Abstract
Intolerance of uncertainty (IU) is an important individual difference factor that may contribute to trait-like aggression. Deficient engagement of the ventrolateral PFC (vlPFC) during social situations may also be a mechanism that links these two constructs. The aim of the current study was to test a proposed mediation model whereby IU is associated with trait aggression through neural activation of the vlPFC during a social exclusion task. Fifty-three adults with a range of impulsive-aggressive traits completed validated assessments of IU and trait aggression, and the ‘Cyberball’ social exclusion task during functional magnetic resonance imaging (fMRI). Results supported the mediation model such that greater levels of IU were associated with greater trait aggression through hypoactivation of the vlPFC during social exclusion. This study is the first to provide evidence suggesting that individuals higher in IU have difficulties engaging regulatory neural processes, which in-turn may increase the propensity for aggression.
Keywords: intolerance of uncertainty, aggression, social exclusion, ventrolateral prefrontal cortex
Recurrent, problematic aggression is highly prevalent and poses enormous public health burden. It is estimated that each year 1.3 million people die from acts of self- and other-directed violence, resulting in substantial economic costs (World Health organization [WHO], 2014). Non-lethal forms of aggression are also detrimental and undermine social relationships, impair academic and occupational achievement, create legal problems, and increase risk for comorbid psychiatric conditions, suicidal behaviors, and physical health problems (McCloskey & Ammerman, 2017; McCloskey, Kleabir, Berman, Chen, & Coccaro, 2010; Moffit, Caspi, Harrington, & Milne, 2002). Importantly, though individual aggressive acts can be situational, the propensity to behave aggressively is conceptualized as a behavioral trait, which emerges early in life and persists through adulthood (Huesmann, Dubow, & Boxer, 2009; Olweus, 1979). There is consequently an ongoing, urgent need to better understand the mechanisms implicated in recurrent aggressive behavior and develop more targeted, effective prevention and treatment strategies.
One individual difference factor that may contribute to trait-like aggression is intolerance of uncertainty (IU), defined as a dispositional characteristic resulting from negative beliefs about uncertainty and its implications (Dugas & Robichaud, 2007). Uncertainty is universally aversive as it diminishes our ability to prepare for future events; resulting in increased anxiety and sustained hypervigilance (see Grupe & Nitschke, 2013 for a review). There are some individuals, however, that are particularly sensitive to uncertainty and hold the belief that all (or most) forms of ambiguity are unacceptable and intolerable (Carleton, Sharpe, & Asmundson, 2007). This negative belief that uncertainty is intolerable can manifest in a variety of ways including excessive worry, avoidance of uncertain situations, and the perception that ambiguous information is threatening (Carleton, 2016). Indeed, factor analytic studies suggest that the IU construct is broad and consists of two sub-factors – inhibitory-IU and prospective-IU. Inhibitory-IU is characterized by behavioral inhibition (e.g., freezing, avoidance) in response to uncertainty whereas prospective-IU is characterized by excessive concerns and worry about future events (Carleton et al., 2007; Hong & Lee, 2015; McEvoy & Mahoney, 2011). Generally, inhibitory-IU reflects behavioral disturbances whereas prospective-IU reflects abnormal cognitive perceptions in response to uncertainty. Across both factors, individuals high in IU tend to find uncertainty distressing and display impaired problem-solving and maladaptive coping responses during times of ambiguity (Dugas, Freeston, & Ladoucer, 1997; Greco & Roger, 2003; Birrell, Meares, Wilkinson, & Freeston, 2011; Carleton, 2012; Carleton, Norton, & Asmundson, 2007).
To date, individual differences in IU have been most often investigated in the context of internalizing psychopathologies and accumulating evidence suggests that IU is a transdiagnostic phenotype of mood and anxiety disorders (Barlow, Sauer-Zavala, Carl, Bullis, & Ellard, 2014). Indeed, studies have shown that individuals with generalized anxiety disorder (Gentes & Ruscio, 2011), major depressive disorder (Yook, Kim, Suh, & Lee, 2010), panic disorder (Carleton et al., 2014), social anxiety disorder (Boelen & Reijntjes, 2009), and obsessive-compulsive disorder (Jacoby, Fabricant, Leonard, Riemann, & Abramowitz, 2013) all exhibit high levels of IU. There is also new and emerging research to suggest that the role of IU in psychopathology extends beyond internalizing psychopathology and may be implicated in maladaptive externalizing behaviors and diagnoses (Carleton, 2016). For instance, our laboratory has repeatedly shown that increased sensitivity to uncertainty is associated with current problematic alcohol use and risk for alcohol use disorder (Gorka et al., 2016a; Gorka, Lieberman, Phan, & Shankman, 2016b).
Aggression, particularly other-directed reactive aggression, often occurs in dynamic social situations. Individuals who are intolerant of uncertainty, and thus find ambiguity unacceptable, may experience distress in social situations as others' internal thoughts and appraisals are never known and consequently, interpersonal interactions always contain elements of uncertainty. Importantly, distress arising from high IU could in-turn increase the propensity for aggressive behavior in some individuals as research suggests that reactive aggression stems from emotional and impulsive responses to frustration and distress (Berkowitz, 1993; Dodge, 1991). The tendency to respond aggressively may be particularly likely in social situations that involve perceived social threat, such as ostracism. In other words, some individuals high in IU may experience social-related distress and exhibit a tendency to behave aggressively, especially when explicitly rejected or provoked. Despite these theoretical links, only one study to our knowledge has directly investigated the role of IU in aggression. In an unselected student sample, Fracalanza et al. (2014) demonstrated that IU mediated the association between generalized anxiety symptoms and self-reported anger expression. Also, though not an examination of aggression per se, it is also worth highlighting that Anderson et al. (2016) randomized undergraduates to an uncertainty induction that was either perceived as avoidable or unavoidable by participants. The researchers found that individuals in the avoidable uncertainty group (only) reported significant increases in state anger pre- to post-induction and it was concluded that beliefs that one's current uncertain state could be avoided potentiates anger.
