Abstract
Study Objectives:
The current archival analyses examine the direct and indirect effects of cognitive behavioral therapy for insomnia (CBT-I) on depression in cancer survivors.
Methods:
We report on 67 cancer survivors from a 2 × 2 randomized controlled trial of CBT-I and armodafinil for insomnia, after collapsing across the noneffective study medication conditions (armodafinil/placebo) to create CBT-I (yes/no). Depression and insomnia were assessed before, during the 7-week CBT-I intervention, at postintervention, and 3 months later by the Patient Health Questionnaire and the Insomnia Severity Index, respectively.
Results:
Mean depression at baseline for all participants was 6.44 (standard error = 0.41, range 0–15). Paired t tests showed that depression improved from baseline to postintervention by 48% (P < .001) in the CBT-I group versus 15% (P = .016) in the non-CBT-I group. Analysis of covariance controlling for baseline found that participants receiving CBT-I had significantly less depression at postintervention (effect size = −0.62; P = .001), compared to those who did not receive CBT-I. These benefits were maintained at the 3-month follow-up. Spearman rank correlations showed that changes in insomnia severity from baseline to postintervention were significantly correlated with concurrent changes in depression (r = .73; P < .001). Path analysis revealed that improvement in depression was mediated by improvement in insomnia severity (P < .001).
Conclusions:
Our findings provide preliminary support that in cancer survivors, CBT-I reduces depression via improvement in insomnia. Further, this reduction in depression remained stable 3 months after completing CBT-I. This suggests that a CBT-I intervention has a meaningful effect on depression.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Title: Cognitive Behavioral Therapy +/- Armodafinil for Insomnia and Fatigue Following Chemotherapy; Identifier: NCT01091974; URL: https://clinicaltrials.gov/ct2/show/record/NCT01091974
Citation:
Peoples AR, Garland SN, Pigeon WR, Perlis ML, Wolf JR, Heffner KL, Mustian KM, Heckler CE, Peppone LJ, Kamen CS, Morrow GR, Roscoe JA. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129–137.
Keywords: cancer, cancer survivors, CBT-I, depression, insomnia, sleep deficiency, sleep-wake disturbances
BRIEF SUMMARY
Current Knowledge/Study Rationale: Depression is a significant and persistent side effect of cancer and its treatment. Depression is strongly correlated with insomnia, allowing for the possibility that cognitive behavioral therapy for insomnia (CBT-I) may also reduce associated depression.
Study Impact: This study shows that in cancer survivors with insomnia, CBT-I has the potential to improve not only insomnia, but also comorbid depression through improvements in insomnia severity. In addition, this study highlights the importance of screening for insomnia in cancer survivors with depression and providing access to CBT-I as a treatment option.
INTRODUCTION
Cancer and its treatment cause short-term and long-term psychological and physical side effects, such as depression and sleep disruption, which are very common and debilitating problems. Depression includes a variety of mood disturbances and clinical presentations and may range in severity, with nonpathologic sadness at the milder end, minor or sub-threshold depression in the middle, and major depression at the more severe end of the spectrum.1 Up to 52% of individuals with cancer report depression spectrum syndromes,2 mostly reflecting differences in patient cohorts and assessment methods. Additionally, the meta-analytical pooled prevalence of major depression in individuals with cancer is approximately 15%, whereas for minor depression it is 19%,3 which is approximately three to five times greater than that found in the general population.4 Untreated depression can become chronic and persist for many years after the completion of cancer treatment, interfering with patients' ability to fully recover.5
In patients with cancer and cancer survivors, most depressive symptoms are in the subthreshold range and therefore are frequently underdiagnosed or underestimated by professionals.1 Underrecognition of depressive symptoms may result in inadequate treatment, which can put patients at risk of the development of a major depressive disorder.5 Individuals with unaddressed and untreated depression are more likely to experience exacerbation of symptom burden and less likely to exercise, and they have worsened quality of life, greater psychological burden on the family, higher suicide risk, and increased health care utilization and expenditures.1,4,6 Depression also negatively affects the body's inflammatory response7 and is an independent risk factor for cancer mortality, with a 39% greater mortality rate among patients in whom major or minor depression has been diagnosed and 26% higher mortality rate among those with depressive symptoms.8 Thus, depression is an important clinical and public health problem that is exacerbated by cancer and its treatment requiring management before it becomes severe and/or contributes to additional morbidity and mortality.
There are limited data on effective pharmacologic and psychotherapeutic treatment options for depression in individuals with cancer. The evidence for the effectiveness of currently available pharmacologic agents for treating depression is also limited in patients with cancer.1 Further, antidepressants are more effective in patients with severe depression, and they are not usually recommended for subthreshold or mild depression.1,9 Behavioral interventions targeting the risk factors of depression may be more feasible strategies, especially for milder cases of depression.10 Thus, more studies on treatment options are warranted.
