Abstract
Study Objectives:
To compare Epworth Sleepiness Scale (ESS) scores of men and women and determine if there is a correlation with sleep-disordered breathing (SDB) based on subsequent polysomnography (PSG).
Methods:
Consecutive adult patients were identified who completed ESS and PSG at Mayo Clinic in Rochester, Minnesota, between January 1, 2013, and January 31, 2015. Apnea-hypopnea index (AHI) ≥ 5 events/h was classified as presence of SDB, and increasing values represented greater severity.
Results:
Among 6,593 patients with valid ESS scores and timely subsequent PSG, 42% were women. Mean (standard deviation [SD]) age of women was 56.2 (15.2) years; men, 58.5 (15.1) years. Mean (SD) ESS score was 9.5 (5.4) for women and 9.5 (5.3) for men. SDB was present in 83.6% of men and 68.3% of women. Mean (SD) AHI of men was 25.9 (26.7) events/h; women, 16.1 (22.4) events/h (P < .001). Each unit increase in ESS score of men was associated with a 0.51-unit increase in AHI (P < .001); women had a 0.16-unit associated increase (P = .04) (effect ratio, threefold greater for men). PSG demonstrated that women had greater sleep efficiency, less respiratory effort-related arousals, and less hypoxemia (all P < .001). Among women, ESS did not correlate with presence of SDB or mild to moderate SDB. There was a small association in women with severe SDB.
Conclusions:
ESS is not correlated with SDB at mild to moderate levels in women and has a smaller association than in men with severe SDB. Further work is necessary to understand sex-specific differences in patients with SDB.
Citation:
Lipford MC, Wahner-Roedler DL, Welsh GA, Mandrekar J, Thapa P, Olson EJ. Correlation of the Epworth Sleepiness Scale and sleep-disordered breathing in men and women. J Clin Sleep Med. 2019;15(1):33–38.
Keywords: obstructive sleep apnea, OSA in women, sleepiness in OSA by sex
BRIEF SUMMARY
Current Knowledge/Study Rationale: Commonly used screening questionnaires such as the Epworth Sleepiness Scale (ESS) use presence of sleepiness as part of the clinical assessment for likelihood of sleep-disordered breathing (SDB). Women with SDB may have different symptoms and thus the condition could be missed with this type of screening tool.
Study Impact: Our study demonstrates that level of sleepiness as measured by the ESS is not associated with presence of SDB in women. A small association was seen only in women with severe SDB. Physiologic and clinical phenotype differences are present in women with SDB compared with men. Further understanding is necessary to develop SDB screening tools specifically validated for women.
INTRODUCTION
Sleep-disordered breathing (SDB) affects 26% of men and 13% of women in the United States population.1,2 Women with obesity, those who are pregnant, or those in the post-menopausal years of life are at highest risk for SDB.3 Up to 56% of women older than 65 years are estimated to have SDB.4 This disease carries serious public health burden as an independent risk factor for cardiovascular and cerebrovascular disease, as well as its association with increased risk of automobile crashes.5–8 Although SDB is relatively common in women, moderate to severe disease is undiagnosed for 93% of women who have SDB.9 Investigators postulate that this discrepancy exists in part because presentation differs in men and women with SDB.
Classic symptoms associated with SDB are loud snoring, witnessed apnea events, snort arousals, and daytime sleepiness.10 These symptoms are typically present in men with SDB; however, women with SDB are more likely to report symptoms of insomnia, depression, and fatigue.11–13 Questionnaires such as the Epworth Sleepiness Scale (ESS) are used to screen for sleepiness associated with SDB. However, they may not identify the female patient with SDB.
In the current study, we report ESS scores of men and women who presented to the Mayo Clinic Center for Sleep Medicine and subsequently underwent attended in-laboratory polysomnography (PSG). ESS scores and PSG results were compared between men and women. We evaluated the correlation of level of reported sleepiness (as ascertained by the ESS) and presence and severity of SDB by sex.
METHODS
ESS Survey
The self-administered ESS questionnaire has eight items that rate a person's likelihood of dozing off in daily situations. An ESS score greater than 10 suggests excessive daytime sleepiness; higher values represent increased severity of sleepiness. This validated questionnaire has a high level of internal consistency (Cronbach α, 0.88).14 Validation studies have shown mean (standard deviation [SD]) ESS scores of patients with mild, moderate, and severe obstructive SDB to be 9.5 (3.3), 11.5 (4.2), and 16.0 (4.4), respectively.15 Pooled data from 23 trials showed that ESS scores with SDB treatment (ie, continuous positive airway pressure) decreased by 2.7 (95% confidence interval [CI], −3.5 to −2.0).16 ESS indeed seems to be correlated with SDB; however, these trials consisted primarily of male patients. Specific validation studies have not been performed in the female population.