Taken together, IU is an important transdiagnostic individual difference factor that may be implicated in trait aggression; though, direct empirical research is greatly needed to support this hypothesis. Beyond just establishing a relation between IU and aggression, there is also the need to better understand the mechanisms that link IU with aggressive behaviors to develop mechanistically-driven clinical intervention and prevention strategies. As briefly noted above, other-directed, reactive aggression often occurs in social situations, particularly those that are perceived as threating, and individuals high in IU may respond to social stress with aggressive tendencies. In the laboratory, one widely-used experimental paradigm designed to probe responses to interpersonal situations is the Cyberball social exclusion task (Williams, Cheung, & Choi, 2000). During the task, participants are led to believe that they are playing a virtual game of ‘catch’ via an internet connection with two other players. In actuality, the two other players are avatars and their behaviors are pre-programmed to include conditions where the participant is fairly included or socially excluded from the game. As designed, the social exclusion conditions robustly elicit psychological distress and feelings of ostracism (Williams, 2007), and more broadly, acute social rejection has been shown to increase anger and aggressive behaviors (Gaertner, Iuzzini, & O'Mara, 2008; Twenge, Baumeister, Tice, & Stucke, 2001).
With regard to mechanisms, the Cyberball task has been adapted for use during functional magnetic resonance imaging (fMRI) and there is a growing literature focused on elucidating the neural processes underlying response to social exclusion (Eisenberger, Lieberman, &Williams, 2003; Masten et al., 2009; Vijayakumar, Cheng, & Pfeifer, 2017). Converging evidence stemming from this research indicates that the several regions of the prefrontal cortex (PFC) are involved in the experience and regulation of social exclusion, particularly the anterior cingulate cortex (ACC) and ventrolateral prefrontal cortex (vlPFC) (see Kawamoto, Ura, & Nittono, 2015). More specifically, studies have shown that activity within sub-regions of the ACC (i.e., dorsal and subgenual) detect and track the severity of individuals' subjective social pain, whereas activity within the vlPFC exerts top-down regulation of social pain and predicts decreases in social distress and psychophysiological arousal during social exclusion (Eisenberger et al., 2003; Eisenberger, Taylor, Gable, Hilmert, & Lieberman, 2007; Masten et al., 2009). Interestingly, in further support of the vlPFC's regulatory role, recent transcranial direct current stimulation (tDCS) studies have demonstrated that stimulation of the right vlPFC during social exclusion dampens participants' reports of distress and reduces aggressive responding (Riva, Romero Lauro, DeWall, & Bushman, 2012; Riva, Romero Lauro, DeWall, Chester, & Bushman, 2014; Riva, Romero Lauro, Vergallito, DeWall, & Bushman, 2015).
The existing literature suggests that activation of the PFC, especially the vlPFC, may underlie reactivity to threatening social situations and aggressive behaviors. It is therefore possible that vlPFC functioning mediates the association between IU and trait aggression, and is one potential mechanism that links these two dispositional characteristics. The current study was notably designed to test these proposed associations in a sample of adults with a range of impulsive-aggressive tendencies and traits. The sample specifically included individuals both with and without current diagnoses of intermittent explosive disorder (IED) – a disorder characterized by recurrent impulsive acts of verbal and/or physical aggression and difficulties regulating strong negative emotions such as anger (Coccaro, 2012). The inclusion of individuals with IED is well-suited for the study aims as this subgroup displays affective lability and impaired emotion regulation capabilities, rendering them particularly prone to distress and subsequent reactive aggression during social threat (Fettich, McCloskey, Look, & Coccaro, 2014). In addition, a sample that includes IED and non-IED individuals provides a full and normal distribution of trait aggression, which would not be captured in the general population or in other diagnostic groups (e.g., depressed or anxious patients), allowing for a dimensional assessment of study hypotheses. As our initial aim, we sought to establish a positive association between individual differences in IU and trait aggression in a diverse sample. Second, we tested whether IU is associated with trait aggression through activation of the PFC during social exclusion using the Cyberball paradigm during fMRI. Given that the IU construct can be parsed into two sub-factors, we notably probed relationship with inhibitory-IU and prospective-IU separately.
Method
Participants and Procedure
Participants were drawn from a larger study on the correlates and treatment of IED. To be included as a patient, participants were required to have a current full-threshold Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition (DSM-IV) diagnosis of IED. Controls or non-patients had no lifetime Axis I disorders. Exclusionary criteria for both groups included an inability to provide consent and read and write in English, a lifetime history of psychosis or bipolar disorder, and current (past month) depression, substance dependence or psychotropic medication use. This study was approved by the Temple University Institutional Review Board, and informed consent was obtained from all participants.
All data analyzed in the current study was taken from the baseline assessment. A total of 53 participants (27 controls; 26 patients) had no missing self-report data and good quality fMRI data, defined as adequate BOLD signal coverage of the whole brain and <3mm displacement in any one direction across the scan. Demographics and clinical characteristics of the entire sample are presented in Table 1.
Table 1.
Demographics and clinical characteristics of the sample.
| Mean (SD) or % (n = 53) |
|
|---|---|
| Demographics | |
| Age (years) | 34.0 (10.9) |
| Sex (male) | 52.8% |
| Hispanic Ethnicity | 9.4% |
| Race | |
| Caucasian | 39.6% |
| African American | 39.6% |
| Asian | 17.0% |
| ‘Other’ | 3.8% |
| Study Variables | |
| Inhibitory IU | 8.4 (3.7) |
| Prospective IU | 19.5 (6.2) |
| Total IU | 27.9 (8.8) |
| LHA Total Score | 10.4 (9.8) |
| Current Diagnoses | |
| Mood Disorder | 0.0% |
| Anxiety Disorder | 1.9% |
| Alcohol Use Disorder | 1.9% |
| Personality Disorder other than IED | 20.8% |
Note. IU = intolerance of uncertainty; LHA = Life History of Aggression.
