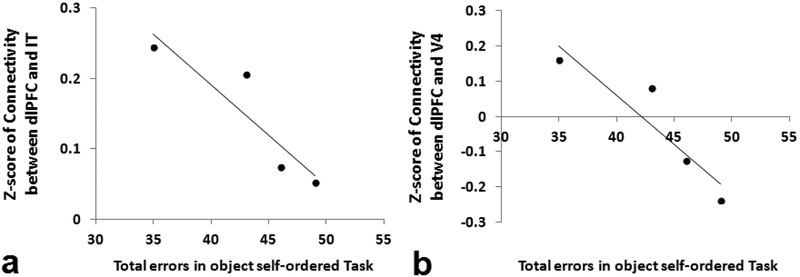
A one-way ANOVA test was applied to test the difference of the correlations for the three behavioral measurement groups. There was no significant difference in the homogeneity of variance test (p=0.85) but the correlations were significantly different in the three groups. Furthermore, as shown in Table 1, correlation analysis showed that the total errors in the object self-order task significantly decreased with the functional connectivity of dlFPC with IT and V4 (see also Fig. 3). However, such decreasing trend with the functional connectivity was observed but did not reach significance in other areas. It was also found that the scores in visual memory tasks (VPC-delay and VPC-spatial) were not significantly correlated with the functional connectivity of dlPFC with any other areas (Table 1).
Table 1.
Pearson’s correlations between memory measures and the dlPFC functional connectivity with different cortical areas (*p < 0.05)
| Areas | Self-order | VPC-delay | VPC-spatial | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neo-C | Neo-H | Neo-C | Neo-H | Neo-C | Neo-H | |||||||
| r | p | r | p | r | p | r | p | r | p | r | p | |
| mPFC | −0.35 | 0.39 | −0.50 | 0.25 | −0.01 | 0.49 | 0.06 | 0.47 | −0.55 | 0.22 | −0.69 | 0.16 |
| IT | −0.23 | 0.43 | −0.91 | 0.04* | 0.08 | 0.46 | 0.59 | 0.21 | −0.40 | 0.30 | 0.05 | 0.48 |
| MST | −0.54 | 0.32 | −0.65 | 0.18 | 0.52 | 0.24 | 0.19 | 0.40 | 0.16 | 0.42 | 0.03 | 0.49 |
| FST | −0.09 | 0.47 | −0.53 | 0.24 | 0.04 | 0.48 | 0.10 | 0.45 | −0.31 | 0.35 | 0.26 | 0.37 |
| PPC | −0.36 | 0.38 | −0.73 | 0.14 | 0.26 | 0.37 | 0.33 | 0.34 | −0.20 | 0.40 | 0.17 | 0.41 |
| V4 | −0.01 | 0.50 | −0.92 | 0.04* | −0.03 | 0.49 | 0.60 | 0.20 | −0.34 | 0.33 | −0.11 | 0.45 |
Abbreviations. Neo-C: animals received sham lesions; Neo-H: animals received neurotoxic lesions of the hippocampus; FST: fundus of superior temporal area; IT: inferotemporal cortex; mPFC: medial prefontal cortex; MST: medial superior temporal cortex; PPC: posterior parietal cortex; V4: visual cortical area; Self-order: object self-order working memory; VPC: visual paired-comparison task at delays of 120s; VPC-spatial: object-in-Place visual paired-comparison task.
Figure 3.
Total errors on the object self-order task in animals with Neo-Hibo lesions were significantly correlated with functional connectivity (Fisher Z-scores) between dorsolateral prefrontal cortex (dlPFC) and the areas of (a) infero-temporal cortex (IT) and (b) visual cortical area (V4).
4. Discussion
The present results revealed significant differences in the patterns of dlPFC functional networks between animals with neonatal hippocampal lesions and sham-operated controls. The within-group maps and the between-group comparisons yielded a highly coherent picture showing altered interactions of core regions of the working memory network (mPFC and PPC) as well as the dorsal (FST and MST) and ventral (V4 and IT) visual processing areas in animals with neonatal hippocampal lesions. Yet, correlations between functional connectivity changes and working memory impairment were found only between the dlPFC and visual cortical areas (IT and V4).
