Abstract
Study Objectives:
The aim of this study is to generate and validate supervised machine learning algorithms to detect patients with Chiari malformation (CM) 1 or 1.5 at high risk of the development of sleep-related breathing disorders (SRBD) using clinical and neuroradiological parameters.
Methods:
We prospectively included two independent datasets. A training dataset (n = 90) was used to obtain the best model, whereas a second dataset was used to validate it (n = 74). In both cohorts, the same clinical, neuroradiological, and sleep studies were carried out. We used two supervised machine learning approaches, multiple logistic regression (MLR) and the unbiased recursive partitioning technique conditional inference tree (URP-CTREE), to detect patients at high risk of SRBD. We then compared the accuracy, sensitivity, and specificity of the two prediction models.
Results:
Age (odds ratio [OR] 1.1 95% confidence interval [CI] 1.05–1.17), sex (OR 0.19 95% CI 0.05–0.67), CM type (OR 4.36 95% CI 1.14–18.5), and clivus length (OR 1.14 95% CI 1.01–1.31) were the significant predictor variables for a respiratory disturbance index (RDI) cutoff that was ≥ 10 events/h using MLR. The URP-CTREE model predicted that patients with CM-1 who were age 52 years or older and males with CM-1 who were older than 29 years had a high risk of SRBD. The accuracy of predicting patients with an RDI ≥ 10 events/h was similar in the two cohorts but in the URP-CTREE model, specificity was significantly greater when compared to MLR in both study groups.
Conclusions:
Both MLR and URP-CTREE predictive models are useful for the diagnosis of SRBD in patients with CM. However, URP-CTREE is easier to apply and interpret in clinical practice.
Citation:
Ferré Á, Poca MA, de la Calzada MD, Moncho D, Urbizu A, Romero O, Sampol G, Sahuquillo J. A conditional inference tree model for predicting sleep-related breathing disorders in patients with chiari malformation type 1: description and external validation. J Clin Sleep Med. 2019;15(1):89–99.
Keywords: Chiari malformation type 1, craniovertebral junction malformation, logistic regression, machine learning, magnetic resonance imaging, morphometric analysis, posterior cranial fossa, sleep apnea, sleep disorders, sleep-related breathing disorders
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleep studies are not routinely considered in the workup of patients with Chiari malformation. However, evidence collected over the past decade shows that sleep disorders are highly prevalent in these patients. In the routine clinical workup for patients with Chiari malformation, clinicians should therefore identify those with a high likelihood of presenting clinically relevant sleep-related breathing disorders.
Study Impact: The current study proposes an easy algorithm than can facilitate the high pretest probability of sleep-related breathing disorders in patients with Chiari malformation to decide whether to recommend sleep study.
INTRODUCTION
Chiari malformation type 1 (CM-1) is traditionally defined as a congenital hindbrain anomaly characterized by a descent of the cerebellar tonsils (TD) through the foramen magnum (FM) of at least 3 mm.1 Experimental work and morpho-metric studies have shown that CM-1 results mainly from a small posterior cranial fossa (PCF) due to a short/dysplastic occipital bone, which is a consequence of paraxial mesodermal underdevelopment.2,3 Together, small PCF and tonsillar herniation cause compression of the lower brainstem, the lower cranial nerves, the upper cervical spinal cord, and the cerebellar components. CM-1 symptoms are heterogeneous and may include headache and neck pain, paresthesia, motor deficits, dysphagia, and nocturnal respiratory disorders.4–6 In some patients with CM-1, dysfunction of the respiratory drive has also been described, in addition to severe and potentially fatal complications that include respiratory failure, postoperative susceptibility to respiratory failure, and even sudden death.7–18 Surgical treatment of CM-1 with posterior fossa decompression or reconstruction is effective in reducing clinical symptoms.19–21
Sleep-related breathing disorders (SRBDs) are highly prevalent among adults and are a well-documented independent risk factor for reduced vitality, impaired quality of life (QoL), and increased incidence of hypertension, cardiovascular disease, all-cause mortality, stroke, occupational and traffic accidents, and sudden cardiac death.22–26 Patients with CM-1 have a higher prevalence of SRBD than the prevalence described in population-based studies or control patients.6,27–31 In a previous study, we found a very high prevalence of SRBD (50%) in adult patients with CM-1, which was moderate to severe in ∼30% of cases. In most of these patients, we found obstructive hypopneas or apneas and poor sleep efficiency and sleep quality.27 Currently, few studies have addressed the clinical and radiological biomarkers that may help to detect patients with CM-1 at high risk of SRBD, and such studies are limited by small cohorts and contradictory results.6,27,29 Previous work has shown that age, male sex, excess weight, and the presence of hydrocephalus could increase the risk of SRBD. However, severity of SRBD was not related to any traditional clinical CM-1 symptom or the degree of TD.27
Statistics, data mining, and artificial intelligence have improved significantly in the past two decades with the aid of parallel increases in computer storage and processing power, which allow the application of repetitive complex mathematical calculations to big data. Statistical learning or machine learning techniques are based on complex algorithms that have been successfully applied in predicting either quantitative or categorical outcomes.32 A traditional classification of machine learning techniques is to divide them into supervised and unsupervised techniques. A supervised learning problem in medicine starts with a dataset of patients in which we want to predict a categorical outcome (ie, apnea, no apnea) based on a set of variables, for example, demographic, clinical, radiological, biomarkers, etc.32 In supervised machine learning techniques, researchers have the outcome variable measured and so the algorithms are guided in the learning process. In unsupervised machine learning, researchers observe only the variables but do not have the outcome measurement; the algorithms try to detect some structure within the data and identify patterns and trends.32 Multiple logistic regression (MLR) is considered a classic algorithm of supervised machine learning because MLR uses a classification algorithm and learns from data when constructing the model to predict the outcome in binary classification problems. However, MLR assumes linearity, does not account for interactions, and does not provide direct decision rules for the stratification of patients.33,34 Hothorn et al. and Seibold et al. developed an unbiased recursive partitioning regression tool called the Conditional Inference Tree (URP-CTREE).33,35 The main advantage of this method over MLR is that it provides direct decision rules for stratification, its visual representation is directly interpretable, and it can be easily implemented in a clinical setting.33–36
The goal of the current study is to develop and apply two supervised machine learning approaches, MLR and URPCTREE, to screen for patients with CM-1 and identify those with a high risk of SRBD based on clinical and neuroradiological characteristics. In addition, we compared the accuracy of the prediction models and conducted an external validation in a second independent cohort of patients.
METHODS
Our study was based on two independent datasets: a training dataset (n = 90) to obtain the best model and a second dataset for validating the model (n = 74). Both cohorts were studied in a single institution and underwent the same neuroradiological studies.
