Abstract
Study Objectives:
Delirium is a postoperative complication accompanied by disturbances in attention, cognition, arousal, and psychomotor activity. Wrist actigraphy has been advocated to study inactivity and inferred sleep patterns during delirium. We hypothesized that altered patterns of motor activity or immobility, reflective of disordered sleep and wakefulness patterns, would serve as predictive markers of hypoactive postoperative delirium.
Methods:
Eighty-four elderly surgical patients were classified into three groups based on the timing of hypoactive delirium following surgery: intact with no delirium throughout postoperative days (POD) 0–5 (n = 51), delirium during POD 0–1 (n = 24), and delirium during POD 2–5 (n = 13). Delirium was detected on daily Confusion Assessment Method evaluations and chart review. Actigraphy measures were calculated from accelerometry signals acquired on the first postoperative day (POD 0, 16:00–23:00) and night (POD 0, 23:00–POD 1, 06:00).
Results:
Actigraphy metrics showed substantial interpatient variability. Among the three patient groups, only those without delirium showed greater movement during the day compared to night and also fewer minutes of night immobility (P = .03 and P = .02, Wilcoxon rank-sum tests). These patients were poorly discriminated from those with delirium during either POD 0–1 or POD 2–5, using differences in day and night activity (C-statistic, 95% confidence interval [CI]: 0.66 [0.53–0.79] and C-statistic, 95% CI: 0.71 [0.55–0.87], respectively). Inclusion of low-frequency signals improved performance of immobility measures without affecting those based on activity. Cognitively intact patients during POD 0–5 were distinguished from those with delirium during POD 0–1, based on differences in the number of day and night immobile minutes (C-statistic 0.65, 95% CI: [0.53–0.78]). Actigraphy metrics with the strongest association to delirium incidence were not reliably correlated with an increased risk during POD 0–5, when accounting for patient age, sex, intensive care unit admission, and Charlson Comorbidity Index (adjusted odds ratio of 1.7, 95% CI: [1.0–3.0], P = .09, likelihood ratio test).
Conclusions:
Early postoperative wrist actigraphy metrics that serve as markers of sleep and wakefulness offer limited capacity as sole predictors or markers of hypoactive delirium.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Title: Electroencephalography Guidance of Anesthesia to Alleviate Geriatric Syndromes (ENGAGES) Study; Identifier: NCT02241655; URL: https://clinicaltrials.gov/ct2/show/NCT02241655
Citation:
Maybrier HR, King CR, Crawford AE, Mickle AM, Emmert DA, Wildes TS, Avidan MS, Palanca BJ; ENGAGES Study Investigators. Early postoperative actigraphy poorly predicts hypoactive delirium. J Clin Sleep Med. 2019;15(1):79–87.
Keywords: actigraphy, anesthesia, arousal, postoperative delirium, sleep, surgery
BRIEF SUMMARY
Current Knowledge/Study Rationale: Delirium is associated with alterations of sleep and psychomotor activity. Immobility and activity measures derived from wrist actigraphy in the early postoperative period have unknown utility for predicting delirium in high-risk patients.
Study Impact: Markers of activity and immobility had only weak discriminative capacity for concurrent delirium and poorly predicted those with subsequent delirium. These data suggest that actigraphy within the first 24 hours after surgery is unlikely to be useful for delirium prediction.
INTRODUCTION
Delirium is a frequent surgical complication defined by acute impairments in attention and cognition.1 This syndrome is also commonly accompanied by disruptions in psychomotor activity2 and sleep architecture. Putative risk factors for delirium after surgery include preoperative3,4 and postoperative5 disruptions of sleep architecture and circadian rhythms.6 In a small case series, postoperative patients with delirium show similar levels of motor activity during day and night, providing more evidence that sleep disturbances may accompany this complication.7,8 Sleep quality may be a modifiable risk factor that can be intervened upon in the perioperative period, as temporal relationship to the onset of delirium has mechanistic and clinical implications.
Actigraphy can reveal altered sleep-wake patterns that may precede or coincide with delirium.9–11 This inexpensive, noninvasive technique has been validated against polysomnography for identifying periods of sleep and wakefulness. Wrist actigraphy can also detect circadian motor activity patterns10 that may reflect delirium risk.3,7,8 It is unknown whether wrist actigraphy measurements acquired within the first 24 hours after surgery can predict subsequent delirium. Standardized approaches to analyzing actigraphy data in the early postoperative period would also need to account for potential activity restrictions, due to pain, monitoring and intravenous devices, or sanctioned bed rest. Although the inclusion of lower frequency of accelerometer signals has been explored in patients with limited mobility,12–17 it is not known whether this approach improves discriminative capacity of actigraphy markers for delirium.
The goal of this exploratory study is to evaluate whether actigraphy measures based on the first day and night after surgery, would mark or predict hypoactive delirium. The prevalent hypoactive delirium subtype is accompanied by psychomotor retardation, rather than the agitation2 associated with nocturnal hyperactivity or “sundowning.”18 A group with hypoactive delirium may provide a more homogenous motor phenotype, with slower or fewer movements compared to other subtypes. We hypothesized that altered patterns of motor activity or im-mobility, that reflect sleep and wakefulness patterns, would allow us to discriminate the presence and timing of hypoactive postoperative delirium. Therefore, we used metrics of activity and immobility as surrogate measures of sleep, in lieu of software-based scoring.
