Abstract
Inequity aversion (negative feelings induced by outcome differences between the self and other) plays a key role in human social behaviors. The neurotransmitters oxytocin and GABA have been implicated in neural responses to inequity. However, it remains poorly understood not only how individual genetic factors related to oxytocin and GABA affect the neural mechanisms behind inequity aversion, but also how these genes interact. To address these issues, we examined relationships between genotypes, behavioral decisions and brain activities during the ultimatum game. We identified interactive effects between the polymorphisms of the oxytocin receptor gene (OXTR) and glutamate decarboxylase 1 gene for GABA synthesis (GAD1) on envy aversion (i.e., disadvantageous inequity aversion) and on envy-induced activity in the dorsal ACC (dACC). Thus, our integrated approach suggested interactive genetic effects between OXTR and GAD1 on envy aversion and the underlying neural substrates.
Introduction
Inequity aversion plays a key role in human social behaviors, such as cooperation and donation. Using economic games, functional magnetic resonance imaging (fMRI) studies have established that many brain regions, including the anterior cingulate cortex (ACC) [1–4], medial prefrontal cortex (mPFC) [1,5,6], dorsolateral prefrontal cortex (dlPFC) [1,3,7–9], insula [1,9], amygdala [2,10–11] and striatum [6–7,11], are involved in inequity aversion.
Over the last decade, the neuromodulator oxytocin has gained attention as an influencer on human social behaviors [12–24] and human emotional brain networks [25]. Oxytocin is a peptide hormone and neuropeptide produced in the hypothalamus. The axons of hypothalamic oxytocin neurons project to several regions associated with inequity aversion, including the amygdala, hippocampus, ACC and mPFC [26]. Several reports have investigated the effect of oxytocin on prosocial behaviors related to inequity aversion [27]. The administration of oxytocin was found to alter the subjective evaluation of unfairness [28] and money allocation with others in economic games such as the trust game and ultimatum game [13,17,24,29]. It is also reported that intranasal oxytocin increases envy (i.e., disadvantageous inequity) aversion and guilt (i.e., advantageous inequity) aversion [30]. In addition, relationships between the polymorphisms of the oxytocin receptor gene (OXTR) and social behaviors have been reported, including correlations of OXTR polymorphisms with trust and altruism [31] and with mental disorders such as autism [32–35]. However, the effects of OXTR polymorphisms on behaviors and brain activities associated with inequity aversion remain poorly understood [13,36].
GABA is the primary inhibitory neurotransmitter in the central nervous system and is also important for inequity aversion. One study using the ultimatum game found the administration of benzodiazepine, which increases the efficacy of GABA at the GABA A receptor, reduces the rejection ratio and activity in the amygdala, dACC and mPFC in response to unfair offers [2]. Like OXTR, polymorphisms of the genes coding the subunits of the GABA A receptor were reported to be correlated with altruism [37] and autism [38]. It was also shown that a polymorphism on the promoter region of the enzyme for GABA synthesis modulates ACC activity in humans [39]. On the other hand, many GWAS (Genome Wide Association Studies) have shown that the influence of each single nucleotide polymorphism is small and that most reported genetic associations could be false positives [40–41]. However, some GWAS indicated that there is an association between social traits and genetic variants [42]. In particular, Linnér and colleagues [43] suggested that the genes involved in GABAergic neurotransmission influence personality traits.
In addition, the influence of the GABA A receptor on oxytocin was recently reported in rodents. Blockage of the receptor suppressed the effects of oxytocin on freezing behavior as well as amygdala activity in fear conditioning [26,44]. Sabihi and colleagues [45] showed that the administration of oxytocin to the mPFC was accompanied by increased activation of GABA neurons through the GABA A receptor in the mPFC and altered neuronal activation of the amygdala following the anxiety test [45].
Based on these previous studies, we hypothesized that GABA may also interact with the function of oxytocin in inequity aversion. To address this issue, we conducted a model-based fMRI study of the ultimatum game, a widely-used task in inequity-aversion literature, and quantified the effects of single nucleotide polymorphisms (SNPs) and interactions of oxytocin receptor gene (OXTR) and GABA-related genes on human behaviors and brain activities in inequity aversion. We focused on the genes for OXTR, GABAA receptor gene clusters (GABA A receptor subunit clusters on chromosomes 5q34-q35, 4p12, 6q14-16 and 15q11-q13), and enzymes for GABA synthesis. In addition, for our analysis, we conducted the Triple-Dominance measure task, which measures a participant’s egalitarianism in resource allocation [46].
Materials and methods
Participants
The ethical committees of the National Institute of Information and Communication Technology (NICT), Japan, Tamagawa University, Japan, and University of Tokyo approved this study, and written informed consent to the behavioral, saliva collection and MRI experiments was obtained from all participants before the experiments were done. The individual in this manuscript has given written informed consent to publish the face image (PLOS consent form).
Two hundred and fourteen Japanese students (111 males, age = 19.5±0.12; 103 females, age = 19.6±0.12) who did not declare any history of neurological or psychiatric disorders participated in the first saliva sample collection for the SNP analysis and the Triple-Dominance measure task to identify their social value orientation (i.e., prosocial, individualistic or competitor). All participants were invited to the fMRI experiments. Adjusting for the availability of the participants and MRI scanning slots, 97 participants (56 males, age = 19.3±0.17; 41 females, age = 19.4±0.22) took part in the fMRI experiments.
Tasks
Triple-Dominance measure task: Day1
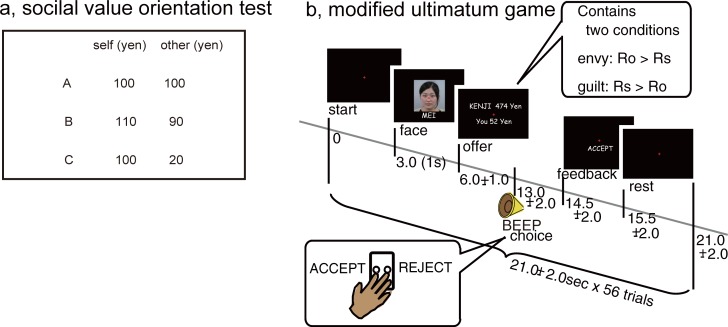
The Triple-Dominance measure task is a forced three-choice form of money distribution between the self and an unknown other, and has been used to identify a participant’s social value orientation [10–11,46]. Thirteen to forty participants in a room received a sheet of paper on which two numbers were written. One number represented the identity of the participant and the other number the identity of the other participant who was randomly paired with the participant. Participants were presented with 8 Triple-Dominance measure tasks, which asked them to choose the most preferable money distribution for the self and the other from three options within 10 s (Fig 1A). In this particular example, one option (A; prosocial) maximizes the sum of outcomes for the self and other and minimizes the difference of outcomes. It is therefore associated with inequity aversion. A second option (B; individualistic) maximizes the outcome for the self. The third option (C; competitive) maximizes the difference between the outcomes for the self and the other. A participant was assigned a social value orientation (i.e., prosocial, individualist, or competitor) when the participant made more than six consistent choices out of eight. Participants never knew who was paired with them and no feedback was given during the task. Participants received the amount of money based on their choices.