Study Population
Consecutive men and women who completed ESS assessment and subsequently underwent attended in-laboratory PSG at Mayo Clinic between January 1, 2013, and January 31, 2015, were identified from the database of the Mayo Clinic Center for Sleep Medicine. The pre-PSG ESS score was verified through chart review. Only patients who had a valid ESS score obtained within 3 months of PSG were included. Detailed PSG parameters were recorded for each patient, including presence and severity of SDB.
SDB Classification
SDB is classified into a severity level in accordance with the apnea-hypopnea index (AHI), which indicates the frequency of disordered breathing events per hour. The reference AHI is less than 5 (no SDB). Mild disease is defined as an AHI of at least 5 but less than 15; moderate disease, at least 15 but less than 30; and severe disease, 30 or greater.17 Disordered breathing events were scored in accordance with The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, using the alternate hypopnea definition (nasal pressure signal drop of ≥ 30% from pre-event baseline for a duration of at least 10 seconds with ≥ 4% oxygen desaturation from pre-event baseline).18
Study Participants
In total, 7,619 patients were identified in the database. Of these, 1,026 patients were excluded from the study because (1) PSG was performed more than 3 months after ESS completion, (2) a titration-only PSG was performed (rather than a diagnostic or split-night study), or (3) consent to participate in research was not available.
Statistical Analysis
Descriptive summaries were reported as frequency and percentage for categorical variables and mean (SD) or median (range) for continuous variables. Subgroup analysis compared primary clinical end-point data with use of χ2 test or Fisher exact test for categorical variables and two-sample t test or Wilcoxon rank-sum test for continuous variables.
Patient characteristics were compared by sex with analysis of variance for continuous variables and χ2 test for categorical variables. PSG was reviewed and AHI collected for each patient. Logistic regression analysis models were used to conduct correlation analysis between ESS score and AHI.
A sample size of 70 provides an 80% power to detect a difference of 4.4 SD between ESS scores from the 2 groups of patients. Our study was adequately powered. The Mayo Clinic Institutional Review Board approved this study.
RESULTS
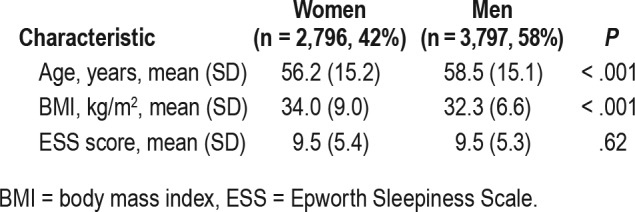
Of 6,593 patients with valid ESS scores and timely subsequent PSG, 42% were women. Mean (SD) age of the women was 56.2 (15.2) years; men, 58.5 (15.1) years (Table 1).
Table 1.
Clinical characteristics of women and men.
SDB was present in 83.6% of men and 68.3% of women. Mean AHI was 25.9 events/h for men and 16.1 events/h for women (P < .001). Table 2 outlines the mean (SD) of sleep efficiency, respiratory effort-related arousal (RERA) index, and oxygen saturation as measured by pulse oximetry (SpO2) in women and men. These data points showed statistically signifi-cant sex-specific differences (P < .001).
Table 2.
Polysomnography differences between women and men.