METHODS
Patients and Recruitment
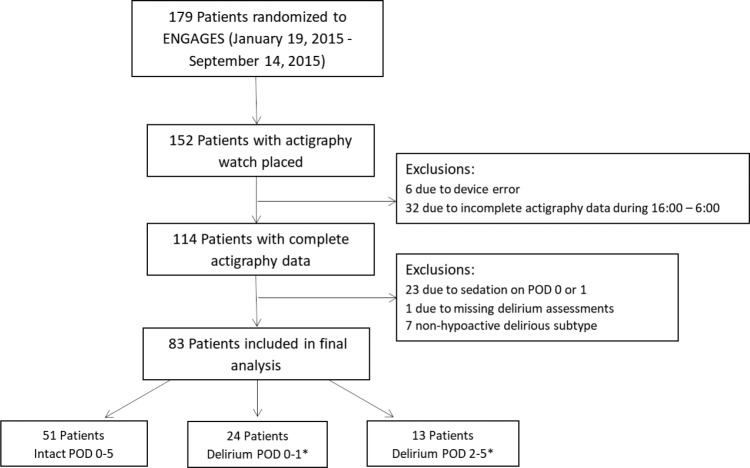
All patients were enrolled in the recently completed study of Electroencephalography Guidance of Anesthesia to Alleviate Geriatric Syndromes (ENGAGES, NCT02241655). This prospective randomized controlled trial was designed to test whether avoidance of electroencephalographic suppression during surgery is associated with a lower incidence of postoperative delirium.19 ENGAGES was approved by the Human Research Protection Office at the Washington University School of Medicine in St. Louis. Patients enrolled in ENGAGES were at least 60 years of age and scheduled for major elective surgery under general anesthesia. This substudy of ENGAGES was conducted between January 19, 2015 and September 14, 2015 and halted due to equipment availability. Complete actigraphy recordings were collected from 114 patients. Eighty-three patients were included in our final data set; reasons for exclusion are detailed in Figure 1. Those who demonstrated only hyperactive or mixed subtypes were excluded from analyses. Patients were not withdrawn due to arm restraints (n = 6).
Figure 1. Enrollment flow chart.
* = 5 patients included in both groups. POD = postoperative day.
Delirium Assessments and Classification
Determination of delirium was based on validated bedside clinical instruments and review of medical records. Trained research staff determined whether delirium was present, using the Confusion Assessment Method (CAM) or the Intensive Care Unit (CAM-ICU) version.20,21 The CAM was used for all verbally responsive patients, whereas the CAM-ICU was used for those who were intubated or otherwise nonverbal. Assessments on postoperative day (POD) 0 were completed at least 2 hours after completion of surgery. Daily assessments were then performed between 13:00 and 20:00 through postoperative day 5 (POD 5) or hospital discharge. We supplemented the sensitivity for our primary outcome by employing a validated review of participants' electronic medical record for evidence of delirium.22 Nursing CAM-ICU assessments from each 12-hour shift and Richmond Agitation-Sedation Scale23 scores were incorporated in the review to assess abnormal psychomotor activity.
Patients were also grouped according to the presence and timing of delirium (Figure 1). Delirium motor subtypes include hypoactive, hyperactive, and mixed.2 Patients were categorized as hypoactive if they displayed psychomotor retardation or a decreased level of consciousness. Those with psychomotor agitation or hypervigilance were categorized with the hyperactive form. The infrequent mixed subtype was reserved for patients who displayed both hyperactive and hypoactive characteristics within the same interview. Patients with delirium were further categorized relative to the day of surgery, as either concurrent (POD 0–1) or after actigraphy measurements (POD 2–5). Our three groups included: no delirium on all postoperative assessments (Intact POD 0–5, n = 57), hypoactive delirium incident on the day of surgery or on postoperative day 1 (Delirium POD 0–1, n = 19), hypoactive delirium during the interval from postoperative days 2 through 5 (Delirium POD 2–5, n = 13). Five patients were included in both Delirium groups, given presentation of hypoactive delirium during both POD 0–1 and POD 2–5.
Actigraphy Acquisition and Preprocessing
Although actigraphy is relatively inexpensive to acquire, a short time window within the first 24 hours after surgery was selected based on the rationale that early changes in motor activity patterns would lend clinical utility for intervention. The following three actigraphy bracelet models provided accelerometry signals: ASPW wActiSleep Plus, ASPB, and wGT3XBT (ActiGraph Corp., Pensacola, Florida, United States). Patients wore one of these devices on their nondominant wrist immediately following their procedure until removal on POD 1. Accelerometer signals, acquired by these devices at 30 Hz sampling rate, were both preprocessed and exported through ActiLife software (v6.11.5, ActiGraph Corp). Specifics of data filtering and detection of counts in ActiLife have not been fully disclosed by the manufacturer. Counts were calculated from the filtered accelerometry time-series,24 summed within 1-minute bins, and exported in comma-separated value (CSV) formatted data files.
We considered whether the exclusion of low frequencies during preprocessing of accelerometry signals would reduce the discriminability of actigraphy markers for hypoactive delirium. Prior investigations suggested that the inclusion of low frequency signals might increase sensitivity of these devices for detecting movement.12–17 These lower frequencies are routinely excluded during the processing of raw accelerometer signals to filter out noise and signal drift, prior to quantification of activity counts. For ActiGraph devices, activation of the Low Frequency Extension (LFE) reduces the high-pass filter cutoff frequency for attenuating lower frequencies. If lower amplitude motion is contained in these lower frequencies, either sensitivity for detecting movement or specificity for quantifying immobility may be improved. Relatively impaired limb movements may be expected in elderly patients who have recently undergone major surgery. Thus, we performed analyses, with and without the LFE active, to determine if discriminability among patient groups could be improved through the inclusion of lower signal frequencies.