4.1. Dorsolateral prefrontal functional networks in control animals
As illustrated in Figure 2a, the dlPFC functional networks in Neo-C animals include a large number of brain areas, mPFC, PPC, PCC, IT, FST, MST, and V4. Such extended functional network is consistent with the demonstration of widespread monosynaptic and multisynaptic connections between dlPFC and other cortical areas reported in anatomical tracer studies (see for reviews (Yeterian et al., 2012)) and more recently in studies of white matter tracts using DTI (see for review (Thiebaut de Schotten et al., 2012)). Thus, the results from the control animals strengthen the view that the dlPFC is part of a broader network of interconnected brain areas and this network is known to be involved in working memory (see for review (Constantinidis and Procyk, 2004)). Working memory is believed to require the coordination of multiple regions associated with the dlPFC, such as the PPC and posterior unimodal association areas (V4, IT, FST, MST) within the ventral and dorsal visual streams. These regions do not function in isolation but rather interact to maintain and manipulate sensory percepts that are no longer present in the environment (Gazzaley et al., 2004) and are used to guide subsequent behavior. For example, the functional connectivity observed between the dlPFC and posterior parietal areas (PPC) in control animals is consistent with the strong bidirectional connectivity linking these two cortical areas as demonstrated by neuroanatomical tracer studies (Pandya et al., 1981) as well as projections from PPC to visual cortical areas (Katsuki et al., 2012). This functional coordination appears to reflect activity within the dorsal attentional network involved with top-down control of attention and monitoring processes regardless of stimulus modality (Corbetta et al., 2002). At the same time, performance on visual WM tasks requires the functional coordination of primary and associative visual areas (V4, FST, MST, IT) in which spatial and object information of visual stimuli are processed, encoded and maintained (Ungerleider et al., 1998; Zimmer, 2008). Such maintenance of stimulus information is likely to be mediated in part by feedback projections from prefrontal cortex (PFC) to posterior association cortices, through reciprocal connections between these visual areas (Boussaoud et al., 1990; Chafee et al., 1998; Miller et al., 1996; Ungerleider et al., 1989; Webster et al., 1994). In addition, the dlPFC is also directly linked with both the anterior and posterior cingulate cortex (ACC and PCC, respectively) (Goldman-Rakic et al., 1984; Hatanaka et al., 2003; Pandya et al., 1981; Paus et al., 2001; Petrides et al., 1999; Vogt et al., 1987; Wang et al., 2001). Although the participation of the ACC in WM is still debated, co-activation of the dlPFC and ACC during WM tasks has been demonstrated by brain imaging in humans (Inoue et al., 2004) and its functional interactions with the dlPFC in WM memory processes seem to be related to error detection and behavior evaluation rather than memory per se (Hadland et al., 2003; Rushworth et al., 2003).
4.2. Dorsolateral-PFC functional connectivity is altered after neonatal hippocampal lesions
Neonatal hippocampal lesions had a profound and extensive impact on the dlPFC functional connectivity network. As shown in Fig. 2a–b, the Neo-Hibo animals showed fewer brain areas functionally interconnected with dlPFC as compared to controls. Decreased functional connectivity was observed in the mPFC in the frontal lobe, PPC in the parietal lobe, FST and MST in the dorsal visual stream, and IT and V4 in the ventral visual stream (Fig. 2c). Decreased dlPFC-mPFC connectivity is consistent with our earlier observation of alterations of fractional anisotropy (FA) and diffusivity indices in the mPFC in the same animals with neonatal hippocampal lesions as detected by DTI (Meng et al., 2014). The present results indicate that the impact of the neonatal hippocampal lesions extends to multiple cortical areas interconnected with the dlPFC.