Depression rarely occurs in isolation in cancer populations but commonly coexists with other symptoms, such as sleep disruption, sleep-wake disturbances, and sleep deficiency. For instance, between 30% to 87% of newly diagnosed or recently treated patients with cancer report sleep disruption.11 Additionally, 30% to 60% experience insomnia symptoms, whereas at least 20% meet the diagnostic criteria for insomnia12 with high rates of insomnia persisting for years after the completion of cancer treatments.11 Considerable data in the general population have shown that insomnia is not only associated with depression, but is an independent risk factor for its development.13 Further, a growing body of literature has shown a strong association between sleep disruption and depression in patients with cancer.14,15 One study in particular found that patients with cancer and insomnia had significantly more depression than patients without insomnia.15 The relationship between sleep disturbance and depression could be because of shared common biopsy-chosocial mechanisms, such as neuroendocrine imbalances, systemic and neuroinflammation, dysregulation of circadian rhythms, and hypothalamic-pituitary-adrenal (HPA) axis dys-function.4,10,16,17 Further, feelings of helplessness or hopelessness and the reduced ability to cope with stressors of life because of cancer diagnosis and treatment may also be common underlying factors for both the symptoms.16,17 Although the understanding of the causal relationship between insomnia and depression in individuals with cancer is limited, these two symptoms can exacerbate as well as maintain each other. Therefore, interventions targeting sleep disruption could provide a promising approach to treating depression among patients with cancer.
In the general population, cognitive behavioral therapy for insomnia (CBT-I) is considered the gold standard treatment for insomnia, resulting in sustained sleep improvements over time, and its acceptability by participants is high.18 CBT-I is a sleep-focused, multimodal intervention consisting of sleep restriction, stimulus control, cognitive restructuring, and education to reestablish a regular sleep pattern.19 Further, a recent meta-analysis of studies in the general population found that insomnia treatments, such as CBT-I, had moderate to large effects on depressive symptoms in individuals with both disorders.20 There is also growing evidence from randomized controlled trials (RCTs) that CBT-I is an effective method for reducing insomnia in individuals with cancer.21 A recent systematic review and meta-analysis of RCTs in cancer survivors showed a large effect size for the pooled self-reported insomnia severity (Cohen's d = 0.77) for those who underwent CBT-I compared to those who did not.21 Some of these studies in cancer survivors evaluated the effect of CBT-I on secondary measures such as depression, but revealed mixed results.22–26 Additionally, though one study in the general population with comorbid insomnia and depression did find that improvement in insomnia mediated the improvement in depression with no direct effects of CBT-I on depression,27 no study to date in cancer survivors has evaluated whether the effects of CBT-I on depression are mediated by improvements in insomnia.
The objectives of the current archival data analysis of a phase II RCT in cancer survivors are to: (1) examine the effect of CBT-I on depression, and (2) determine whether change in insomnia severity mediates the effects of CBT-I on depression.
METHODS
Study Design
This study is a secondary analysis from a previously completed multicenter, randomized, placebo-controlled trial evaluating interventions for insomnia in cancer survivors with chronic insomnia, where insomnia severity was the primary outcome measure.28 The parent trial was a four-arm factorial study of CBT-I (yes/no) versus armodafinil (yes/no), in which participants were randomized to one of the four intervention arms: (1) CBT-I+placebo; (2) CBT-I+armodafinil; (3) armodafinil; and (4) placebo. Randomization was performed using an online computer-generated random table with block size 8 and stratified by city and sex.
The institutional review boards of the University of Rochester and University of Pennsylvania approved the protocol, and all participants provided written informed consent. This trial is registered with ClinicalTrials.gov, number NCT01091974.
Participants
The participants in the parent trial were recruited in Rochester, New York and Philadelphia, Pennsylvania, between September 2008 and November 2012 as previously described in full detail.28 Briefly, eligible participants were survivors with any cancer type who had completed all cancer treatments not less than 1 month before study start, had no measurable disease, met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition diagnostic criteria for insomnia and that the insomnia began or became worse with the onset of cancer or treatment, and were required to discontinue any prescribed or over-the-counter sleep medications for an 11-week study period (including 2 weeks at baseline). Participants who had previous exposure to CBT-I; had taken modafinil or armodafinil; or had a history of seizures, severe headaches, hypertension, sleep apnea, substance abuse, or an unstable medical or psychiatric illness (including suicidality) were not eligible.
Intervention
CBT-I Group
The participants were randomized to CBT-I with either armodafinil or placebo. The CBT-I intervention is a multicomponent intervention and included sleep restriction, stimulus control, cognitive restructuring, and sleep hygiene guidelines. The participants randomized to CBT-I received seven weekly one-on-one sessions with therapists trained in CBT-I. The CBT-I intervention was individually tailored based on baseline sleep diaries, and closely followed a published treatment manual.19 Sessions 1, 2, and 4 (30 to 60 minutes) were conducted in person, and sessions 3, 5, 6, and 7 (15 to 30 minutes) were conducted over the telephone to reduce burden and increase retention of participants.
Non-CBT-I Group
The participants who were not randomized to the CBT-I intervention also received either armodafinil or placebo along with the sleep hygiene guidelines. The sleep hygiene guidelines (eg, keep bedroom cool and free of light, avoid naps, avoid using alcohol as sleep aid, etc.) were provided only at the time of consent and was considered as part of the waitlist condition. The non-CBT-I group did not have any sessions with the therapists during the 7-week intervention period.
Measures
On-study questionnaires and clinical record information forms were used to obtain demographic and clinical information, such as age, sex, race and ethnic background, marital status, educational background, cancer type, and previous treatments.