Actigraphy Measures
Following data import of ActiLife-exported CSV data files, subsequent analyses were performed using MATLAB software (Mathworks, Natick, Massachusetts, United States) toolboxes and custom-written scripts. Measures of root mean-squared activity (RMSactivity) were calculated by combining counts (binned in 1-minute intervals) across all three accelerometer axes (X, Y, and Z):
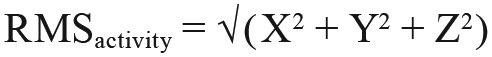 |
Actigraphy metrics from both day and night periods may show aberrant patterns indicating altered sleep-wake cycles. Using similar boundaries as other investigators, we defined the daytime epoch as POD 0 16:00–23:00, and the nighttime epoch as POD 0 23:00 to POD 1 06:00.8 For each patient and epoch, we calculated actigraphy metrics to quantify both the extent of movement and immobility. In contrast to our prior approach that combined epochs of inactivity (RMSactivity equal to 0) and periods of activity (RMSactivity greater than 0),25 immobility and activity metrics were assayed separately for direct comparison to existing literature. Median activity count (MAC) was calculated from all minutes with nonzero RMS activity within each epoch. For each patient, median rather than mean26,27 was taken given the likely skew in the activity count distribution during each patient's recording. MACDay-Night assesses the difference in activity between day and night.28 Patients active during the day and with minimal movement and sleep microarousals at night would have strongly positive MACDay-Night measures.
As the hypoactive subtype of delirium is associated with psychomotor retardation and a reduced level of arousal,29 we reasoned that the extent of inactivity would also serve as a useful marker. We quantified inactivity using the number of immobile minutes (NOIM), defined as the total number of minutes with an RMSactivity count of zero.7,8 Analogous to our measures for movement within each time epoch, NOIM were calculated for the day (NOIMDay) and night (NOIMNight). The total duration over both periods (NOIMTotal) and the difference in nighttime and daytime minutes (NOIMNight-Day) were also derived. We assumed that NOIM would serve as a reasonable surrogate for minutes of sleep and that NOIMNight-Day would be positive for patients who slept less in the daytime.
Statistical Analyses
Nonparametric statistical approaches were applied using MATLAB functions and custom-written scripts. Because this was an exploratory hypothesis-generating study, no sample size calculations were performed. Median and interquartile ranges were calculated for activity and immobility measurements.30 In comparing patient characteristics, Wilcoxon rank-sum tests were used to assess differences in age or Charlson Comorbidity Index, whereas chi-square tests were applied to evaluate differences in proportions of females or postoperative admission to the ICU. Wilcoxon rank-sum tests assessed differences in median actigraphy measures, without correction of α for multiple comparisons. We used the concordance statistic (C-statistic) to discriminate actigraphy metrics between groups. To calculate the C-statistic, we calculated the area under the curve (AUC) generated by receiver operating characteristic (ROC) analysis. The C-statistic indicates the performance at distinguishing two groups across varied thresholds of sensitivity and specificity, ranging from chance (0.5) to ideal (1.0). Ninety-five percent confidence intervals (CI) were calculated using bootstrapping (MATLAB “bootci” function, 1,000 iterations, normal approximated interval with bootstrap bias and standard error). CI excluding 0.5 would be consistent with discriminability above chance.
Odds ratios were also computed using logistic regression to assess the relationships between standardized (z-scored) actigraphy measures and delirium at any point during POD 0–5. Univariate models included all activity and immobility measures, with and without LFE. Multivariable logistical regression models were then constructed to determine the optimal performance based on the top three measures. We used L1 penalized (lasso) logistic regression with z-scored actigraphy measures as predictors and the outcome being delirium at any point during the interval of POD 0–5. These analyses were implemented in the “glmnet” R package. The penalty parameter was chosen by leave-one-out cross-validation. This approach yielded a top set of actigraphy metrics. We report estimated joint odds ratio (OR) for these variables by usual logistic regression, along with CIs and likelihood ratio P values.
RESULTS
Differential Activity Between Day and Night May Predict Hypoactive Delirium
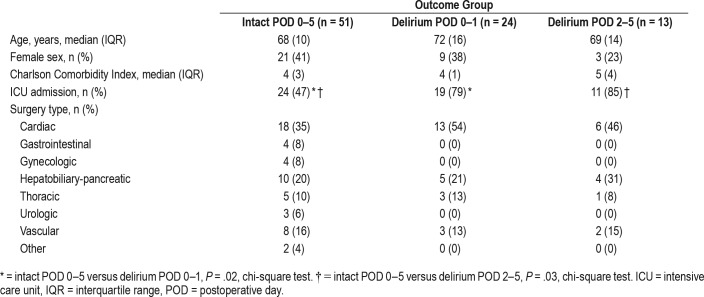
We compared motor activity measures among three groups of patients designated by presence and timing of delirium: no hypoactive postoperative delirium during POD 0–5 (Intact POD 0–5 group, n = 51), hypoactive postoperative delirium in the POD 0–1 interval (Delirium POD 0–1 group, n = 24), and hypo-active delirium during the POD 2–5 period (Delirium POD 2–5 group, n = 13). Demographic characteristics of these patients are provided in Table 1. These groups had no significant differences in median Charlson Comorbidity Index31 or age (P > .05 for all comparisons, Wilcoxon rank-sum test). There were no significant differences in sex between groups (P > .05 for all comparisons, chi-square test). Compared to those intact on POD 0–5, a higher proportion of postoperative ICU admission was noted in those with delirium on POD 0–1 (P = .02, chi-square test) and POD 2–5 (P = .03, chi-square test).
Table 1.
Patient demographics.
