Abstract
Study Objectives:
Evaluate consequences of intermediate to high risk of undiagnosed obstructive sleep apnea (OSA) among individuals with chronic obstructive pulmonary disease (COPD).
Methods:
Using data from the Long Term Oxygen Treatment Trial (LOTT), we assessed OSA risk at study entry among patients with COPD. We compared outcomes among those at intermediate to high risk (modified STOP-BANG score ≥ 3) relative to low risk (score < 3) for OSA. We compared risk of mortality or first hospitalization with proportional hazard models, and incidence of COPD exacerbations using negative binomial regression. We adjusted analyses for demographics, body mass index, and comorbidities. Last, we compared St. George Respiratory Questionnaire and Quality of Well-Being Scale results between OSA risk groups.
Results:
Of the 222 participants studied, 164 (74%) were at intermediate to high risk for OSA based on the modified STOP-BANG score. Relative to the 58 low-risk individuals, the adjusted hazard ratio of mortality or first hospitalization was 1.61 (95% confidence interval 1.01–2.58) for those at intermediate to high risk of OSA. Risk for OSA was also associated with increased frequency of COPD exacerbations (adjusted incidence rate ratio: 1.78, 95% confidence interval 1.10–2.89). Respiratory symptoms by St. George Respiratory Questionnaire were 5.5 points greater (P = .05), and Quality of Well-Being Scale scores were .05 points lower (P < .01) among those at intermediate to high risk for OSA, indicating more severe respiratory symptoms and lower quality of life.
Conclusions:
Among individuals with COPD, greater risk for undiagnosed OSA is associated with poor outcomes. Increased recognition and management of OSA in this group could improve outcomes.
Citation:
Donovan LM, Feemster LC, Udris EM, Griffith MF, Spece LJ, Palen BN, He K, Parthasarathy S, Strohl KP, Kapur VK, Au DH. Poor outcomes among patients with chronic obstructive pulmonary disease with higher risk for undiagnosed obstructive sleep apnea in the LOTT cohort. J Clin Sleep Med. 2019;15(1):71–77.
Keywords: chronic obstructive pulmonary disease, obstructive sleep apnea
BRIEF SUMMARY
Current Knowledge/Study Rationale: Among those with chronic obstructive pulmonary disease (COPD) and diagnosed obstructive sleep apnea (OSA), a lack of treatment for OSA is associated with poor outcomes. However, the consequences of unrecognized OSA among those with COPD is unclear.
Study Impact: The current study finds that most of those sampled with COPD (74%) were at intermediate to high risk of undiagnosed OSA based on modified STOP-BANG criteria, and these individuals were also found to have a greater risk of poor outcomes. The results indicate a potentially high burden of undiagnosed OSA among those with COPD, and further research will be needed to evaluate the effect of identification and treatment of OSA in this population.
INTRODUCTION
As both are relatively common, a convergence of obstructive sleep apnea (OSA) and chronic obstructive pulmonary disease (COPD) is often present, and individuals in whom both conditions have been diagnosed have greater morbidity and mortality than with either condition alone.1 These poor outcomes may relate to augmentation of poor oxygenation and fragmented sleep, as both COPD and untreated OSA have been associated with sleep-related hypoxemia and resultant increase in pulmonary artery pressures.2–5 Moreover, observational studies suggest worse quality of life,6,7 increased all-cause mortality, and more frequent exacerbations among patients with COPD and OSA overlap when compared to those with COPD alone.6–9 Although the prevalence of diagnosed OSA among patients with COPD enrolled in the recent Long Term Oxygen Treatment Trial (LOTT) was 22%,10 the true prevalence of OSA is likely to be significantly higher. Among a recent sample of patients with COPD enrolled in pulmonary rehabilitation, 65.9% had at least mild OSA by polysomnography.11 These findings, combined with prior research demonstrating that approximately 84% to 93% of patients with moderate to severe OSA remain undiagnosed in the general population, suggest that many patients with COPD are likely to have undiagnosed, and therefore untreated OSA, which may predispose these individuals to poor outcomes.11–13 Among these undiag-nosed individuals, OSA risk can be assessed using risk assessment questionnaires. The STOP-BANG questionnaire is one example and consists of eight yes or no questions that can be completed quickly.14 A STOP-BANG score of ≥ 3 has a sensitivity of 88% to 93% and specificity of 23% to 43% for moderate to severe OSA in perioperative and general populations,14–17 and among patients with moderate to severe COPD, had a sensitivity of 80% and specificity of 50%.13
Among individuals with COPD participating in LOTT, we sought to estimate the prevalence of OSA in patients at intermediate to high risk, and compare clinical outcomes to those at low risk. OSA risk was ascertained using LOTT entry questionnaire information mapped to components of the STOP-BANG questionnaire. Our primary outcome was a composite of mortality or first hospitalization, which was also the primary outcome in LOTT.10 We also sought to assess the association between OSA risk and frequency of COPD exacerbations and quality of life. We hypothesized that intermediate to high risk of OSA, as detected by a composite of question responses at trial enrollment, would be associated with a combined endpoint of mortality or first hospitalization among patients with COPD. We also hypothesized that individuals with a higher risk of undiagnosed OSA would have a greater COPD exacerbation frequency, and reduced quality of life.
