Abstract
Study Objectives:
The aim of this study was to test the efficacy of fully automated Internet-delivered cognitive behavioral therapy for insomnia (CBT-I) 18 months after the intervention period on sleep, daytime functioning, and beliefs about sleep for adults with chronic insomnia.
Methods:
Participants in this study had participated in a randomized controlled trial comparing the efficacy of unguided Internet CBT-I with web-based patient education. Participants who had received Internet CBT-I (n = 95) completed online questionnaires and online sleep diaries 18 months after the intervention period. We used linear mixed models to study changes from baseline to postassessment and to 18-month follow-up, and a separate mixed-models analysis to study changes from postassessment to 18-month follow-up.
Results:
Mean age of the participants was 45.5 years (standard deviation = 12.6) and 64% were females. Sixty-six participants (70%) completed the 18-month follow-up assessment. There were significant improvements from baseline to 18-month follow-up on the Insomnia Severity Index (ISI) (Cohen d = 2.04 [95% confidence interval (CI) 1.66–2.42]) and the Bergen Insomnia Scale (BIS) (d = 1.64 [95% CI 1.30–1.98]), levels of daytime fatigue (d = 0.85 [95% CI 0.59–1.11]), psychological distress (d = 0.51 [95% CI 0.29–0.73]), and beliefs about sleep (d = 1.44 [95% CI 1.15–1.73]). Moderate to large effect size improvements were also shown on the diary-derived sleep variables. All improvements from baseline to postassessment were essentially maintained to 18-month follow-up.
Conclusions:
Unguided Internet CBT-I appears to have sustained effects on sleep, daytime functioning, and beliefs about sleep up to 18 months after the intervention period.
Clinical Trial Registration:
This study presents long-term follow-up data of a previous clinical trial. Registry: ClinicalTrials.gov, Title: Internet-based Treatment for Insomnia in Norway, Identifier: NCT02261272, URL: https://clinicaltrials.gov/ct2/show/NCT02261272
Citation:
Vedaa Ø, Hagatun S, Kallestad H, Pallesen S, Smith OR, Thorndike FP, Ritterband LM, Sivertsen B. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101–110.
Keywords: cognitive behavioral therapy, daytime functioning, insomnia, Internet, long-term
BRIEF SUMMARY
Current Knowledge/Study Rationale: Internet-delivered cognitive behavioral therapy for insomnia (CBT-I) is efficacious as treatment for chronic insomnia. However, the long-term effectiveness of such treatment on sleep and daytime symptoms has received little attention in previous research. We investigated whether improvements attained during Internet CBT-I on sleep and daytime functioning were maintained 18 months after the intervention period.
Study Impact: Internet CBT-I led to improved sleep and reduced symptoms of fatigue and psychological distress 18 months after the intervention period. These results add to the growing evidence verifying the efficacy of sustained improvements after self-guided Internet-delivered treatment for chronic insomnia.
INTRODUCTION
A recent meta-analysis established the efficacy of both guided and unguided Internet-delivered cognitive behavioral therapy for insomnia (CBT-I) and reported significant and robust effects on insomnia severity and on several sleep diary outcomes.1 It was concluded that the effects, which in terms of effect sizes (Hedges g) ranged from 0.21 to 1.09, were comparable to those of in-person–delivered CBT-I.1 The meta-analysis further showed that the effects of Internet CBT-I were maintained for insomnia severity and sleep quality at follow-up assessments (up to 48 weeks). However, for other sleep diary characteristics (ie, sleep onset latency [SOL], wake after sleep onset [WASO], and total sleep time [TST]), maintenance of treatment effects was less consistently found. The authors recommended that future research should now focus on the long-term efficacy of Internet CBT-I.1
Three previous trials have examined the long-term outcomes of unguided Internet CBT-I. Lancee et al.2 studied treatment gains of Internet CBT-I 48 weeks after treatment completion, and found sustained effects. However, the design of that study intended to investigate whether the same self-help treatment given online or by paper-and-pencil material produced differential results. Due to this they needed to keep the two treatments as similar as possible, resulting in significant restrictions to the extent interactive and personalized treatment elements were offered in the online treatment compared to other Internet CBT-I programs (eg, the two studies by Ritterband et al.3,4). Ritterband et al.4 conducted a study comparing Internet CBT-I to a control group and included a 1-year follow-up assessment. Results demonstrated superior effects of Internet CBT-I on insomnia severity and on sleep diary outcomes. That paper, however, did not report the effects of the treatment on outcome measures other than sleep, such as daytime fatigue and psychological well-being, which often are reported consequences of insomnia.5 Using the same Internet CBT-I intervention as Ritterband et al.4 (SHUTi, BeHealth Solutions, LLC, Charlottesville, Virginia), Batterham et al.6 investigated whether the efficacy of Internet CBT-I on depressive symptoms and insomnia severity were sustained over 18 months compared to a control group. They found symptoms of depression, anxiety, and insomnia decreased significantly after the intervention period and remained significantly lower compared to the control group for more than 18 months.6 Notably, the between-group effect sizes on insomnia severity in that study were reduced from 1.27 after the intervention period to 0.55 at 18 months follow-up assessment.6 This may suggest an increase in sleep problems for the intervention group after the intervention period (or an improvement in the control group), but the power calculations are also likely influenced by the fact that only 19% of participants completed the 18-month assessment in that study. This level of attrition also makes the generality of the results on the long-term effects in that study somewhat uncertain.