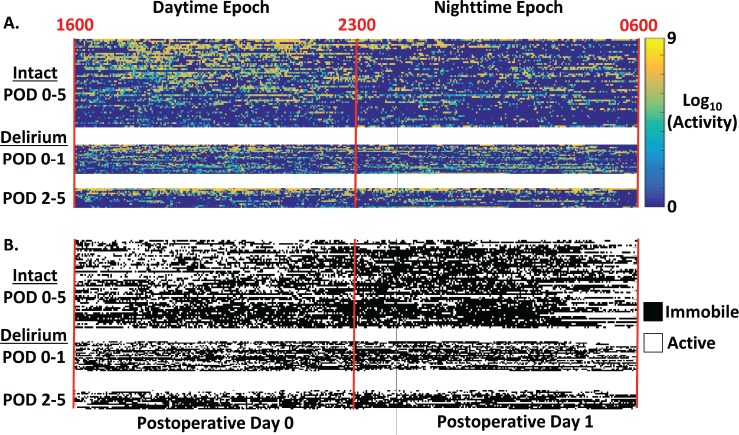
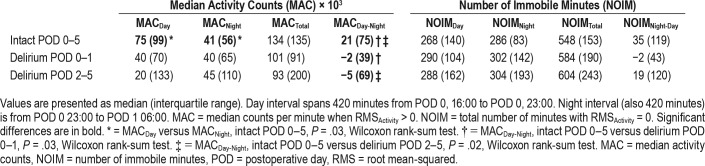
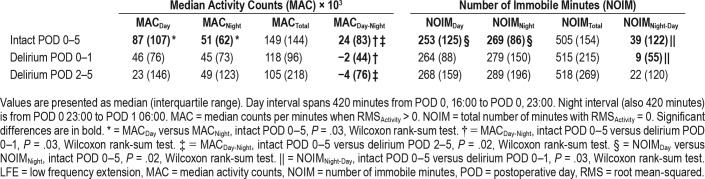
Activity counts over 1-minute epochs showed substantial variability in the timing and number of counts across patients within the first 14 hours after surgery (over eight orders of magnitude, Figure 2A). For the Intact POD 0–5 group, greater MACs are observed during the daytime (16:00–23:00) than for the nighttime (23:00–06:00, Figure 2A). Only the Intact POD 0–5 cohort showed a significant difference between day and night activity (MACDay-Night, 75 × 103 counts compared to 41 × 103 counts, P = .03, Wilcoxon rank-sum test). Thus, this surrogate marker of an intact sleep-wake cycle was only observed in the group without detectable delirium throughout POD 0–5 (Table 2).
Figure 2. Temporal profiles of activity and immobility for patients categorized based on delirium onset relative to postoperative day.
Each row depicts measures from an individual, with patients separated into three groups: Intact POD 0–5, Delirium POD 0–1, and Delirium POD 2–5. Participants in each group are arranged in descending order from top to bottom by total daytime activity counts. Color scale indicates the magnitude of activity counts within a 1-minute interval. (A) Actigraphy measures were calculated for two epochs, Daytime (1600–2300, POD 0) and Nighttime (2300, POD 0 - 0600, POD 1). Activity counts at 1-minute increments are plotted on a logarithmic scale due to the range across measurements. MN = midnight. (B) Time course of 1-minute increments classified as patients being either immobile (black) or active (white). POD = postoperative day.
Table 2.
Median activity counts and number of immobile minutes during daytime, nighttime, and combined epochs.
Compared to cognitively intact patients, those with hypoactive delirium during POD 0–1 were expected to show reduced motor activity, related to psychomotor retardation during concurrent actigraphy. No significant differences were observed for MACDay, MACNight, or MACTotal when comparing patients in the Intact POD 0–5 and Delirium POD 0–1 groups. MACDay-Night differed between patients who were intact during POD 0–5 and patients with delirium over the interval from POD 0–1 (21 × 103 compared to −2 × 103 counts, P = .03, Wilcoxon rank-sum test).
Patients presenting with hypoactive delirium during POD 2–5 could have a prodromal period of reduced motor activity. Instead, comparisons between patients Intact POD 0–5 and Delirium POD 2–5 groups showed no significant differences in MAC between time epochs (P > .05, Wilcoxon rank-sum test). However, the difference between day and night (MACDay-Night) was greater among those of the Intact POD 0–5 compared to Delirium POD 2–5 (21 × 103 compared to −5 × 103 counts, P = .02, Wilcoxon rank-sum test).
Greater Durations of Immobility May Accompany Hypoactive Delirium
We reasoned that the NOIM28 would complement graded continuous measures of motor activity. Like our measures of motor activity, NOIM measures showed substantial inter-patient variability (Figure 2B). In contrast to the MAC measures, there were no significance differences between NOIMDay and NOIMNight within patients who were intact during POD 0–5 (Table 2, Wilcoxon rank-sum test, all P > .05). Furthermore, there were no significant differences in NOIM measures among any of the groups (Wilcoxon rank-sum test, all P > .05). Thus, with the default actigraphy signal preprocessing, NOIM did not distinguish epochs of day and night, nor groups defined by the presence of postoperative delirium.
Inclusion of Low Frequencies May Enhance Immobility Measures
Activation of the low frequency extension (LFE) allowed the processing of lower signal frequencies that are conventionally attenuated prior to the detection of activity counts. Inclusion of these lower frequencies could potentially affect the specificity of immobility metrics (see Methods). Differences in temporal profiles of activity and immobility at an individual patient level were difficult to discern (Figure S1 in the supplemental material). Relationships of MAC measures within or between patient groups were unchanged with LFE active (Table 3) compared to identical analyses with LFE inactive (Table 2). High variability persisted in NOIMDay and NOIMNight epochs (Table 3). In contrast to our previous analysis with the LFE inactive, paired comparisons for patients intact during POD 0–5 showed a greater NOIMNight compared to NOIMDay (269 compared to 253 minutes, Wilcoxon rank-sum test, P = .02). This finding was mirrored by lower interquartile ranges for NOIMDay (LFE inactive: 140 minutes, LFE active: 125 minutes) and greater intra-individual NOIMNight-Day for patients in this group. Overall, these results are consistent with LFE activation conferring greater NOIM specificity for detecting immobility and sleep.
Table 3.
Median activity counts and number of immobile minutes during daytime, nighttime, and combined epochs with LFE active.
NOIMNight-Day was greater in patients without delirium compared to those with delirium on POD 0–1 (Table 3, P = .03, Wilcoxon rank-sum test). Otherwise, there were no differences in the median NOIMNight, NOIMTotal, or NOIMDay between groups with the LFE (Wilcoxon rank-sum test, all P < .05). Similarly, with the LFE active, comparisons of NOIM measures between patients with delirium during POD 2–5 and those without delirium POD 0–5 yielded no significant differences. Thus, overall, while implementation of the LFE may aid in detecting immobile periods, the magnitude of the effects between groups remain small and of questionable clinical utility.