METHODS
This is a secondary analysis of the data collected prospectively from participants in LOTT, a longitudinal randomized parallel group trial of supplemental oxygen versus no supplemental oxygen among patients with COPD and moderate resting- or exertion-related oxygen desaturation. Greater details regarding LOTT are described elsewhere.10 Patients were recruited from 47 centers nationwide and were included if they had a resting room air oxygen saturation of 89% to 93% or moderate desaturation with exercise as measured by the 6-minute walk test (< 90% for ≥ 10 seconds and ≥ 80% for ≥ 5 minutes).
Our exposure of interest was risk of OSA as estimated by a risk profile using certain questions that parallel those in the STOP-BANG questionnaire.14 Because neck circumference was not measured in the LOTT trial, we used the remaining seven of the eight STOP-BANG items. However, we still incorporated a cutoff of ≥ 3, which would be expected to decrease sensitivity relative to the complete instrument. The items used in this modified STOP-BANG score and their source in the LOTT questionnaire are included in Table 1. The primary outcome of interest was a composite of death or first hospitalization, as was used in the primary analysis of LOTT. Secondary outcomes included the individual risk of death or first hospitalization, and overall incidence of COPD exacerbations. Finally, we sought to assess disease-specific and overall quality of life in those at intermediate to high versus low risk of OSA using the St. George Respiratory Questionnaire and Quality of Well-Being Scale at baseline, 1, 2, and 3 years of follow-up. Minimum important differences for the St. George Respiratory Questionnaire and the Quality of Well-Being Scale are 4 and 0.03 points, respectively.18–20
Table 1.
Definitions used in assessment of OSA risk.
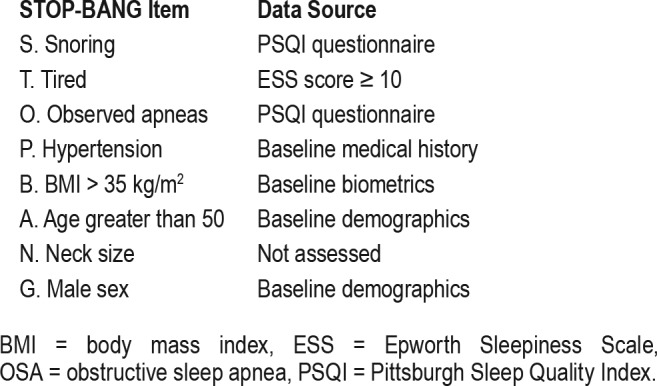
Comorbidities were assessed from baseline screening in LOTT by patients' report of diagnoses made by their physicians. For the purposes of our investigation, we included comorbidities hypothesized to confound the relationship between OSA and death or health care utilization. We included hyper-tension, coronary artery disease, heart failure, diabetes, and stroke.21–24 We defined smoking status as current versus not current smoking of tobacco cigarettes. We also accounted for use of supplemental oxygen as defined by patient's randomized assignment, no oxygen, 24-hour oxygen use, or oxygen use only with exercise and sleep.