In the current study, we report long-term results (18-month follow-up) from a randomized controlled trial comparing unguided Internet CBT-I (SHUTi) with web-based patient education. In previous publications we have demonstrated that the SHUTi intervention was superior to the patient education (control condition) in terms of improving sleep, daytime functioning, and psychological well-being after the intervention period and that the treatment gains were relatively well maintained at 6-month follow-up.7,8 The aim of the current study is to examine whether the observed improvements in sleep and daytime functioning from baseline to after the intervention period are maintained at 18-month follow-up in the experimental group.
METHODS
Participants and Procedure
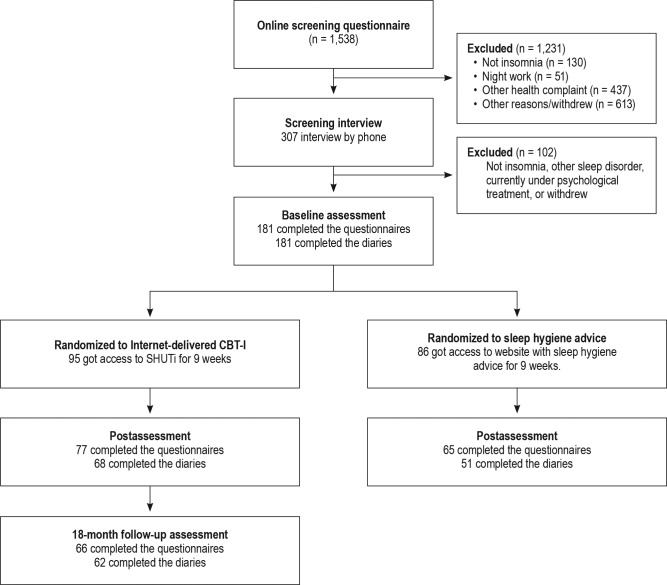
The participants in this study had participated in a randomized controlled trial comparing the effects of unguided Internet CBT-I (n = 95) to web-based patient education (n = 86).7,8 Those who were randomized to receive unguided Internet CBT-I were invited to complete another set of online questionnaires and 10 days of sleep diaries 18 months following the intervention period (Figure 1). Those who participated in the patient education control group were given access to Internet CBTI after postassessment, and thus could not be included in the 18-month follow-up study.
Figure 1. Consort diagram and participant flow.
Only the results from the 18-month follow-up assessment are reported in this study. The results from the randomized controlled trial are reported in previous publications.7,8
The inclusion procedure is detailed in a previous study7 and is therefore only briefly described here. Recruitment took place between November 2013 and March 2014. Eligible candidates were screened sequentially by a set of online screening questions, followed by a 15-minute telephone interview performed by a clinician. The online screening questionnaire was anonymous and noncommittal, where candidates received automated feedback based on their answers. Eligible candidates were encouraged to contact the research team through a dedicated email if they wanted to be evaluated further for eligibility through a telephone interview. Candidates had to be 18 years or older, be fluent in Norwegian, have Internet access, and report sleep difficulties according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (Text Revision) (DSM-IV-TR)9 criteria for insomnia (difficulty initiating sleep, difficulty maintaining sleep, and/or early morning awakenings; in addition to daytime impairment due to sleep disturbance) 3 or more nights per week and for at least 3 months. Candidates were ineligible if they reported working night shifts, if they had another known sleep disorder such as obstructive sleep apnea, hypersomnia, narcolepsy, or who had a diagnosis of severe mental illness (e.g., current major depressive disorder, bipolar disorder, schizophrenic disorder). A single-item question was used to assess sleep apnea in the online screening questionnaire: “During the last four weeks, how often have you experienced interrupted breathing during your sleep?” Those who answered “usually” or “always” were excluded from the study. Depression was assessed in the online screening questionnaire using MADRS-S,10 in which a score higher than 19 points indicates moderate/severe depression and was set as exclusion criterion. Further assessment of the candidates' sleep problems and mental health was done during the telephone interview; where those who reported a diagnosis of hypersomnia, narcolepsy, a major depressive disorder, bipolar disorder, or schizophrenic disorder were excluded. Use of hypnotics for sleep problems or other medications was not an exclusion criterion.
Treatment
The Internet CBT-I program (SHUTi)11 consists of 6 online sessions designed to be completed weekly during a 9- week period. The weekly sessions covered the basic topics of CBT-I,12 including sleep restriction, stimulus control, cognitive restructuring, sleep hygiene, and relapse prevention. The treatment recommendations provided by the program are personalized for patients based on each individual's input in the program throughout the treatment (for more details about the SHUTi program, see Thorndike et al.13). After the 9-week treatment period, participants were reassessed with online questionnaires and online sleep diaries (10 diaries in a 14-day period). Participants were contacted at 6-month follow-up,7,8 and in terms of the current study, also at 18-month follow-up and asked to complete the same assessment (questionnaires and 10 days of sleep diaries).
Ethics
The study was approved by the Regional Committee for Medical and Health Research Ethics in SouthEast Norway (2012/1934 REK, SouthEast B). Informed consent was provided and participants were compensated 500 Norwegian krone (approximately 60 United States dollars) for completing the 18-month follow-up assessment. ClinicalTrials.gov identifier: NCT02261272.