Sleep Studies and Scoring Criteria
Excessive daytime sleepiness in patients in both cohorts was estimated with the Epworth Sleepiness Scale (ESS).37 ESS is considered abnormal in adults when the ESS score is ≥ 11.37 Conventional nocturnal polysomnography (PSG) recordings (PROFUSION, Compumedics, Abbotsford, Victoria, Australia) were performed and evaluated by the same investigator according to the American Academy of Sleep Medicine (AASM) standard criteria (version 2.2).38 Respiratory measurements were made using an oronasal airflow thermistor and nasal pressure cannula, inductive thoracic and abdominal movements, diaphragmatic electromyography, arterial oxygen saturation (SaO2) using a pulse oximeter, inductive snoring, electrocardiogram, piezoelectric sensor for leg movements, body position recordings, and pulse transient time. Cardiorespiratory polygraph (CRP) (SOMNEA, Compumedics, Abbotsford, Victoria, Australia) included analysis of the nasal pressure cannula, inductive thoracic and abdominal movements, SaO2 using a pulse oximeter, and body position recordings. Video and audio recordings were obtained for each study, and all PSG and CRP data were collected and stored in a digital system.
Apnea was defined as a decrease of 90% in pre-event baseline airflow for at least 10 seconds detected by the oronasal thermal sensor. A differentiation was made between obstructive and central apneas according to respiratory effort channels (the presence or absence of thoracoabdominal movement). Hypopnea was defined as a ≥ 30% reduction in flow amplitude with respect to the baseline using a nasal cannula pressure sensor for a duration of at least 10 seconds and associated with either a drop in SaO2 of at least 3% or arousal. Respiratory effort-related arousal (RERA) was defined as a sequence of breaths lasting 10 seconds and characterized by increasing respiratory effort or flattening of nasal pressure waveforms, leading to arousal when the sequence of breaths did not meet criteria for apnea or hypopnea. The apnea-hypopnea index (AHI) was defined as the sum of apnea and hypopnea divided by total sleep time. The respiratory disturbance index (RDI) was defined as the sum of the number of apneas plus hypopneas and RERAs divided by total sleep time. The diagnosis of SRBD by PSG, established according to the definitions of the AASM,39 required an RDI ≥ 5 events/h, with a stratification of mild (RDI 5–14.9 events/h), moderate (RDI 15–29.9), or severe (RDI ≥ 30 events/h). We also calculated the oxygen desaturation index as a reduction in SaO2 of ≥ 3% and hypoventilation, which was defined when the cumulative percentages of time spent at a SaO2 of < 90% (CT90) were ≥ 30% of the total sleep time.
Training Dataset
This study was approved by the ethics committee and written informed consent was obtained from all patients. The 90 patients included in the study were prospectively selected from a group of nonsurgically treated patients in whom primary CM-1 was diagnosed and who had been referred to the department of neurosurgery from 2006 to 2013.27 Inclusion criteria consisted of primary CM-1 in patients aged 18 to 68 years in whom a TD ≥ 3 mm below the foramen magnum had been diagnosed in midsagittal T1W1 magnetic resonance imaging (MRI).1 The study protocol for these patients included clinical evaluation, neurological examination, cranial and spinal MRI, computed tomography of the craniovertebral junction with coronal, sagittal, and three-dimensional reconstructions, and neurophysiological examination (brainstem auditory evoked potentials, somatosensory evoked potentials, and nocturnal PSG). Medical history included clinical manifestations and a systematic interview about sleep complaints, which focused mainly on excessive daytime sleepiness. Sleepiness was evaluated using the ESS and was considered abnormal when the ESS score was higher than 11.
Patients were excluded if they presented with complex craniovertebral junction malformation, defined as the coexistence of tonsillar herniation, and at least three of the following abnormalities: significant retroflexed odontoid, basilar invagination (BI), platybasia, severe bone abnormalities in the C0-C2 complex, unilateral or bilateral occipital condyle hypoplasia, atlanto-occipital assimilation, and other abnormalities that cause anterior compression of the cervicomedullary junction. Additional exclusion criteria were: adenoidal or tonsillar hypertrophy (tonsil size ≥ 3 of the Friedman stage classification),40 advanced congestive failure, the use of psychotropic medication—or other medications—that affect sleep and/or SRBDs, or previous surgery at the craniovertebral junction.
Validation Dataset
Our validation cohort was selected from 190 patients with CM-1 admitted to the Neurosurgery Department between January 1, 2013 and June 31, 2017. Of these 190 patients, 63 were excluded because they were children (age younger than 18 years) and 48 because they did not meet the inclusion criteria. Therefore, the potential number of patients was 79. Of them, only 5 patients declined to participate; therefore, 74 were included in the final validation cohort. Sixty patients underwent in-home CRP, and 14 were studied by conventional PSG. This study was approved by the ethics committee of the Vall d'Hebron University Hospital.
Brain and Spinal Magnetic Resonance Image Protocol
In Text S1 in the supplemental material, we show the neuroradiological studies conducted and a reduced version of the more comprehensive craniometric parameters, previously used and published by our group, that were evaluated in the two statistical models3,41,42 (Figure 1).
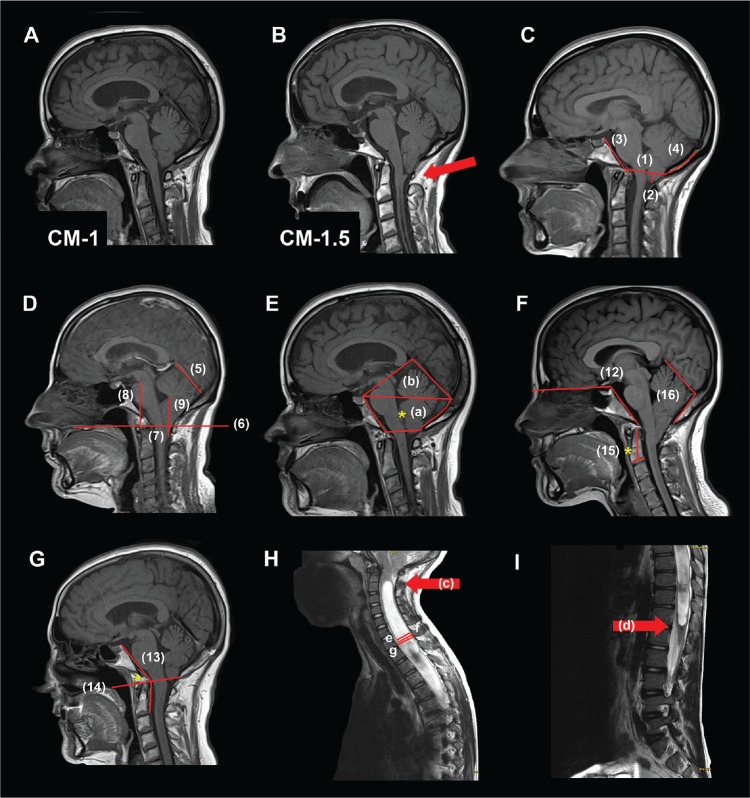
Figure 1. Measurements.