During their initial visit, participants completed a battery of questionnaires as well as a 3-hour diagnostic interview conducted by trained graduate-level diagnosticians who were blind to study hypotheses. Interview measures included the Structured Clinical Interview for the DSM-IV (First, Gibbon, Spitzer, & Williams, 1996), the Structured Interview for Disorders of Personality (Pfohl, Blum, & Zimmerman, 1995), the Life History of Aggression (Coccaro, Berman, & Kavoussi, 1997), and the IED-Interview (McCloskey & Coccaro, 2003). Diagnoses were confirmed using a best estimate procedure (Klein, Ouimette, Kelly, Ferro, & Riso, 1994), where a written diagnostic report for each participant is presented and reviewed by a team of diagnosticians, and is supervised by a licensed clinical psychologist. Participants who met study eligibility were scheduled for their fMRI session. Upon arrival to the laboratory for their fMRI visit, all participants provided negative drug and alcohol screens. Participants then completed an MRI safety screening form. Prior to the scanning portion of the experiment, participants were familiarized with the MRI environment and experimental protocol. Participants were then taken to the imaging center, where they completed the fMRI paradigm, including the Cyberball task. After the scan, subjects were debriefed and compensated for their participation.
Intolerance of Uncertainty
Participants completed the widely used Intolerance of Uncertainty Scale (IUS; Freeston, Rhéaume, Letarte, Dugas, & Ladouceur, 1994) to assess the degree to which individuals find uncertainty to be distressing, frustrating, and undesirable. Items are rated on a five-point Likert scale ranging from 1 (not at all characteristic of me) to 5 (entirely characteristic of me). The present study utilized the 12-item version of the IUS (Carleton et al., 2007), which consists of items that are not specific to any psychiatric disorder and has better psychometric properties than the original 27-item version. The 12-item IUS produces a total score, as well as the two factor-analytically derived subscales: a 7-item prospective-IU scale measuring fear and anxiety in response to future events, and a 5-item inhibitory-IU scale measuring the propensity for uncertainty to inhibit one's actions. Both the IUS subscales, and the total score, were normally distributed across participants.
Aggression
To measure lifetime history of aggressive behaviors, participants completed the Life History of Aggression measure (LHA; Coccaro et al., 1997). The LHA assesses the frequency of 11 different aggressive and antisocial behaviors since adolescence and each behavior is rated on a 5-point scale based on the number of times of occurrence. In addition to a total score, three subscales can be derived: aggression, antisocial behavior, and self-directed aggression. Behaviors that are assessed by the LHA include physical fights, verbal aggression, disciplinary problems, and self-injurious behavior and suicide attempts. In the current study, the LHA was administered as a clinician-rated instrument and LHA total scores were normally distributed across participants.
Experimental Task
Cyberball is a virtual ball-tossing game developed for the study of interpersonal rejection and exclusion (Williams et al., 2000; Williams & Jarvis, 2006). For the purposes of this study, the task was adapted for fMRI to be presented on a computer screen and viewed through MRI compatible goggles during the scan. The task was 7 minutes in duration and included four trials of one minute each. At the start of the task, participants were told that they were playing a game with two other participants whom they had not met previously. During each trial, they saw a cartoon of the two other virtual players in the upper part of the screen, labeled “Participant 1” and “Participant 3”. In the lower-center portion of the screen was a cartoon of the main participant, labeled “You.” Unbeknownst to the participant the actions of the virtual players were fixed and controlled by the computer program. A virtual player started the game by throwing the ball to another player, and the order in which the ball was tossed was predetermined except for the participant's own choices. The participant was able to toss the ball to either of the players by pressing one of two keys on a button box. The virtual players waited between 0.5 and 3 seconds to throw the ball to enhance deception. The first two sequential trials were inclusion blocks, during which the subject received the ball one third of the time. The remaining two blocks were exclusion blocks, in which the subject received the ball twice within the first 20 seconds and was excluded thereafter (i.e., the virtual players tossed the ball exclusively amongst themselves). The order of the inclusion/exclusion blocks was always inclusion first, in order to avoid influences of exclusion on inclusion blocks. A 30-second fixation cross was presented in between blocks. Each trial was also preceded by a 5-second screen stating that a network connection was being established, to further enhance the deception.
After scanning, a manipulation check questionnaire was administered to assess the degree to which the Cyberball games elicited feelings of social rejection. This questionnaire has been used in previous experimental paradigms involving the Cyberball task (e.g., Eisenberger et al., 2003) and includes questions on self-esteem (“I felt liked.”), belongingness (“I felt rejected.”), meaningfulness (“I felt invisible.”), and control (“I felt powerful.”) Participants were asked how much they agreed with each statement, on a scale from 1 (not at all) to 9 (very much).
fMRI Data Acquisition and Preprocessing
Neuroimaging was performed on a 3-Tesla Verio Siemens system at the Temple University MRI Center (TUMRIC) using a 12-channel phased array head coil. A high-resolution T1-weighted magnetization-prepared rapid-acquisition gradient echo (MPRAGE) image was acquired in the sagittal plane and provided high-resolution structural images for co-registration of functional images and inter-subject normalization. Each functional scan of the task included 210 acquisitions collected with a whole-brain T2*-weighted gradient echoplanar imaging (EPI) sequence in 30 contiguous oblique axial slices approximately parallel to the AC-PC line acquired in interleaved order (TR=2000ms, TE=30ms, flip=90°, 5mm slice thickness with no gap, FOV 220 mm, 3.44 mm × 3.44 mm in-plane resolution).
Images were processed using SPM12 (Wellcome Trust Centre for Neuroimaging). The first four volumes were discarded to allow for T1 equilibration effects. Standard preprocessing of functional images included slice-time correction, spatial realignment to correct for head motion, coregistration to the participant's T1 image and warping to MNI space, resampling to 2mm/side voxels and smoothing with an 8mm FWHM isotropic Gaussian kernel. The general linear model was applied to the time series, convolved with the canonical hemodynamic response function and included a 128s high-pass filter. Inclusion (or fair-play) and exclusion conditions were modeled using block-related regressors. Nuisance covariates representing each of the 6 estimated motion time series provided during the motion correction were also included. Effects were estimated at each voxel and for each subject. Individual participant contrast maps for exclusion > inclusion were created, consistent with prior Cyberball studies (e.g., Masten et al., 2009, 2011).