Patient Health Questionnaire
Depression was assessed by the Patient Health Questionnaire (PHQ-9), a 9-item patient self-report questionnaire corresponding to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition diagnostic criteria for major depressive disorder29 with well-established psychometric properties.30 The PHQ-9 is commonly used in patients with cancer for screening, diagnosing, monitoring, and measuring the severity of depression.31 For each item, the participants are asked to assess how much they were bothered by the symptoms over the past 2 weeks on a scale of 0 (not at all) to 3 (nearly every day). The total score ranges from 0 to 27 measuring the severity of depression with the following commonly used cutoffs: 0 to 4 no depression, 5 to 9 mild depression, and ≥ 10 moderate to severe depression.29 A score of > 4 on the PHQ-9 total score indicates any level of depression. Because one of the items on the PHQ-9 includes a sleep question “trouble falling asleep/staying asleep, sleeping too much,” this sleep item was excluded from PHQ-9 to also calculate PHQ-8, that is, summation of eight items.
Insomnia Severity Index
Insomnia severity was assessed by the Insomnia Severity Index (ISI), a commonly utilized seven-item psychometrically validated instrument for rating insomnia and recommended for use in cancer trials.32 Items are rated on a scale of 0 to 4, with higher summed scores (0 to 28) indicating worse insomnia.33
Participants had the option of completing the questionnaires either using paper and pen on scannable forms or using an Internet data portal. Both PHQ-9 and ISI were completed by all the participants at consent, preintervention (average of weeks 1 and 2), weekly during the intervention period, postintervention (average of weeks 10 and 11), and 3 months after the completion of the intervention, that is, 3-month follow-up (average of weeks 23 and 24). Baseline was considered average of weeks 1 and 2, because all participants were required to discontinue any sleep medications from week 1 onward as some of them were on sleep medication until consent. Treatment adherence in the parent trial was assessed by sleep diaries.
Sample Aggregation
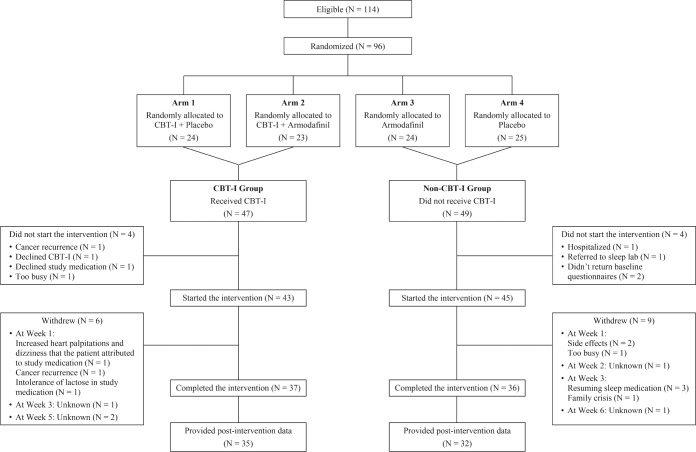
In the parent trial, 114 participants were eligible, 96 were randomized, 88 began the interventions, and 73 completed the interventions.28 We conducted an initial analysis of covariance (ANCOVA) to assess differences in depression, with CBT-I (yes/ no) and armodafinil (yes/no) as main effects, but found no significant effect of armodafinil on depression. Therefore, for the current secondary analysis, the data were collapsed across the ineffective armodafinil and placebo conditions and two groups were created: participants who received CBT-I versus those who did not (Figure 1). We included 67 participants (out of 73 who completed the interventions) in the current analysis who had no missing postintervention data for the variables of interest.
Figure 1. CONSORT diagram.
CBT-I = cognitive behavioral therapy for insomnia.
Statistical Analyses
The main outcome variable for this secondary analysis was depression at postintervention, as measured by PHQ-9 total score. Descriptive statistics were performed for all variables of interest. Percentages were calculated for categorical variables, and means and standard error (SE) for continuous variables. t tests for continuous variables and chi-square tests for categorical variables were performed on all baseline characteristics to determine if significant baseline differences existed. Means and SE were calculated for baseline, postintervention, follow-up, and change scores (baseline-post). Paired t tests were performed to assess within-group changes in depression. We used ANCOVA to assess differences in mean change in depression between the two groups (CBT-I [yes/no]), consisting of a linear model including postintervention depression as a dependent variable, baseline depression as a covariate, and CBT-I (yes/ no) as a fixed effect. The stratification variables were initially entered, but were insignificant, and therefore dropped. We also re-ran the ANCOVA analysis for the PHQ-8 (after excluding the single sleep item from PHQ-9) to assess if differences in mean change in depression between the CBT-I (yes/no) groups were consistent with the PHQ-9 analysis. Paired t tests were performed to examine changes in depression from postintervention to 3-month follow-up among the two intervention groups in order to assess if changes in depression were maintained at the 3-month follow-up after the completion of the intervention.
Spearman rank correlations were used to examine the association between depression and insomnia severity change scores from baseline to postintervention. To determine if CBT-I affected depression directly or whether its effect on depression was mediated by insomnia severity, we performed a path analysis using a structural equation model with maximum likelihood estimation. In the model, direct effects from CBT-I (yes/no) on both insomnia severity change score and depression change score were included. For the indirect effects, insomnia severity change score as a predictor of depression change score was included. Two-sided value of P ≤ .05 was considered statistically significant in all analyses. All statistical analyses were performed using SPSS version 24 (IBM Corp., Armonk, New York, United States) and STATAIC version 14 (StataCorp, College Station, Texas, United States) for analyses as appropriate.