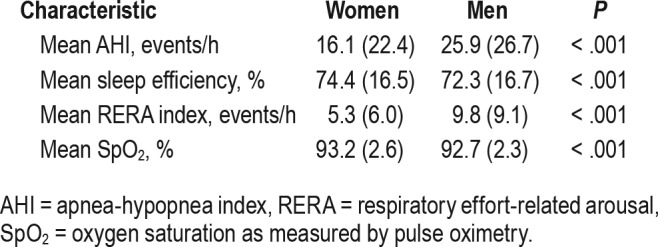
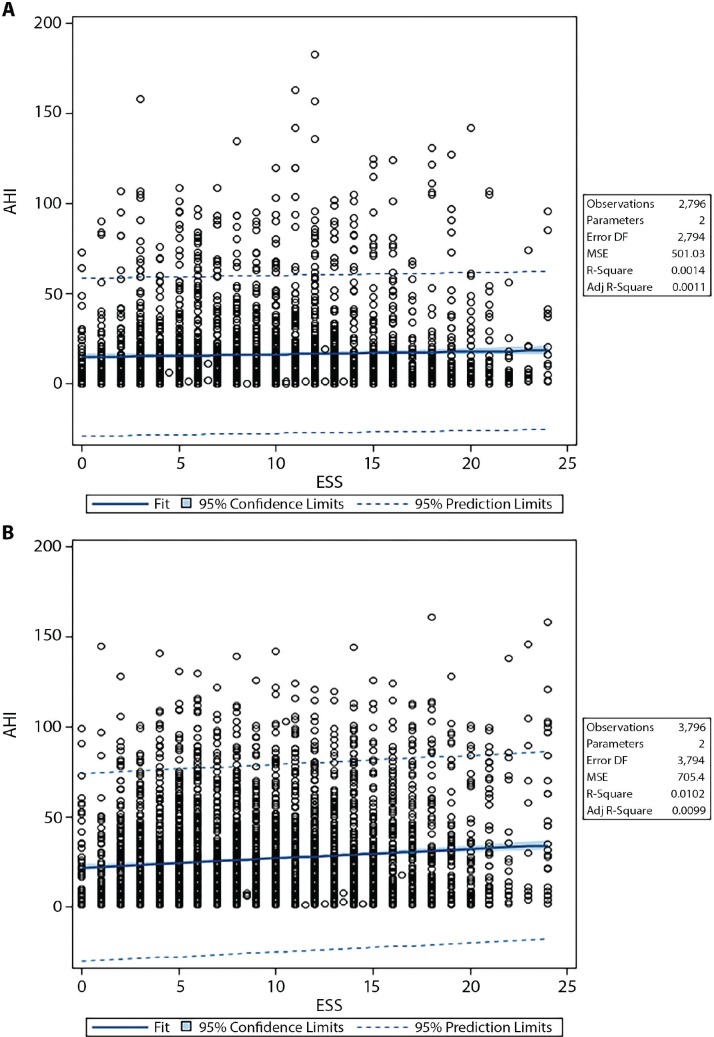
Figure 1 shows fit plots demonstrating relationships between AHI and ESS in women and in men. Each unit increase in ESS score for men was associated with a 0.51-unit increase in AHI (P < .001); for women, the increase in ESS was associated with only a 0.16-unit increase in AHI (P = .04) (effect ratio, threefold greater in men).
Figure 1. Fit plots showing relationship between AHI and ESS in women vs men.
(A) In women, each unit increase in ESS corresponds with a 0.16-unit increase in AHI (P = .04). (B) In men, each unit increase in ESS corresponds with a 0.51-unit increase in AHI (P < .001). The effect ratio was approximately three times greater in men. Adj = adjusted, AHI = apnea-hypopnea index, DF = degrees of freedom, ESS = Epworth Sleepiness Scale, MSE = mean squared error.
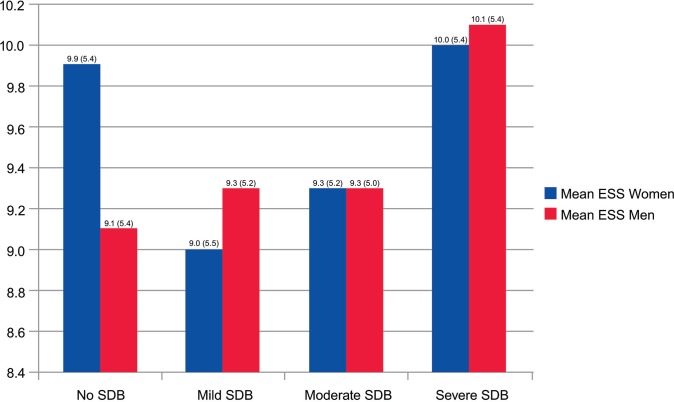
Mean ESS score of women without SDB was higher than that of men without SDB. In this group of women with normal nocturnal breathing, the mean (SD) ESS score was 9.9 (5.4) versus 9.1 (5.4) in men (P = .006) (Figure 2). In patients with severe SDB, there was no significant difference between ESS scores in men and women; mean ESS score in women was 10.0 (5.3) versus 10.1 (5.4) in men (P = .82). Multinomial logistic regression models demonstrated that among men, each unit increase in ESS increases odds of having SDB by 2% (P = .03). With each 5-unit increase in ESS, the odds of having severe SDB increase by 19% (P = .003). Among women, ESS did not correlate with the presence of SDB or AHI values in the mild to moderate range. However, there was a much smaller association compared with men for severe SDB (with each 5-unit increase in ESS in women, odds of having severe SDB increased by 1.5% [P = .003]).