Statistical Analyses
The exposure of interest in all analyses was intermediate to high risk of OSA as assessed by a score on our modified version of the STOP-BANG questionnaire of ≥ 3. We used Cox proportional hazard models to assess the association between increased risk of OSA and time to first hospital admission or death. The proportional hazards assumption was met.25 As a secondary analysis, we compared the overall incidence of COPD exacerbations, including inpatient and outpatient exacerbations between OSA risk groups by negative binomial regression. Overdispersion of counts precluded use of Poisson distribution. In all models, to account for confounding by characteristics associated with the exposure of OSA risk and our outcomes, we adjusted for age, sex, body mass index (BMI), race, oxygen assignment, postbronchodilator forced expiratory volume in 1 second (FEV1), and smoking status and the comorbidities of hypertension, coronary artery disease, diabetes mellitus, heart failure, and stroke. We also assessed interactions between each covariate and the primary exposure. We assessed differences between OSA risk groups in Quality of Well-Being Scale and St. George Respiratory Questionnaire at baseline and 1, 2, and 3 years of follow-up with unpaired t tests assuming unequal variances. All analyses were performed with the STATA statistical package (version 14.0; StataCorp LLC, College Station, Texas, United States).
RESULTS
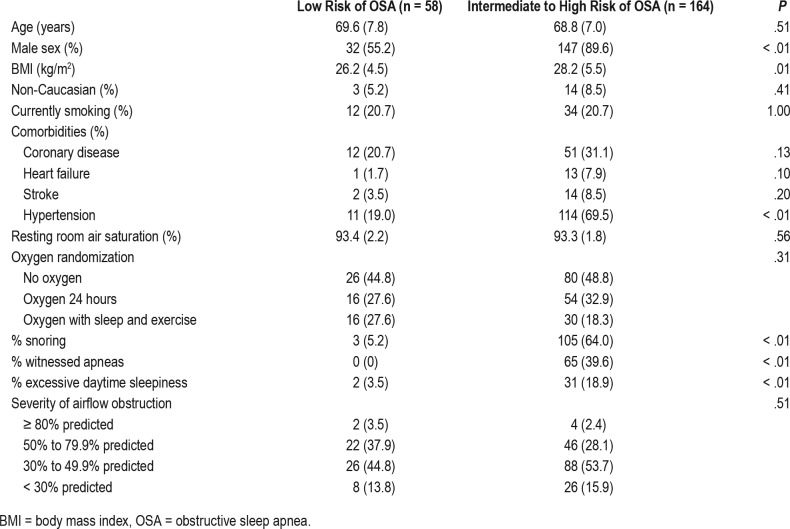
Of the 738 patients included in LOTT, 305 had bed partners available to answer questions from the Pittsburgh Sleep Quality Index pertaining to snoring and witnessed apneas, and therefore ascertainment of the modified STOP-BANG score was limited to these individuals. Of the 305 participants, 83 had previously diagnosed OSA at baseline, and therefore 222 patients were included in our analytic dataset. Of these 222 patients, 164 (74%) had an intermediate to high risk of OSA (modified STOP-BANG ≥ 3). As expected based on the elements of the modified STOP-BANG instrument, those at intermediate to high risk for OSA were more likely to be men (89.6% versus 55.2%), have a higher BMI (28.2 kg/m2 versus 26.2 kg/m2), have hypertension (69.5% versus 19.0%), report excessive daytime sleepiness (18.9% versus 3.5%), bed partner acknowledgement of disruptive snoring (64.0% versus 5.2%), and observed apneas (39.6% versus 0%). More details regarding baseline characteristics and comorbidities are included in Table 2.
Table 2.
Baseline characteristics of cohort.