Data Analysis Plan
We first examined whether the Cyberball task elicited the expected feelings of social exclusion using the manipulation check post-scan questionnaire. Specifically, we conducted a series of paired-sample t-tests for the six subjective states (feeling liked, rejected, invisible, powerful, included, and excluded) assessed during the inclusion and exclusion blocks of the fMRI task. Four participants were missing self-report ratings due to technical errors and were therefore excluded from these analyses.
With regard to our hypotheses, as part of aim one, we first assessed whether there were significant associations between individual differences in IU and lifetime aggression scores (model c path) using a series of partial Pearson's correlations, Bonferroni-corrected for multiple comparisons. Total IU, prospective-IU, and inhibitory-IU were examined separately to assess for potential differential relationships. For the IU subscales (i.e., prospective- and inhibitory-IU), the impact of one subscale was tested while covering for the other, in-order to determine unique associations with aggression. Age and biological sex were also included as covariates in the partial correlations, as well as all subsequent analyses, given the potential impact of these factors on study measures (e.g., Archer, 2004; Filkowski, Olsen, Duda, Wanger, & Sabatinelli, 2016; Silvers et al., 2016). For aim two, we then tested whether there was an association between IU and PFC activation during social exclusion during the Cyberball task (model b path). As noted above, individual contrast maps of social exclusion > fair play during Cyberball were created for each subject. Contrast maps were then entered into a second-level one sample t-test in SPM whereby prospective-IU and inhibitory-IU were simultaneously included as separate regressors, along with the covariates age and sex. The effect of each IU subscale on neural responses to social exclusion, while controlling for the other IU subscale, can therefore be estimated in addition to the average effect of both subscales (total IU). Of note, several recent studies have suggested that the two IU subscales should be entered into statistical models simultaneously to prevent potential mutual suppressor effects in which two correlated predictor variables have the opposite effect on the criterion/outcome variable, causing the associations to be obscured when each subscale is examined separately (Gorka, Nelson, Phan, & Shankman, 2016c; Jackson, Nelson, & Hajcak, 2016; Watson, Clark, Chmielewski, & Kotov, 2013). To determine our fMRI significance threshold, we applied an anatomically-derived (AAL atlas) partial brain mask of the entire PFC to our data (search volume=451,840 mm3, encompassing 56,480 voxels). Cluster-based significance thresholding was used to adjust for multiple comparisons within the search volume. Results were restricted to the PFC given our strong a priori hypotheses and the well-established role of the PFC in responding to social exclusion (Eisenberger et al., 2003; Kawamoto et al., 2015). Based on simulations (10,000 iterations) performed with 3DClustSim, an adaptation of AlphaSim (10,000 iterations; updated and ‘bug-free’ on December 2015; [https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html]), a family wise error correction at α<0.05 was achieved for voxel threshold of p<0.005 with minimum cluster size of 228 contiguous voxels.
To test our mediation model for aim three, we next extracted parameter estimates from 10mm (radius) spheres surrounding peak activations identified in the model described above. A test of mediation was then conducted using PROCESS – an SPSS macro for path-analysis based modeling (Hayes, 2013). PROCESS provides bootstrapped bias-corrected 95% confidence intervals of the indirect effect using 1000 bootstrapped samples (Hayes, 2012). IU was specified as the independent variable, extracted neural parameter estimates as the mediator, and trait aggression scores as the dependent variable. Age and sex were included as covariates.
Results
Experimental Task Manipulation Check
Participants reported that they felt more liked (t[48] = 6.67, p < .01), powerful (t[48] = 4.17, p < .01), and included (t[48] = 8.14, p < .01) during the inclusion relative to exclusion task blocks. They also reported feeling more rejected (t[48] = -5.30, p < .01), invisible t[48] = -5.81, p < .01), and excluded (t[48] = -6.30, p < .01) during exclusion relative to inclusion task blocks. The task therefore elicited self-reported feelings of social exclusion as designed.
IU is associated with Aggression and Neural Response to Social Exclusion
Partial correlations indicated that greater LHA total scores were associated with greater total IU (r = .49, p < .001). While controlling for the other IU subscale, results also indicated that greater LHA total scores were associated with prospective-IU (r = .45, p < .001) but not inhibitory-IU (r = .05, ns; see Fig. 1). Results of the SPM regression analyses also revealed that individual differences in IU were associated with neural response during social exclusion. Specifically, greater prospective-IU was associated with less left vlPFC activation during social exclusion (MNI peak [-40, 32, -14], Z = 3.77, k = 1952 mm3, pcorrected < .05; Fig. 2). There were no significant associations between inhibitory-IU, or total IU, and neural activation.
Figure 1.

Scatter plots depicting the partial correlations between trait aggression and total IU (A), prospective-IU (B), and inhibitory-IU (C). All correlations are adjusted for age and biological sex. Correlations for each IU subscale are adjusted for the other IU subscale. IU = intolerance of uncertainty; LHA = Life History of Aggression.
Figure 2.
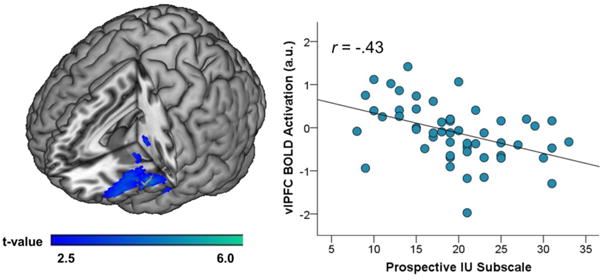
On the left, a voxel-wise statistical t-map on a canonical brain displaying significant associations between prospective-IU and neural activations during social exclusion vs. fair play. On the right, scatter plot which illustrates the association between left vlPFC activation and prospective-IU. IU = intolerance of uncertainty; vlPFC = ventrolateral prefrontal cortex.