(A) Chiari malformation type 1 (CM-1): tonsillar descent (TD) ≥ 3 mm below the foramen magnum (FM) and the obex located above the level of the FM. (B) Chiari malformation type 1.5 (CM-1.5): TD ≥ 3 mm below the FM and obex located below the level of the FM. (C, D, E) Morphometric measurements made on mid-sagittal T1WI. Linear and planimetric parameters: (C-1) diameter FM; (C-2) cerebellar TD respect to the McRae line; (C-3) clivus length; (C-4) suboccipocium length; (D-5) tentorium length; (D-6) basal line (BL); (D-7) cerebellar TD respect to BL; (D-8) pons length; (D-9) fastigium length; (E-a) the osseous area of the posterior cranial fossa (PCF); (E-a+b) total PCF area. Angular measurements: (F) (F-12) basal angle; (G) (G-13) Wackenheim angle: (G-14*) basilar impression respect to the Chamberlain (F-15*) odontoid angle; (F-16) tentorium-occipital angle. Syringomyelia and spinal measurements: (H) (H-c, arrow) syringomyelia superior limit; (I) (I-d, arrow) syringomyelia inferior limit. Syringomyelia length = distance between superior and inferior limit. (H-e) Syringomyelia antero-posterior (AP) diameter; (H-f) spinal cord diameter: maximal diameter of the cord in the same slice that maximal diameter of the cavity in millimeters; (H-g) maximum spinal canal diameter. More specifications and descriptions of the morphometric measures are included in Text S1 in the supplemental material.
Statistical Analysis
Descriptive statistics were obtained for each variable. The Shapiro-Wilk test and inverse probability plot were used to test whether data followed a normal distribution. To compare between-group differences (in categorical variables) χ2 statistics or the Fisher exact test were used as appropriate. Between-group differences were determined by an independent two-sample t test or the Mann-Whitney U test, depending on assumptions on statistical distribution. To correlate two continuous variables, Kendall tau (when data did not follow a normal distribution) or Pearson correlation test (for data following a normal distribution) was used. Unless otherwise specified, differences were considered statistically significant when P ≤ .05. Statistical analyses were carried out with Microsoft enhanced R distribution (Microsoft R Open 3.4.1, Microsoft corporation, Redmond, Washington, United States; https://mran.microsoft.com) and the integrated development environment R Studio v1.0.153 (RStudio, Inc., Boston, Massachusetts, United States). The following R packages were used in the analysis: XLConnect 0.2.13, gmodels 2.16.2, dplyr 0.7.2, rcompanion 1.10.1, caret 6.0.76, and partykit.
Supervised Machine Learning Approaches
Logistic Regression Model
The purpose of MLR in this study was to isolate the relationship between predictors and the outcome variable from the effects of covariates. The outcome variable was described as an RDI equal to or above 10 events/h, corresponding to a mild SRBD (RDI 5–15 events/h) according to the AASM.39 The rationale for this threshold is explained in the Discussion section. For this model, the absence of a clinically significant sleep disorder (RDI < 10 events/h) was coded as 0 and coded as 1 when the RDI was above or equal to 10 events/h. To conduct this analysis, we applied the general linear model (glm) function in R using the binomial family. Preselected input variables (Table S2 and Table S3 in the supplemental material) were introduced into the model according to the method suggested by Hosmer et al.43 Our goal was to obtain the best-fitting model while minimizing the number of parameters.43 In brief, risk factors in a continuous scale for the preselected RDI cutoff were individually tested by univariate analysis. Categorical variables were tested for significance via a standard contingency table analysis of the outcome (y = 0, 1) versus the k levels of the independent variable. Significance was tested with the Pearson chi-square test. Independent predictors for an RDI ≥ 10 events/h were age as well as all morphometric parameters, planimetric parameters, and syringomyelia measurements. All variables with P < .25 in the univariate analysis were then entered in an MLR analysis.43 Variables that were not statistically significant at P < .05 were eliminated and a new model was generated without them.
In the third step, variables excluded in the univariate analysis were added individually to the final model to test statistical significance. According to Hosmer et al., this step is crucial for identifying variables that by themselves were not significantly related to the outcome, but could be important contributors to the final model in the presence of other variables.43 In the final model, the original coefficients, their statistical significance, the 95% confidence intervals (CI), and the odds ratio (OR) were reported. A two-tailed value of P < .05 was considered statistically significant for the MLR. Nagelkerke pseudo R-squared values were used as a goodness-of-fit measurement for the final model. Pseudo R-squared values range from 0 to 1, with higher values indicating a better model fit.
Conditional Inference Trees
In addition to the conventional logistic regression method, we used URP-CTREE technique developed by Hothorn et al.33 This method is an unbiased recursive partitioning tree-structured regression tool that identifies homogeneous subgroups from within an initial heterogeneous population. To conduct this analysis, we used the ctree function implemented in the partykit R package, a toolkit for representing, summarizing, and visualizing tree-structured regression and classification models.44 In brief, ctree performs an exhaustive search of all possible splits of the input variables and selects the covariates that show the best split.33 For this model, we used an RDI ≥ 10 events/h to define the binary outcome. R code is available upon request from the corresponding author.
The accuracy of the two models was evaluated both internally and externally in a second validation cohort using the following metrics: (1) confusion matrices with accuracy, sensitivity, specificity, and negative and positive predictive values; (2) the calculated area under the curve (AUC); and (3) the root mean squared error for evaluating the difference between the predicted values by a model and the observed values.
RESULTS
Training and Validation Cohorts
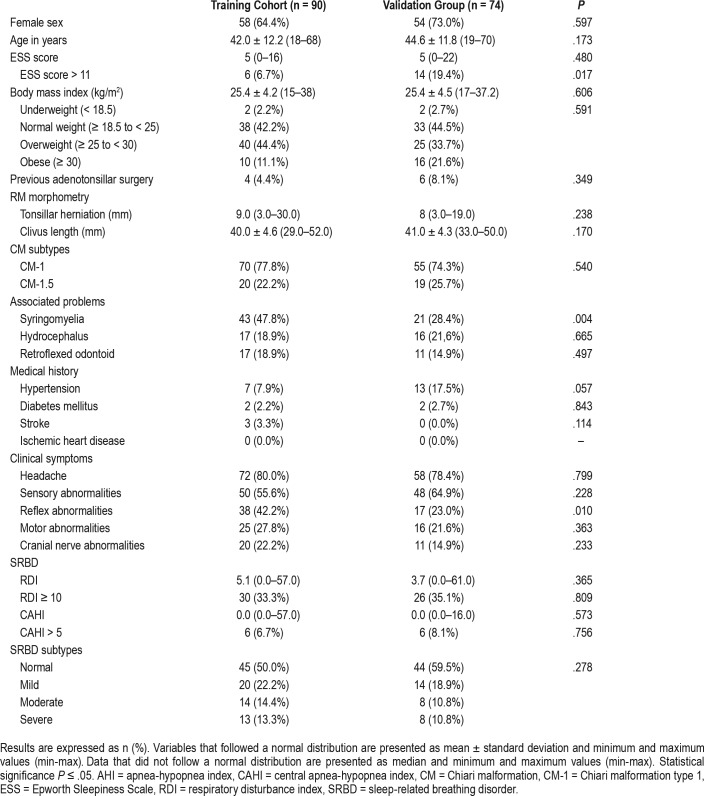
The demographic, clinical, and craniometric data obtained from the CM training group (n = 90) are summarized in Table 1. Briefly, 64.4% of the participants were women, with a mean age of 42.0 ± 12.2 years. The median TD of the entire group was 9 mm (min: 3, max: 30). Of the entire cohort, 77.8% of patients (n = 70) were classified as CM-1 and 22.2% (n = 20) were classified as CM-1.5. Only two patients in both groups received a previous diagnosis of SRBD and were on continuous positive airway pressure (CPAP) therapy. In these patients, our studies were conducted after CPAP treatment was temporarily cancelled for 2 weeks. Previous adenotonsillectomy was reported in four patients in the training group and six in the validation group (Table 1).