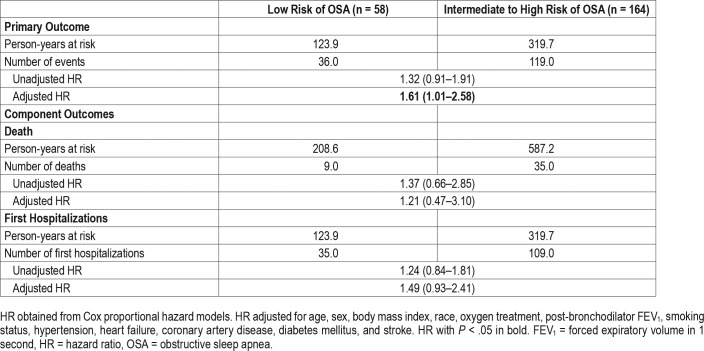
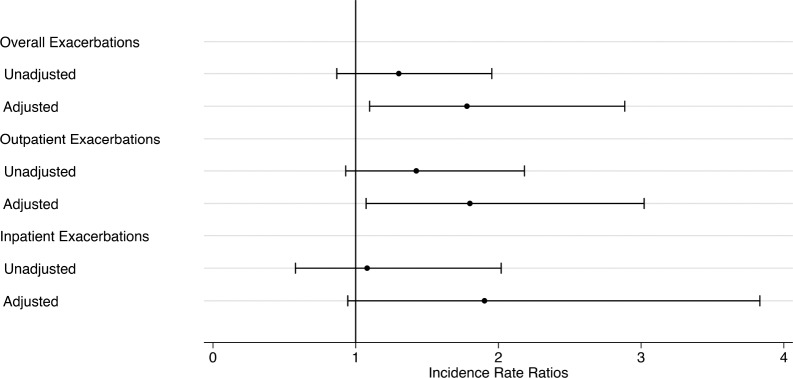
Overall, there was a statistically significant difference in the composite outcome of death or first hospitalization between groups with 73% (n = 119) of the intermediate to high-risk and 62% (n = 36) of low-risk individuals experiencing the composite outcome (adjusted hazard ratio [HR] 1.61, 95% confidence interval [CI] 1.01–2.58, P = .044). An unadjusted Kaplan-Meier curve is presented in Figure 1. However, intermediate to high risk of OSA was not significantly associated with the outcomes separately (death, HR 1.21, 95% CI 0.47–3.10; hospital admission, HR 1.49, 95% CI 0.93–2.41, Table 3). With regard to secondary outcomes, individuals at intermediate to high risk of OSA had a greater frequency of exacerbations with an incidence rate ratio (IRR) of 1.78 (95% CI 1.10–2.89) relative to those at low risk for OSA. Broken down further, this increased risk for COPD exacerbations was statistically significant for outpatient exacerbations (IRR 1.80, 95% CI 1.07–3.02), but not for exacerbations requiring hospital admission (IRR 1.90, 95% CI 0.94–3.83; Figure 2). No evidence of effect modification was observed between OSA risk and any of the covariates in either model.
Figure 1. Kaplan-Meier curve of hospitalization-free survival by OSA risk level.
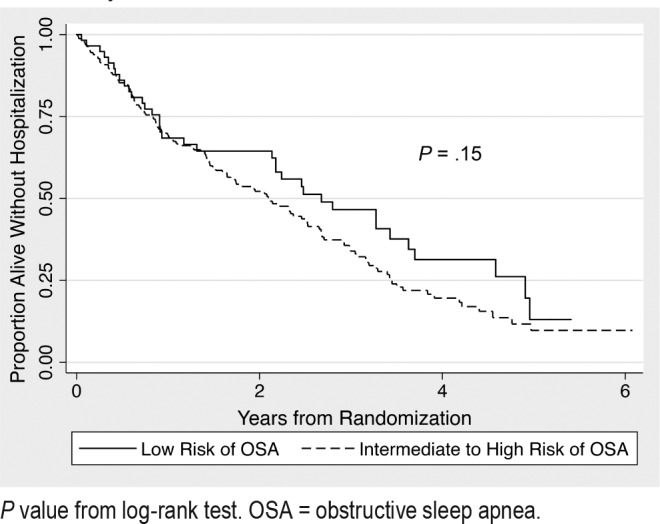
P value from log-rank test. OSA = obstructive sleep apnea.
Table 3.
Primary endpoint of death or first hospitalization by OSA risk group category.
Figure 2. Incidence rate of exacerbations for those at intermediate to high risk for OSA relative to low-risk individuals.
Incidence rate ratios and 95% confidence intervals from negative binomial regression of exacerbation counts. HR adjusted for age, sex, body mass index, race, oxygen treatment, post-bronchodilator FEV1, smoking status, hypertension, heart failure, coronary artery disease, diabetes mellitus, and stroke. FEV1 = forced expiratory volume in 1 second, HR = hazard ratio, OSA = obstructive sleep apnea.