Fig 1. Design of tasks.
(A,B) Participants make decisions in (A) the Triple-Dominance Measure task and (B) the modified ultimatum game. Each participant played the role of the responder. The modified ultimatum game contained both advantageous (guilt) and disadvantageous (envy) proposals unlike the standard ultimatum game, which only contains disadvantageous offers.
Modified ultimatum game: Day2
We used a modified version of the ultimatum game [47] to examine brain responses to inequity (Fig 1B). In comparison with the standard ultimatum game, both disadvantageous (envy; reward for other was larger than reward for self) and advantageous (guilt; reward for self was larger than reward for other) conditions were included. After a short display (1 s) of the name and face of a proposer, the participant was asked to decide whether to accept or reject the offered division of 500 yen (equivalent to 5 US dollars) by a button press within 1 s after a beep. Base offers were one of 7:3, 6:4, 5:5, 4:6, 3:7, 2:8 or 1:9 for the participant (responder) and proposer. Each base offer appeared 8 times in one session in pseudo random order. Therefore, one session comprised 56 trials. Uniform random numbers ranging from -25 yen to 25 yen were added to the base offer in each trial in order to maximize the participant’s involvement in the task. Because each name and face was utilized once, this task was a sequential one-shot game. We instructed the participants that the faces and offers in the ultimatum game were collected from students at a nearby university whose rewards would depend on the participants’ choices. In fact, all faces (neutral facial expressions) were selected from the facial expression database [48] released by the Advanced Telecommunications Research Institute (ATR), Japan. The total time of a session was 1176 s.
Because the ultimatum game is an asymmetric game for a proposer and a responder, rejection behaviors by the responder might partly correspond to costly punishment as well as inequity aversion. Therefore, our SNP analysis considered both the Triple-Dominance measure task and ultimatum game.
SNP analysis
Saliva sampling was done using the DNA collection kit Oragene·DNA (OG-500) (DNA Genotek Inc.). Using commercially available TaqMan probes and ABI PRISM 7900HT and following the protocol recommended by the manufacturer (Thermo Fisher Scientific, Waltham, MA, USA), we selected and genotyped the SNPs of genes for enzymes involved in GABA synthesis and major subunits of the GABA A receptor: rs3791878 and rs2236418 (GAD1 and GAD2, respectively), and rs3811991, rs2617503, rs1912960, rs2351299, rs279858, rs9362632, rs140682 and rs878960 (GABA A receptor subunit genes clusters on chromosomes 5q34-q35, 4p12, 6q14-16 and 15q11-q13). We did the same for the oxytocin-receptor (OXTR) genes: rs237924, rs75775, rs4686302, rs1042778 and rs53576 (SNPs upstream of the gene, on the protein coding region, exon and long intron). We divided the genotypes of these SNPs into two groups by combining heterozygotes and minor allele homozygotes, since the frequencies of some minor alleles were not sufficiently high.
Statistical analysis
We conducted statistical tests of the genetic effects by using the functions ‘ranksum’ and ‘anovan’ in MATLAB R2014a, and the correction for multiple comparisons by using the Benjamini-Hochberg method [49] in R version 3.0.2 (https://www.r-project.org). For the analysis of interactions, a representative SNP on each GABA A receptor cluster was included in ANOVA.
MRI acquisition
MRI scanning was conducted with a Siemens Trio TIM 3T scanner at Tamagawa University (Japan). The parameters used were: repetition time 2 s, echo time 25 ms, flip angle 90°, field of view 192 mm, and resolution 3 × 3 × 3 mm. High-resolution (T1 [1 × 1 × 1 mm] and T2 [0.6 × 0.4 × 3 mm]) structural images were also acquired for each participant. In addition to the experimental trials, the session contained three initial dummy scans.
GLM analysis
Imaging data were analyzed using standard procedures in Statistical Parametric Mapping (SPM12 http://www.fil.ion.ucl.ac.uk/spm) on MATLAB R2014a. Before the analysis, we performed motion correction and non-linear transformation into the standard space of the Montreal Neurological Institute (MNI) coordinates using a T2 template. These normalized EPI images were re-sliced into 2 × 2 × 2 mm voxels and then smoothed with a 6 mm FWHM isotropic Gaussian kernel. The data were high-passed filtered (cut-off frequency, 128 s).
First-level analysis: In the main analysis, for each participant, eight functional regressors were included in the general linear model analysis of the fMRI data. The standard event-related regressors were constructed at the time of the proposer’s face presentation, offer presentation, button press (choice) and feedback presentation. For the offer presentation, four reward-related regressors (parametric modulators) were also included: reward for self (Rs), reward for proposer (Ro), envious difference (the reward difference when Rs < Ro), and guilty difference (the reward difference when Ro < Rs). Since the range of the reward variables is continuous and wide (i.e., between 0 and 450), common logarithm (base = 10) was used for these four parametric modulators. In addition to these eight regressors, we included six head movement parameters that were calculated from the realignment.
Second-level analysis: To contrast neural correlates with envy and guilt aversions for polymorphisms, we conducted a second-level group analysis using a multiple-regression for analyzing the effects of polymorphisims on the OXTR and GABA-related genes, and using a full factorial design to analyze the interaction between the genotypes of GAD1 rs3791878 (GG, GT/ TT) and OXTR rs53576 (AA, AG/GG). The neural correlates of envy and guilt aversions were defined as brain activity correlated with the envious reward difference between other and self (Ro-Rs > 0) and with the guilty reward difference between self and other (Rs-Ro > 0), respectively.
Utility function
Three utility functions were considered to analyze envy and guilt aversions as described below.
| Eq. 1 |
| Eq. 2 |
| Eq. 3 |
The weights (β) in the equations were estimated from behaviors during the ultimatum game by the maximum likelifood estimation (MLE) using the internal point method in MATLAB R2014a. Eq 1 comes from Fehr & Schmidt [50]. Envious reward differences and guilty reward differences contribute to judgements separately in this model. Eq 2 and Eq 3 come from the inequity-aversion model, which represents the reward difference (containing both envious and guilty differences) by a single term. The amount of the reward for others is considered in Eq 3, but not in Eq 2. βself was set to 1 in all three equations.
ROIs for small volume correction
We used the functional ROIs defined by Shen and colleagues when we conducted small volume corrections, which were produced from the resting-state fMRI data of 79 healthy participants and parcellated by group-wise graph theory-based analysis [51]. These ROIs have functional homogeneity within each node and good parcellation reproducibility across multiple groups of healthy volunteers. To select the ROIs for small volume correction, we defined two criteria: 1) previous reports showed the importance of the regions for the inequity aversion or decisions in the ultimatum game (e.g. amygdala and dACC) and 2) the whole brain analysis detected correlation between activity and the inequity aversion or decision (the statistical threshold was p < 0.001 uncorrected).