Table 1.
Demographic and clinical data in the training and validation groups of patients with CM-1.
Logistic Regression Analysis
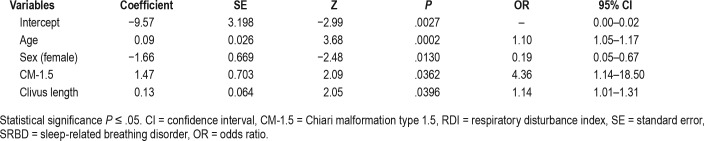
As described in the Material and Methods section, all variables were statistically significant when P < .25 in a univariate analysis and some clinically relevant variables (age, sex, BMI, Chiari type, ESS score, etc.) were included in the first multivariate logistic regression model. In total, 39 covariates were entered (Table S2 and Table S3 in the supplemental material). Only 18 covariates were statistically significant and added to the full model. These variables consisted of age, sex, BMI, ESS score, Chiari type, Evans index, total bone area of the posterior fossa, basal angle, clivus length, fastigium and pons distances to the basal line, Wackenheim angle, and the superior limit of the syringomyelia. In a second iteration, a new reduced model fit was tested using the covariates that were statistically significant (P ≤ .05) in the full model. Deleting, refitting, and verifying covariates was continued until all relevant variables were included in the model, with those excluded being clinically and/ or statistically irrelevant.43 The final model retained only four variables: age, sex, Chiari subtype, and clivus length. The adequacy of the final model gave a log-likelihood of −44.18 (df = 5) and an AIC of 98.35. The Nagelkerke pseudo R-squared value was 0.35 for the final model. A summary of the coefficients and the OR for the final model is shown in Table 2.
Table 2.
Multiple logistic regression predicting the probability of SRBD (RDI ≥ 10 events/h).
Post Hoc Logistic Regression Analysis With an RDI Cutoff of 5 events/h
Despite our primary endpoint being the detection of patients with an RDI ≥ 10 events/h, we conducted an additional MLR post hoc analysis using the lower traditional cutoff of 5 events/h that corresponds to a mild sleep disturbance according to the AASM.39 The methods and results are shown in Text S1 and Table S4 in the supplemental material.
Conditional Inference Trees
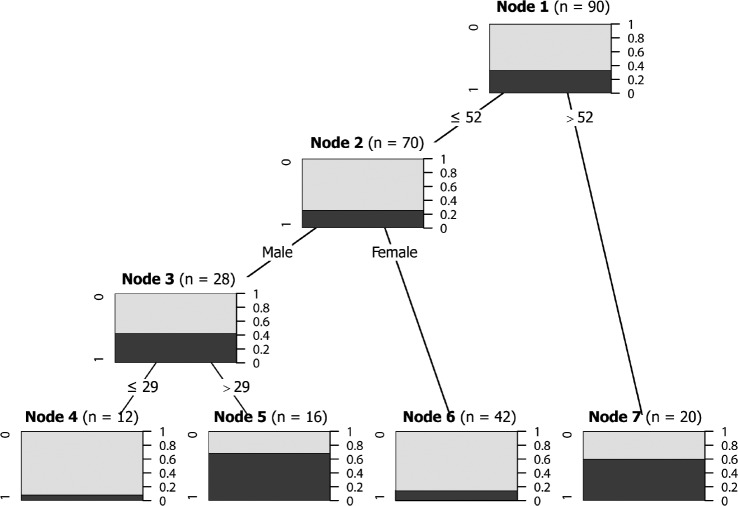
The first approach to build this model included the same 18 variables entered in the logistic regression model. However, when the entire set of 18 variables entered in the ctree function was implemented in the partykit R package, the algorithm was unable to fit any model. In a second iteration, the covariates found in the final MLR were entered, and the ctree function developed a conditional inference tree with four terminal nodes (Figure 2). The model retained age and sex as relevant variables, but excluded Chiari type and clivus length as irrelevant in the recursive partitioning tree. The URP-CTREE algorithm led to a partition of the initial cohort into three subgroups based on age and sex, with four final nodes (nodes 4 to 7).
Figure 2. Conditional inference tree for the training dataset for predicting RDI ≥ 10 events/h.
For each node, the Bonferroni-adjusted P values are given and the fraction of patients with CM-1 with RDI > 10 events/h is displayed for each terminal node. In the terminal nodes, black shading indicates the probability of an RDI ≥ 10 events/h for a specific subgroup of patients. A multiple testing-adjusted P value is given, which describes the strength of the statistical association between the early predictor characteristic (age and sex) and the outcome (RDI ≥ 10 events/h). The four plots at the bottom show the sample size and the distribution of the clinical endpoint (RDI ≥ 10 events/h) for each subgroup. In these plots, 0 equals absence of the clinical endpoint and 1 equals RDI ≥ 10 events/h. CM-1 = Chiari malformation type 1, RDI = respiratory disturbance index.
Internal and External Validation of the Models
Both MLR and URP-CTREE were validated by a second independent cohort (n = 74). The demographics and clinical and craniometric parameters of the validation cohort are also shown in Table 1. The only statistically significant differences between the training and validation groups were frequency of an ESS score > 11 (higher in the validation group), abnormal reflexes (higher in the training group), and a lower frequency of syringomyelia in the validation group (Table 1).
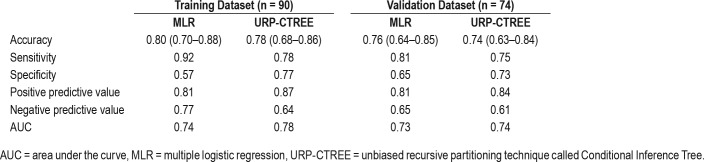
Goodness of fit was evaluated internally and externally using the following metrics: confusion matrices with accuracy, sensitivity, specificity, and negative and positive predictive values, and the calculated area under the curve of the predicted values versus the true responses with both models. These metrics are summarized in Table 3. The accuracy in the training dataset was 0.80 (95% CI 0.70–0.88) for the MLR model and 0.78 (95% CI 0.68–0.86) for URP-CTREE. Sensitivity was somewhat lower in URP-CTREE (0.78). However, specificity of the model was much greater in the URP-CTREE model (0.77) when compared to the MLR model (0.57). These changes were also reflected in the positive and negative predictive values, as shown in Table 3. Both models showed a similar acceptable discrimination, shown by the area under the receiver operating characteristic curve of 0.74 for MLR and 0.78 for URP-CTREE in the training cohort. According to Hosmer, an AUC ≥ 0.80 indicates excellent discrimination.43
Table 3.
Summary of metrics used to evaluate the goodness of fit of the multiple logistic regression and the unbiased recursive partitioning technique called Conditional Inference Tree models.
External validation was conducted in both models by applying them to the second independent cohort (n = 74). As in the training cohort, both MLR and URP-CTREE models were accurate in predicting patients with an RDI ≥ 10 events/h. However, when using the URP-CTREE, the specificity was significantly better (0.73) when compared to MLR (0.65) (Table 3).