Using the same animals, our previous diffusion tensor MR imaging studies have shown a significant increase in MD values in dlPFC, but not in the vlPFC, after Neo-H lesions (Meng et al., 2013). Increased diffusivity (or reduced FA) is usually associated with reduction of axonal membrane and fibers, or disruptions of myelin sheath of axons (Schwartz et al., 2003). The increased diffusivity suggested abnormal microstructural changes in dlPFC after the neonatal hippocampal lesions. Thus, it is possible that the decreased dlPFC functional connectivity with several cortical areas found in the present study may have resulted from a compromised dlPFC architecture during development following the early-onset damage. Although there exist no empirical studies in monkeys that have assessed the development of the dorsolateral prefrontal cortex after neonatal hippocampal lesions, results from our laboratory have already demonstrated that large medial temporal lobe lesions, including the hippocampus, in infant monkeys yielded alterations of dorsolateral prefrontal cortex morphology (Chlan-Fourney et al., 2000; Chlan-Fourney et al., 2003) and functioning (Bertolino et al., 1997; Saunders et al., 1998). None of these changes occurred in adult monkeys that had received the same medial temporal lobe lesions in adulthood. In addition, behavioral, electrophysiological, neurochemical, and ultrastructural studies in rodents have provided mounting evidence that damage to the ventral hippocampus a few days after birth result in protracted and widespread brain alterations, including the prefrontal cortex (for a review, see (Tseng et al., 2009)). Considering the small sample size, future studies will need to replicate the present findings with a larger number of animals.
4.3. Relationships between dlPFC functional connectivity and working memory performance
Given that animals with neonatal hippocampal lesions are severely impaired at the long delays of object and spatial recognition tasks (Zeamer and Bachevalier, 2013; Zeamer et al., 2010) as well as in working memory tasks, especially those requiring monitoring WM processes (Heuer and Bachevalier, 2011; 2013), it was interesting to determine whether the memory deficits correlated with some of the changes in the dlPFC functionality. A shown on Table 1 and Figure 3, significant correlations were found only between working memory deficits and decreased functional connectivity between dlPFC and visual cortical areas, such as IT (r = −0.91, p = 0.04) and V4 (r = −0.92, p = 0.04). Such correlations were not observed on performance on any of the recognition tasks that the animals received (Table 1). The critical interactions between IT cortex and the ventrolateral prefrontal cortex (vlPFC) in maintenance of visual information and memory processes have previously been demonstrated (Eacott et al., 1992; Fiebach et al., 2006; Gutnikov et al., 1997; Petrides, 2000; Woloszyn et al., 2009) and a top-down model between the two cortical areas has been proposed (Renart et al., 2001). Interestingly, our findings point to significant functional interactions between IT and the dlPFC and functional disconnection between the two cortical areas is associated with impairment in WM tasks specifically tapping onto monitoring processes (Heuer and Bachevalier, 2011). Furthermore, given that the same monkeys with neonatal hippocampal lesions (J. Bachevalier, unpublished data) are unimpaired in tasks measuring object discrimination, requiring cortical/striatal interactions, and in devaluation tasks requiring amygdala/orbitofrontal interactions, it seems reasonable to suggest that the impact of the neonatal hippocampal lesions may be specific to neural networks linked to the dlPFC and not other neural networks.