Results
Genotype distribution
The genotype distributions of the 97 fMRI-experiment participants are shown in S1 Table. The distributions of all SNPs we examined were not different from the Hardy-Weinberg equilibrium (p > 0.01), yielding a result consistent with previous reports [52–53] and datasets, including the HapMap (international HapMap Project) and 1000 Genome project for Asian populations.
Egalitarianism and SNPs on OXTR and GABA-related genes
The effects of oxytocin on prosocial behaviors such as trust and generosity have been previously reported [13,17,24,29]. For egalitarianism, a previous study reported no significant correlation between the donation money and an OXTR genetic variation in the dictator game [54]. However, Israel and colleagues [31] reported correlations between SNPs on the long intron region of OXTR and social value orientation using the Triple-Dominance measure task. Therefore, we first conducted the Triple-Dominance measure task (Fig 1A) and evaluated the effects of OXTR SNPs on egalitarianism. As shown in Table 1, rs53576 and rs4686302 was significantly associated with the number of prosocial choices (p = 0.0027 and 0.0373, respectively, N-way ANOVA). We examined the correlation between social value orientation and OXTR SNPs and found that only rs53576, an SNP located at the long intron, was significantly correlated with the type of social value orientation (prosocial or individualist; Table 2, p = 0.046, Chi-squared test).
Table 1. Correlation of the SNPs on OXTR and the number of prosocial choices in social value orientation test.
| SNP | P | F | test |
|---|---|---|---|
| rs53576 | 0.0027 | 9.53 | N-way ANOVA |
| rs4686302 | 0.037 | 4.47 | |
| rs75775 | 0.33 | 0.94 | |
| rs237924 | 0.57 | 0.33 | |
| rs1042778 | 0.31 | 1.06 |
Table 2. Interactions between Social Value Orientation (SVO) and SNPs on OXTR.
| SNP | Location | Type | P | |
|---|---|---|---|---|
| rs53576 | 3rd intron | SNVa | 0.046 | AA/AG.GG |
| rs4686302 | 3rd exon | SNV(missense) | 0.93 | CC/CT.TT |
| rs75775 | Upstream | SNV | 0.45 | GG/GT.TT |
| rs237924 | Upstream | SNV | 0.45 | CC/CT.TT |
| rs1042778 | Downstream 3’-UTRb | SNV | 0.55 | GG/GT.TT |
aSNV, single nucleotide variance
bUTR, untranslated region.
We also examined relationships between the genotypes of SNPs of GABA-related genes and social value orientation, because a previous study showed that the injection of benzodiazepine, which facilitates the GABA A receptor, decreased the rejection rates of unfair offers in the ultimatum game without changing sensitivity to fairness [2]. However, we found neither a significant effect of SNPs of GABA-related genes (S2 Table) nor an interactive effect between SNPs of GABA-related genes and rs53576 of OXTR on social value orientation. These results suggested that only rs53576 had a main effect on the sensitivity to egalitarianism.
Behaviors in the ultimatum game
In the context of economic games, inequity aversion can be decomposed into envy (disadvantageous; rewards for others are higher than for self) and guilt (advantageous; rewards for self are higher than for others) aversions [50]. To quantify inequity aversion, the weights for inequity aversion were estimated and compared. We introduced three models (Eqs1 to 3 in Materials and Methods) and, upon applying the Akaike information criterion (AIC) [55] and the Bayesian information criterion (BIC) [56] (S3 Table), found that the envy-guilt model (Eq 1) was most suitable for the present study based on the modified ultimatum game (Fig 1B). Because -βenvy and -βguilt are indices that correspond to behavioral decisions based on envy and guilt aversions, we defined the Decision Index (DI) for envy and guilt as -βenvy and -βguilt, respectively, for the following analyses. The ‘minus’ sign of β means that the feeling is negative (aversive).
Brain activities in the ultimatum game
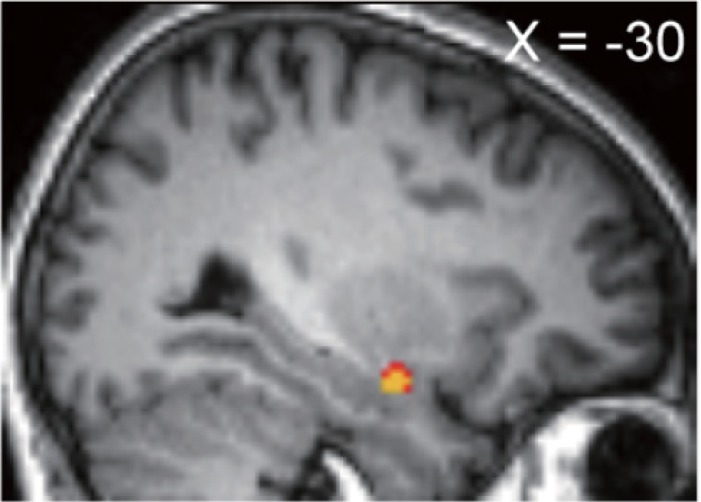
To identify the brain activities that correlated with the decisions induced by envy and guilt aversions, we conducted second-level GLM analysis of the brain activations that correlated with envy (Ro > Rs) and guilt (Rs > Rs) using DIenvy and DIguilt as second-level regressors (Fig 2). DIenvy was found to be correlated with envy-correlated activity in the amygdala (Table 3; p = 2.9 x 10−2, small volume corrected, MNI coordinates -30, -6, -18), consistent with previous studies [10–11,57]. On the other hand, we did not find any brain activity correlated with DIguilt.
Fig 2. Activation in amygdala correlated with DIenvy.
Amygdala responses to disadvantageous inequity (envy) were correlated with each participant’s DIenvy (PFWE_SVC = 2.9 x 10−2 small volume corrected, peak MNI coordinate -30, -6, -18).
Table 3. The effects of DIenvy on whole brain envy-correlated activity.
| Region | peak positiona | t score |
z score |
Puncb | PFWE_svcc | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| amygdala | -30 | -6 | -18 | 4.02 | 3.86 | 5.8 x 10−5 | 2.9 x 10−2 |
aPeak locations are shown as MNI coordinates.
bp values at the peak are shown.
csvc; small volume correction.
Solitary effects of SNPs on envy aversion
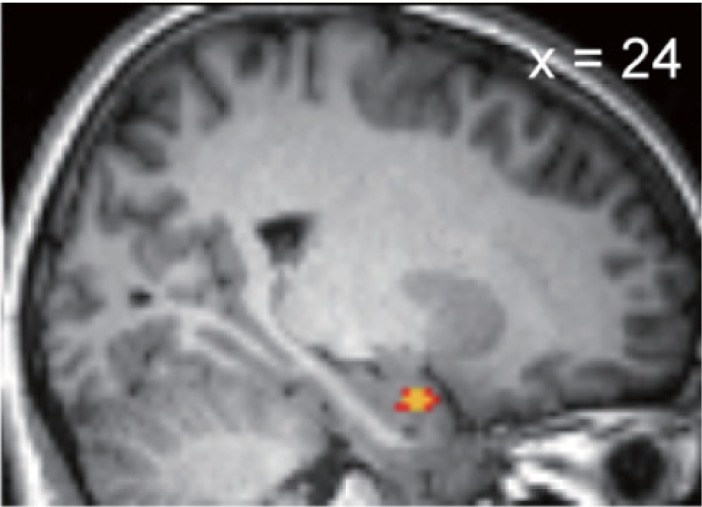
We looked for the effects of SNPs on DIenvy. Neither significant solitary (main) effects of SNPs nor interactive effects of SNPs with gender [20,58] were identified (Panels A-B in S4 Table; Wilcoxon rank-sum test). However, the envy-induced amygdala activity was affected by rs53576. More specifically, amygdala activity was found to be larger in A carriers of rs53576 (Fig 3 and Table 4; p = 1.0 x 10−2 at the peak position, small volume corrected, MNI coordinates 24, -2, -22). There were no other significant effects of single SNPs on envy-induced brain responses.