Discriminability for Early and Late Hypoactive Delirium Based on Activity and Immobility Measures
To address how well actigraphy measures could discriminate between patient outcome groups, we calculated C-statistics following ROC analyses. We focused on the day-night difference metrics that previously showed differences between groups. C-statistic and confidence intervals for MAC and NOIM measures (LFE active) are provided in Table 4. MACDay-Night poorly discriminated patients who were Intact POD 0–5 from those with delirium (POD 0–1: C-statistic 0.66, 95% CI: [0.53–0.79]; POD 2–5: C-statistic 0.71, 95% CI: [0.55–0.87]). All other comparisons of MACDay, MACDay-Night, MACNight, and MACTotal had confidence intervals overlapping 0.5, suggesting no capacity for distinguishing between patients intact during POD 0–5 and those with delirium during the same period. Overall, the contrast of activity between day and night was poorly predictive for the presence of delirium during either POD 0–1 or POD 2–5.
Table 4.
Discriminability between groups based on median activity counts or number of immobile minutes.
Measures of immobility were also assessed for discriminative capacity. Using NOIMNight-Day, patients with delirium during POD 0–1 were discriminated from patients intact during POD 0–5 (C-statistic for either 0.65, 95% CI: [0.53–0.78]) However, NOIMNight, NOIMDay and NOIMTotal did not effectively distinguish actigraphy measures among these two groups (data not shown), as the CIs for C-statistic included 0.5. Thus, NOIM based on night actigraphy measures alone may be informative but is only a poor marker for early delirium during POD 0–1. Without the LFE active, measures of immobility did not allow discrimination between patient groups while activity measures remained poorly effective (Table S1 in the supplemental material). Comparisons of NOIM measures for patients in the Delirium POD 2–5 and Intact POD 0–5 groups show discriminative performance no better than chance. Use of LFE, as a technical advancement to improve detection of immobility periods, allowed discriminability as a marker of hypoactive delirium without affecting discriminability based on MAC. Paralleling measures of activity, only NOIM differences between day and night epochs passed statistical thresholds during comparisons between study groups.
We also assessed how each activity and immobility measure related to the risk of delirium any time between POD 0 and 5. Table 5 displays the unadjusted ORs of a standard deviation change in each actigraphy variable. Although immobility measures were not related to an increased likelihood of delirium, a reduced risk of this complication (per standard deviation of metric) was only associated with MACDay-Night with LFE (OR 0.53, 95% CI: [0.31–0.91], P = .02) or without LFE (OR 0.53, 95% CI: [0.31–0.91], P = .02). When these estimates were adjusted for age, sex, Charlson Comorbidity Index, and ICU admission, however, neither passed significance testing (Table S2 in the supplemental material). Thus, the differential of day and night activity may only have modest value as an indicator of delirium risk in the postoperative period.
Table 5.
Univariate classification odds ratio for one standard deviation change in the respective actigraphy variable for the presence of delirium during the interval from POD 0–5.
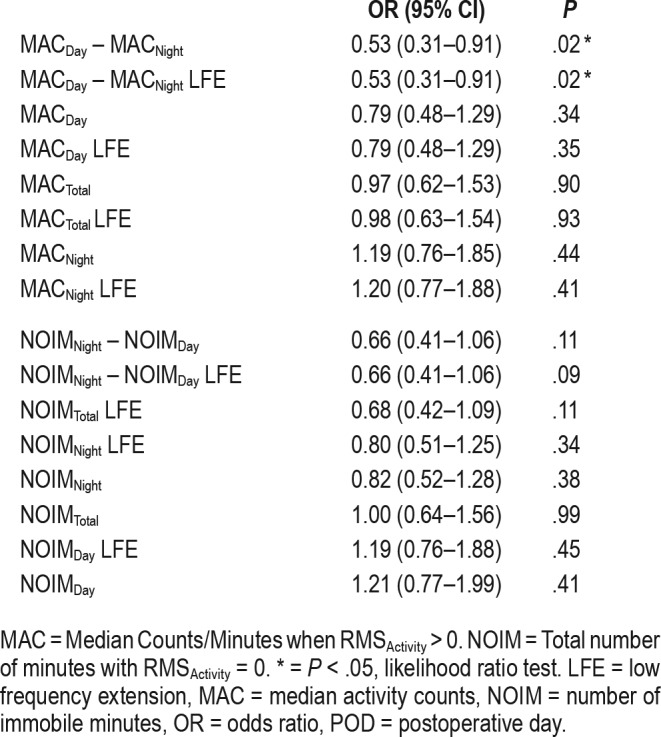
Last, multivariable regression models were constructed to assess whether combinations of the top three performing metrics provided additional discriminability beyond the poorly predictive measures that would not survive multiple comparisons corrections. These models were adjusted for covariates of age, ICU admission, and medical comorbidities. The actigraphy measures most strongly predictive of delirium during POD 0–5 included NOIMNight with LFE, NOIMNight-Day with LFE, and MACDay-Night with LFE. When considered together, a C-statistic for the combined measures was only 0.67 (95% CI: [0.56–0.77]. A 90% increase in odds of delirium would be predicted by 1-unit change in the weighted versions of these three metrics (unadjusted OR: 1.9, 95% CI: [1.2–3.4]), but did not survive significance testing when adjusted for covariates (adjusted OR 1.7 [1.0–3.0] P = .09, likelihood ratio test). Thus, even combinations of actigraphy measures perform poorly at discriminating individuals with delirium against those without this complication during POD 0–5.