Concurrent with the aforementioned findings, disease-specific limitations were associated with overall lower quality of life among those at intermediate to high risk of OSA. St. George Respiratory Questionnaire scores were 5.5 points higher among those intermediate to high risk for OSA (P = .046), and scores for the Quality of Well-Being Scale were .05 points lower (P = .009). These differences relative to scores of patients at low risk for OSA were meaningful and significantly different at baseline, and this significant difference persisted for both measures at 12 months. However, differences between groups were no longer significantly different by 36 months (Figure 3).
Figure 3. Quality of life and disease-specific impairment at baseline and over time in groups at low and intermediate to high risk of OSA.
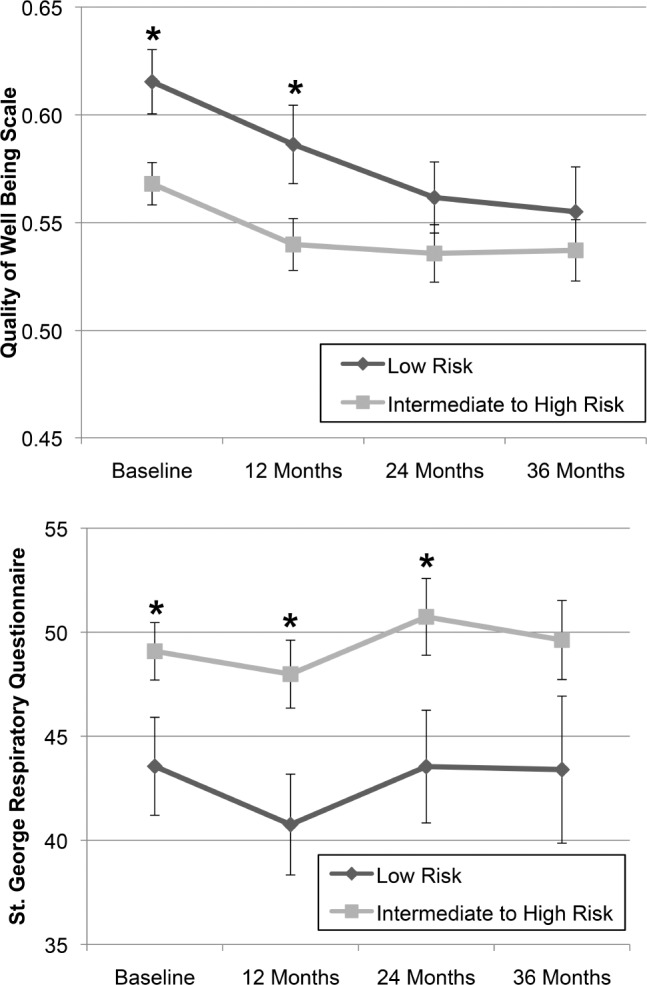
* = P < .05 according to unpaired t test assuming unequal variances. The number of respondents for low risk individuals was 58 at baseline, 53 at 12 months, 44 at 24 months, and 34 at 36 months, whereas the number of respondents for intermediate to high risk individuals was 164 at baseline, 139 at 12 months, 109 at 24 months, and 89 at 36 months. Error bars refer to the standard error of each measure. OSA = obstructive sleep apnea.
DISCUSSION
Among patients with COPD and a moderate degree of oxygen desaturation at rest or with exertion, we found intermediate to high risk of OSA to be associated with greater risk of the composite outcome of death or hospitalization. These participants also experienced a higher incidence of COPD exacerbations and had lower condition- specific and general quality of life. Collectively, our results suggest those with COPD at higher risk for unrecognized OSA are also more likely to have poor outcomes.
Our findings also indicate that clinical indicators for OSA are common among those with COPD but without a diagnosis of OSA. Using the specificity of STOP-BANG among patients with COPD found by Soler et al. (50%), 82 of 164 patients at intermediate to high risk in our sample would be expected to have OSA.13 Even using the most conservative estimate of the full STOP-BANG's specificity for OSA in the more general population, 23%,17 38 of the 164 patients at intermediate to high risk would be expected to have OSA. This conservative estimate would increase the estimated minimum prevalence of diagnosed OSA from 83 of 305 (27.2%) to 121 of 305 (39.7%). Given the paucity of patients in the community who are asked at all about sleep-related symptoms,26 this degree of underdiagnosis is not surprising. Furthermore, it is worth noting that our findings regarding OSA risk among patients with COPD may be underestimated, as an exclusion for entry into LOTT was severe excessive daytime sleepiness (Epworth Sleepiness Scale score > 15).