DISCUSSION
In the current study, we demonstrate that the URP-CTREE model is not only as accurate as the MLR model for the prediction of SRBD, but it is also easier for the clinician to interpret and apply to predict SRBD in patients with CM-1. This approach may be useful in conducting patient stratification to more precisely determine the indications for surgery and screen patients at high risk of postoperative respiratory depression, thus supporting clinical decision making and implementing targeted care in these patients.
What RDI Cutoff is Clinically Relevant in CM-1?
The definition of SRBD is still arbitrary and widely heterogeneous. As Hudgel recently remarked, “as polysomnogram scoring criteria have changed over time, severity classification categories have remained constant.”45 The most widely used classification is still based on a report by an AASM Task Force published in 1999 that has not been updated since.39 In this classification, SRBDs were considered to be mild (5 to 15 events/h), moderate (15 to 30 events/h), or severe (> 30 events/h).39 However, the separation between mild and moderate degrees—as recognized by the authors of the current classification—is especially arbitrary and this split is not supported by substantial evidence.39 In addition, the scoring of apneas is quite consistent across laboratories but the metrics for scoring hypopneas may yield significantly different estimates of AHI.46 We followed the recommendations of the AASM Sleep Apnea Definitions Task Force guidelines to score hypopneas, which requires “at least a 30% decrease in a measure of airflow accompanied by either a 3% decrease in oxyhemoglobin saturation or an event-related arousal.”38
The body's response to apnea induces brain arousal and blood oxygen desaturation, and may result in sleep fragmentation and nonrestorative sleep. In this screening process, the key issue is to define clinically relevant SRBD and determine whether the screening algorithms should be fine-tuned to detect patients with mild or moderate SRBD. The health effects and the appropriate management of patients with mild SRBD in the general population (RDI of 5 to 15 events/h) is still a matter of considerable debate. However, the Sleep Heart Health Study showed that a significant relationship exists between RDI and excessive sleepiness in middle-aged and older adults.47 Excessive sleepiness increased from 21% in participants with RDI < 5 events/h to 28% in those with an RDI of between 5 and 15 events/h.47 In the general population, sleepiness has a clear effect on job performance and QoL, and it is a causal factor in motor vehicle accidents.47 In addition, two population studies have shown a clear correlation between mild SRBD and adverse cardiovascular and metabolic outcomes.47–49 Another source of confusion is that some reviews use RDI and AHI interchangeably; however, RDI includes not only apneas and hypopneas but may also include other types of breathing irregularities and therefore a patient's RDI can be higher than the AHI.
Some studies have shown that CM-1 has a significant effect on self-perceived QoL.50,51 Mueller and Oro reported that 68% of patients with CM-1 reported difficulty sleeping and 61% memory and concentration problems.50 Whether or not SRBDs are a significant contributor to many of the less objective complaints in patients with CM-1 (fatigue, lack of concentration, insomnia, etc.) is still unknown and merits further clarification. In designing the model for screening patients, we decided to establish the cutoff at the middle of the mild category, with an RDI ≥ 10 events/h. This cutoff represents a tradeoff between referring too many patients to the sleep specialist and missing patients with relevant SRBD. Although it is widely accepted that in the management of patients with mild RDI CPAP is not indicated, there is a general consensus that in these patients interventions such as weight loss, sleep hygiene, avoidance of alcohol and respiratory depressant drugs, positional therapy, etc. should be considered.52 We found the combined prevalence of mild SRBD was ∼50% in both training and validation groups. This prevalence is consistent with other reports in more selected populations.27 In a prospective study, Botelho et al. found in 32 patients with craniovertebral junction malformations— including patients with CM-1—that 59% had, minimally, mild SRBD (AHI > 5 events/h).29 In another cohort of patients with CM that included both CM-1 and CM-2, Dauvilliers et al. found an AHI ≥ 5 events/h in 70% of patients with CM-1.53
Comparing Screening Models
The URP-CTREE methodology developed by Hothorn et al. is an unbiased recursive partitioning tree-structured regression tool that can identify homogeneous subgroups from an initial heterogeneous population.33,36,44 This method has been shown to have a prediction accuracy that is similar to that of MLR in stratifying a cohort of patients with traumatic spinal cord injury.34 In our study, the main advantages of this technique over MLR were the simplification of the model obtained and improved specificity.
Both MLR and URP-CTREE models were accurate in predicting patients with an RDI ≥ 10 events/h. However, the URP-CTREE had a moderate increase in specificity (0.73) when compared with MLR (0.65), and it was also easier to apply to the clinical setting. Therefore, we believe that patients with CM-1 and risk factors for SRBD (mainly age and sex) require a comprehensive assessment of sleep and breathing, even if they are asymptomatic, do not report sleepiness, and have a normal BMI. The model defined by URP-CTREE showed that any patient with CM-1 with an age of 52 years or older should be referred for sleep studies (PSG or CRP). In addition, in males older than 29 years who have CM-1, a sleep study should be indicated regardless of BMI or symptoms. Males younger than 29 and females younger than 52 years without symptoms (a normal ESS score) may not require screening for SRBD. In our study, age was a strong predictor of SRBD in patients with CM-1. Males younger than 29 years and females younger than 52 years without symptoms (a normal ESS score) may not require screening for SRBD. In our study, age was a strong predictor of SRBD in patients with CM-1. This is consistent with Dauvilliers et al., who reported that age was the only independent predictor of SRBD.53 Our study also supports the finding that BMI did not account for the ability to predict a respiratory event index in patients with CM, which, as suggested by these authors, provides indirect evidence for a causal relationship between CM and SRBD.53 In both models, age and male sex were significant predictors of SRBD. This finding is in line with the general literature. In a population study conducted in Spain in noninstitutionalized individuals aged 30 to 70 years and with an RDI ≥ 10 events/h, the prevalence was 19% for men and 14.9% in women.22 A recent systematic review, conducted in the general adult population aged 18 years or older using an RDI ≥ 5 events/h as a criterion showed that the prevalence of SRBD ranged from 13% to 33% in men and 6% to 19% in women, with an increased risk with age in both sexes.54
Pathophysiology of Sleep Apneas in CM-1
Central apneas are infrequent in patients with CM-1, and this was reconfirmed in the current study. Most apneas in patients with CM-1 or CM-2 have been reported to be predominantly obstructive or mixed.6,27,28,31 Central apneas in CM-1 would appear to be easily explained by the depression of the respiratory centers, the reticular activating system, and reduced ventilatory chemosensitivity of the peripheral chemoreceptors or interference with their afferent pathways.55,56 However, the etiology of obstructive sleep apnea (OSA) is much more difficult to justify in the absence of obesity and its pathophysiology remains unclear. As remarked in the crucial studies on the embryogenesis of CM-1 conducted by Marin-Padilla, cephalic axial skeletal-neural dysraphic disorders of any severity involve abnormalities in the viscerocranium and therefore the facial skeleton and the oropharynx.2,57 He showed that children with CM have a small oral cavity and an apparently large tongue that “seems to fill the entire mouth.”57 In these specimens, he also found that the pharyngeal cavity was small and short and the larynx and epiglottis were slightly elevated.57 Marin-Padilla hypothesized that these oropharyngeal defects were secondary deformations resulting from the adaptation of the facial skeleton to a primarily short axial basicranium.57 In healthy children, SRBD is less frequent than in adults. It affects 1% to 4% of healthy children and the criteria for diagnosis differ from those used in adults.58 Selvadurei et al. conducted a retrospective study of syndromic and non-syndromic children with SRBD that underwent MRI.58 They found that in nonsyndromic children with abnormal brain MRI studies, the most common abnormal brain MRI finding was CM-1 (88% of the group).58 In another retrospective study, Amin et al. found that in children with CM-1, the prevalence of SRBD was 49%, and most of them were obstructive.59
In a case-control study involving 76 adult patients with CM-1, Urbizu et al. showed that the oropharynx and oral cavity were abnormal in these patients.60 This study found in these patients that the soft palate was longer and thinner, with a marked reduction in the oral cavity area, and the epiglottis had a lower position when compared to participants in the control group.60 Based on these findings, the structural anomalies of the oropharynx may explain the frequent obstructive SRBD observed in these patients, but because they do not change a few months after surgery, they cannot therefore explain the postsurgical improvement of symptoms.