Despite the small sample size, the present results clearly demonstrate that resting-state fMRI is sufficiently sensitive to detect the effects of cell body-selective, neurotoxic lesions of the hippocampus on the functional interactions between the dlPFC with several brain regions in monkeys. Our observations are consistent with the proposal that working memory deficits following neonatal damage restricted to the hippocampus arise from disrupted network interactions that include the prefrontal cortex. Whether the widespread disruption of neural networks is specific to the early timing of the hippocampal lesions or could also be associated with adult-onset hippocampal lesions will need to be empirically tested. There exist only few studies that have assessed the effects of adult-onset hippocampal lesions on working memory processes, and none have used the Obj-SO task, restricted neurotoxic lesions, or have used functional neuroimaging techniques to assess disruption of neural networks. Thus, monkeys with adult-onset nonselective hippocampal lesions were impaired in variations of working memory paradigms (Kimble et al., 1963; Mahut, 1971; Waxler et al., 1970). Furthermore, findings of a recent diffusion tensor imaging study performed on the monkeys described in the present report (Meng et al., 2014) indicated that neonatal hippocampal lesions resulted in significant and enduring white matter alterations in the hippocampal projection system similar to the white matter changes earlier reported after adult-onset hippocampal lesions (Shamy et al., 2010). But interestingly, the early-onset hippocampal lesions resulted in additional white matter changes in the temporal stem and optic radiation that have not been described after adult-onset hippocampal lesions. Thus, it is possible that the impact of the neonatal hippocampal lesions on other brain structures may be far more widespread than that found after the adult-onset hippocampal lesions.
Acknowledgements
The authors are grateful to Ruth Connelly and Doty Kempf (DVM) at Yerkes Imaging Center for animal handling, Sudeep Patel and Dr. Chunxia Li for MRI data acquisition. This project was funded by NIH/NIMH grant MH0588446 (JB), the National Center for Research Resources P51RR000165, currently supported by the Office of Research Infrastructure Programs / OD P51OD011132.
References
- Bachevalier J, & Nemanic S (2008). Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus, 18, 64–80. [DOI] [PubMed] [Google Scholar]
- Baddeley A (1992). Working memory. Science, 255, 556–559. [DOI] [PubMed] [Google Scholar]
- Baker JT, Holmes AJ, Masters GA, Yeo BT, Krienen F, Buckner RL, & Ongur D (2014). Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry, 71, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Saunders RC, Mattay VS, Bachevalier J, Frank JA, & Weinberger DR (1997). Altered development of prefrontal neurons in rhesus monkeys with neonatal mesial temporo-limbic lesions: a proton magnetic resonance spectroscopic imaging study. Cerebral Cortex, 7, 740–748. [DOI] [PubMed] [Google Scholar]
- Blue SN, Kazama AM, & Bachevalier J (2013). Development of memory for spatial locations and object/place associations in infant rhesus macaques with and without neonatal hippocampal lesions. Journal of the International Neuropsychological Society, 19, 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussaoud D, Ungerleider LG, & Desimone R (1990). Pathways for motion analysis: cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. Journal of Comparative Neurology, 296, 462–495. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, & Weinberger DR (2000). Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cerebral Cortex, 10, 1078–1092. [DOI] [PubMed] [Google Scholar]
- Chafee MV, & Goldman-Rakic PS (1998). Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. Journal of Neurophysiology, 79, 2919–2940. [DOI] [PubMed] [Google Scholar]
- Chlan-Fourney J, Webster MJ, Felleman DJ, & Bachevalier J (2000). Neonatal medial temporal lobe lesions alter the distribution of tyrosine hydroxylase immunoreactive varicosities in the macaque prefrontal cortex. Washington D.C. Society for Neuroscience Abstract, 228.18. [Google Scholar]
- Chlan-Fourney J, Webster MJ, Jung J, & Bachevalier J (2003). Neonatal medial temporal lobe lesions decrease GABAergic interneuron densities in macaque prefrontal cortex: Implications for schizophrenia and Autism. Washington D.C. Society for Neuroscience Abstract, 315.9. [Google Scholar]