Fig 3. Whole-brain effects of the polymorphisms on OXTR (rs53576) on envy-correlated activity.
Regression analysis showed that amygdala activity induced by inequity was correlated with rs53576 ‘A’. (PFWE_SVC = 1.0 x 10−2, peak MNI coordinates 24, -2, -22). The cluster was small-volume corrected using the ROI defined by Shen et al. [50], and the threshold of the image was p < 0.001 and p < 0.005 (uncorrected, yellow and red, respectively) for display purposes.
Table 4. The effects of rs53576 (OXTR) on whole brain envy-correlated activity.
| Region | peak positiona | t score |
z score |
Puncb | PFWE_svcc |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| amygdala | 24 | -2 | -22 | 4.36 | 4.15 | 1.7 x 10−5 | 1.0 x 10−2 |
aPeak locations are shown as MNI coordinates.
bp values at the peak are shown.
csvc, small volume correction.
Effects of GAD1-OXTR interaction on envy aversion
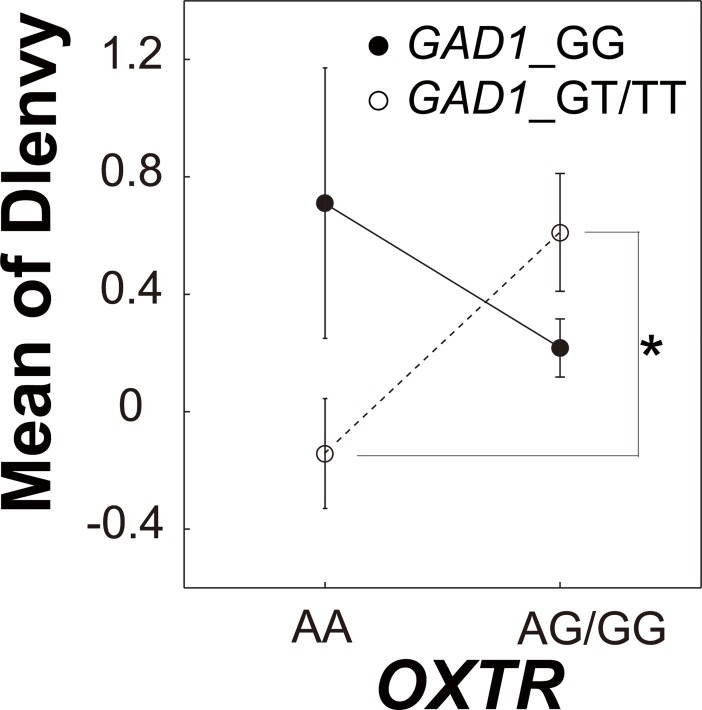
Motivated by reports on oxytocin-GABA interactions in rodents [26,45,59], we investigated the interactive effects between OXTR and SNPs of GABA-related genes on DIenvy. Because rs53576 was the only gene correlated with social value orientation and the envy-induced amygdala activity, we chose it as the candidate SNP of OXTR. We found significant interactive effects of SNPs (rs3791878 (GAD1)-rs53576 (OXTR), rs2236418 (GAD2)-rs140682 (chr 15q), rs1912960 (chr 4p)-rs53576 (OXTR), rs1912960 (chr 4p)-gender, and rs9362632 (chr 6q)-gender) on DIenvy by using the N-way ANOVA (Table 5). We then compared DIenvy among the participant groups, which were determined by the SNP subtype in a post-hoc manner (Panels A-E in S5 Table). We found a significant interactive effect between GAD1 and OXTR (Fig 4 and Panel A in S5 Table; p = 4.2 x 10−2, Wilcoxon ranksum test, correction for multiple comparisons by the number of comparisons in each condition (= 6) was done by the Benjamini and Hochberg method), but not between any other combination (Panels B-E in S5 Table). This result indicates that GABA synthesis and oxytocin presence coordinately modulate the behavioral decisions induced by envy aversion.
Table 5. Effects of SNPs or gender on DIenvy.
| Interactive effects between SNPs and gender | |||
|---|---|---|---|
| SNP | p | F | Test |
| rs3791878 (GAD1) x rs2236418 (GAD2) | 0.87 | 0.03 | N-way ANOVA |
| rs3791878 (GAD1) x rs3811991 (chr5q) | 0.79 | 0.07 | |
| rs3791878 (GAD1) x rs1912960 (chr4p) | 0.18 | 1.81 | |
| rs3791878 (GAD1) x rs9362632 (chr6q) | 0.98 | 0 | |
| rs3791878 (GAD1) x rs140682 (chr15q) | 0.99 | 0 | |
| rs3791878 (GAD1) x rs53576 (OXTR) | 0.0039 | 9.05 | |
| rs3791878 (GAD1) x gender | 0.30 | 1.1 | |
| rs2236418 (GAD2) x rs3811991 (chr5q) | 0.52 | 0.42 | |
| rs2236418 (GAD2) x rs1912960 (chr4p) | 0.072 | 3.36 | |
| rs2236418 (GAD2) x rs9362632 (chr6q) | 0.21 | 1.62 | |
| rs2236418 (GAD2) x rs140682 (chr15q) | 0.045 | 4.2 | |
| rs2236418 (GAD2) x rs53576 (OXTR) | 0.29 | 1.13 | |
| rs2236418 (GAD2) x gender | 0.43 | 0.64 | |
| rs3811991 (chr5q) x rs1912960 (chr4p) | 0.64 | 0.21 | |
| rs3811991 (chr5q) x rs9362632 (chr6q) | 0.29 | 1.16 | |
| rs3811991 (chr5q) x rs140682 (chr15q) | 0.77 | 0.08 | |
| rs3811991 (chr5q) x rs53576 (OXTR) | 0.22 | 1.56 | |
| rs3811991 (chr5q) x gender | 0.94 | 0.01 | |
| rs1912960 (chr4p) x rs9362632 (chr6q) | 0.72 | 0.13 | |
| rs1912960 (chr4p) x rs140682 (chr15q) | 0.052 | 3.95 | |
| rs1912960 (chr4p) x rs53576 (OXTR) | 0.031 | 4.92 | |
| rs1912960 (chr4p) x gender | 0.042 | 4.35 | |
| rs9362632 (chr6q) x rs140682 (chr15q) | 0.45 | 0.57 | |
| rs9362632 (chr6q) x rs53576 (OXTR) | 0.21 | 1.60 | |
| rs9362632 (chr6q) x gender | 0.028 | 5.08 | |
| rs140682 (chr15q) x rs53576 (OXTR) | 0.11 | 2.63 | |
| rs140682 (chr15q) x gender | 0.13 | 2.27 | |
| rs53576 (OXTR) x gender | 0.32 | 1.00 | |
Fig 4. Mean values of the DIenvy.