RESULTS
Participant Characteristics
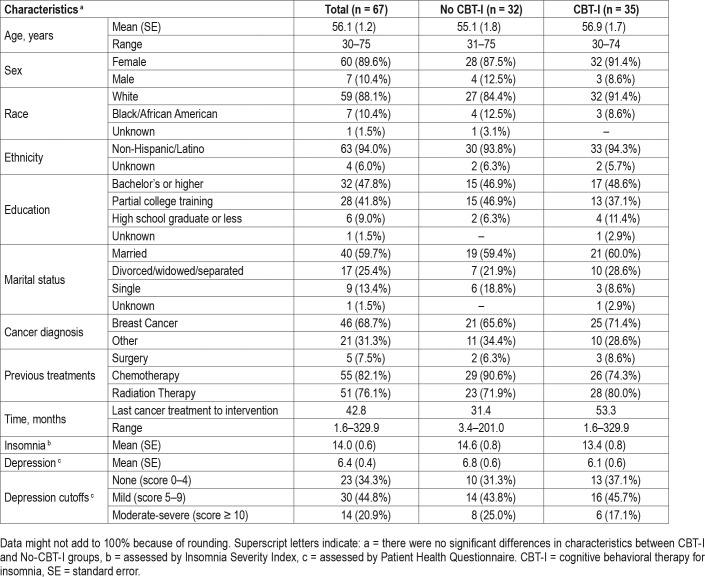
Table 1 shows the baseline characteristics of the 67 participants included in the current analyses, who had no missing postintervention data, with 35 participants who underwent the CBT-I intervention and 32 who did not. Briefly, for all 67 participants, mean age was 56 years (range 30–75), 90% were female, 88% were white, 94% were non-Hispanic or Latino, 69% were treated for breast cancer, and mean time since last cancer treatment to intervention was 43 months. Sixty-six percent of the 67 participants (n = 44) reported at least some depression (ie, score > 4) at baseline, with 21% (n = 14) having moderate to severe depression (ie, score ≥ 10). Overall mean depression at baseline was 6.4, indicating mild depression. These characteristics were not significantly different between the two groups at baseline.
Table 1.
Baseline characteristics of participants.
Effect of CBT-I on Depression
The unadjusted means for depression from baseline to follow-up for the two groups are shown in Figure 2. Post-intervention depression and insomnia severity means for the CBT-I group were 3.16 (SE = 0.55) and 4.51 (SE = 0.75), respectively; whereas for the non-CBT-I group they were 5.81 (SE = 0.66) and 11.27 (SE = 0.96), respectively. For the CBT-I group, 74% (n = 26) had no depression, 17% (n = 6) had mild depression, and 9% (n = 3) had moderate to severe depression at postintervention; for the non-CBT-I group, 38% (n = 12) had no de -pression, 50% (n = 16) had mild depression, and 13% (n = 4) had moderate to severe depression. Overall, only 26% (n = 9) of survivors reported having any depression (ie, score > 4) at postintervention in the CBT-I group compared to 63% (n = 20) in the non-CBT-I group (P = .002). Paired t tests showed that the CBT-I group had 48% reduction in depression from baseline to postintervention (mean change = 2.93; SE = 0.43; 95% confidence interval = 2.05, 3.81; P < .001), whereas the reduction in the non-CBT-I group was only 15% (mean change = 1.02; SE = 0.40; 95% confidence interval = 0.20, 1.83; P = .016).
Figure 2. Unadjusted mean depression, assessed by PHQ-9, over time by intervention groups.
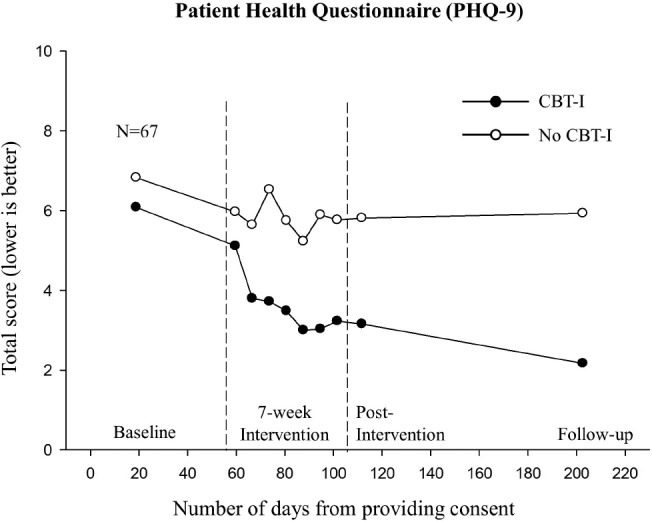
Numbers per group at baseline, postintervention, and 3-month follow-up were as follows: CBT-I group, 35, 35, and 30; non-CBT-I group, 32, 32, and 30, respectively. CBT-I = cognitive behavioral therapy for insomnia, PHQ-9 = Patient Health Questionnaire.