Instruments
Insomnia Severity Index
Insomnia Severity Index (ISI) is a brief self-report instrument measuring the patient's perception of his or her insomnia. It contains seven questions that target the participants' symptoms and consequences of insomnia, and the level of distress they experience in relation to these difficulties. Each item is rated on a Likert scale of 0 to 4, hence the composite scores range from 0 to 28, where higher scores suggest more severe insomnia. The ISI is a reliable self-report measure and has demonstrated adequate sensitivity to changes in response to treatment, where participants are considered responders to treatment if the ISI score decreases by 8 or more points.14,15 An ISI score of less than 8 is the most widely used remission criterion.16 The instrument has previously demonstrated excellent internal consistency.15 Cronbach alphas at pretreatment, post-treatment, and 18-month follow-up in the current study were .51, .73, and .87, respectively.
Bergen Insomnia Scale
The Bergen Insomnia Scale (BIS)17 comprises six items that assesses symptoms of insomnia based on the American Psychiatric Association's DSM-IV-TR.18 Individuals are asked to indicate how many days a week (0–7) during the past month they have struggled with six specified symptoms of insomnia. The scale can be used as a continuous measure or as a dichotomous/diagnostic measure. Higher scores indicate more symptoms of insomnia when BIS is used as a continuous measure. The diagnostic criteria of insomnia are met with a score of ≥ 3 on at least one of the first four items (nighttime symptoms) and a score of ≥ 3 on at least one of the last two items (daytime symptoms). Cronbach alpha of BIS was .58 at pretreatment, .82 at posttreatment, and .85 at 18-month follow-up.
Brief Dysfunctional Beliefs and Attitudes Scale
The Brief Dysfunctional Beliefs and Attitudes Scale (DBAS-16)19 is a self-report questionnaire designed to identify various maladaptive sleep- and insomnia-related cognitions. Participants are given a list of 16 statements reflecting different beliefs and attitudes about sleep, and they are asked to indicate on a 10-point scale how much they agree with the statements. A high score indicates more maladaptive beliefs that are assumed to have a potential perpetuating effect on insomnia symptoms. The scale has proven adequate internal consistency both for clinical and research samples.19 Also, in the current study, internal consistency was high, where Cron-bach alphas for the DBAS-16 at the different assessment points were .81 at baseline, .89 at posttreatment, and .89 at 18-month follow-up, respectively.
Sleep Diaries
Sleep diaries20 that were completed online were used to register self-reported sleep data, where participants provided daily estimates of their sleep the previous night during the baseline assessment, posttreatment assessment, and at 18-month follow-up (10 days of diaries in a 14-day window were required at each assessment point). Throughout the assessment periods, participants received automated daily reminders via email to complete the sleep diary. The following measures were derived from the diary: SOL, WASO (time awake during the night), early morning awakening (EMA, time spent in bed after final wake-up), time in bed (TIB), TST, and sleep efficiency (SE, total sleep time as a percentage of time in bed). In the current study, the analyses were based on mean scores of the 10 days for each of the respective sleep diary parameters, in line with previous publications.7,8
Hospital Anxiety and Depression Scale
The Hospital Anxiety and Depression Scale (HADS) is a widely used measure of general psychological distress.21 The scale comprises 14 questions pertaining to non-vegetative symptoms of anxiety and depression. Each item is rated on a Likert scale of 0 to 4. A higher score indicates greater symptom severity. The HADS can be used to separately assess symptom severity of anxiety and depression. However, in the current study the subscales were combined into one general factor measuring psychological distress, adhering to previous studies that have demonstrated superior factor loadings on one general factor.22,23 Scores on the HADS using the general factor range from 0 to 56. The recommended cutoff point for identifying clinical cases of anxiety and depression is ≥ 8 on both subscales.21,22 Cronbach alphas at the different assessment points were .82 at pretreatment, .83 at posttreatment, and .82 at 18-month follow-up.
Chalder Fatigue Questionnaire
Chalder Fatigue Questionnaire (CFQ)24 is a self-administered questionnaire used to measure the extent and severity of participants' fatigue complaints. CFQ comprises 11 items addressing physical and psychological fatigue, and 2 items addressing the duration (from “less than one week” through “six months or more”) and the frequency of fatigue complaints (ie, how much of the time they feel fatigued; from “25% of the time” through “all the time”). Each item is answered on a four-point scale ranging from asymptomatic to maximum symptomatology. A composite score including all 13 items was used in the current study with scores ranging from 0 to 39. Reliability measures of CFQ have been high both in clinical samples25 as well as in the general population.26 CFQ was chosen as a measure of fatigue in the current study since a Norwegian validated version was available.26 In the current study, Cronbach alphas for CFQ at the different assessment points were .84 at pretreatment, .85 at posttreatment, and .86 at 18-month follow-up.
Statistics
All analyses were performed using IBM SPSS Statistics, version 23 (IBM SPSS Statistics, New York, United States). The long-term effects (18-month follow-up) of Internet CBT-I were examined using linear mixed models for repeated-measures analysis, where all participants with baseline data were included in the analysis in line with the intention-to-treat principle. Two consecutive mixed-model analyses were performed. In the first, baseline assessments were used as reference and compared to posttreatment and 18-month follow-up assessments; in the second, posttreatment assessments were used as reference and compared to the 18-month follow-up assessments. Furthermore, we used independent samples t tests and chi-square test for independence to investigate differences between those who completed follow-up and those lost to follow-up.
No constraints were imposed on the covariance structure for repeated measures (type = unstructured). Mixed-model analysis uses maximum likelihood estimation and can handle data that are missing at random (MAR) on dependent variables. Although there are no conclusive tests to prove the assumption of MAR, it is generally considered a more realistic assumption as compared to missing completely at random (MCAR). Under the assumption of MAR (missing at random), the estimates are still valid, and provide valuable information about the extent to which the changes from baseline to posttreatment are maintained at 18-month follow-up.