Mediation Model
The results above revealed a specific relation between the prospective-IU subscale and left vlPFC activation during social exclusion and therefore, prospective-IU and left vlPFC were tested in the mediation model with LHA total scores. As expected, greater prospective-IU was associated with less vlPFC activation (a path: b = -0.05, SE=0.01, p < .01, 95% CI: -0.07 – -0.02). Less vlPFC activation was also associated with greater LHA total scores while controlling for prospective-IU (b path: b = -4.09, SE=1.81, p < .05, 95% CI: -7.75– -0.43). As expected given the results above, there was a direct, positive effect of prospective-IU on LHA total scores (c path: b = 0.58, SE=0.19, p < .01, 95% CI: 0.19 –0.97). Most importantly, the overall mediation model was significant and there was an indirect effect of prospective-IU on LHA total scores through vlPFC activation (c′ path: b = 0.18, SE=0.11, 95% CI: 0.03 – 0.46; Fig. 3).
Figure 3.
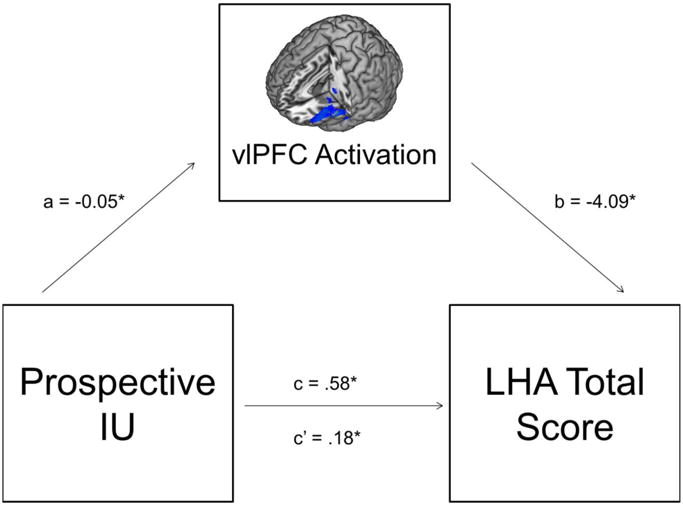
The proposed mediation model including path estimates. IU = intolerance of uncertainty; vlPFC = ventrolateral prefrontal cortex; LHA = Life History of Aggression.
Discussion
Findings from the current study provide several novel contributions that advance theory and research on the mechanisms implicated in trait aggression. In a sample of individuals with a full range of impulsive-aggressive tendencies and traits, we first found that greater levels of total IU and prospective-IU, but not inhibitory-IU, were associated with greater trait aggression. This suggests that there is a link between IU (particularly prospective-IU) and aggression, as hypothesized. Our findings also revealed that during social exclusion, relative to fair play, greater levels of prospective-IU (only) were associated with less vlPFC activation. Lastly, decreased vlPFC activity during social exclusion mediated the association between prospective-IU and trait aggression, which supports our overall proposed model. The findings together provide initial evidence to suggest that individuals with high IU may have difficulties regulating their affect, including social distress, which in-turn may increase the propensity for aggression. The current study is the first to provide direct evidence of an association between prospective-and total-IU and trait aggression in a clinically representative sample. As briefly discussed, IU is a broad construct that reflects the belief that uncertainty is unacceptable and intolerable (Carleton, 2012, 2016; Shihata, McEvoy, Mullan, & Carleton, 2016). Individuals high in IU are known to display exaggerated threat reactivity (e.g., Jackson et al., 2016; Nelson, Liu, Sarapas, & Shankman, 2016), and maladaptive coping responses during times of distress and uncertainty (Buhr & Dugas, 2006). It is therefore possible that individuals higher in IU (relative to lower in IU) are more vigilant of potential threats during social situations and experience difficulty regulating IU-related distress, leading to increased anger and the potential for aggression. For instance, during a hostile interpersonal exchange, individuals high in IU may find others' words and actions excessively threatening and in-turn experience high levels of unregulated anger and the tendency to display other- and/or self-directed hostility and aggression. Notably, the link between IU and trait aggression was specific to prospective-IU – the facet of IU capturing maladaptive cognitive processes such as subjective distress and worry (Carleton, 2007; McEvoy and Mahoney, 2011). This IU distinction importantly fits, theoretically, as inhibitory-IU reflects behavioral inhibition of action, which is antithetical to aggressive behavior. The present findings also indicated that greater levels of prospective-IU were associated with less vlPFC activation during exclusion from a virtual ball tossing game – an aversive social situation. Given that the vlPFC has been identified as a key region implicated in the regulation of negative affect and pain, particularly during the Cyberball paradigm (Eisenberger et al., 2003), this finding suggests that individuals higher in IU may have more difficulties regulating situational distress and exerting top-down inhibitory control over affective and behavioral processes. This finding also indicates that individual differences in IU may be linked to broader emotion regulation deficits, beyond just social pain, which could account for why IU is a transdiagnostic construct, related to multiple forms of psychopathology and maladaptive behavior. To date, high IU has been implicated in anxiety (Carleton et al., 2012), depression (Gentes & Ruscio, 2011), eating disorders (Frank et al., 2012), autism (Boulter, Freeston, South, & Rodgers, 2014), substance abuse (Gorka et al., 2016b), and now aggression, which all share dysfunction in affect regulation. Decreased engagement of the vlPFC could be one common underlying mechanism shared across each of these syndromes.
It is important to highlight that we did not initially hypothesize that prospective-IU and inhibitory-IU would have divergent associations with neural reactivity; although as mentioned above, the two factors do reflect different manifestations of IU and have been shown to have different associations with other affective psychophysiological indices (Gorka et al., 2016c; Jackson et al., 2016). It is important to highlight that the Cyberball fMRI paradigm does not tap into behavioral responding or afford the opportunity for avoidance, which may have contributed to the lack of associations between inhibitory-IU and neural reactivity. Prospective-IU, on the other hand, is likely to be probed during Cyberball as social exclusion inherently elicits cognitive and affective reactions. From a conceptual standpoint, high prospective-IU is also more likely to map onto vlPFC activation given that worry and intense emotional reactions can be reflections of poor or insufficient emotion regulation.