Our results showing worse outcomes among those at increased risk for undiagnosed OSA are consistent with prior reports of patients with overlapping OSA and COPD that demonstrated reduced mortality, exacerbations, and hospitalizations among patients adherent to OSA therapy.9,27 Furthermore, a recent large study involving administrative claims data showed a substantial reduction in hospitalization rates in patients following initiation of positive airway pressure therapy (PAP).28 A major strength of our study is avoidance of the healthy user effect.29 Prior work assessing risk of untreated OSA in COPD has relied on assessments of PAP use versus non-use, for which the healthy user effect is a major concern; the patients capable of using continuous PAP are likely healthier than nonusers.9 The mechanism of poor outcomes associated with overlapping OSA and COPD is unclear, but potential explanations may include greater nocturnal hypoxemia, right heart strain, and increased airway inflammation related to superimposed OSA.2,30–32
Our study has several limitations. First, although the focus on those at higher risk of undiagnosed OSA allows us to avoid the healthy user bias in assessing the risk of untreated OSA, only a portion of the intermediate to high risk group actually has OSA.14,15,17 This misclassification would tend to underestimate the true effect of OSA in this setting and bias toward null findings. Furthermore, at the standard cutoff score of ≥ 3,15,33 our modified version of the STOP-BANG would be expected to be less sensitive than the standard instrument. For instance, our definition of “tired” uses an Epworth Sleepiness Scale score ≥ 10, which would be expected to be less sensitive than the standard item, a single yes-no item assessing patient's perspective of feeling tired or sleepy.14 Similarly, missing neck circumference would limit the ability of some truly high-risk individuals to be classified as likely to have intermediate to high risk of OSA. For instance, a man with hypertension and a large neck circumference but no other STOP-BANG risk factors would be classified as intermediate to high risk on the formal STOP-BANG but would be low-risk on our version. This misclassification of intermediate to high risk individuals as low risk would also bias our results toward the null. It is also worth mentioning that given our small cohort size of 222, we may have been underpowered to detect differences in the separate endpoints of mortality and hospitalization, and due to attrition of patients completing questionnaires by 36 months (n = 123), we may also have been underpowered to detect longer-term differences in quality of life. Furthermore, it is possible that OSA risk status shifted for individuals over time due to weight changes; this potential crossover would also be expected to bias our results toward the null. Finally, although we attempted to account for confounding between OSA risk and our outcomes of interest, patients with elevated risk for OSA may have had adverse health behaviors such as unreported smoking or medication non-adherence that contributed to residual confounding. Balancing these potential limitations are this study's strengths, which include rigorous data collection in LOTT, strong quality assurance procedures, and a diverse population spread across multiple centers.
The current study shows that many individuals with COPD and a moderate degree of hypoxemia are at increased risk for OSA, and demonstrates poor outcomes and a trend toward long-term reduced quality of life among these individuals. This study underscores the importance of OSA recognition and management among patients with COPD. Although randomized trials are necessary to fully elucidate the benefits of OSA identification and management in COPD, we suggest that providers evaluate for OSA symptoms and risk in patients with COPD to facilitate OSA therapy among affected patients.