An interaction between the polymorphisms of GAD1 (rs3791878: GG, filled solid; GT/TT, open dashed) and OXTR (rs53576) was revealed by ANOVA (p = 3.9 x 10−3, Table 5). DIenvy was larger in rs3791878GT/TT-rs53576AG/GG carriers than in rs3791878GT/TT-rs53576AA carriers (asterisk, p = 4.2 x 10−2, S5 Table). Error bars represent standard errors.
We next wished to identify envy-correlated brain activity that paralleled the interactive effect between GAD1 (GG, GT/TT) and OXTR (AA, AG/GG) by conducting a full factorial design analysis (see Materials and Methods). We identified a significant interactive effect in the dACC (Fig 5A and Table 6; F = 17.02, p = 4.3 x 10−2 at the peak position, small volume corrected, MNI coordinates 8, 14, 28). This brain region has been consistently highlighted in inequity aversion [2,11,60].
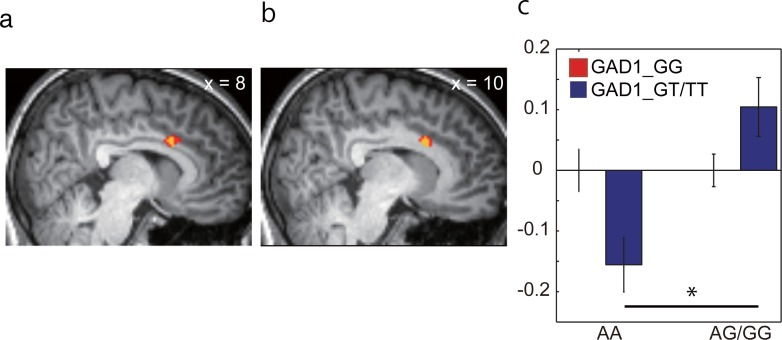
Fig 5. Whole-brain interactive effects between polymorphisms of GAD1 (rs3791878) and OXTR (rs53576) on envy-correlated activity.
(A,B) An interactive effect was found in the dACC (A, F = 17.02, PFWE_SVC = 4.3 x 10−2, MNI coordinates 8, 14, 28). More specifically, the response to envy in the dACC was larger in rs3791878GT/TT-rs53576AG/GG carriers and rs3791878GG-rs53576AA carriers (B, PFWE_SVC = 2.8 x 10−2). Peak locations in the MNI coordinates are shown in Table 6. Each cluster was small-volume corrected using the ROI defined by Shen et al. [50], and the threshold of the image was p < 0.001 and p < 0.005 (uncorrected, yellow and red respectively) for display purposes. The envy-correlated activity at the peak location in B (MNI coordinates 10, 14, 28) is displayed separately for the different groups shown in Fig 4 (rs3791878GT/TT-rs53576AG/GG > rs3791878GT/TT-rs53576AA). (C) Mean envy-induced activities in the rs3791878GG and rs3791878 GT/TT groups are shown in blue and red, respectively.
Table 6. Interactive effects between GAD1 and OXTR on whole brain envy-correlated activity.
| All groups | |||||||
| Region | peak positiona | F |
z score |
Puncb | PFWE_svcc | ||
| x | y | z | |||||
| dACC | 8 | 14 | 30 | 17.02 | 3.77 | 8.0 x 10−5 | 4.3 x 10−2 |
| rs3791878GT/TT-rs53576AG/GG > rs3791878GT/TT-rs53576AA | |||||||
| Region | peak positiona |
t score |
z score |
Puncb | PFWE_svcc | ||
| x | y | z | |||||
| dACC | 10 | 14 | 28 | 4.04 | 3.87 | 5.4 x 10−5 | 2.8 x 10−2 |
aPeak locations are shown as MNI coordinates.
bp values at the peak are shown.
csvc, small volume correction.
We further compared the envy-correlated dACC activity between two groups whose DIenvy were different (i.e., rs3791878GT/TT-rs53576AG/GG > rs3791878GT/TT-rs53576AA). Whole brain analysis revealed that dACC activity was higher in the higher DIenvy group (Fig 5B and 5c and Table 6; p = 2.8 x 10−2 at the peak position, small volume corrected, MNI coordinates 10, 14, 28). These results strongly indicated that the genetic interaction between GAD1 and OXTR had an influence on the envy-induced activation of the dACC.
Discussion
In this study, we reported the interactive effect between GABA- and oxytocin-related genes on human envy aversion. We found that each participant’s DIenvy calculated from accept/reject behavior during the ultimatum game was correlated with the interaction effect between GAD1 and OXTR (Fig 4) and that this interactive effect was correlated with the envy-induced activity of the dACC (Fig 5), which has been implicated to play a crucial role in social information processing [61].
The response to unfair offers consists of at least two process: the manipulation of aversive feelings and the decision-making based on the aversive feelings. In our task, unfair proposals induced aversive feelings. The aversive feelings were larger in people with prosocial traits. We confirmed that the correlation between social value orientation and the polymorphism on OXTR (Table 1 indicated that rs53576 ‘A’ correlated with the prosocial trait). The aversive feelings induced by inequity were previously reported to correlate with the amygdala response to inequity [10,11], and we found the correlation between the type of OXTR and the amygdala activity correlated with envy aversion (inequity) (Fig 3). These findings suggested contributions by oxytocin and the amygdala in the first process.
On the other hand, in the second process, decisions (accept/reject) were made by taking aversive feelings into consideration. In our procedure, decision indices calculated from the rejection behaviors in the envy condition correlated with the OXTR-GAD1 interaction (Fig 4). This observation suggested that envy aversion depends on interactive effects between the sensitivity to inequity (which was related to the type of OXTR) and the function of GABA (which was related to the type of GAD1). We also found that this interactive effect was correlated with envy-related brain activity in the dACC (Fig 5). In this task, participants had to compensate aversive feelings to accept envious proposals, and this discrepancy between inequity aversion and the accepted decision was larger in prosocials who disliked inequity (rs53576 ‘A’). Researchers have repeatedly reported the contribution of the dACC to resolving conflicts [62,63]. Especially in the context of the ultimatum game, it was suggested that activity in the dACC decreases when participants forgive unfair partners [64]. Our results may suggest that the dACC activity controlled by GAD1 has an effect on resolving conflicts between inequity aversion and the accepted decision. This hypothesis is consistent with a report that shows correlation between the polymorphism of the GAD1 gene and the change in GABA concentration in human dACC [65].