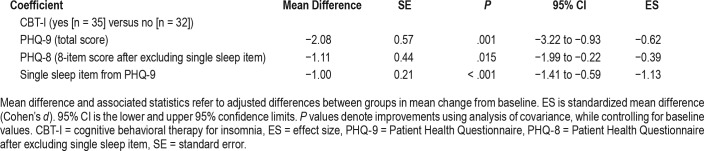
ANCOVA, controlling for baseline depression, showed that participants receiving CBT-I had significantly less depression (PHQ-9 total score) at postintervention (by 2.08 units, ie, a 38% improvement) compared to those who did not receive CBT-I (P = .001). Even after excluding the single sleep item from the PHQ-9, there was greater improvement for the PHQ-8 (8-item score) at postintervention in the CBT-I group, while controlling for baseline score, compared to those who did not receive CBT-I (P = .015). Details on the ANCOVA are pro -vided in Table 2. Descriptively, 23% (n = 8) of survivors in the CBT-I group achieved a clinically important reduction in depression (ie, ≥ 5-point reduction on the PHQ-9 total score) at postintervention, compared to 6% (n = 2) of survivors in the non-CBT-I group.
Table 2.
Comparison of depression at postintervention.
At the 3-month follow-up after completion of the intervention, the unadjusted mean depression and insomnia severity for the CBT-I group were 2.17 (SE = 0.44) and 3.80 (SE = 0.66), respectively; for the non-CBT-I group they were 5.93 (SE = 0.88) and 11.57 (SE = 1.14), respectively. For the CBT-I group, 71% (n = 25) had no depression and 14% (n = 5) had mild depression at 3-month follow-up; for the non-CBTI group, 47% (n = 15) had no depression, 25% (n = 8) had mild depression, and 22% (n = 7) had moderate to severe depression. Overall, only 14% of survivors (n = 5) reported having any depression (ie, score > 4) at 3-month follow-up in the CBT-I group versus 47% (n = 15) in the non-CBT-I group. Paired t tests showed that there was no statistically significant difference between depression at postintervention and 3-month follow-up for either group (both Ps ≥ .18). Thus, these findings indicate that improvements in depression assessed at postintervention for participants in the CBT-I were sustained at 3-month follow-up even after the completion of CBT-I.
Mediating Effect of Insomnia Severity on CBT-I's Effect on Depression
Correlational analysis showed that change in insomnia severity from baseline to postintervention was significantly associated with concurrent change in depression (r = .73, P < .001), indicating that improvement in insomnia is associated with improvement in depression. Similarly, change in insomnia severity from baseline to postintervention was significantly associated with the PHQ-8 (r = .59, P < .001), after excluding the single sleep item from PHQ-9, indicating that improvement in insomnia is associated with improvement in depression independent of the single sleep item in the PHQ-9 measure.
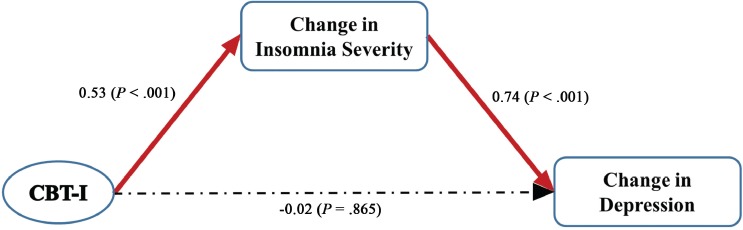
The path analysis (Figure 3) revealed that the only statistically significant direct effects (standardized path coefficient, B) were from CBT-I on the insomnia severity change score (B = 0.53; P < .001) and from the insomnia severity change score on the depression change score (B = 0.74; P < .001). CBT-I did not have a significant direct effect on the depression change score (B = −0.02; P = .865). However, CBT-I had significant indirect effect on the depression change score via the insomnia severity change score (B = 0.39; P < .001). This indicates that CBT-I has only indirect beneficial effects on depression, through its positive effect on insomnia severity. The path model fit the data very well with a root mean square error of approximation (RMSEA) < 0.001 (RMSEA < 0.01 is considered ideal). Similar results were found with PHQ-8, that is, after the single sleep item was removed from the PHQ-9 (data not shown).
Figure 3. Path diagram for the structural equation model.
Changes in insomnia severity and depression are from baseline to postintervention. Statistically significant paths are in red with standardized path coefficients marked (P ≤ .05). Dotted lines indicate nonsignificant paths. CBT-I = cognitive behavioral therapy for insomnia.
DISCUSSION
We have previously reported for this trial that our 7-week CBT-I treatment program was effective in improving insomnia severity and sleep quality,28 sleep continuity,34 fatigue,35 and quality of life36 in cancer survivors who have chronic insomnia. The goal of this secondary analysis was to assess the ability of CBT-I to alleviate depression in cancer survivors and determine whether positive effects of CBT-I on depression were mediated by reduced insomnia severity. These additional analyses showed that the intervention for insomnia also improved comorbid depression.