DISCUSSION
Summary of Findings
We explored actigraphy metrics as concurrent or predictive markers for altered sleep/wakefulness patterns in the context of hypoactive postoperative delirium. We assumed that im-mobility would mark periods of sleep or hypoactive delirium while activity would primarily mirror wakeful intervals. Patients in whom delirium did not develop during POD 0–5 showed greater activity and lower duration of immobility during the day compared to during the night. This pattern during the first 24 hours following surgery presumes a cycle of daytime wakefulness and nocturnal sleep continued from the preoperative period. Within-individual differences in daytime and nighttime measures for both activity and immobility only poorly discriminated patients who were intact on POD 0–5 from those with delirium during the intervals of POD 0–1. Measures based on activity, but not immobility, detected those in whom delirium subsequently developed on POD 2–5. Last, inclusion of lower frequency signals, through activation of the LFE, augmented sensitivity for detecting within-group and between-group differences in immobility metrics. This extra preprocessing did not adversely affect discriminability based of activity measures. We caution that these effects are small, merely useful for hypothesis generation, and would not survive correction for multiple comparisons. The large variance of activity across patients suggests difficulty in employing these measures for prognostic purposes at an individual level. Overall, our data suggest that this variance, coupled with the nonspecific nature of wrist activity, highlights substantial impediments in using actigraphy alone as surrogate measures of sleep and wakefulness to predict hypoactive delirium in the early postoperative period.
Comparison to Previous Studies
Multiple lines of evidence suggest a relationship between sleep disruption and postoperative delirium. Evidence of this relationship is supported by the (1) independent risk for delirium associated with sleep disturbances, such as obstructive sleep apnea,31,32 (2) reduction of delirium incidence with sleep promotion interventions (ie, exogenous melatonin, earplugs, improved sleep hygiene, etc.),33–35 and (3) shared features of circadian rhythm disorders and delirium.5,36,37 Our results are consistent with a correlation between sleep disturbance and delirium but suggest that components of clinical polysomnography may more directly address this putative relationship in patients with delirium and critical illness.37,38
Our study extends earlier investigations of postoperative delirium and actigraphy in the surgical population.7,8,28 Jacobson and colleagues28 compared actigraphy measures of 13 postsurgical patients, 6 of whom had delirium. Results showed that patients with delirium had greater median activity at night, a lower difference between night and day activity, and fewer resting minutes during both night and day. A subsequent study7 reported on 79 patients who had undergone cardiac surgery who were stratified by duration and timing of delirium over the first 5 postoperative days: no delirium or delirium only on the first postoperative day (n = 46), delirium of 2 to 3 days duration (n = 16), or delirium lasting 4 or more days (n = 17). The principal findings included greater day-night amplitude (MACDay-Night) and lower duration of daytime immobility for patients with delirium of short duration.7 A separate report from the same group also compared actigraphy between 32 patients who had undergone cardiac surgery and who did not have delirium and 38 with delirium on POD 1.8 The cohort of patients with delirium showed no significant differences in immobility duration for day or night when compared to controls. Instead, the group with delirium showed a lower median daytime restlessness index (ratio of number of minutes with activity to the number of immobile minutes) on POD 1 and less activity during the 5-hour epoch of the lowest activity (L5) during the first 24 hours after surgery. Our findings based on activity and immobility measures are consistent with these reports.
We are aware of only one investigation that has evaluated whether preoperative actigraphy measures predict subsequent delirium.3 With a smaller sample size of patients with delirium (n = 7), Leung and colleagues reported that wakefulness after sleep onset, a measure of sleep fragmentation, was greater in the preoperative period in 7 patients in whom delirium developed during POD 1–3, compared to 43 patients who were CAM negative, over the postoperative period.3 We are unable to easily account for the discrepancy in the robustness of findings relative to our study. Based on our data, the onset of poor postoperative variability in sleep-wake cycles compared to baseline could serve as a clinical tool for detecting hypoactivity in prodromal or subclinical cases of delirium.
Study Limitations
We acknowledge the various limitations of our analyses. First, our actigraphy metrics were primarily based on two 7-hour epochs within the first 24-hours of surgery, to more closely compare our data to prior investigations. There are no biological constraints to justify these temporal demarcations, however. Second, our sample size was modest. This raises the potential of type II error with our group analyses. Additionally, delirium outcome groups had a higher incidence of initial postoperative intensive care unit admission compared to those without this complication, such that postsurgical care could account for group differences. Finally, we did not correct for the number of statistical tests applied in this exploratory study. Any positive findings require caution, given the possibility of type I errors.
The delirium categorization of patients is also a possible source of uncertainty. Due to the inherent fluctuations in the severity and character of delirium, discrete daily CAM assessments combined with subsequent delirium chart review may not have identified all patients with delirium in our cohort. Additionally, we attempted to study a homogenous subset of delirium through the exclusion of patients with identified hyperactive or mixed forms of delirium. It is probable that some hyperactive features may have escaped detection due to the frequency of our delirium assessments. Furthermore, clinical use of actigraphy for predicting delirium would not be based on prior identification of motoric subtypes. Finally, factors that independently affect both mobility and delirium incidence, including residual general anesthesia, opioid administration, and acute postoperative pain, could have confounded our results.
Implications for Future Research
The use of actigraphy to assess sleep-wake patterns in the postoperative period is limited by both technique and patient characteristics. Actigraphy is unable to distinguish passive motion of the device from voluntary motor activity of the wrist. Accelerometers within these devices are unable to distinguish immobility due to sleep, restful wakefulness, or inattention during delirium. Furthermore, physical restraints due to intravenous lines, monitoring equipment, or activity restrictions pose an additional impediment in this population. However, given the technical difficulty and cost associated with polysomnography, actigraphy will continue to be a useful objective tool for estimating patterns of sleep and motor activity in the clinical setting.