In a pivotal study in patients with craniovertebral junction malformations, Botelho et al. showed that surgery resulted in improved respiratory events during sleep, lowered sleep fragmentation, and decreased the sleep apnea index in a significant number of patients.21 In a pilot study conducted at our institution (results in preparation), we have confirmed their findings. In 62% of our patients: SRBD improved significantly or even disappeared after surgery. This make the causal relationship between SRBD and CM-1 very likely, but considering that the abnormalities described by Marin Padilla and confirmed by Urbizu et al. do not change in the months following surgery, explaining the pathophysiology of sleep apneas in patients with CM remains a challenge. An alternative hypothesis, as suggested by Botelho et al., is that the etiopathogenesis of OSAS could be related to direct dysfunction of the motor efferent cranial nerves (IX, X, XI and/or XII), possibly leading to muscle atrophy and increasing the tendency toward pharyngeal collapse.6
Study Limitations
The main limitation of our study is that both our training and validation cohorts were referred to our neurosurgical department, which has a research program in CM-1. Consequently, many patients were referred because of an incidentally found Chiari malformation when studying headache, through familiar screening, or because of other reasons. These patients were asymptomatic and neurologically intact or had vague complaints, without any specific symptom potentially related to the diagnosis of CM-1. Thus, the prevalence of SRBD may be underestimated in patients with CM-1 with neurological abnormalities. Another limitation is that in the validation cohort, CRP was for the most part performed instead of PSG. CRP is an accepted method for SRBD diagnosis but cannot be used to determine RERAs.61 However, in our previous study, the prevalence of RERA in the CM-1 population was negligible, and therefore we believe this difference does not affect our results.27 An additional limitation is that objective measurements of the craniofacial structures were not included in either the training or in the validation cohort despite their wide acceptance as relevant factors in the pathophysiology of OSA. We did not include these parameters in the analysis because our main goal was to correlate the volume of the posterior cranial fossa and the neural compression of the brainstem with the SRDBs in patients with CM-1. Further studies in this population should include these parameters to clarify the complex pathophysiology of the SRBD in these patients. In addition, both cohorts in the current study were adults, and therefore our screening algorithms are only valid for adults, and not children, with CM-1.
CONCLUSIONS
Our study agrees with other reports that show that the prevalence of SRBD in adults with CM-1 is high. Undiagnosed SRBD in these patients is associated with significant morbidity and risks in patients that are not candidates for or do not accept surgical treatment. In addition, it is crucial for neurosurgeons and anesthesiologists to evaluate the presence and severity of SRDB in order to optimize perioperative management (such as ventilator support, reducing the use of drugs that depress the respiratory drive, and other factors). The potential effect on their clinical evolution, selecting patients for surgery, and reducing the risk of postsurgical complications has raised awareness of SRBD in these patients. Respiratory depression and failure, with some fatal outcomes, have been reported as a frequent complication of surgical treatment.62
Despite this, PSG is still not routinely considered in the workup of patients with CM-1. One of the reasons for this is that many institutions have limited access to PSG. Therefore, in the routine clinical workup for patients with CM-1, clinicians should identify patients with a high risk of clinically relevant SRBD when recommending referral for a sleep study—ideally PSG—based on this pretest likelihood. The current study proposes an algorithm than can guide the triage of patients with CM-1 using the age and sex of the patient. The URP-CTREE model found that patients with CM-1 with an age older than or equal to 52 years and patients with CM-1 who are male and older than 29 years, regardless of BMI, had a high risk of SRBD. Males younger than 29 years and females younger than 52 years had a low risk of SRBD, and therefore sleep studies to screen for SRBD may not be indicated.
DISCLOSURE STATEMENT
The Neurotraumatology and Neurosurgery Research Unit is supported by a grant from the Departament d'Universitats, Recerca i Societat de la Informació de la Generalitat de Catalunya (SGR 2014-844). This work has also been supported in part by the Fondo de Investigación Sanitaria (Instituto de Salud Carlos III), grant number PI13/02397, which was co-financed by the European Regional Development Fund (ERDF) and awarded to Dr. M.A. Poca. Dr. Urbizu was the recipient of a postdoctoral fellowship from Fundación Ramón Areces (Spain). The nongovernmental organization Asociación Nacional de Amigos de Arnold-Chiari generously donated money to support this research. All authors declare that they have no financial conflicts of interest. All work associated with this study was performed at Vall d′Hebron University Hospital, Barcelona, Spain.