It was reported that the administration of oxytocin in humans changes the anxiety trait [66] and that the anxiety trait is related to the microstructural property of the amygdala-ACC pathway [67]. The GABA concentration in the dACC was reported to correlate with amygdala activation during the processing of emotional stimuli [68]. Our study may extend these findings and suggest the possibility that the amygdala activity in social tasks is generally linked with GABA levels in the dACC.
Animal studies have indicated that oxytocin neurons project to both the amygdala and ACC [26]. OXTR is also expressed in both the amygdala and ACC in humans [69]. Although it is difficult for the present study to clarify whether oxytocin works on the ACC directly or indirectly through the amygdala, we found that oxytocin contributes to the aversive feelings to inequity that are mainly expressed in the amygdala, while the interaction between oxytocin and GABA synthesis affects the decision-making that is based on inequity aversion, which is principally represented in the dACC. This observation is comparable with a previous report that showed rejection behavior was not influenced by oxytocin administration [24].
Several studies have reported that the GABA A receptor is essential for oxytocin function in fear and anxiety conditions [26,45]. In the present study, no SNPs of GABA A receptor-related genes had an interactive effect with the SNP of OXTR, but the SNP of GAD1 did. One potential explanation for this observation is that the GABA A receptor consists of five subunits, each encoded by a distinct gene. SNPs of the individual subunit genes might have only a small effect, as Benjamin and colleagues [40] stated, while GAD1 encodes the enzyme that synthesizes GABA and has a direct effect on the amount of GABA that could control neural activities.
With respect to the SNPs of OXTR, several contradictory results have been reported between Asian and Caucasian populations regarding the long intron region [32–34]. We reported here that the ‘A’ allele of rs53576 was correlated with the prosocial trait in Japanese, but the ‘A’ allele was correlated with the antisocial trait in Caucasians [70]. Therefore, the SNP itself may not be the real cause of the phenotype variation. Differences in social culture or physical environment might account for the opposite effects of the same allele type between different populations. Since all participants in our experiments were Japanese university students, we could not assess regional or age differences. Regarding gender differences, we did not find a gender difference in the present study (neither social value orientation nor decision indices in the envy condition) despite contradictory evidence regarding the effects of intranasal oxytocin injection and OXTR polymorphisms in social contexts [20,71–74]. However, we did find a significant correlation between the guilt decision index and the type of OXTR in females (p = 0.025, Wilcoxon ranksum test, correction for multiple comparisons by the number of comparisons in each condition was done by the Benjamini and Hochberg method). This finding may indicate not only that the effect of oxytocin on inequity aversion is different for envy and guilt conditions, but also that the effect of oxytocin on guilty feelings is different between males and females. Further studies are necessary to validate this hypothesis.
Supporting information
Distributions of the SNPs are shown.
(DOCX)
There was no interactive effect between SNPs of GABA-related genes.
(DOCX)
The envy-guilt model (Eq 1) was most suitable for the present study based on the modified ultimatum game.
(DOCX)
No significant solitary (main) effects of SNPs nor interactive effects of SNPs with gender were identified.
(DOCX)
We found a significant interactive effect between GAD1 and OXTR.
(DOCX)
Acknowledgments
We are grateful to Satoshi Tada and Tomoki Haji for technical assistance and Peter Karagiannis for editing an early version of the manuscript.
Data Availability
The data underlying the results presented in the study are available from the OPEN ICPSR database and may be accessed at the following URL: http://doi.org/10.3886/E107923V1.
Funding Statement
This work was supported by Core Research for Evolutional Science and Technology (CREST), Japan Science and Technology Agency (JST), the Center of innovation at Osaka University, Grant-in-Aid for Scientific Reserch (KAKENHI)(17H06314 and 26242087) and JST AIP-PRISM (JPMJCR18ZR). These funders had roles in data collection, analysis, and preparation of manuscripts.
References
- 1.Feng C, Luo YJ, Kruger F. Neural signatures of fairness-related normative decision making in the ultimatum game: a coordinated-based meta-analysis. Human Brain Mapping. 2015;36: 591–602. 10.1002/hbm.22649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gospic K, Mohlin E, Fransson P, Petrovic P, Johannesson M, Ingvar M. Limbic justice–amygdala involvement in immediate rejection in the ultimatum game. PLoS Biol. 2011;9: e1001054 10.1371/journal.pbio.1001054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kodaka F, Takahashi H, Yamada M, Takano H, Nakayama K, Ito H, et al. Effect of cooperation level of group on punishment for non-coorerators: a functional magnetic resonance imaging study. PLoS ONE. 2012;7: e41338 10.1371/journal.pone.0041338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lockwood PL, Apps MAJ, Roiser JP, Viding E. Encoding of vicarious reward prediction in anterior cingulate cortex and relationship with trait empathy. J Neurosci. 2015;35: 13720–13727. 10.1523/JNEUROSCI.1703-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: evidence from the ultimatum game. J Neurosci. 2007;27: 951–956. 10.1523/JNEUROSCI.4606-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tricomi E, Rangel A, Camerer CF, O'Doherty JP. Neural evidence for inequality-averse social preferences. Nature. 2010;463: 1089–1091. 10.1038/nature08785 [DOI] [PubMed] [Google Scholar]
- 7.Fliessbach K, Phillips CB, Trautner P, Schnabel M, Elger CE, Falk A, et al. Neural responses to advantageous and disadvantageous inequity. Front Hum Neurosci. 2012;6: 165 10.3389/fnhum.2012.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nihonsugi T, Ihara A, Haruno M. Selective increase of intention-based economic decisions by noninvasive brain stimulation to the dorsolateral prefrontal cortex. J Neurosci. 2015;35: 3412–3419. 10.1523/JNEUROSCI.3885-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the ultimatum game. Science. 2003;300: 1755–1758. 10.1126/science.1082976 [DOI] [PubMed] [Google Scholar]
- 10.Haruno M, Frith CD. Activity in the amygdala elicited by unfair divisions predicts social value orientation. Nat Neurosci. 2010;13: 160–161. 10.1038/nn.2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haruno M, Kimura M, Frith CD. Activity in the nucleus accumbens and amygdala underlies individual differences in prosocial and individualistic economic choices. J Cogn Neurosci. 2014;26: 1861–1870. 10.1162/jocn_a_00589 [DOI] [PubMed] [Google Scholar]
- 12.Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15: 301–309. 10.1016/j.tics.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 13.Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58: 639–650. 10.1016/j.neuron.2008.04.009 [DOI] [PubMed] [Google Scholar]
- 14.Declerck S, Boone C, Kiyonari T. Oxytocin and cooperation under conditions of uncertainty: The modulating role of incentives and social information. Horm Behav. 2010;57: 368–374. 10.1016/j.yhbeh.2010.01.006 [DOI] [PubMed] [Google Scholar]