DISCLOSURE STATEMENT
Dr. Au reports personal fees from Novartis for service on a data monitoring committee, personal fees from American Board of Internal Medicine for service on the exam writing committee, personal fees from Annals of the American Thoracic Society for service as a deputy editor, he also reports research grants from the Department of Veterans Affairs, NIH/NHLBI, and the American Lung Association, outside of the submitted work. Dr. Donovan reports grants from NIH T32HL007287-38, 1F32HL140685 – 01, and F32HL142125-01; and the ASPIRE (Academic Sleep Pulmonary Integrated REsearch/Clinical) Fellowship during the conduct of the study. Dr. Feemster reports grants from the NIH/NHLBI, HL111116, during the conduct of this study, and grants from the American Lung Association and Veteran's Health Administration, outside of the submitted work. Dr. Parthasarathy reports grants from NIH/NHLBI, grants from Patient Centered Outcomes Research Institute, grants from US Department of Defense, grants from NIH (National Cancer Institute) NCI, grants from Johrei Institute, personal fees from American Academy of Sleep Medicine, non-financial support from National Center for Sleep Disorders Research of the NIH (NHLBI), personal fees from UpToDate Inc., grants from Younes Sleep Technologies, Ltd., grants from Niveus Medical Inc., personal fees from Vapotherm, Inc., personal fees from Merck, Inc., grants from Philips-Respironics, Inc., personal fees from Philips-Respironics, Inc., personal fees from Bayer, Inc., personal fees from Nightbalance, Inc, outside the submitted work; In addition, Dr. Parthasarathy has a patent UA 14-018 U.S.S.N. 61/884,654; PTAS 502570970 (Home breathing device) issued. Dr. Spece reports grants from NIH, T32HL007287-38 and F32HL142125-01, during the conduct of the study. Dr. Strohl is a site PI for Jazz Pharmaceuticals and Inspire Medical Therapy, and a consultant to Sommetrics, Seven Dreamers, and Galvani Bioelectronics, outside of the submitted work. The other authors do not report any conflicts of interest. None of the funding agencies or institutions had any role in the planning or execution of this work. The views expressed here are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. All authors approve of the manuscript.
ABBREVIATIONS
- BMI
body mass index
- COPD
chronic obstructive pulmonary disease
- FEV1
forced expiratory volume in 1 second
- LOTT
Long Term Oxygen Treatment Trial
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
REFERENCES
- 1.Zamarrón C, García Paz V, Morete E, del Campo Matías F. Association of chronic obstructive pulmonary disease and obstructive sleep apnea consequences. Int J Chron Obstruct Pulmon Dis. 2008;3(4):671–682. doi: 10.2147/copd.s4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaouat A, Weitzenblum E, Krieger J, Ifoundza T, Oswald M, Kessler R. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med. 1995;151(1):82–86. doi: 10.1164/ajrccm.151.1.7812577. [DOI] [PubMed] [Google Scholar]
- 3.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owens RL, Macrea MM, Teodorescu M. The overlaps of asthma or COPD with OSA: a focused review. Respirology. 2017;22(6):1073–1083. doi: 10.1111/resp.13107. [DOI] [PubMed] [Google Scholar]
- 5.Sandek K, Andersson T, Bratel T, Hellström G, Lagerstrand L. Sleep quality, carbon dioxide responsiveness and hypoxaemic patterns in nocturnal hypoxaemia due to chronic obstructive pulmonary disease (COPD) without daytime hypoxaemia. Respir Med. 1999;93(2):79–87. doi: 10.1016/s0954-6111(99)90295-0. [DOI] [PubMed] [Google Scholar]
- 6.Zohal MA, Yazdi Z, Kazemifar AM, Mahjoob P, Ziaeeha M. Sleep quality and quality of life in COPD patients with and without suspected obstructive sleep apnea. Sleep Disord. 2014;2014:508372. doi: 10.1155/2014/508372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mermigkis C, Kopanakis A, Foldvary-Schaefer N, et al. Health-related quality of life in patients with obstructive sleep apnoea and chronic obstructive pulmonary disease (overlap syndrome) Int J Clin Pract. 2007;61(2):207–211. doi: 10.1111/j.1742-1241.2006.01213.x. [DOI] [PubMed] [Google Scholar]
- 8.Stanchina ML, Welicky LM, Donat W, Lee D, Corrao W, Malhotra A. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: the overlap syndrome. J Clin Sleep Med. 2013;9(8):767–772. doi: 10.5664/jcsm.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325–331. doi: 10.1164/rccm.200912-1869OC. [DOI] [PubMed] [Google Scholar]