Although our data suggest that measures of immobility may be useful for predicting delirium at a group level, the likelihood of clinical diagnostic capability on a single patient level remains remote. The possibility that gross measures of motor activity alone can serve as markers of concurrent or future delirium appears unlikely due to a lack of specificity. In this sense, one could propose that wrist actigraphy could instead play a role as a screening rather than confirmatory diagnostic tool. For example, patients without differential activity or immobility between day and night could be considered at risk for delirium and followed with greater surveillance. Our study provides emphasis on measures of immobility to complement graded measures motor activity for future prospective investigations.
In summary, our data suggest that early postoperative wrist actigraphy measures were poorly effective as markers for concurrent or subsequent hypoactive delirium. High variance across patients and low discriminative capacity represent substantial limitations for the use of actigraphy in predicting this common neurological complication of major surgery.
DISCLOSURE STATEMENT
Work for this study was performed at the Department of Anesthesiology, Washington University School of Medicine in St. Louis. All authors have read and approved the manuscript. Authors HR Maybrier, CR King, A Crawford, AM Mickle, TS Wildes, MS Avidan, and BJA Palanca report no conflicts of interest. Research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448, sub-award KL2TR000450 (BJP), from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH, Bethesda, MD, USA), the National Institute on Aging (Bethesda, MD, USA) 1UH2AG050312-01, 4UH3AG050312-02 (MSA, TSW), 1R21AG052821-01 (BJP, MSA), and 1R01AG057901-01 (BJP). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH. This was a sub-study of the ENGAGES trial, which was funded by the National Institute of Health and National Institute on Aging Grant 1UH2AG050312-01. Additional efforts were supported by R21AG052821 and R01AG057901.
ACKNOWLEDGMENTS
The authors appreciate the efforts of the ENGAGES research group, particularly: GP Apakama, T Budelier, JK Burton, DA Emmert, TJ Graetz, S Gupta, EJ Lenze, SL N Lin, McKinnon, MR Muench, MR Murphy, JD Oberhaus, D Park, A Patel, S Stark, BA Torres, RT Upadhyayula, and AC Winter.
ABBREVIATIONS
- AUC
area under the curve
- CAM
Confusion Assessment Method
- CAM-ICU
Confusion Assessment Method for the Intensive Care Unit
- CI
confidence interval
- C-statistic
concordance statistic
- ENGAGES
Electroencephalography Guidance of Anesthesia to Alleviate Geriatric Syndromes
- L5
five cumulative hours of lowest amplitude activity
- LFE
low frequency extension
- MAC
median activity counts
- NOIM
number of immobile minutes
- POD
postoperative day
- RMS
root mean-squared
- ROC
receiver operating characteristic
REFERENCES
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 2.Meagher DJ, Trzepacz PT. Motoric subtypes of delirium. Semin Clin Neuropsychiatry. 2000;5(2):75–85. doi: 10.153/SCNP00500075. [DOI] [PubMed] [Google Scholar]
- 3.Leung JM, Sands LP, Newman S, et al. Preoperative sleep disruption and postoperative delirium. J Clin Sleep Med. 2015;11(8):907–913. doi: 10.5664/jcsm.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Todd OM, Gelrich L, MacLullich AM, Driessen M, Thomas C, Kreisel SH. Sleep disruption at home as an independent risk factor for postoperative delirium. J Am Geriatr Soc. 2017;65(5):949–957. doi: 10.1111/jgs.14685. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald JM, Adamis D, Trzepacz PT, et al. Delirium: a disturbance of circadian integrity? Med Hypotheses. 2013;81(4):568–576. doi: 10.1016/j.mehy.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 6.Ángeles-Castellanos M, Ramírez-Gonzalez F, Ubaldo-Reyes L, Rodriguez-Mayoral O, Escobar C. Loss of melatonin daily rhythmicity is associated with delirium development in hospitalized older adults. Sleep Sci. 2016;9(4):285–288. doi: 10.1016/j.slsci.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osse RJ, Tulen JH, Bogers AJ, Hengeveld MW. Disturbed circadian motor activity patterns in postcardiotomy delirium. Psychiatry Clin Neurosci. 2009;63(1):56–64. doi: 10.1111/j.1440-1819.2008.01888.x. [DOI] [PubMed] [Google Scholar]
- 8.Osse RJ, Tulen JH, Hengeveld MW, Bogers AJ. Screening methods for delirium: early diagnosis by means of objective quantification of motor activity patterns using wrist-actigraphy. Interact Cardiovasc Thorac Surg. 2009;8(3):344–348. doi: 10.1510/icvts.2008.192278. discussion 348. [DOI] [PubMed] [Google Scholar]
- 9.Bisgaard T, Kjaersgaard M, Bernhard A, Kehlet H, Rosenberg J. Computerized monitoring of physical activity and sleep in postoperative abdominal surgery patients. J Clin Monit Comput. 1999;15(1):1–8. doi: 10.1023/a:1009930026753. [DOI] [PubMed] [Google Scholar]
- 10.Madsen MT, Rosenberg J, Gögenur I. Actigraphy for measurement of sleep and sleep-wake rhythms in relation to surgery. J Clin Sleep Med. 2013;9(4):387–394. doi: 10.5664/jcsm.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gogenur I, Bisgaard T, Burgdorf S, van Someren E, Rosenberg J. Disturbances in the circadian pattern of activity and sleep after laparoscopic versus open abdominal surgery. Surg Endosc. 2009;23(5):1026–1031. doi: 10.1007/s00464-008-0112-9. [DOI] [PubMed] [Google Scholar]
- 12.Wallen MB, Nero H, Franzen E, Hagströmer M. Comparison of two accelerometer filter settings in individuals with Parkinson's disease. Physiol Meas. 2014;35(11):2287–2296. doi: 10.1088/0967-3334/35/11/2287. [DOI] [PubMed] [Google Scholar]