The results showed that survivors receiving CBT-I had a 38% greater improvement in depression at postintervention compared with those who were not treated with CBT-I. We also found that the improvement in depression remained stable 3 months after the completion of CBT-I. These findings are consistent with previous studies that have also shown a secondary benefit of CBT-I on reducing depression in cancer survivors in addition to ameliorating subjective sleep difficulties22–24 and that treatment benefits were sustained over time. One study showed the benefits were maintained up to 1 year after the intervention.23
Another important finding was that improvement in depression was due to improvement in insomnia severity via CBT-I and not due to CBT-I having a direct effect on depression. This finding is consistent with a study in breast cancer survivors by Dirksen and Epstein,37 which found through mediational analysis that CBT-I did not have a direct effect on psychosocial outcomes, such as depression, although they did not assess the indirect effect of the intervention. In their study, they only found significant improvement in depression in the CBT-I group, whereas there was no significant group difference between CBT-I and control conditions.
There could be several plausible reasons why an insomnia intervention, namely CBT-I, could lead to an improvement in depression in cancer survivors. Chief among these is that insomnia is not only comorbid with depression, but has been shown to predict new onset depression and blunt the effect of depression care.38,39 Further, the development of insomnia and depression in individuals with cancer may share common bio-behavioral mechanisms. From a psychological perspective, chronic insomnia is associated with increased fatigue and cognitive difficulties, which may reduce the ability to cope with stressors of life, thus being perceived as a lack of control.16,17 These, in turn, may lead to depressive symptoms related to helplessness and hopelessness, and may trigger neuroendocrine imbalances.16,17
Studies have shown that both sleep disruption and depression are also associated with increased inflammation, dysregulation of circadian rhythms, and HPA axis dysfunction.4,10 Cancer treatments, such as chemotherapy and radiotherapy, are known to elevate proinflammatory cytokines resulting in systemic inflammation, which may persist long after the completion of treatment.40 Sleep disruption can also further increase proinflammatory cytokines, contributing to systemic inflammation.4,10,41 These proinflammatory cytokines can dysregulate cortisol levels, cause HPA axis dysfunction, and cross the blood-brain barrier, resulting in oxidative stress and inflammation in the brain.1,10 This can lead to alternations in neurotransmitter and neuroendocrine function1,10; thereby, causing depression and behavioral alterations.42 Indeed, some studies have shown that administration of cytokine immunotherapy for cancer treatment, such as interluken-2 and/or interferon-α-2b, results in the development of depression.43 Behavioral therapies for treating insomnia may have positive effects on inflammation, immune regulation, and HPA axis activity via its positive effects on sleep.10 In an RCT in older adults with insomnia, CBT-I significantly reduced systemic inflammation and expression of genes encoding proinflammatory mediators.44 Further, Savard et al. found preliminary evidence that successful treatment of insomnia with CBT-I resulted in immune improvement in breast cancer survivors.45 Additional research is needed to determine the relationship between insomnia and depression in individuals with cancer.
The following limitations should be considered when interpreting the results from this study. First, this was a secondary data analysis of a completed multicenter randomized controlled trial that did not have treatment of depression as a focus. Despite this, participants entered the trial with depressive symptomatology worthy of clinical attention. Future longitudinal RCTs in individuals with cancer specifically designed to investigate the effect of CBT-I on clinically relevant depression as well as the links between depression and insomnia are needed. Also, future studies should examine the effect of CBT-I on mood, coping, and stress management. Second, the participants were predominantly white women of non-Hispanic ethnicity, which may limit the generalizability of these findings to men and to those with different racial and ethnic backgrounds. Further, these analyses were limited by the small sample size of the parent trial and the high dropout rates, that is, only 75% and 65% of the participants in the CBT-I and non-CBT-I groups, respectively, completed the intervention and provided postintervention data. Also, the non-CBT-I group was a waitlist condition in regard to the CBT-I and its participants did not receive an equivalent amount of attention from research staff compared to those in the CBTI group. Thus, the time and attention the participants in the CBT-I group received may be a potential confounder. Last, improvements in depression and insomnia severity were measured at the same time, thus limiting firm conclusions about the directionality of the relationship. Nonetheless, there were several strengths, namely treatment randomization, the use of reliable and validated measures, and longitudinal data with 3-month follow-up.
Clinical Implications
Considerable advances in the treatment of cancer have been made during the past few decades, but attention to the physical and psychological symptoms of cancer and its treatment has been given less priority in clinics. The current study has important clinical implications. It provides additional evidence of the link between depression and insomnia, indicating the importance of screening for both symptoms in cancer survivors, as survivors who have insomnia may be more likely to have comorbid depression and vice versa. More importantly, this study strengthens the evidence that CBT-I, through its positive effects on insomnia, may be effective in reducing depression in survivors with insomnia. Thus, insomnia interventions should be offered on a more routine basis to cancer survivors with insomnia, as targeting insomnia may have a meaningful effect on other associated symptoms such as depression.
CONCLUSIONS
The findings from the current analyses suggest that CBT-I is both efficacious and durable for reducing depression in cancer survivors who have chronic insomnia. These beneficial effects of CBT-I on depression appear to be mediated by improvement of insomnia severity. Because of the high prevalence of depression and insomnia in cancer survivors and their profound detrimental effect on quality of life, these results highlight the importance of increasing awareness of early screening and access to evidence-based insomnia interventions for alleviating these symptoms.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This study was supported by National Cancer Institute grants 5 R01 CA126968, 2 R25 CA102618-01A1, and UG1 CA189961 and National Center for Advancing Translational Sciences grant TL1 TR002000. Study medication, armodafinil, was provided by Teva Pharmaceuticals, USA. The sponsors had no role in the design or conducting of this research. Dr. Perlis receives royalties for a variety of educational materials related to CBT-I (Books & DVDs); has received federal and industry grant support for CBT-I related studies from NIH, Cephalon, Teva, and Sanofi-Aventis; has done consulting activities related to CBT-I from Lumosity, Sleep Easily, InsomniSolv, NYU, UB, and UCSD; and has received honoraria for multiple speaking engagements related to CBT-I. He also coordinates and lectures for three nonprofit CBT-I activities via the Penn BSM program including: annual Basic and Advanced CBT-I courses and a monthly BSM Mini-fellowship. The remaining authors declare that they have no conflicts of interest.