In addition to individual pathways linking IU, aggression, and vlPFC activation, our results notably demonstrated that vlPFC reactivity during social exclusion mediated the association between high IU and trait aggression. This suggests that vlPFC activity is one potential mechanism through which IU influences aggression. Support for the proposed model importantly coincides with recent tDCS studies which have demonstrated that direct stimulation of the vlPFC can reduce both distress and aggressive behaviors (Riva et al., 2012, 2014, 2015). Specifically, Riva et al. (2014) applied tDCS or sham stimulation over the right vlPFC while participants were socially excluded or included during the Cyberball task and afterwards, provided the opportunity to aggress against the fellow task ‘players’ by objecting them to drink a participant-determined amount of hot sauce. Excluded participants who received tDCS displayed less behavioral aggression relative to individuals who received sham stimulation. The findings from the Riva et al. studies, as well as the current findings, together suggest that the vlPFC could be an important neural treatment target for the development of strategies aimed at preventing and/or attenuating aggression. Direct stimulation of the vlPFC is one promising novel treatment approach; however, it is also important to consider whether other cognitive-behavioral and/or pharmacological approaches can similarly enhance vlPFC functioning in high-risk situations.
It is worth noting that the current fMRI findings were specific to the left vlPFC whereas past research, particularly the above tDCS studies (e.g., Riva et al., 2014), have implicated the right vlPFC. Given these potentially conflicting findings, and the overall paucity of studies examining neural mechanisms implicated in aggression, it is still unclear whether vlPFC hypoactiviation of both, or just one, hemisphere influences trait aggression; though more broadly, both left and right vlPFC have been shown to be involved in the successful regulation of negative emotions (e.g., Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008).
The current study had numerous strengths and addressed several important gaps in the existing aggression literature. There were also several limitations. First, the study was cross-sectional and individual differences in IU, vlPFC activation, and trait aggression were all measured close in time. Despite evidence of mediation, causal relationships amongst study variables cannot be inferred. Second, as was briefly noted above, the Cyberball task may not have been best-suited for tapping into individual differences in inhibitory-IU and future studies using more dynamic ambiguous contexts and behavioral paradigms may yield novel results. Third, the current study focused on a trait measure of aggression (i.e., LHA scores). Although aggression has been conceptualized as a stable individual difference factor (Huesmann, Eron, Lefkowitz, & Walder, 1984) that is observed across a variety of contexts, the proposed links between vlPFC hypoactivation and impulsive aggression are situational in nature. Therefore, future studies should further test the current model using state measures of aggressiveness following social exclusion (e.g., the hot sauce paradigm used by Riva et al. [2014]). Lastly, the study enrolled IED patients and healthy controls. Although this resulted in a sample with a normal distribution of IU and impulsive-aggressive traits, it is possible that the present findings do not generalize to other populations (e.g., other psychiatric groups, unselected community samples). In sum, the present study demonstrated that higher levels of IU are associated with more trait aggression, and this association is mediated by decreased vlPFC engagement during social exclusion. The findings converge with existing research suggesting that high IU is an important, transdiagnostic clinical construct that can manifest as numerous maladapative conditions and behaviors (Carleton, 2016), and extends this work for the first time to trait aggression. The findings also highlight the important mechanistic role of the vlPFC in both IU and trait aggression and indicate that enhancing vlPFC functioning within high-risk populations may be a valuable novel treatment target.
Acknowledgments
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R01MH086525 (PI: McCloskey). Dr. Stephanie Gorka was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number K23AA025111 (PI: Gorka). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Authorship: M.S.M was the study principal investigator and therefore developed the concept and design. E.Y.C. was also involved in the study design and project management. S.M.G and B.H. performed the data analyses in collaboration with K.L.P. All authors assisted in data interpretation. S.M.G. drafted the paper and M.S.M., K.L.P, E.Y.C. and B.H. provided critical revisions. All authors approved the final version of the paper for submission.
All authors declare no conflicts of interest.
References
- Anderson KG, Deschênes SS, Dugas MJ. Experimental manipulation of avoidable feelings of uncertainty: Effects on anger and anxiety. Journal of Anxiety Disorders. 2016;41:50–58. doi: 10.1016/j.janxdis.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Archer J. Sex differences in aggression in real-world settings: a meta-analytic review. Review of General Psychology. 2004;8(4):291–322. [Google Scholar]
- Barlow DH, Sauer-Zavala S, Carl JR, Bullis JR, Ellard KK. The Nature, Diagnosis, and Treatment of Neuroticism: Back to the Future. Clinical Psychological Science. 2014;2:344–365. [Google Scholar]
- Berkowitz L. Aggression: Its causes, consequences, and control. Philadelphia, PA: Temple University Press; 1993. [Google Scholar]
- Birrell J, Meares K, Wilkinson A, Freeston M. Toward a definition of intolerance of uncertainty: A review of factor analytical studies of the Intolerance of Uncertainty Scale. Clinical Psychology Review. 2011;31(7):1198–1208. doi: 10.1016/j.cpr.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Boelen PA, Reijntjes A. Intolerance of uncertainty and social anxiety. Journal of Anxiety Disorders. 2009;23(1):130–135. doi: 10.1016/j.janxdis.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Boulter C, Freeston M, South M, Rodgers J. Intolerance of uncertainty as a framework for understanding anxiety in children and adolescents with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2014;44(6):1391–1402. doi: 10.1007/s10803-013-2001-x. [DOI] [PubMed] [Google Scholar]