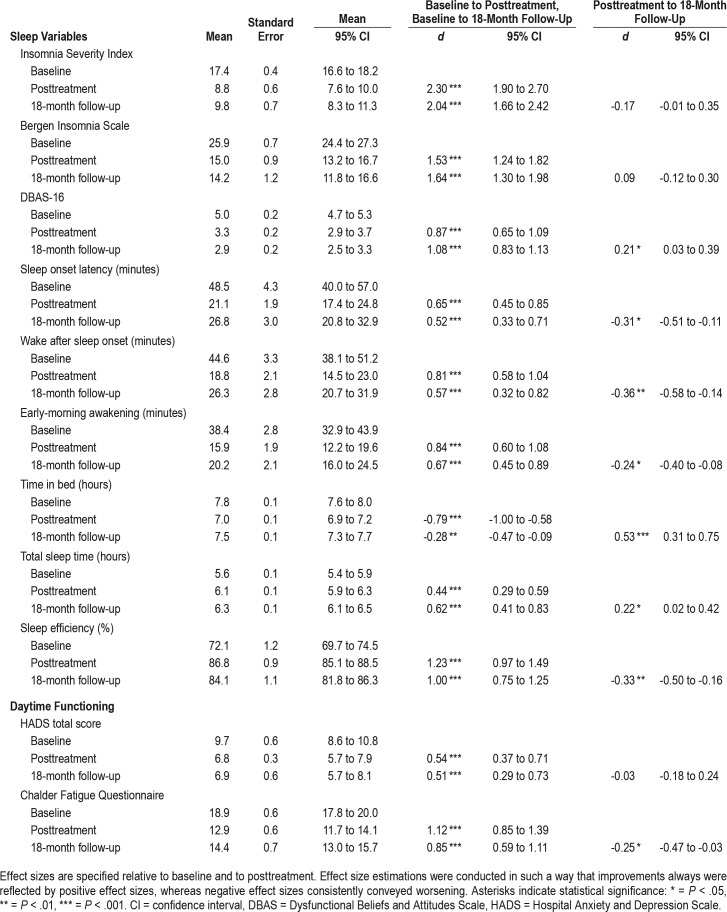
Effect sizes (Cohen d) were calculated in line with the recommendations of Carlson and Schmidt27 and Morris28 and were based on the results from the mixed-model analyses (estimated means and their standard errors). Effect size estimations were conducted in such a way that improvements always were reflected by positive effect sizes, whereas negative effect sizes consistently conveyed worsening. Effect sizes are shown in Table 1. Effect sizes are regarded as large (d = 0.8), moderate (d = 0.5), or small (d = 0.2) according to recognized guidelines.29
Table 1.
Results from linear mixed models (n = 95).
Sample Size Calculation
Previous studies on face-to-face CBT-I have yielded between-group effect sizes (Cohen d) commonly in the range from 0.5 to 1.0 on important sleep diary outcomes (SOL, WASO, number of awakenings, EMA, TIB, TST, and SE) at postassessment, whereas the first study that was carried out on the Internet CBT-I SHUTi program compared to a waitlist control group yielded between-group effect sizes in the range of 0.4 (TST) to 1.0 (SOL) on sleep diary-derived variables at postassessment.
The current study was originally powered to be able to detect a difference between Internet CBT-I and a patient education group at posttreatment of 0.45 (for greater description, see first paper from this clinical trial7). With a power of .80 at P = .05 (two-tailed), the number of participants needed in each group in order to detect differences on the sleep diary outcomes was estimated to be 79.30 The sample size was increased to account for an anticipated dropout rate of approximately 20%, and 205 participants with insomnia were finally included in the original study, where 95 of these were allocated to the Internet CBT-I condition investigated in the current study. Furthermore, the current study has a repeated-measures design using within-subject analyses only, which further strengthens the statistical power.
RESULTS
Sample Characteristics
The sample characteristics have been described previously7 and are therefore only briefly described here. Mean age of the 95 participants was 45.5 years (standard deviation [SD] = 12.6) upon enrollment, and most were female (64%) and married/ cohabiting (64%). Mean years of education was 16.3 (SD = 3.2), which corresponds to a completed bachelor's degree. Sixteen percent of the participants had been suffering from insomnia for 3 to 11 months, 58% for 1 to 10 years, and 26% for more than 10 years. Sixty-six of the 95 participants (70%) first enrolled in the study completed the 18-month follow-up assessment (Figure 1). No significant differences between those who completed the 18-month follow-up and those lost to follow-up were observed on the aforementioned parameters. However, those lost to 18-month follow-up had a somewhat higher baseline ISI score (mean = 18.6, SD = 4.5) than those who completed the follow-up (mean = 16.9, SD = 3.3), t93 = 1.83, P = .041 (two-tailed). There were no significant differences between those who completed the follow-up and dropouts on any of the other variables.
The total number of individuals with clinical anxiety or depression identified with the HADS at baseline was 33 out of 95 (34.7%). Of these 33 participants, 78.8% completed the postassessment and 72.7% completed the 18-month follow-up. Of the 62 participants who scored below the threshold for anxiety/depression at baseline, 82.3% completed the postassessment and 67.7% completed the 18-month follow-up. Furthermore, we calculated the corresponding assessment completion rates based on those who reported taking any form of medication for sleep problems (including z-hypnotics, antidepressants, antihistamines, and melatonin) at baseline versus those who did not. In total, 23 of 95 participants (24.2%) used some form of sleep medication at baseline, of which 65.2% completed the postassessment and 52.2% completed the 18-month follow-up. In comparison, of those who did not use any sleep medication at baseline, 86.1% completed the postassessment and 75.0% completed the 18-month follow-up. However, those who did not complete the 18-month follow-up were not more likely (although close to significant) to use sleep medication at baseline compared to those who did not use medication, χ21, 95 = 3.82, P = .051, phi = −.23.