ACKNOWLEDGMENTS
This study was carried out as part of a doctoral thesis by one of the authors (A.F.) at the Universitat Autònoma de Barcelona. The authors thank T. Hothorn, Professor of the Division of Biostatistics at the Institute for Social and Preventive Medicine, University of Zurich, Switzerland and author of the partykit package, for helping us in resolving questions and applying URP-CTREE to our data. The statistical analysis and the potential error are however the sole responsibility of the manuscript authors. Author contributions to the study and manuscript preparation include the following: (1) conception and design by Ferré, Poca, and Sahuquillo; (2) acquisition of data: Ferré, Moncho, Poca, and Sahuquillo; (3) analysis and interpretation of data: Ferré, Sahuquillo, Poca, and Moncho; (4) statistical analysis: Ferré, Sahuquillo; (5) drafting of the article: Ferré, Sahuquillo, Moncho, and Poca; (6) critical revision of the article: all authors; (7) review of the submitted version of manuscript: all authors; (8) approval of the final version of the manuscript on behalf of all authors: Sahuquillo; and (9) study supervision: Sahuquillo.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- AUC
area under the curve
- BI
basilar impression
- BMI
body mass index
- CI
confidence interval
- CM
Chiari malformation
- CPAP
continuous positive airway pressure
- CRP
cardiorespiratory polygraph
- CT90
cumulative percentages of time spent at an SaO2 of < 90%
- EMG
electromyogram
- ESS
Epworth Sleepiness Scale
- FM
foramen magnum
- MLR
multiple logistic regression
- OR
odds ratio
- OSA
obstructive sleep apnea
- PCF
posterior cranial fossa
- PSG
polysomnography
- QoL
quality of life
- RDI
respiratory disturbance index
- RERA
respiratory effort-related arousal
- SaO2
arterial oxygen saturation
- SRBD
sleep-related breathing disorders
- TD
descent of the cerebellar tonsils
- URP-CTREE
Unbiased Recursive Partitioning Technique Conditional Inference Tree
REFERENCES
- 1.Barkovich A, Wippold F, Sherman J, Citrin C. Significance of cerebellar tonsillar position on MR. AJNR Am J Neuroradiol. 1986;7(5):795–799. [PMC free article] [PubMed] [Google Scholar]
- 2.Marin-Padilla M, Marin-Padilla T. Morphogenesis of experimentally induced Arnold-Chiari malformation. J Neurol Sci. 1981;50(1):29–55. doi: 10.1016/0022-510x(81)90040-x. [DOI] [PubMed] [Google Scholar]
- 3.Urbizu A, Poca MA, Vidal X, Rovira A, Sahuquillo J, Macaya A. MRI-based morphometric analysis of posterior cranial fossa in the diagnosis of chiari malformation type I. J Neuroimaging. 2014;24(3):250–256. doi: 10.1111/jon.12007. [DOI] [PubMed] [Google Scholar]
- 4.Sahuquillo J, Poca MA. [Current surgical treatment of Chiari type I malformation and Chiari I- syringomyelia complex] Neurologia (Barcelona, Spain) 1998;13(5):223–245. [PubMed] [Google Scholar]
- 5.Milhorat TH, Chou MW, Trinidad EM, et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44(5):1005–1017. doi: 10.1097/00006123-199905000-00042. [DOI] [PubMed] [Google Scholar]
- 6.Botelho RV, Bittencourt LRA, Rotta JM, Tufik S. Adult Chiari malformation and sleep apnoea. Neurosurgical Rev. 2005;28(3):169–176. doi: 10.1007/s10143-005-0400-y. [DOI] [PubMed] [Google Scholar]
- 7.Ali MM, Russell N, Awada A, McLean D. A cranio-cervical malformation presenting as acute respiratory failure. J Emerg Med. 1996;14(5):569–572. doi: 10.1016/s0736-4679(96)00129-1. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez D, Requena I, Arias M, Valdes L, Pereiro I, De lT. Acute respiratory failure as the first sign of Arnold-Chiari malformation associated with syringomyelia. Eur Respir J. 1995;8(4):661–663. [PubMed] [Google Scholar]
- 9.Tsao TC, Juang YC, Chiang YC, Tsai YH, Lan RS, Lee CH. Pneumonia preceding respiratory failure. A rare, easily misleading clinical manifestation in adult Arnold-Chiari malformation. Chest. 1991;99(5):1294–1295. doi: 10.1378/chest.99.5.1294. [DOI] [PubMed] [Google Scholar]
- 10.Nogues MA, Gene R, Encabo H. Risk of sudden death during sleep in syringomyelia and syringobulbia. J Neurol Neurosurg Psychiatry. 1992;55(7):585–589. doi: 10.1136/jnnp.55.7.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwabuchi K, Miyauchi T, Kyuuma Y, Hosaka H, Kunimi Y, Yagishita S. [A sudden-death in a case of Arnold-Chiari malformation (type I) with sleep apnea] No To Shinkei. 1985;37(6):575–581. [PubMed] [Google Scholar]
- 12.Quebada PB, Duhaime AC. Chiari malformation Type I and a dolichoodontoid process responsible for sudden cardiorespiratory arrest. Case report. J Neurosurg. 2005;103(6 Suppl):567–570. doi: 10.3171/ped.2005.103.6.0567. [DOI] [PubMed] [Google Scholar]
- 13.Stephany JD, Garavaglia JC, Pearl GS. Sudden death in a 27-year-old man with Chiari I malformation. Am J Forensic Med Pathol. 2008;29(3):249–250. doi: 10.1097/PAF.0b013e31817efaf6. [DOI] [PubMed] [Google Scholar]
- 14.Rocker GM, Macaulay MA, Sangalang V. Sudden death and Chiari malformations. Intensive Care Med. 1995;21(7):621. doi: 10.1007/BF01700174. [DOI] [PubMed] [Google Scholar]
- 15.Martinot A, Hue V, Leclerc F, Vallee L, Closset M, Pruvo JP. Sudden death revealing Chiari type 1 malformation in two children. Intensive Care Med. 1993;19(2):73–74. doi: 10.1007/BF01708364. [DOI] [PubMed] [Google Scholar]
- 16.Adelman S, Dinner DS, Goren H, Little J, Nickerson P. Obstructive sleep apnea in association with posterior fossa neurologic disease. Arch Neurol. 1984;41(5):509–510. doi: 10.1001/archneur.1984.04050170055017. [DOI] [PubMed] [Google Scholar]
- 17.Krieger AJ. Respiratory failure as a surgical risk in patients with hindbrain anomalies. Heart Lung. 1973;2(4):546–551. [PubMed] [Google Scholar]
- 18.Zolty P, Sanders MH, Pollack IF. Chiari malformation and sleep-disordered breathing: a review of diagnostic and management issues. Sleep. 2000;23(5):637–643. [PubMed] [Google Scholar]
- 19.Sahuquillo J, Rubio E, Poca MA, Rovira A, Rodriguez-Baeza A, Cervera C. Posterior fossa reconstruction: a surgical technique for the treatment of Chiari I malformation and Chiari I/syringomyelia complex--preliminary results and magnetic resonance imaging quantitative assessment of hindbrain migration. Neurosurgery. 1994;35(5):874–884. doi: 10.1227/00006123-199411000-00011. discussion 884–875. [DOI] [PubMed] [Google Scholar]
- 20.Sahuquillo J, Poca MA, Rovira A, Raspall G, Chasampi A. Skull Base Surgery: Anatomy, Diagnosis and Treatment. Basel, Switzerland: Karger; 1994. A new surgical technique for the treatment of Chiari I malformation and Chiari I/ syringomyelia complex: preliminary results in 10 patients. In: Samii M, ed; pp. 1126–1129. [Google Scholar]
- 21.Botelho RV, Bittencourt LR, Rotta JM, Tufik S. The effects of posterior fossa decompressive surgery in adult patients with Chiari malformation and sleep apnea. J Neurosurg. 2010;112(4):800–807. doi: 10.3171/2009.7.JNS09174. [DOI] [PubMed] [Google Scholar]
- 22.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3 Pt 1):685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 23.Baldwin CM, Griffith KA, Nieto FJ, O'Connor GT, Walsleben JA, Redline S. The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep. 2001;24(1):96–105. doi: 10.1093/sleep/24.1.96. [DOI] [PubMed] [Google Scholar]