- 15.De Dreu CKW, Greer LL, Handgraaf MJ, Shalvi S, Van Kleef GA, Baas M, et al. The Neuropeptide Oxytocin Regulates Parochial Altruism in Intergroup Conflict Among Humans. Science. 2010;328: 1408–1411. 10.1126/science.1189047 [DOI] [PubMed] [Google Scholar]
- 16.Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, et al. Oxytocin enhances amygdala-dependent, social reinforced learning and emotional empathy in humans. J Neurosci. 2010;30: 4999–5007. 10.1523/JNEUROSCI.5538-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435: 673–676. 10.1038/nature03701 [DOI] [PubMed] [Google Scholar]
- 18.Klack J, Pfundmair M, Agroskin D, Jones E. Who in to blame? Oxytocin promotes nonpersonalistct attribution in response to a trust betrayal. Biol Psychol. 2013;92: 387–394. 10.1016/j.biopsycho.2012.11.010 [DOI] [PubMed] [Google Scholar]
- 19.Mayer-Linedenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neuroscience. 2011;12: 524–538. 10.1038/nrn3044 [DOI] [PubMed] [Google Scholar]
- 20.McCall C, Singer T. The animal and human neuroendocrinology of social cognition, motivation and behavior. Nat Neurosci. 2012;15: 681–688. 10.1038/nn.3084 [DOI] [PubMed] [Google Scholar]
- 21.Riem MME, van Ijzendoorn MH, Tops M, Boksem MAS, Rombouts SARB, Bakermans-Kranenburg MJ. Oxytocin effects on complex brain networks are moderated by experiences of maternal love withdrawal. European Neuropsychopharmacology. 2013;23: 1288–1295. 10.1016/j.euroneuro.2013.01.011 [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc Nati Acad Sci USA. 2009;106: 21437–21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theodoridou A, Rowe AC, Penton-Voak IS, Rogers PJ. Oxytocin and social perception: Oxytocin increases perceived facial trustworthiness and attractiveness. Horm Behav. 2009;56: 128–132. 10.1016/j.yhbeh.2009.03.019 [DOI] [PubMed] [Google Scholar]
- 24.Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PLoS One. 2007;2: e1128 10.1371/journal.pone.0001128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eckstein M, Markett S, Kendrick KM, Ditzen B, Liu F, Hurlemann R, et al. Oxytocin differentially alters resting state functional connectivity between amygdala subregions and emotional control networks: Inverse correlation with depressive traits. Neuroimage. 2017;149: 458–467. 10.1016/j.neuroimage.2017.01.078 [DOI] [PubMed] [Google Scholar]
- 26.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73: 553–566. 10.1016/j.neuron.2011.11.030 [DOI] [PubMed] [Google Scholar]
- 27.Chang SW, Fagan NA, Toda K, Utevsky AV, Pearson JM, Platt ML. Neural mechanisms of social decision-making in the primate amygdala. Proc Natl Acad Sci USA. 2015;112: 15012–16017. 10.1073/pnas.1520704112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radke S, de Bruin ER. The other side of the coin: oxytocin decreases the adherence to fairness norms. Front Hum Neurosci. 2012;6: 193 10.3389/fnhum.2012.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikolajczak M, Gross JJ, Lane A, Corneille O, de Timary P, Luminet O. Oxytocin makes people trusting, not gullible. Psychol Sci. 2010;21: 1072–1074. 10.1177/0956797610377343 [DOI] [PubMed] [Google Scholar]
- 30.Shamay-Tsoory SG, Fisher M, Dash J, Harari H, Perach-Bloom N, Levkovtz Y. Intranasal administration of oxytocin increases envy and schadenfreude (Gloating). Biol Psychiatry. 2009;66: 864–870. 10.1016/j.biopsych.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 31.Israel S, Lerer E, Shalev I, Uzefovsky F, Riebold M, Laiba E, et al. The oxytocin receptor (OXTR) contributes to prosocial fund allocations in the dictator game and the social value orientations task. PLoS One. 2009;4: e5535 10.1371/journal.pone.0005535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, et al. Positive association of the oxytocin receptor gene (OXTR) with Autism in the Chinese Han population. Biol Psychiatry. 2005;58: 74–77. 10.1016/j.biopsych.2005.03.013 [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Kawamura Y, Shimada T, Otowa T, Koishi S, Sugiyama T, et al. Association of the oxytocin receptor (OXTR) gene polymorphisms with autism spectrum disorder (ASD) in the Japanese population. J Hum Genet. 2010;55: 137–141. 10.1038/jhg.2009.140 [DOI] [PubMed] [Google Scholar]
- 34.Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH Jr. Association of the oxytocincin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett. 2007;417: 6–9. 10.1016/j.neulet.2007.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saito Y, Suga M, Tochigi M, Abe O, Yahata N, Kawakubo Y, et al. Neural correlate of autistic-like traits and common allele in the oxytocin receptor gene. Soc Cogn Affect Neurosci. 2013;9: 1443–1450. 10.1093/scan/nst136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu Y, Scheele D, Becker B, Voos G, David B, Hurlemann R, et al. The effect of oxytocin on third-party altruistic decisions in unfair situations: an fMRI study. Sci Rep. 2016;6: 20236 10.1038/srep20236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsang SY, Zhong S, Mei L, Chen J, Ng SK, Pun FW, et al. Social cognitive role of schizophrenia candidate gene GABRB2. PLoS One. 2013;8: e62322 10.1371/journal.pone.0062322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sesarini CV, Costa L, Granada N, Coto MG, Pallia RC, Argibay PF. Association between GABA(A) receptor subit polymorphisms and autism specterum disorder (ASD). Psychiatry Res. 2015;229: 580–582. 10.1016/j.psychres.2015.07.077 [DOI] [PubMed] [Google Scholar]
- 39.Colic L, Li M, Demenescu LR, Li S, Müller I, Richter A, et al. GAD65 promoter polymorphism rs2236418 modulates harm avoidance in women via inhibition/excitation balance in the rostral ACC. J. Neurosci. 2018; 10.1523/JNEUROSCI.1985-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benjamin DJ, Cesarini D, van der Loos MJ, Dawes CT, Koellinger PD, Magnusson PK, et al. The genetic architecture of economic and political preferences. Proc Natl Acad Sci USA. 2012;109: 8026–8031. 10.1073/pnas.1120666109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chabiris CF, Hebert BM, Benjamin DJ, Beauchamp J, Cesarini D, van der Loos MJ, et al. Most reported genetic associations with general intelligence are probably false positive. Psychol. Sci. 2012;23: 1314–1323. 10.1177/0956797611435528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okbay A, Baselmans BM, De Neve JE, Turley P, Nivard MG, Fontana MA, et al. Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nature Genetics. 2016;48: 624–633. 10.1038/ng.3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linnér RK, Biroli P, Kong E, Meddens SFW, Wedow R, Fontana MA, et al. Genome-wide study identifies 611 loci associated with risk tolerance and risky behaviors. bioRxiv 261081;doi: 10.1101/261081. [DOI] [Google Scholar]
- 44.Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308: 245–248. 10.1126/science.1105636 [DOI] [PubMed] [Google Scholar]
- 45.Sabihi S, Dong SM, Maurer SD, Post C, Leuner B. Oxytocin in the medial prefrontal cortex attenuates anxiety: Anatomical and receptor specificity and mechanism of action. Neuropharmacology. 2017;125: 1–12. 10.1016/j.neuropharm.2017.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Lange PAM. The pursuit of joint outcomes and equality in outcomes: an integrative model of social value orientation. Journal of Personality and Social Psychology. 1999;77: 337–349. [Google Scholar]
- 47.Güth W, Schmittberger R, Schwarze B. An experimental analysis of ultimatum bargaining. J Econ Behav Organ. 1982;3: 367–388. [Google Scholar]
- 48.Ogawa T, Oda M. Construction and evaluation of the facial expression database. ATR Technical Report. 1998; TR-H-244.