Baseline to Posttreatment/18-Month Follow-Up
Table 1 presents a summary of the results from the linear mixed models. Results for the primary outcome measures of ISI and BIS showed large and significant improvements from baseline to posttreatment (ISI, P < .001; BIS, P < .001) and from baseline to 18-month follow-up assessment (ISI, P < .001; BIS, P < .001).
On secondary sleep outcomes, significant improvements were observed across all sleep diary parameters from baseline: SOL (to posttreatment, P < .001; to 18-month follow-up, P < .001), WASO (to posttreatment, P < .001; to 18-month follow-up, P < .001), and EMA (to posttreatment, P < .001; to 18-month follow-up, P < .001) with moderate to large effect sizes. Baseline SE of 72% (SD = 12.0) improved to 87% (SD = 5.8) at posttreatment (P < .001), and 84% (SD = 10.9) at 18-month follow-up (P < .001) both with large effect sizes. Mean TST increased from 5.6 hours (SD = 1.4) at baseline to 6.1 hours (SD = 0.9) at posttreatment (P < .001), and 6.3 hours (SD = 1.0) at 18-month follow-up (P < .001), both with small to moderate effect sizes.
Participants scored significantly lower on the DBAS-16 at posttreatment (P < .001) and at 18-month follow-up (P < .001) relative to baseline, both with large effect sizes. Psychological distress (HADS) improved significantly from baseline to post-treatment (P < .001) and to 18-month follow-up (P < .001) with moderate effect sizes. The fatigue scores showed large effect size improvements from baseline to posttreatment (P < .001) and to 18-month follow-up (P < .001).
Posttreatment to 18-Month Follow-Up
Overall, the improvements obtained at posttreatment were maintained at 18-month follow-up (Table 1). We found no significant changes on the ISI or the BIS from posttreatment to 18-month follow-up. Patient scores on DBAS-16 further improved from posttreatment to 18-month follow-up (P = .030) with a small effect size. As for the sleep diary measures SOL (P = .035), WASO (P = .012), and EMA (P = .010), small but significant setbacks were observed. Significant setbacks were also observed in terms of SE, where mean SE dropped from 87% (SD = 8.4) at posttreatment to 84% (SD = 10.7) at 18-month follow-up (P = .002). TST significantly increased from posttreatment to 18-month follow-up (P = .025), from 6.1 hours (SD = 0.9) to 6.3 hours (SD = 1.0), respectively. The improvements observed on the measure of psychological distress (HADS) after the intervention period remained stable at 18-month follow-up. In terms of fatigue (CFQ), a small setback was observed from posttreatment to 18-month follow-up (P = .047).
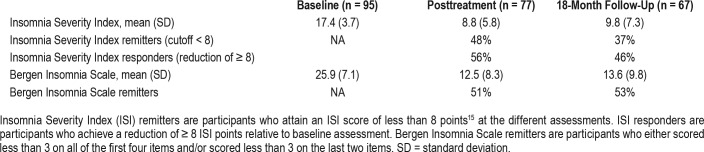
Insomnia Remitters and Responders
Baseline data indicated a mean ISI score of 17.4 (SD = 3.7) (Table 2), which is within the range of moderate to severe clinical insomnia. At baseline, all participants reported an ISI score of 8 points or more, referring to the threshold for remission used in previous studies.15 At 18-month follow-up, 37% reported an ISI score of less than 8 points, indicating that they were in remission. A decrease of ≥ 8 ISI points has been suggested as an indicator of a responder to the treatment,15 and, at 18-month follow-up, 46% fulfilled this criterion. All participants meet the BIS criteria for insomnia at baseline, whereas 53% were in remission at 18-month follow-up.
Table 2.
Insomnia remitters and responders (n = 95).
DISCUSSION
The purpose of the current study was to investigate the extent to which the observed improvements from baseline to after the intervention period are also maintained at 18 months after the intervention period. The study sample comprised the experimental group of a previously published randomized controlled trial comparing Internet CBT-I to web-based patient education, where superiority of Internet CBT-I over patient education was demonstrated short term.7,8 Findings in the current study indicate that the effects for the Internet CBT-I group overall were good at 18-month follow-up evaluation, reflected by effect sizes ranging from 0.5 (SOL) to 2.0 (ISI) on all outcome measures. Compared to studies with shorter follow-up time, the overall effects in the current study appeared slightly higher than those reported in recent meta-analysis on self-help interventions for insomnia,1,31 and are comparable in magnitude to those found in similar studies on fully automated Internet CBT-I3,4,32 and in-person delivered CBT-I.33
It is particularly important to establish the long-term effects of unguided self-help interventions because of the potential for cost-effective dissemination on a population level. Current evidence suggests that interventions including elements of therapist support are somewhat more effective than those without.1 However, we found that large and sustained effects also could be achieved using fully automated Internet CBT-I. This is especially notable as only those with severe psychiatric comorbidity were excluded, whereas disorders such as mild to moderate depression and anxiety disorders were included. The current report adds to the list of studies showing promising effects of treatments that can replicate effects of in-person CBT-I, both in terms of individualizing treatment and making the therapeutic concepts accessible for participants through interactive educational tools.3,4,32,34,35
On most parameters, participants showed the greatest improvement immediately after completing the treatment. If small setbacks occurred, they were observed at 18-month follow-up. Still, the setbacks from posttreatment to 18-month follow-up on sleep diary measures appeared negligible considering that mean SOL, WASO, and EMA still remained below the clinically meaningful threshold of 30 minutes at 18-month follow-up,36 and SE decreased from 87% after the intervention period to 84% at 18-month follow-up, which is only marginally below the clinical threshold of 85%.37 Importantly, a significant improvement on TST from treatment completion to 18-month follow-up was observed. The sleep diary data results are interesting as the recent meta-analysis by Zachariae et al.1 render the long-term effects of Internet CBT-I on sleep diary outcomes somewhat elusive, particularly after adjusting for possible publication bias.