- Buhr K, Dugas MJ. Investigating the construct validity of intolerance of uncertainty and its unique relationship with worry. Journal of Anxiety Disorders. 2006;20(2):222–236. doi: 10.1016/j.janxdis.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Carleton RN. The intolerance of uncertainty construct in the context of anxiety disorders: theoretical and practical perspectives. Expert Review of Neurotherapeutics. 2012;12(8):937–947. doi: 10.1586/ern.12.82. [DOI] [PubMed] [Google Scholar]
- Carleton RN. Into the unknown: a review and synthesis of contemporary models involving uncertainty. Journal of Anxiety Disorders. 2016;39:30–43. doi: 10.1016/j.janxdis.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Carleton RN, Duranceau S, Freeston MH, Boelen PA, McCabe RE, Antony MM. “But it might be a heart attack”: Intolerance of uncertainty and panic disorder symptoms. Journal of Anxiety Disorders. 2014;28(5):463–470. doi: 10.1016/j.janxdis.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Carleton RN, Mulvogue MK, Thibodeau MA, McCabe RE, Antony MM, Asmundson GJ. Increasingly certain about uncertainty: Intolerance of uncertainty across anxiety and depression. Journal of Anxiety Disorders. 2012;26(3):468–479. doi: 10.1016/j.janxdis.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Carleton RN, Norton MPJ, Asmundson GJ. Fearing the unknown: A short version of the Intolerance of Uncertainty Scale. Journal of Anxiety Disorders. 2007;21(1):105–117. doi: 10.1016/j.janxdis.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Carleton RN, Sharpe D, Asmundson GJ. Anxiety sensitivity and intolerance of uncertainty: requisites of the fundamental fears? Behaviour Research and Therapy. 2007;45(10):2307–2316. doi: 10.1016/j.brat.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Coccaro EF. Intermittent explosive disorder as a disorder of impulsive aggression for DSM-5. American Journal of Psychiatry. 2012;169(6):577–588. doi: 10.1176/appi.ajp.2012.11081259. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Berman ME, Kavoussi RJ. Assessment of life history of aggression: development and psychometric characteristics. Psychiatry Research. 1997;73(3):147–157. doi: 10.1016/s0165-1781(97)00119-4. [DOI] [PubMed] [Google Scholar]
- Dodge KA. The structure and function of reactive and proactive aggression. In: Pepler DJ, Rubin KH, editors. The development and treatment of childhood aggression. Hillsdale, NJ: Lawrence Erlbaum Associates; 1991. pp. 201–218. [Google Scholar]
- Dugas MJ, Robichaud M. Cognitive-behavioral treatment for generalized anxiety disorder: From science to practice. Taylor & Francis; 2007. [Google Scholar]
- Dugas MJ, Freeston MH, Ladouceur R. Intolerance of uncertainty and problem orientation in worry. Cognitive Therapy and Research. 1997;21(6):593–606. [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35(4):1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filkowski MM, Olsen RM, Duda B, Wanger TJ, Sabatinelli D. Sex differences in emotional perception: Meta-analysis of divergent activation. NeuroImage. 2016;147:925–933. doi: 10.1016/j.neuroimage.2016.12.016. [DOI] [PubMed] [Google Scholar]
- Fettich KC, McCloskey MS, Look AE, Coccaro EF. Emotion regulation deficits in intermittent explosive disorder. Aggressive behavior. 2015;41(1):25–33. doi: 10.1002/ab.21566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JB. User's Guide for the SCID-I (Research Version) New York: Biometrics Research; 1996. [Google Scholar]
- Fracalanza K, Koerner N, Deschênes SS, Dugas MJ. Intolerance of uncertainty mediates the relation between generalized anxiety disorder symptoms and anger. Cognitive Behaviour Therapy. 2014;43(2):122–132. doi: 10.1080/16506073.2014.888754. [DOI] [PubMed] [Google Scholar]
- Frank GK, Roblek T, Shott ME, Jappe LM, Rollin MD, Hagman JO, Pryor T. Heightened fear of uncertainty in anorexia and bulimia nervosa. International Journal of Eating Disorders. 2012;45(2):227–232. doi: 10.1002/eat.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeston MH, Rhéaume J, Letarte H, Dugas MJ, Ladouceur R. Why do people worry? Personality and Individual Differences. 1994;17:791–802. [Google Scholar]
- Gaertner L, Iuzzini J, O'Mara EM. When rejection by one fosters aggression against many: Multiple-victim aggression as a consequence of social rejection and perceived groupness. Journal of Experimental Social Psychology. 2008;44(4):958–970. doi: 10.1016/j.jesp.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentes EL, Ruscio AM. A meta-analysis of the relation of intolerance of uncertainty to symptoms of generalized anxiety disorder, major depressive disorder, and obsessive–compulsive disorder. Clinical Psychology Review. 2011;31(6):923–933. doi: 10.1016/j.cpr.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Gorka SM, Hee D, Lieberman L, Mittal VA, Phan KL, Shankman SA. Reactivity to uncertain threat as a familial vulnerability factor for alcohol use disorder. Psychological Medicine. 2016a;46(16):3349–3358. doi: 10.1017/S0033291716002415. [DOI] [PubMed] [Google Scholar]
- Gorka SM, Lieberman L, Phan KL, Shankman SA. Association between problematic alcohol use and reactivity to uncertain threat in two independent samples. Drug and Alcohol Dependence. 2016b;164:89–96. doi: 10.1016/j.drugalcdep.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Nelson BD, Phan KL, Shankman SA. Intolerance of uncertainty and insula activation during uncertain reward. Cognitive, Affective, & Behavioral Neuroscience. 2016c;16(5):929–939. doi: 10.3758/s13415-016-0443-2. [DOI] [PubMed] [Google Scholar]
- Greco V, Roger D. Uncertainty, stress, and health. Personality and Individual Differences. 2003;34(6):1057–1068. [Google Scholar]
- Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nature Reviews Neuroscience. 2013;14(7):488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. PROCESS SPSS Macro Computer software and manual 2013 [Google Scholar]
- Hong RY, Lee SS. Further clarifying prospective and inhibitory intolerance of uncertainty: Factorial and construct validity of test scores from the Intolerance of Uncertainty Scale. Psychological Assessment. 2015;27(2):605–620. doi: 10.1037/pas0000074. [DOI] [PubMed] [Google Scholar]
- Huesmann LR, Dubow EF, Boxer P. Continuity of aggression from childhood to early adulthood as a predictor of life outcomes: Implications for the adolescent-limited and life-course-persistent models. Aggressive Behavior. 2009;35(2):136–149. doi: 10.1002/ab.20300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huesmann LR, Eron LD, Lefkowitz MM, Walder LO. Stability of aggression over time and generations. Developmental Psychology. 1984;20(6):1120–1134. [Google Scholar]