- 13.Feito Y, Garner HR, Bassett DR. Evaluation of ActiGraph's low-frequency filter in laboratory and free-living environments. Med Sci Sports Exerc. 2015;47(1):211–217. doi: 10.1249/MSS.0000000000000395. [DOI] [PubMed] [Google Scholar]
- 14.Korpan SM, Schafer JL, Wilson KC, Webber SC. Effect of ActiGraph GT3X+ position and algorithm choice on step count accuracy in older adults. J Aging Phys Act. 2015;23(3):377–382. doi: 10.1123/japa.2014-0033. [DOI] [PubMed] [Google Scholar]
- 15.Ried-Larsen M, Brønd JC, Brage S, et al. Mechanical and free living comparisons of four generations of the Actigraph activity monitor. Int J Behav Nutr Phys Act. 2012;9:113. doi: 10.1186/1479-5868-9-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cain KL, Conway TL, Adams MA, Husak LE, Sallis JF. Comparison of older and newer generations of ActiGraph accelerometers with the normal filter and the low frequency extension. Int J Behav Nutr Phys Act. 2013;10:51. doi: 10.1186/1479-5868-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Webber SC, St John PD. Comparison of ActiGraph GT3X+ and StepWatch step count accuracy in geriatric rehabilitation patients. J Aging Phys Act. 2016;24(3):451–458. doi: 10.1123/japa.2015-0234. [DOI] [PubMed] [Google Scholar]
- 18.Assad S, Ghani U, Sulehria T, Mansoor T, Ameer MA. Intensive care unit psychosis-sundowning: a challenging phenomenon. Indian J Crit Care Med. 2017;21(2):112–113. doi: 10.4103/ijccm.IJCCM_390_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wildes TS, Winter AC, Maybrier HR, et al. Protocol for the Electroencephalography Guidance of Anesthesia to Alleviate Geriatric Syndromes (ENGAGES) study: a pragmatic, randomised clinical trial. BMJ Open. 2016;6(6):e011505. doi: 10.1136/bmjopen-2016-011505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 21.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 22.Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST, Jr, Leslie DL, Agostini JV. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53(2):312–318. doi: 10.1111/j.1532-5415.2005.53120.x. [DOI] [PubMed] [Google Scholar]
- 23.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 24.ActiGraph White Paper: What is a Count? Actigraph website. [Accessed December 24, 2018]. https://s3.amazonaws.com/actigraphcorp.com/wp-content/uploads/2017/11/26205758/ActiGraph-White-Paper_What-is-a-Count_.pdf.
- 25.Maybrier HR, Mickle AM, Murphy MR, et al. Actigraphy for diagnosing and predicting hypoactive postoperative delirium. Presented at: 48th Annual Meeting of the International Anesthesia Research Society; May 22, 2016; San Francisco, CA. [Google Scholar]
- 26.Bisgaard T, Klarskov B, Kehlet H, Rosenberg J. Recovery after uncomplicated laparoscopic cholecystectomy. Surgery. 2002;132(5):817–825. doi: 10.1067/msy.2002.127682. [DOI] [PubMed] [Google Scholar]
- 27.Redeker NS, Mason DJ, Wykpisz E, Glica B, Miner C. First postoperative week activity patterns and recovery in women after coronary artery bypass surgery. Nurs Res. 1994;43(3):168–173. [PubMed] [Google Scholar]
- 28.Jacobson SA, Dwyer PC, Machan JT, Carskadon MA. Quantitative analysis of rest-activity patterns in elderly postoperative patients with delirium: support for a theory of pathologic wakefulness. J Clin Sleep Med. 2008;4(2):137–142. [PMC free article] [PubMed] [Google Scholar]
- 29.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 30.Zutshi M, Delaney CP, Senagore AJ, Fazio VW. Shorter hospital stay associated with fastrack postoperative care pathways and laparoscopic intestinal resection are not associated with increased physical activity. Colorectal Dis. 2004;6(6):477–480. doi: 10.1111/j.1463-1318.2004.00692.x. [DOI] [PubMed] [Google Scholar]
- 31.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 32.Lam EWK, Chung F, Wong J. Sleep-disordered breathing, postoperative delirium, and cognitive impairment. Anesth Analg. 2017;124(5):1626–1635. doi: 10.1213/ANE.0000000000001914. [DOI] [PubMed] [Google Scholar]
- 33.Flannery AH, Oyler DR, Weinhouse GL. The impact of interventions to improve sleep on delirium in the ICU: a systematic review and research framework. Crit Care Med. 2016;44(12):2231–2240. doi: 10.1097/CCM.0000000000001952. [DOI] [PubMed] [Google Scholar]
- 34.Litton E, Carnegie V, Elliott R, Webb SA. The efficacy of earplugs as a sleep hygiene strategy for reducing delirium in the ICU: a systematic review and meta-analysis. Crit Care Med. 2016;44(5):992–999. doi: 10.1097/CCM.0000000000001557. [DOI] [PubMed] [Google Scholar]
- 35.Chen S, Shi L, Liang F, et al. Exogenous melatonin for delirium prevention: a meta-analysis of randomized controlled trials. Mol Neurobiol. 2016;53(6):4046–4053. doi: 10.1007/s12035-015-9350-8. [DOI] [PubMed] [Google Scholar]
- 36.Oldham MA, Lee HB, Desan PH. Circadian rhythm disruption in the critically ill: an opportunity for improving outcomes. Crit Care Med. 2016;44(1):207–217. doi: 10.1097/CCM.0000000000001282. [DOI] [PubMed] [Google Scholar]
- 37.Scott BK. Disruption of circadian rhythms and sleep in critical illness and its impact on the development of delirium. Curr Pharm Des. 2015;21(24):3443–3452. doi: 10.2174/1381612821666150706110656. [DOI] [PubMed] [Google Scholar]
- 38.Watson PL, Pandharipande P, Gehlbach BK, et al. Atypical sleep in ventilated patients: empirical electroencephalography findings and the path toward revised ICU sleep scoring criteria. Crit Care Med. 2013;41(8):1958–1967. doi: 10.1097/CCM.0b013e31828a3f75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.