- Constantinidis C, & Procyk E (2004). The primate working memory networks. Cognitive, Affective, & Behavioral Neuroscience, 4, 444–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3, 201–215. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, & Haxby JV (1997). Transient and sustained activity in a distributed neural system for human working memory. Nature, 386, 608–611. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, & Gaffan D (1992). Inferotemporal-frontal Disconnection: The Uncinate Fascicle and Visual Associative Learning in Monkeys. European Journal of Neuroscience, 4, 1320–1332. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Rissman J, & D’Esposito M (2006). Modulation of inferotemporal cortex activation during verbal working memory maintenance. Neuron, 51, 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, & D’Esposito M (2004). Functional connectivity during working memory maintenance. Cognitive, Affective, & Behavioral Neuroscience, 4, 580–599. [DOI] [PubMed] [Google Scholar]
- Glavis-Bloom C, Alvarado MC, & Bachevalier J (2013). Neonatal hippocampal damage impairs specific food/place associations in adult macaques. Behavioral Neuroscience, 127, 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1988). Topography of cognition: parallel distributed networks in primate association cortex. Annual Review of Neuroscience, 11, 137–156. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, & Schwartz ML (1984). Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience, 12, 719–743. [DOI] [PubMed] [Google Scholar]
- Gutnikov SA, Ma YY, & Gaffan D (1997). Temporo-frontal disconnection impairs visual-visual paired association learning but not configural learning in Macaca monkeys. European Journal of Neuroscience, 9, 1524–1529. [DOI] [PubMed] [Google Scholar]
- Hadland KA, Rushworth MF, Gaffan D, & Passingham RE (2003). The anterior cingulate and reward-guided selection of actions. Journal of Neurophysiology, 89, 1161–1164. [DOI] [PubMed] [Google Scholar]
- Hatanaka N, Tokuno H, Hamada I, Inase M, Ito Y, Imanishi M, Hasegawa N, Akazawa T, Nambu A, & Takada M (2003). Thalamocortical and intracortical connections of monkey cingulate motor areas. Journal of Comparative Neurology, 462, 121–138. [DOI] [PubMed] [Google Scholar]
- Henseler I, Falkai P, & Gruber O (2010). Disturbed functional connectivity within brain networks subserving domain-specific subcomponents of working memory in schizophrenia: relation to performance and clinical symptoms. Journal of Psychiatric Research, 44, 364–372. [DOI] [PubMed] [Google Scholar]
- Heuer E, & Bachevalier J (2011). Neonatal hippocampal lesions in rhesus macaques alter the monitoring, but not maintenance, of information in working memory. Behavioral Neuroscience, 125, 859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer E, & Bachevalier J (2013). Working memory for temporal order is impaired after selective neonatal hippocampal lesions in adult rhesus macaques. Behavioural Brain Research, 239, 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Mikami A, Ando I, & Tsukada H (2004). Functional brain mapping of the macaque related to spatial working memory as revealed by PET. Cerebral Cortex, 14, 106–119. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, & Mintun MA (1993). Spatial working memory in humans as revealed by PET. Nature, 363, 623–625. [DOI] [PubMed] [Google Scholar]
- Kang SS, Sponheim SR, Chafee MV, & MacDonald AW 3rd (2011). Disrupted functional connectivity for controlled visual processing as a basis for impaired spatial working memory in schizophrenia. Neuropsychologia, 49, 2836–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki F, & Constantinidis C (2012). Unique and shared roles of the posterior parietal and dorsolateral prefrontal cortex in cognitive functions. Frontiers in Integrative Neuroscience, 6, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble DP, & Pribram KH (1963). Hippocampectomy and behavior sequences. Science, 139, 824–825. [DOI] [PubMed] [Google Scholar]