Increase in TST from treatment termination to follow-up is a phenomenon also shown in studies on in-person–delivered CBT-I; where TST typically remains unchanged or even reduced during the initial intervention, but increases at follow-up evaluation.37–39 Many participants may at posttreatment still be adhering to a limited TIB (ie, sleep restriction), and spend the following weeks or months reaching a sleep duration that meets their needs. A transient feature of sleep restriction is consequently an elevated sleep drive that may give rise to an artificially high SE (and correspondingly low SOL, WASO, and EMA), which might be expected to ebb somewhat and then to stabilize as TST increases to match the participants' sleep need. However, the finding that fewer participants were in remission at follow-up compared to posttreatment as defined by the ISI or BIS suggests that a few participants experienced relapse of sleep problems (Table 2). In addition, we found a small increase in fatigue scores from treatment completion to 18-month follow-up. These findings are in contrast to those reported from a 3-year follow-up study comparing guided with unguided Internet CBT-I, reporting that more participants in both conditions were in remission at follow-up as compared to posttreatment.40 Similarly, the recent study by Ritterband et al.4 assessing the effects of a fully automated Internet CBT-I also indicated that more people were in remission at 1-year follow-up, as compared to 6-month follow-up and posttreatment.
Even though there was a small but significant setback after the intervention period (posttreatment to 18-month follow-up) in levels of fatigue, the effect-size improvements from baseline to 18-month follow-up evaluation remained large. Thus, we overall conclude that the beneficial effects of the treatment on fatigue complaints were substantial and lasting. These findings are in line with previous studies demonstrating short-term effects,34,41 but the current study extends these results to evidence of sustained effects beyond treatment completion. Furthermore, on general psychological distress, we demonstrated moderate effect-size improvements from baseline to postassessment and to 18-month follow-up, and there was no significant change between postassessment and follow-up evaluation. This is in line with prior results that show treatment targeting insomnia reduces psychological symptoms, both when the treatment is delivered in person42 and when delivered online.6,34,35 One study found that unguided Internet CBT-I compared to a control group reduced symptoms of anxiety and depression with moderate between-group effect sizes, where the effects were maintained and appeared unabated to a 18-month follow-up evaluation.6 The fact that only 19% of participants completed the 18-month assessment in that study makes the results on the long-term effects somewhat uncertain. However, 70% of the participants in the current study completed the 18-month follow-up, and our observations confirm that the effects appear maintained up to 18 months after the intervention period. A cautionary note when interpreting these results is that only 34% of the participants were in the clinical range of anxiety/depression at baseline. These effects are nevertheless noteworthy considering that the Internet CBT-I treatment does not directly address anxiety and depressive symptoms and the intervention does not propound any booster sessions. These findings suggest that the presence of symptoms of mild to moderate psychological distress and fatigue should not be a pretext to postpone insomnia treatment, which delivered online might be a valuable supplement to treatment of comorbid conditions.
Limitations
It should be noted that the 18-month follow-up evaluation in the current study did not include a control group, which precludes firm causal inferences about the long-term effects of Internet CBT-I due to lack of control for several threats to internal validity.43 Participants typically experience a peak in symptoms upon seeking treatment, where it is reasonable to expect a regression toward mean with passage of time. However, chronic insomnia emerges as a persistent disorder where nearly 70% will still have the disorder 1 year later, and 50% 3 years later when left untreated.44 Still, we know little about the prognosis of untreated insomnia. Participants in the current study had long histories of chronic insomnia, which makes spontaneous recovery of the magnitude we observed an unlikely explanation. Participants with a higher ISI score at baseline were more likely not to complete the 18-month follow-up, and those who used sleep medication at baseline had a tendency (close to significant) toward not completing the 18-month follow-up, which potentially can lead to a bias in the current study. It is possible that missing data at follow-up is partly MNAR. This can imply a risk of underestimation or overestimation of the true change. A sensitivity analysis is recommended in order to check how MNAR may affect change estimates. This was nevertheless not carried out due to the relatively low N in the current sample. One limitation of the current study is that other sleep disorders were only assessed through a short online screening questionnaire and a telephone interview, and no objective measures or standardized assessments were used to rule out other sleep disorders. It is also a limitation that no objective sleep measure (eg, actigraphy or polysomnography) was used as an outcome measure. Another limitation pertains to the low internal consistency of the baseline scores of the ISI and the BIS; however, this is most likely a reflection of the restricted range of scores at this measurement time. It should also be noted that the measure of fatigue used in this study (the CFQ) is not among the consensus recommendations for assessments in insomnia.45 CFQ has nevertheless shown good psychometric properties with high reliability also in clinical samples.25 Most participants were recruited after various media appearances of the authors and may thus represent a self-selected group of resourceful and especially motivated individuals. In line with this, the average education level in the current sample was high; 76.2% had completed at least 1 year of higher education (university/university college level), compared to 32.2% in the Norwegian population as a whole (Statistics Norway). As such, we cannot know whether the observed effects in the current study are transferable to participants referred from general practice. However, this is a limitation that pertains to most studies on fully automated Internet CBT-I.3,4,32,34