- 49.Benjamini Y, Hochberg Y. Controlling the false discovery tare: a practical and powerful approach to multiple testing. J Royal Statistical Society Series. 1995; B57: 289–300. [Google Scholar]
- 50.Fehr E, Schmidt KM. A theory of fairness, competition, and cooperation. The Quarterly Journal of Economics. 1999;114: 817–868. [Google Scholar]
- 51.Shen X, Tokoglu F, Papademetris X, Constable RT. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. Neuroimage. 2013;82: 403–415. 10.1016/j.neuroimage.2013.05.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim HS, Sherman DK, Sasaki JY, Xu J, Chu TQ, Ryu C, et al. Culture, distress, and oxytocin receptor polymorphisms (OXTR) interact to influence emotional support seeking. Proc Nati Acad Sci USA. 2010;107: 15717–15721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishina K, Takagishi H, Inoue-Murayama M, Takahashi H, Yamagishi T. Polymorphism of the oxytocin receptor gene modulates behavioral and attitudinal trust among men but not women. PLoS One. 2015;10: e0137089 10.1371/journal.pone.0137089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Apicella CL, Cesarini D, Johannesson M, Dawes CT, Lichtenstein P, Wallace B, et al. No association between oxytocin receptor (OXTR) gene polymorphisms and experimentally elicited social preferences. PLoS ONE. 2010;5: e11153 10.1371/journal.pone.0011153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akaike H. A new look at the statistical model identification. IEEE transaction on Automatic Control. 1974;19: 716–723. [Google Scholar]
- 56.Schwarz G. Estimating the dimension of a model. Annals of Statistics. 1978;6: 461–464. [Google Scholar]
- 57.Yu R, Calder AJ, Mobbs D. Overlapping and distinct representations of advantageous and disadvantageous inequality. Human Brain Mapping. 2014;35: 3290–3301. 10.1002/hbm.22402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Honk J, Schutter DJ, Bos PA, Kruijt W, Lentjes EG, Baron-Cohen S. Testoterone administration impairs cognitive empathy in women depending on second-to-fourth digit ratio. Proc Natl Acad Sci USA. 2011;108: 3448–3452. 10.1073/pnas.1011891108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qi J, Han WY, Yang JY, Wang LH, Dong YX, Wang F, et al. Oxytocin regulates changes of extracellular gulutamate and GABA levels induced by methamphetamine in the mouse brain. Addiction Biol. 2012;17: 758–769. [DOI] [PubMed] [Google Scholar]
- 60.Gabay AS, Radua J, Kempton MJ, Mehta MA. The ultimatum game and the brain: a meta-analysis of neuroimaging studies. Neurosci Biobehav Rev. 2014;47: 549–558. 10.1016/j.neubiorev.2014.10.014 [DOI] [PubMed] [Google Scholar]
- 61.Apps MAJ, Rushworth MGS, Chang SWC. The anterior cingulate gyrus and social cognition: tracking the motivation of others. Neuron. 2016;90: 692–707. 10.1016/j.neuron.2016.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Botvinitck M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for action in anterior cingulate cortex. Nature.1999; 402:179–181. 10.1038/46035 [DOI] [PubMed] [Google Scholar]
- 63.MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000; 288:1835–1838. [DOI] [PubMed] [Google Scholar]
- 64.Fatfouta R, Meshi D, Merkl A, Heekeren HR. Accepting unfairness by a significant other is associated with reduced connectivity between medial prefrontal and dorsal anterior cingulate cortex Soc Neurosci. 2018; 13:61–73, 10.1080/17470919.2016.1252795 [DOI] [PubMed] [Google Scholar]
- 65.Marenco S, Savostyanova AA, van der Veen JW, Geramita M, Stem A, Barnett AS, et al. Genetic modulation of GABA levels in the anterior cingulate cortex by GAD1 and COMT. Neuropsychopharmacology. 2010;35: 1708–1717. 10.1038/npp.2010.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective response to psychosocial stress. Biol Psychiatry. 2003; 54:1389–1398. [DOI] [PubMed] [Google Scholar]
- 67.Eden AS, Schreiber J, Anwander A, Keuper K, Laeger I, Zwanzger P, et al. Emotion regulation and trait anxiety are predicted by the microstructure of fibers between amygdala and prefrontal cortex. J. Neurosci. 2015; 35:6020–6027. 10.1523/JNEUROSCI.3659-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levar N, van Leeuwen JMC, Denys D, van Wingen GA. Divergent influences of anterior cingulate cortex GABA concentrations on the emotion circuitry. Neuroimage. 2017;158: 136–144. 10.1016/j.neuroimage.2017.06.055 [DOI] [PubMed] [Google Scholar]
- 69.Boccia ML, Petrusz P, Suzuki K, Marson L, Pedersen CA. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience. 2013; 253:155–164. 10.1016/j.neuroscience.2013.08.048 [DOI] [PubMed] [Google Scholar]
- 70.Tost H, Kolachana B, Hakimi S, Lemaitre H, Verchinski BA, Mattay VS, et al. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proc Natl Acad Sci USA. 2010;107: 13936–13941. 10.1073/pnas.1003296107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rilling JK, Demarco AC, Hackett PD, Chen X, Gautam P, Stair S, et al. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology. 2014;39: 237–248. 10.1016/j.psyneuen.2013.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stankova T, Eichhammer P, Langguth B, Sand PG. Sexually dimorphic effects of oxytocin receptor gene (OXTR) variants on Harm Avoidance. Biol Sex Differ. 2012;3: 17 10.1186/2042-6410-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu N, Li Z, Su Y. The association between oxytocin receptor gene polymorphism (OXTR) and trait empathy. J Affect Disord. 2012;138: 468–472. 10.1016/j.jad.2012.01.009 [DOI] [PubMed] [Google Scholar]
- 74.Yamasue H, Kuwabara H, Kawakubo Y, Kasai K. Oxytocin, sexually dimorphic features of the social brain, and autism. Psychiatry Clin Neurosci. 2009;63: 129–140. 10.1111/j.1440-1819.2009.01944.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distributions of the SNPs are shown.
(DOCX)
There was no interactive effect between SNPs of GABA-related genes.
(DOCX)
The envy-guilt model (Eq 1) was most suitable for the present study based on the modified ultimatum game.
(DOCX)
No significant solitary (main) effects of SNPs nor interactive effects of SNPs with gender were identified.
(DOCX)
We found a significant interactive effect between GAD1 and OXTR.
(DOCX)
Data Availability Statement
The data underlying the results presented in the study are available from the OPEN ICPSR database and may be accessed at the following URL: http://doi.org/10.3886/E107923V1.