- Kraguljac NV, Srivastava A, & Lahti AC (2013). Memory deficits in schizophrenia: a selective review of functional magnetic resonance imaging (FMRI) studies. Behavioral Sciences, 3, 330–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakopoulos M, Dima D, Roiser JP, Corrigall R, Barker GJ, & Frangou S (2012). Abnormal functional activation and connectivity in the working memory network in early-onset schizophrenia. Journal of the American Academy of Child & Adolescent Psychiatry, 51, 911–920 e912. [DOI] [PubMed] [Google Scholar]
- Lee MH, Smyser CD, & Shimony JS (2013). Resting-state fMRI: a review of methods and clinical applications. American Journal of Neuroradiology, 34, 1866–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CX, Patel S, Auerbach EJ, & Zhang X (2013). Dose-dependent effect of isoflurane on regional cerebral blood flow in anesthetized macaque monkeys. Neuroscience Letters, 541, 58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahut H (1971). Spatial and object reversal learning in monkeys with partial temporal lobe ablations. Neuropsychologia, 9, 409–424. [DOI] [PubMed] [Google Scholar]
- McIntosh AR (1999). Mapping cognition to the brain through neural interactions. Memory, 7, 523–548. [DOI] [PubMed] [Google Scholar]
- Meng Y, Payne C, Hu X, Bachevalier J, & Zhang X (2013). Differential alterations of dorsolateral and ventrolateral prefontal cortex in adult macaques with neonatal hippocampal lesion: a diffusion tensor imaging study. San Diego, California. Society for Neuroscience Abstract, 6033. [Google Scholar]
- Meng Y, Payne C, Li L, Hu X, Zhang X, & Bachevalier J (2014). Alterations of hippocampal projections in adult macaques with neonatal hippocampal lesions: A Diffusion Tensor Imaging study. Neuroimage, 102P2, 828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, & Desimone R (1996). Neural mechanisms of visual working memory in prefrontal cortex of the macaque. The Journal of Neuroscience, 16, 5154–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara K, Adachi Y, Osada T, & Miyashita Y (2007). Exploring the neural basis of cognition: multi-modal links between human fMRI and macaque neurophysiology. Trends in Cognitive Sciences, 11, 84–92. [DOI] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Price RE, Jackson EF, & Bachevalier J (2002). Assessment of locus and extent of neurotoxic lesions in monkeys using neuroimaging techniques: a replication. Journal of Neuroscience Methods, 121, 199–209. [DOI] [PubMed] [Google Scholar]
- O’Donnell P (2012). Cortical disinhibition in the neonatal ventral hippocampal lesion model of schizophrenia: new vistas on possible therapeutic approaches. Pharmacology & Therapeutics, 133, 19–25. [DOI] [PubMed] [Google Scholar]
- Owen AM (2000). The role of the lateral frontal cortex in mnemonic processing: the contribution of functional neuroimaging. Experimental Brain Research, 133, 33–43. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Van Hoesen GW, & Mesulam MM (1981). Efferent connections of the cingulate gyrus in the rhesus monkey. Experimental Brain Research, 42, 319–330. [DOI] [PubMed] [Google Scholar]
- Paus T, Castro-Alamancos MA, & Petrides M (2001). Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. European Journal of Neuroscience, 14, 1405–1411. [DOI] [PubMed] [Google Scholar]
- Petrides M (1991). Monitoring of selections of visual stimuli and the primate frontal cortex. Proceedings of the Royal Society of London B: Biological Sciences, 246, 293–298. [DOI] [PubMed] [Google Scholar]
- Petrides M (1995a). Functional organization of the human frontal cortex for mnemonic processing. Evidence from neuroimaging studies. Annals of the New York Academy of Sciences, 769, 85–96. [DOI] [PubMed] [Google Scholar]
- Petrides M (1995b). Impairments on nonspatial self-ordered and externally ordered working memory tasks after lesions of the mid-dorsal part of the lateral frontal cortex in the monkey. The Journal of Neuroscience, 15, 359–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M (1996). Fronto-hippocampal interactions in mnemonic processing In: The Hippocampus: Functions and Clinical Relevance, Kato N (Ed.), Amsterdam: Elsevier, pp. 289–301. [Google Scholar]
- Petrides M (2000). Dissociable roles of mid-dorsolateral prefrontal and anterior inferotemporal cortex in visual working memory. The Journal of Neuroscience, 20, 7496–7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, & Pandya DN (1999). Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. European Journal of Neuroscience, 11, 1011–1036. [DOI] [PubMed] [Google Scholar]