CONCLUSIONS
Overall, our results add to the small but growing evidence that the effects of unguided Internet CBT-I are of substantial magnitude, comparable to in-person CBT-I and well maintained over the long term.2,40 The overall effects seem largest immediately after the intervention period and appear to become somewhat reduced at follow-up evaluations. It should be noted that booster sessions were not offered in the current study, and we might have seen even better results at 18-month follow-up if this had been offered. Further work on the long-term effects of Internet CBT-I should evaluate the effects of treatment in study designs that also include control groups. Also, future studies should incorporate a wider range of long-term functional outcome measures that go beyond sleep and symptoms of distress, such as sick leave, medication use, use of health care services, and other measures of general health. Studies should also aim to evaluate the effectiveness of Internet CBT-I in clinical settings, both in comparison to in-person delivered CBT-I and in the context of stepped-care models.46
DISCLOSURE STATEMENT
Work for this study was performed at the Department of Health Promotion, Norwegian Institute of Public Health, Bergen, Norway. All authors gave critical revision of the manuscript for important intellectual content and approved the manuscript before submission. The study was funded by the Norwegian Institute of Public Health and the Norwegian Research Council (project number 239985). F.P.T. and L.M.R. have equity ownership in BeHealth Solutions (Charlottesville, VA, USA), a company that develops and makes available products related to the research reported in this manuscript. BeHealth Solutions has licensed the SHUTi program and the software platform on which it was built from the University of Virginia. The terms of this arrangement have been reviewed and approved by the University of Virginia in accordance with its conflicts of interest policy. The other authors report no conflicts of interest.
ABBREVIATIONS
- BIS
Bergen Insomnia Scale
- CBT-I
cognitive behavioral therapy for insomnia
- CFQ
Chalder Fatigue Questionnaire
- DBAS
Dysfunctional Beliefs and Attitudes Scale
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- EMA
early morning awakening
- HADS
Hospital Anxiety and Depression Scale
- ISI
Insomnia Severity Index
- MAR
missing at random
- MCAR
missing completely at random
- REK
regional ethics committee
- SE
sleep efficiency
- SOL
sleep onset latency
- TIB
time in bed
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1.Zachariae R, Lyby MS, Ritterband LM, O'Toole MS. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia–a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2016;30:1–10. doi: 10.1016/j.smrv.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Lancee J, van den Bout J, van Straten A, Spoormaker VI. Internet-delivered or mailed self-help treatment for insomnia?: a randomized waiting-list controlled trial. Behav Res Ther. 2012;50(1):22–29. doi: 10.1016/j.brat.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Ritterband LM, Thorndike FP, Gonder-Frederick LA, et al. Efficacy of an Internet-based behavioral intervention for adults with insomnia. Arch Gen Psychiatry. 2009;66(7):692–698. doi: 10.1001/archgenpsychiatry.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritterband LM, Thorndike FP, Ingersoll KS, et al. Effect of a web-based cognitive behavior therapy for insomnia intervention with 1-year follow-up: a randomized clinical trial. JAMA Psychiatry. 2017;74(1):68–75. doi: 10.1001/jamapsychiatry.2016.3249. [DOI] [PubMed] [Google Scholar]
- 5.Sivertsen B, Salo P, Mykletun A, et al. The bidirectional association between depression and insomnia: the HUNT study. Psychosom Med. 2012;74(7):758–765. doi: 10.1097/PSY.0b013e3182648619. [DOI] [PubMed] [Google Scholar]
- 6.Batterham PJ, Christensen H, Mackinnon AJ, et al. Trajectories of change and long-term outcomes in a randomised controlled trial of internet-based insomnia treatment to prevent depression. BJPsych Open. 2017;3(5):228–235. doi: 10.1192/bjpo.bp.117.005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagatun S, Vedaa Ø, Nordgreen T, et al. The short-term efficacy of an unguided Internet-based cognitive-behavioral therapy for insomnia: a randomized controlled trial with a six-month nonrandomized follow-up. Behav Sleep Med. 2017:1–23. doi: 10.1080/15402002.2017.1301941. [DOI] [PubMed] [Google Scholar]
- 8.Hagatun S, Vedaa Ø, Harvey AG, et al. Internet-delivered cognitive-behavioral therapy for insomnia and comorbid symptoms. Internet Interv. 2018;12:11–15. doi: 10.1016/j.invent.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 10.Svanborg P, Ekselius L. Self-assessment of DSM-IV criteria for major depression in psychiatric out-and inpatients. Nord J Psychiatry. 2003;57(4):291–296. doi: 10.1080/08039480307281. [DOI] [PubMed] [Google Scholar]
- 11.Ritterband LM, Thorndike FP, Gonder-Frederick LA, et al. Efficacy of an internet-based behavioral intervention for adults with insomnia. Arch Gen Psychiatry. 2009;66(7):692–698. doi: 10.1001/archgenpsychiatry.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morin CM. Insomnia: Psychological Assessment and Management. New York, NY: Guilford Publications; 1993. [Google Scholar]