- 10.Albert RK, Au DH, Blackford AL, et al. Long-Term Oxygen Treatment Trial Research Group. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375(17):1617–1627. doi: 10.1056/NEJMoa1604344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soler X, Gaio E, Powell FL, et al. High prevalence of obstructive sleep apnea in patients with moderate to severe chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015;12(8):1219–1225. doi: 10.1513/AnnalsATS.201407-336OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38(6):877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soler X, Liao SY, Marin JM, et al. Age, gender, neck circumference, and Epworth sleepiness scale do not predict obstructive sleep apnea (OSA) in moderate to severe chronic obstructive pulmonary disease (COPD): The challenge to predict OSA in advanced COPD. PloS One. 2017;12(5):e0177289. doi: 10.1371/journal.pone.0177289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812–821. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 15.Chung F, Chau E, Yang Y, Liao P, Hall R, Mokhlesi B. Serum bicarbonate level improves specificity of STOP-Bang screening for obstructive sleep apnea. Chest. 2013;143(5):1284–1293. doi: 10.1378/chest.12-1132. [DOI] [PubMed] [Google Scholar]
- 16.Chung F, Yang Y, Brown R, Liao P. Alternative scoring models of STOP-bang questionnaire improve specificity to detect undiagnosed obstructive sleep apnea. J Clin Sleep Med. 2014;10(9):951–958. doi: 10.5664/jcsm.4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nahapetian R, Silva GE, Vana KD, Parthasarathy S, Quan SF. Weighted STOP-Bang and screening for sleep-disordered breathing. Sleep Breath. 2016;20(2):597–603. doi: 10.1007/s11325-015-1255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respir Med. 1991;85(Suppl B):25–31. doi: 10.1016/s0954-6111(06)80166-6. discussion 33–37. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan RM. The minimally clinically important difference in generic utility-based measures. COPD. 2005;2(1):91–97. doi: 10.1081/copd-200052090. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan RM, Atkins CJ, Timms R. Validity of a quality of well-being scale as an outcome measure in chronic obstructive pulmonary disease. J Chronic Dis. 1984;37(2):85–95. doi: 10.1016/0021-9681(84)90050-x. [DOI] [PubMed] [Google Scholar]
- 21.Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370(24):2276–2285. doi: 10.1056/NEJMoa1306766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172(12):1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stone KL, Blackwell TL, Ancoli-Israel S, et al. Sleep disordered breathing and risk of stroke in older community-dwelling men. Sleep. 2016;39(3):531–540. doi: 10.5665/sleep.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grambsch PM, Therneua TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- 26.Mold JW, Quattlebaum C, Schinnerer E, Boeckman L, Orr W, Hollabaugh K. Identification by primary care clinicians of patients with obstructive sleep apnea: a practice-based research network (PBRN) study. J Am Board Fam Med. 2011;24(2):138–145. doi: 10.3122/jabfm.2011.02.100095. [DOI] [PubMed] [Google Scholar]
- 27.Konikkara J, Tavella R, Willes L, Kavuru M, Sharma S. Early recognition of obstructive sleep apnea in patients hospitalized with COPD exacerbation is associated with reduced readmission. Hosp Pract (1995) 2016;44(1):41–47. doi: 10.1080/21548331.2016.1134268. [DOI] [PubMed] [Google Scholar]
- 28.Vasquez MM, McClure LA, Sherrill DL, et al. Positive airway pressure therapies and hospitalization in chronic obstructive pulmonary disease. Am J Med. 2017;130(7):809–818. doi: 10.1016/j.amjmed.2016.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Platt AB, Kuna ST, Field SH, et al. Adherence to sleep apnea therapy and use of lipid-lowering drugs: a study of the healthy-user effect. Chest. 2010;137(1):102–108. doi: 10.1378/chest.09-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Hu K, Liu K, et al. Obstructive sleep apnea exacerbates airway inflammation in patients with chronic obstructive pulmonary disease. Sleep Med. 2015;16(9):1123–1130. doi: 10.1016/j.sleep.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 31.Weitzenblum E, Krieger J, Apprill M, et al. Daytime pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am Rev Respir Dis. 1988;138(2):345–349. doi: 10.1164/ajrccm/138.2.345. [DOI] [PubMed] [Google Scholar]
- 32.Sharma B, Neilan TG, Kwong RY, et al. Evaluation of right ventricular remodeling using cardiac magnetic resonance imaging in co-existent chronic obstructive pulmonary disease and obstructive sleep apnea. COPD. 2013;10(1):4–10. doi: 10.3109/15412555.2012.719050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiu HY, Chen PY, Chuang LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57–70. doi: 10.1016/j.smrv.2016.10.004. [DOI] [PubMed] [Google Scholar]