- 24.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157(15):1746–1752. [PubMed] [Google Scholar]
- 25.Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. 2014;10(4):355–362. doi: 10.5664/jcsm.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am College Cardiol. 2013;62(7):610–616. doi: 10.1016/j.jacc.2013.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferré Á, Poca MA, de la Calzada MD, et al. Sleep-related breathing disorders in Chiari malformation type 1. A prospective study of 90 patients. Sleep. 2017;40(6) doi: 10.1093/sleep/zsx069. [DOI] [PubMed] [Google Scholar]
- 28.Ferré Masó A, Poca MA, de la Calzada MD, Solana E, Romero Tomás O, Sahuquillo J. Sleep disturbance: a forgotten syndrome in patients with Chiari I malformation. Neurologia. 2014;29(5):294–304. doi: 10.1016/j.nrl.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Botelho RV, Bittencourt LR, Rotta JM, Tufik S. A prospective controlled study of sleep respiratory events in patients with craniovertebral junction malformation. J Neurosurg. 2003;99(6):1004–1009. doi: 10.3171/jns.2003.99.6.1004. [DOI] [PubMed] [Google Scholar]
- 30.Gagnadoux F, Meslier N, Svab I, Menei P, Racineux JL. Sleep-disordered breathing in patients with Chiari malformation: improvement after surgery. Neurology. 2006;66(1):136–138. doi: 10.1212/01.wnl.0000191394.53786.62. [DOI] [PubMed] [Google Scholar]
- 31.Henriques-Filho PS, Pratesi R. Sleep apnea and REM sleep behavior disorder in patients with Chiari malformations. Arq Neuropsiquiatr. 2008;66(2b):344–349. doi: 10.1590/s0004-282x2008000300012. [DOI] [PubMed] [Google Scholar]
- 32.Hastie T, Tibshirani R, Friedman JH. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd ed. New York, NY: Springer; 2001. [Google Scholar]
- 33.Hothorn T, Hornik K, Zeileis A. Unbiased recursive partitioning: a conditional inference framework. J Comput Graph Stat. 2006;15(3):651–674. [Google Scholar]
- 34.Tanadini LG, Steeves JD, Hothorn T, et al. Identifying homogeneous subgroups in neurological disorders: unbiased recursive partitioning in cervical complete spinal cord injury. Neurorehabil Neural Repair. 2014;28(6):507–515. doi: 10.1177/1545968313520413. [DOI] [PubMed] [Google Scholar]
- 35.Seibold H, Zeileis A, Hothorn T. Model-based recursive partitioning for subgroup analyses. Int J Biostat. 2016;12(1):45–63. doi: 10.1515/ijb-2015-0032. [DOI] [PubMed] [Google Scholar]
- 36.Zeileis A, Hothorn T, Hornik K. Model-based recursive partitioning. J Comput Graph Stat. 2008;17(2):492–514. [Google Scholar]
- 37.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 38.Berry R, Brooks R, Gamaldo C, et al. for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Darien, IL: American Academy of Sleep Medicine; 2015. Version 2.2. [Google Scholar]
- 39.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 40.Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngol Head Neck Surg. 2002;127(1):13–21. doi: 10.1067/mhn.2002.126477. [DOI] [PubMed] [Google Scholar]
- 41.Moncho D, Poca MA, Minoves T, Ferre A, Canas V, Sahuquillo J. Are evoked potentials clinically useful in the study of patients with Chiari malformation type 1? J Neurosurg. 2017;126(2):606–619. doi: 10.3171/2015.11.JNS151764. [DOI] [PubMed] [Google Scholar]
- 42.Urbizu A, Martin BA, Moncho D, et al. Machine learning applied to neuroimaging for diagnosis of adult classic Chiari malformation: role of the basion as a key morphometric indicator. J Neurosurg. 2018;129(3):779–791. doi: 10.3171/2017.3.JNS162479. [DOI] [PubMed] [Google Scholar]
- 43.Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3rd ed. Hoboken, New Jersey: Wiley; 2013. [Google Scholar]
- 44.Hothorn T, Zeileis A. partykit: a modular toolkit for recursive partytioning in R. J Mach Learn Res. 2015;16:3905–3909. [Google Scholar]
- 45.Hudgel DW. Sleep apnea severity classification - revisited. Sleep. 2016;39(5):1165–1166. doi: 10.5665/sleep.5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ho V, Crainiceanu CM, Punjabi NM, Redline S, Gottlieb DJ. Calibration model for apnea-hypopnea indices: impact of alternative criteria for hypopneas. Sleep. 2015;38(12):1887–1892. doi: 10.5665/sleep.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gottlieb DJ, Whitney CW, Bonekat WH, et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159(2):502–507. doi: 10.1164/ajrccm.159.2.9804051. [DOI] [PubMed] [Google Scholar]
- 48.Brown LK. Mild obstructive sleep apnea syndrome should be treated. Pro. J Clin Sleep Med. 2007;3(3):259–262. [PMC free article] [PubMed] [Google Scholar]
- 49.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 50.Mueller D, Oro JJ. Prospective analysis of self-perceived quality of life before and after posterior fossa decompression in 112 patients with Chiari malformation with or without syringomyelia. Neurosurg Focus. 2005;18(2):Ecp2. doi: 10.3171/foc.2005.18.2.11. [DOI] [PubMed] [Google Scholar]
- 51.Mestres O, Poca MA, Solana E, et al. [Evaluation of the quality of life of patients with a Chiari type I malformation. A pilot study in a cohort of 67 patients] Rev Neurol. 2012;55(3):148–156. [PubMed] [Google Scholar]
- 52.Littner MR. Mild obstructive sleep apnea syndrome should not be treated. Con. J Clin Sleep Med. 2007;3(3):263–264. [PMC free article] [PubMed] [Google Scholar]
- 53.Dauvilliers Y, Stal V, Abril B, et al. Chiari malformation and sleep related breathing disorders. J Neurol Neurosurg Psychiatry. 2007;78(12):1344–1348. doi: 10.1136/jnnp.2006.108779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Shiihara T, Shimizu Y, Mitsui T, Saitoh E, Sato S. Isolated sleep apnea due to Chiari type I malformation and syringomyelia. Pediatr Neurol. 1995;13(3):266–267. doi: 10.1016/0887-8994(95)00180-n. [DOI] [PubMed] [Google Scholar]
- 56.Keefover R, Sam M, Bodensteiner J, Nicholson A. Hypersomnolence and pure central sleep apnea associated with the Chiari I malformation. J Child Neurol. 1995;10(1):65–67. doi: 10.1177/088307389501000116. [DOI] [PubMed] [Google Scholar]
- 57.Marin-Padilla M. Cephalic axial skeletal-neural dysraphic disorders: embryology and pathology. Can J Neurol Sci. 1991;18(2):153–169. doi: 10.1017/s0317167100031632. [DOI] [PubMed] [Google Scholar]
- 58.Selvadurai S, Al-Saleh S, Amin R, et al. Utility of brain MRI in children with sleep-disordered breathing. Laryngoscope. 2017;127(2):513–519. doi: 10.1002/lary.26042. [DOI] [PubMed] [Google Scholar]
- 59.Amin R, Sayal P, Sayal A, et al. The association between sleep-disordered breathing and magnetic resonance imaging findings in a pediatric cohort with Chiari 1 malformation. Can Respir J. 2015;22(1):31–36. doi: 10.1155/2015/831569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Urbizu A, Ferre A, Poca MA, et al. Cephalometric oropharynx and oral cavity analysis in Chiari malformation Type I: a retrospective case-control study. J Neurosurg. 2017;126(2):626–633. doi: 10.3171/2016.1.JNS151590. [DOI] [PubMed] [Google Scholar]
- 61.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3(7):737–747. [PMC free article] [PubMed] [Google Scholar]
- 62.Paul KS, Lye RH, Strang FA, Dutton J. Arnold-Chiari malformation. Review of 71 cases. J Neurosurg. 1983;58(2):183–187. doi: 10.3171/jns.1983.58.2.0183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.