- Jackson F, Nelson BD, Hajcak G. The uncertainty of errors: intolerance of uncertainty is associated with error-related brain activity. Biological Psychology. 2016;113:52–58. doi: 10.1016/j.biopsycho.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Jacoby RJ, Fabricant LE, Leonard RC, Riemann BC, Abramowitz JS. Just to be certain: Confirming the factor structure of the Intolerance of Uncertainty Scale in patients with obsessive-compulsive disorder. Journal of Anxiety Disorders. 2013;27(5):535–542. doi: 10.1016/j.janxdis.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Ura M, Nittono H. Intrapersonal and interpersonal processes of social exclusion. Frontiers in Neuroscience. 2015;9:62. doi: 10.3389/fnins.2015.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DN, Ouimette PC, Kelly HS, Ferro T, Riso LP. Test-retest reliability of team consensus best-estimate diagnoses of axis I and II disorders in a family study. American Journal of Psychiatry. 1994;151:1043–1047. doi: 10.1176/ajp.151.7.1043. [DOI] [PubMed] [Google Scholar]
- Koerner N, Dugas MJ. An investigation of appraisals in individuals vulnerable to excessive worry: The role of intolerance of uncertainty. Cognitive Therapy and Research. 2008;32(5):619–638. [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4(2):143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Morelli SA, Eisenberger NI. An fMRI investigation of empathy for ‘social pain’and subsequent prosocial behavior. Neuroimage. 2011;55(1):381–388. doi: 10.1016/j.neuroimage.2010.11.060. [DOI] [PubMed] [Google Scholar]
- McCloskey MS, Ammerman BA. Suicidal behavior and aggression-related disorders. Current Opinion in Psychology. 2017;22:54–58. doi: 10.1016/j.copsyc.2017.08.010. [DOI] [PubMed] [Google Scholar]
- McCloskey MS, Coccaro EF. Questionnaire and Interview Measures of Aggression in Adults. In: Coccaro EF, editor. Aggression : psychiatric assessment and treatment. New York: Marcel Dekker; 2003. pp. 167–193. [Google Scholar]
- McCloskey MS, Kleabir K, Berman ME, Chen EY, Coccaro EF. Unhealthy aggression: intermittent explosive disorder and adverse physical health outcomes. Health Psychology. 2010;29(3):324–332. doi: 10.1037/a0019072. [DOI] [PubMed] [Google Scholar]
- McEvoy PM, Mahoney AE. Achieving certainty about the structure of intolerance of uncertainty in a treatment-seeking sample with anxiety and depression. Journal of Anxiety Disorders. 2011;25(1):112–122. doi: 10.1016/j.janxdis.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Harrington H, Milne BJ. Males on the life-course-persistent and adolescence-limited antisocial pathways: Follow-up at age 26 years. Development and Psychopathology. 2002;14(1):179–207. doi: 10.1017/s0954579402001104. [DOI] [PubMed] [Google Scholar]
- Nelson BD, Liu H, Sarapas C, Shankman SA. Intolerance of uncertainty mediates the relationship between panic and the startle reflex in anticipation of unpredictable threat. Journal of Experimental Psychopathology. 2016;7(2):172–189. [Google Scholar]
- Olweus D. Stability of aggressive reaction patterns in males: A review. Psychological Bulletin. 1979;86(4):852–875. [PubMed] [Google Scholar]
- Pfohl B, Blum N, Zimmerman M. Structured Clinical Interview for DSM-IV Personality. Iowa City: University of Iowa College of Medicine; 1995. [Google Scholar]
- Riva P, Romero Lauro LJ, DeWall CN, Bushman BJ. Buffer the pain away: stimulating the right ventrolateral prefrontal cortex reduces pain following social exclusion. Psychological Science. 2012;23(12):1473–1475. doi: 10.1177/0956797612450894. [DOI] [PubMed] [Google Scholar]
- Riva P, Romero Lauro LJ, DeWall CN, Chester DS, Bushman BJ. Reducing aggressive responses to social exclusion using transcranial direct current stimulation. Social Cognitive and Affective Neuroscience. 2014;10(3):352–356. doi: 10.1093/scan/nsu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva P, Romero Lauro LJ, Vergallito A, DeWall CN, Bushman BJ. Electrified emotions: Modulatory effects of transcranial direct stimulation on negative emotional reactions to social exclusion. Social Neuroscience. 2015;10(1):46–54. doi: 10.1080/17470919.2014.946621. [DOI] [PubMed] [Google Scholar]
- Shihata S, McEvoy PM, Mullan BA, Carleton RN. Intolerance of uncertainty in emotional disorders: What uncertainties remain? Journal of Anxiety Disorders. 2016;41:115–124. doi: 10.1016/j.janxdis.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin RE, Weber J, Mischel W, Casey BJ, Ochsner KN. vlPFC–vmPFC–amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cerebral Cortex. 2016:3502–3514. doi: 10.1093/cercor/bhw073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twenge JM, Baumeister RF, Tice DM, Stucke TS. If you can't join them, beat them: effects of social exclusion on aggressive behavior. Journal of Personality and Social Psychology. 2001;81(6):1058–1069. doi: 10.1037//0022-3514.81.6.1058. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N, Cheng TW, Pfeifer JH. Neural correlates of social exclusion across ages: A coordinate-based meta-analysis of functional MRI studies. NeuroImage. 2017 doi: 10.1016/j.neuroimage.2017.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Chmielewski M, Kotov R. The value of suppressor effects in explicating the construct validity of symptom measures. Psychological Assessment. 2013;25:929–941. doi: 10.1037/a0032781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KD. Ostracism. Annual Review of Psychology. 2007;58:425–452. doi: 10.1146/annurev.psych.58.110405.085641. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KD, Cheung CK, Choi W. Cyberostracism: effects of being ignored over the Internet. Journal of Personality and Social Psychology. 2000;79(5):748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Williams KD, Jarvis B. Cyberball: A program for use in research on interpersonal ostracism and acceptance. Behavior Research Methods. 2006;38(1):174–180. doi: 10.3758/bf03192765. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Global Status Report on violence prevention. Geneva, Switzerland: Inis Communication; 2014. [Google Scholar]
- Yook K, Kim KH, Suh SY, Lee KS. Intolerance of uncertainty, worry, and rumination in major depressive disorder and generalized anxiety disorder. Journal of Anxiety Disorders. 2010;24(6):623–628. doi: 10.1016/j.janxdis.2010.04.003. [DOI] [PubMed] [Google Scholar]