ABBREVIATIONS
- ANCOVA
analysis of covariance
- B
standardized path coefficient
- CBT-I
cognitive behavioral therapy for insomnia
- HPA
hypothalamic-pituitary-adrenal
- ISI
Insomnia Severity Index
- PHQ-8
Patient Health Questionnaire after excluding single sleep item
- PHQ-9
Patient Health Questionnaire
- RCT
randomized controlled trial
- RMSEA
root mean square error of approximation
- SE
standard error
REFERENCES
- 1.Li M, Fitzgerald P, Rodin G. Evidence-based treatment of depression in patients with cancer. J Clin Oncol. 2012;30(11):1187–1196. doi: 10.1200/JCO.2011.39.7372. [DOI] [PubMed] [Google Scholar]
- 2.Massie MJ, Lloyd-Williams M, Irving G, Miller K. Depression and Cancer. Hoboken, NJ: John Wiley & Sons, Ltd; 2011. The prevalence of depression in people with cancer. In: Kissane DW, Maj M, Sartorius N, eds; pp. 1–36. [Google Scholar]
- 3.Mitchell AJ, Chan M, Bhatti H, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12(2):160–174. doi: 10.1016/S1470-2045(11)70002-X. [DOI] [PubMed] [Google Scholar]
- 4.Irwin MR, Olmstead RE, Ganz PA, Haque R. Sleep disturbance, inflammation and depression risk in cancer survivors. Brain Behav Immun. 2013;30(Suppl):S58–S67. doi: 10.1016/j.bbi.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krebber AM, Buffart LM, Kleijn G, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23(2):121–130. doi: 10.1002/pon.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mausbach BT, Irwin SA. Depression and healthcare service utilization in patients with cancer. Psychooncology. 2017;26(8):1133–1139. doi: 10.1002/pon.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M, Kouzmina E, McCusker M, et al. Pro- and anti-inflammatory cytokine associations with major depression in cancer patients. Psychooncology. 2017;26(12):2149–2156. doi: 10.1002/pon.4316. [DOI] [PubMed] [Google Scholar]
- 8.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115(22):5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 9.Howell D, Keshavarz H, Esplen MJ, et al. on behalf of the Cancer Journey Advisory Group of the Canadian Partnership Against Cancer. Pan-Canadian Practice Guideline: Screening, Assessment and Management of Psychosocial Distress, Major Depression and Anxiety in Adults with Cancer. Toronto: Canada: Canadian Association of Psychosocial Oncology; 2015. Version 2, 2015. [Google Scholar]
- 10.Bortolato B, Hyphantis TN, Valpione S, et al. Depression in cancer: the many biobehavioral pathways driving tumor progression. Cancer Treat Rev. 2017;52:58–70. doi: 10.1016/j.ctrv.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Palesh O, Peppone L, Innominato PF, et al. Prevalence, putative mechanisms, and current management of sleep problems during chemotherapy for cancer. Nat Sci Sleep. 2012;4:151–162. doi: 10.2147/NSS.S18895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mercier J, Savard J, Bernard P. Exercise interventions to improve sleep in cancer patients: A systematic review and meta-analysis. Sleep Med Rev. 2017;36:43–56. doi: 10.1016/j.smrv.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1–3):10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Ho SY, Rohan KJ, Parent J, Tager FA, McKinley PS. A longitudinal study of depression, fatigue, and sleep disturbances as a symptom cluster in women with breast cancer. J Pain Symptom Manage. 2015;49(4):707–715. doi: 10.1016/j.jpainsymman.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28(2):292–298. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pigeon WR. The effect of sleep disturbances on major depressive disorder. Depress Mind Body. 2009;4(3):102–110. [PMC free article] [PubMed] [Google Scholar]
- 17.Pigeon WP, Perlis ML. Insomnia and depression: birds of a feather? Int J Sleep Disorders. 2007;1(3):82–91. [Google Scholar]
- 18.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–133. doi: 10.7326/M15-2175. [DOI] [PubMed] [Google Scholar]
- 19.Perlis ML, Jungquist C, Smith MT, Posner D. Cognitive Behavioral Treatment of Insomnia: A Session-By-Session Guide. New York, NY: Springer-Verlag; 2005. [Google Scholar]
- 20.Gebara MA, Siripong N, DiNapoli EA, et al. Effect of insomnia treatments on depression: a systematic review and meta-analysis. Depress Anxiety. 2018;35(8):717–731. doi: 10.1002/da.22776. [DOI] [PubMed] [Google Scholar]
- 21.Johnson JA, Rash JA, Campbell TS, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27:20–28. doi: 10.1016/j.smrv.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Espie CA, Fleming L, Cassidy J, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol. 2008;26(28):4651–4658. doi: 10.1200/JCO.2007.13.9006. [DOI] [PubMed] [Google Scholar]
- 23.Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: sleep and psychological effects. J Clin Oncol. 2005;23(25):6083–6096. doi: 10.1200/JCO.2005.09.548. [DOI] [PubMed] [Google Scholar]