- 13.Thorndike FP, Saylor DK, Bailey ET, Gonder-Frederick LA, Morin CM, Ritterband LM. Development and perceived utility and impact of an Internet intervention for insomnia. E J Appl Psychol. 2008;4(2):32–42. doi: 10.7790/ejap.v4i2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 15.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morin CM, Espie CA. Insomnia: A Clinician's Guide to Assessment and Treatment. New York, NY: Springer; 2003. [Google Scholar]
- 17.Pallesen S, Bjorvatn B, Nordhus IH, Sivertsen B, Hjørnevik M, Morin CM. A new scale for measuring insomnia: the Bergen Insomnia Scale. Percept Mot Skills. 2008;107(3):691–706. doi: 10.2466/pms.107.3.691-706. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 19.Morin CM, Vallières A, Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16) Sleep. 2007;30(11):1547–1554. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The Consensus Sleep Diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 22.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale: an updated literature review. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 23.Kjærgaard M, Arfwedson Wang CE, Waterloo K, Jorde R. A study of the psychometric properties of the Beck Depression Inventory-II, the Montgomery and Åsberg Depression Rating Scale, and the Hospital Anxiety and Depression Scale in a sample from a healthy population. Scand J Psychol. 2014;55(1):83–89. doi: 10.1111/sjop.12090. [DOI] [PubMed] [Google Scholar]
- 24.Chalder T, Berelowitz G, Pawlikowska T, et al. Development of a fatigue scale. J Psychosom Res. 1993;37(2):147–153. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- 25.Morriss R, Wearden A, Mullis R. Exploring the validity of the chalder fatigue scale in chronic fatigue syndrome. J Psychosom Res. 1998;45(5):411–417. doi: 10.1016/s0022-3999(98)00022-1. [DOI] [PubMed] [Google Scholar]
- 26.Loge JH, Ekeberg Ø, Kaasa S. Fatigue in the general Norwegian population: normative data and associations. J Psychosom Res. 1998;45(1):53–65. doi: 10.1016/s0022-3999(97)00291-2. [DOI] [PubMed] [Google Scholar]
- 27.Carlson KD, Schmidt FL. Impact of experimental design on effect size: Findings from the research literature on training. J Appl Psychol. 1999;84:851–862. [Google Scholar]
- 28.Morris SB. Estimating effect sizes from pretest–posttest-control group designs. Organ Res Methods. 2008;11:364–386. [Google Scholar]
- 29.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 30.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 31.Ho FY-Y, Chung K-F, Yeung W-F, et al. Self-help cognitive-behavioral therapy for insomnia: a meta-analysis of randomized controlled trials. Sleep Med Rev. 2015;19:17–28. doi: 10.1016/j.smrv.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Espie CA, Kyle SD, Williams C, et al. A randomized, placebo-controlled trial of online cognitive behavioral therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep. 2012;35(6):769–781. doi: 10.5665/sleep.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191–204. doi: 10.7326/M14-2841. [DOI] [PubMed] [Google Scholar]
- 34.Thorndike FP, Ritterband LM, Gonder-Frederick LA, Lord HR, Ingersoll KS, Morin CM. A randomized controlled trial of an internet intervention for adults with insomnia: effects on comorbid psychological and fatigue symptoms. J Clin Psychol. 2013;69(10):1078–1093. doi: 10.1002/jclp.22032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christensen H, Batterham PJ, Gosling JA, et al. Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight Study): a randomised controlled trial. Lancet Psychiatry. 2016;3(4):333–341. doi: 10.1016/S2215-0366(15)00536-2. [DOI] [PubMed] [Google Scholar]
- 36.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2013. [Google Scholar]
- 37.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004) Sleep. 2006;29(11):1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 38.Espie CA, Inglis SJ, Tessier S, Harvey L. The clinical effectiveness of cognitive behaviour therapy for chronic insomnia: implementation and evaluation of a sleep clinic in general medical practice. Behav Res Ther. 2001;39(1):45–60. doi: 10.1016/s0005-7967(99)00157-6. [DOI] [PubMed] [Google Scholar]
- 39.Friedman L, Benson K, Noda A, et al. An actigraphic comparison of sleep restriction and sleep hygiene treatments for insomnia in older adults. J Geriatr Psychiatry Neurol. 2000;13(1):17–27. doi: 10.1177/089198870001300103. [DOI] [PubMed] [Google Scholar]
- 40.Blom K, Jernelöv S, Ruck C, Lindefors N, Kaldo V. Three-year follow-up of insomnia and hypnotics after controlled internet treatment for insomnia. Sleep. 2016;39(6):1267–1274. doi: 10.5665/sleep.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ritterband LM, Bailey ET, Thorndike FP, Lord HR, Farrell-Carnahan L, Baum LD. Initial evaluation of an Internet intervention to improve the sleep of cancer survivors with insomnia. Psychooncology. 2012;21(7):695–705. doi: 10.1002/pon.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manber R, Edinger J, Gress J, San Pedro-Salcedo M, Kuo T, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489–495. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campbell DT, Stanley JC. Experimental and Quasi-Experimental Designs for Research 1963. Dallas, TX: Houghton Mifflin Company; 1963. [Google Scholar]
- 44.Morin CM, Benca R. Chronic insomnia. Lancet. 2012;379(9821):1129–1141. doi: 10.1016/S0140-6736(11)60750-2. [DOI] [PubMed] [Google Scholar]
- 45.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29(9):1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 46.Vincent N, Walsh K. Stepped care for insomnia: An evaluation of implementation in routine practice. J Clin Sleep Med. 2013;9(3):227–234. doi: 10.5664/jcsm.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]