- Ralchle ME, & Snyder AZ (2007). A default mode of brain function: A brief history of an evolving idea. Neuroimage, 37, 1083–1090. [DOI] [PubMed] [Google Scholar]
- Renart A, Moreno R, de la Rocha J, Parga N, & Rolls ET (2001). A model of the IT-PF network in object working memory which includes balanced persistent activity and tuned inhibition. Neurocomputing, 38, 1525–1531. [Google Scholar]
- Rushworth MF, Hadland KA, Gaffan D, & Passingham RE (2003). The effect of cingulate cortex lesions on task switching and working memory. Journal of Cognitive Neuroscience, 15, 338–353. [DOI] [PubMed] [Google Scholar]
- Saleem KS, & Logothetis NK (2006). A combined MRI and histology atlas of the rhesus monkey brain in stereotaxic coordinates. Academic Press. [Google Scholar]
- Saunders RC, Kolachana BS, Bachevalier J, & Weinberger DR (1998). Neonatal lesions of the medial temporal lobe disrupt prefrontal cortical regulation of striatal dopamine. Nature, 393, 169–171. [DOI] [PubMed] [Google Scholar]
- Schwartz ED, & Hackney DB (2003). Diffusion-weighted MRI and the evaluation of spinal cord axonal integrity following injury and treatment. Experimental Neurology, 184, 570–589. [DOI] [PubMed] [Google Scholar]
- Shamy JL, Carpenter DM, Fong SG, Murray EA, Tang CY, Hof PR, & Rapp PR (2010). Alterations of white matter tracts following neurotoxic hippocampal lesions in macaque monkeys: a diffusion tensor imaging study. Hippocampus, 20, 906–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell’Acqua F, Valabregue R, & Catani M (2012). Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex, 48, 82–96. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, & Lipska BK (2009). The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behavioural Brain Research, 204, 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Courtney SM, & Haxby JV (1998). A neural system for human visual working memory. Proceedings of the National Academy of Sciences of the United States of America, 95, 883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Gaffan D, & Pelak VS (1989). Projections from inferior temporal cortex to prefrontal cortex via the uncinate fascicle in rhesus monkeys. Experimental Brain Research, 76, 473–484. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, & Raichle ME (2007). Intrinsic functional architecture in the anaesthetized monkey brain. Nature, 447, 83–86. [DOI] [PubMed] [Google Scholar]
- Vogt BA, & Pandya DN (1987). Cingulate cortex of the rhesus monkey: II. Cortical afferents. Journal of Comparative Neurology, 262, 271–289. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shima K, Sawamura H, & Tanji J (2001). Spatial distribution of cingulate cells projecting to the primary, supplementary, and pre-supplementary motor areas: a retrograde multiple labeling study in the macaque monkey. Neuroscience Research, 39, 39–49. [DOI] [PubMed] [Google Scholar]
- Waxler M, & Rosvold HE (1970). Delayed alternation in monkeys after removal of the hippocampus. Neuropsychologia, 8, 137–146. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Bachevalier J, & Ungerleider LG (1994). Connections of inferior temporal areas TEO and TE with parietal and frontal cortex in macaque monkeys. Cerebral Cortex, 4, 470–483. [DOI] [PubMed] [Google Scholar]
- Woloszyn L, & Sheinberg DL (2009). Neural dynamics in inferior temporal cortex during a visual working memory task. The Journal of Neuroscience, 29, 5494–5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN, Tomaiuolo F, & Petrides M (2012). The cortical connectivity of the prefrontal cortex in the monkey brain. Cortex, 48, 58–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeamer A, & Bachevalier J (2013). Long-term effects of neonatal hippocampal lesions on novelty preference in monkeys. Hippocampus, 23, 745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeamer A, Heuer E, & Bachevalier J (2010). Developmental trajectory of object recognition memory in infant rhesus macaques with and without neonatal hippocampal lesions. The Journal of Neuroscience, 30, 9157–9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Jiang TZ, Tian LX, Liu Y, Liu ZN, Liu HH, & Kuang F (2007). Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neuroscience Letters, 417, 297–302. [DOI] [PubMed] [Google Scholar]
- Zimmer HD (2008). Visual and spatial working memory: from boxes to networks. Neuroscience & Biobehavioral Reviews, 32, 1373–1395. [DOI] [PubMed] [Google Scholar]