- 24.Savard J, Ivers H, Savard MH, Morin CM. Is a video-based cognitive behavioral therapy for insomnia as efficacious as a professionally administered treatment in breast cancer? Results of a randomized controlled trial. Sleep. 2014;37(8):1305–1314. doi: 10.5665/sleep.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ritterband LM, Bailey ET, Thorndike FP, Lord HR, Farrell-Carnahan L, Baum LD. Initial evaluation of an Internet intervention to improve the sleep of cancer survivors with insomnia. Psychooncology. 2012;21(7):695–705. doi: 10.1002/pon.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthews EE, Berger AM, Schmiege SJ, et al. Cognitive behavioral therapy for insomnia outcomes in women after primary breast cancer treatment: a randomized, controlled trial. Oncol Nurs Forum. 2014;41(3):241–253. doi: 10.1188/14.ONF.41-03AP. [DOI] [PubMed] [Google Scholar]
- 27.Ashworth DK, Sletten TL, Junge M, et al. A randomized controlled trial of cognitive behavioral therapy for insomnia: an effective treatment for comorbid insomnia and depression. J Couns Psychol. 2015;62(2):115–123. doi: 10.1037/cou0000059. [DOI] [PubMed] [Google Scholar]
- 28.Roscoe JA, Garland SN, Heckler CE, et al. Randomized placebo-controlled trial of cognitive behavioral therapy and armodafinil for insomnia after cancer treatment. J Clin Oncol. 2015;33(2):165–171. doi: 10.1200/JCO.2014.57.6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee PW, Schulberg HC, Raue PJ, Kroenke K. Concordance between the PHQ-9 and the HSCL-20 in depressed primary care patients. J Affect Disord. 2007;99(1–3):139–145. doi: 10.1016/j.jad.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Hinz A, Mehnert A, Kocalevent RD, et al. Assessment of depression severity with the PHQ-9 in cancer patients and in the general population. BMC Psychiatry. 2016;16:22. doi: 10.1186/s12888-016-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redeker NS, Pigeon WR, Boudreau EA. Incorporating measures of sleep quality into cancer studies. Support Care Cancer. 2015;23(4):1145–1155. doi: 10.1007/s00520-014-2537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savard MH, Savard J, Simard S, Ivers H. Empirical validation of the Insomnia Severity Index in cancer patients. Psychooncology. 2005;14(6):429–441. doi: 10.1002/pon.860. [DOI] [PubMed] [Google Scholar]
- 34.Garland SN, Roscoe JA, Heckler CE, et al. Effects of armodafinil and cognitive behavior therapy for insomnia on sleep continuity and daytime sleepiness in cancer survivors. Sleep Med. 2016;20:18–24. doi: 10.1016/j.sleep.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heckler CE, Garland SN, Peoples AR, et al. Cognitive behavioral therapy for insomnia, but not armodafinil, improves fatigue in cancer survivors with insomnia: a randomized placebo-controlled trial. Support Care Cancer. 2016;24(5):2059–2066. doi: 10.1007/s00520-015-2996-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peoples AR, Garland SN, Perlis ML, et al. Effects of cognitive behavioral therapy for insomnia and armodafinil on quality of life in cancer survivors: a randomized placebo-controlled trial. J Cancer Surviv. 2017;11(3):401–409. doi: 10.1007/s11764-017-0597-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dirksen SR, Epstein DR. Efficacy of an insomnia intervention on fatigue, mood and quality of life in breast cancer survivors. J Adv Nurs. 2008;61(6):664–675. doi: 10.1111/j.1365-2648.2007.04560.x. [DOI] [PubMed] [Google Scholar]
- 38.Pigeon WR, Bishop TM, Krueger KM. Insomnia as a precipitating factor in new onset mental illness: a systematic review of recent findings. Curr Psychiatry Rep. 2017;19(8):44. doi: 10.1007/s11920-017-0802-x. [DOI] [PubMed] [Google Scholar]
- 39.Pigeon WR, Hegel M, Unutzer J, et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;31(4):481–488. doi: 10.1093/sleep/31.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Irwin MR. Depression and insomnia in cancer: prevalence, risk factors, and effects on cancer outcomes. Curr Psychiatry Rep. 2013;15(11):404. doi: 10.1007/s11920-013-0404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166(16):1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 42.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capuron L, Ravaud A, Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J Clin Oncol. 2000;18(10):2143–2151. doi: 10.1200/JCO.2000.18.10.2143. [DOI] [PubMed] [Google Scholar]
- 44.Irwin MR, Olmstead R, Breen EC, et al. Cognitive behavioral therapy and tai chi reverse cellular and genomic markers of inflammation in late-life insomnia: a randomized controlled trial. Biol Psychiatry. 2015;78(10):721–729. doi: 10.1016/j.biopsych.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part II: immunologic effects. J Clin Oncol. 2005;23(25):6097–6106. doi: 10.1200/JCO.2005.12.513. [DOI] [PubMed] [Google Scholar]