Figure 2. ESS score among women and men.
ESS was less associated with the presence of SDB in women than men. The correlation is improved in women with moderate to severe SDB. Y-axis begins at 8.4. ESS = Epworth Sleepiness Scale, SDB = sleep-disordered breathing.
PSG data showed that women had increased sleep efficiency, fewer RERAs, and less hypoxemia than men (all P < .001). Overall arousal indices in women with and without SDB were lower than in men. Mean arousal index in women without SDB was 21.5 (14.4); in women with SDB, mean arousal index increased to 39.4 (25.7) (P < .001). In men, mean arousal index in those without SDB was 24.2 (14.8), and in those with SDB, mean arousal index rose to 46.8 (26.2) (P < .001). Within the SDB cohort, mean arousal indices were lower in women with mild SDB (28.4 [15.8] versus 32.1 [17.5]; P < .001). Mean arousal index was also slightly lower in women with moderate SDB, but the difference was not significant (39.0 [19.0] versus 41.1 [20.0]; P = .10). Mean arousal index in women with severe SDB was slightly higher than in their male counterparts, but this difference was not significant (65.5 [30.9] versus 64.9 [25.9]; P = .62). Analysis of covariance calculations dem -onstrated that arousal index has a significant effect on ESS (P < .001). Each unit increase in arousal index contributes to 0.01-unit increase in ESS.
Mean body mass index (BMI) was higher in the overall cohort of women than in men (Table 1). BMI in women was higher than in men within all of the following subgroups:
Without SDB: women 30.6 (7.8) versus men 29.0 (5.2), P = .003
Mild SDB: women 34.7 (8.6) versus men 31.3 (5.7), P < .001
Moderate SDB: women 35.0 (8.5) versus men 32.3 (6.1), P < .001
Severe SDB: women 38.1 (10.4) versus men 34.9 (7.4), P < .001
Univariate logistic regression revealed that BMI was associated with presence of SDB with an odds ratio (OR) of 1.087 (95% CI, 1.077–1.098). The relationship was stronger in men (OR, 1.129) compared with women (OR, 1.078). After adjustment for ESS, age, and sex, BMI remained associated with higher AHI. The AHI increased by 12% with each unit increase in BMI. The magnitude of association was stronger in men than women (OR, 1.148 versus 1.112).
Mean total AHI values were lower in women with mild to moderate SDB than men (mild SDB, 8.2 [2.8] versus 8.7 [3.0]; P < .001 and moderate SDB, 20.6 [4.2] versus 21.2 [4.3]; P = .02). However, AHI values during rapid eye movement (REM) sleep were higher in women than in men with mild to moderate SDB (mild SDB REM, AHI, 25.0 [22.8] versus 19.6 [20.2]; P < .001, moderate SDB 34.7 [31.1] versus 27.5 [25.6]; P = .004).
In women with severe SDB, mean AHI was higher in women, but the difference was not significant (59.1 [26.6] versus 58.2 [23.7], P = .90). Mean AHI during REM sleep was also higher in women with severe SDB than men, but the difference was not significant (44.3 [39.1] versus 39.1 [33.3], P = .10). SDB events were predominantly obstructive in this cohort. The mean (SD) central apnea index of all women with SDB was 1.3 (5.4); of all men, 3.6 (9.6) (P < .001). Central apnea indices remained small in all subgroups of SDB and were lower in women than men: (mild SDB, 0.4 [1.0] versus 0.7 [1.4] [P < .001]; moderate SDB, 1.1 [2.7] versus 2.3 [4.0] [P < .001]; severe SDB 3.4 [10.4] versus 7.3 [14.3] [P < .001]).
DISCUSSION
Our study showed that ESS is less associated with the presence of SDB in women compared with men. Several studies have reported that men and women answer questions about sleepiness differently and that the ESS is more likely to detect sleepiness of men than of women. Women with sleepiness are less likely to have an abnormal ESS score.18,19 Additionally, women with SDB may have no sleepiness and instead report symptoms of insomnia, mood disturbance, or fatigue.20,21 These symptoms would not be picked up by screening tools such as ESS. In fact, 40% of women with moderate to severe SDB do not report classic SDB symptoms such as snoring and daytime sleepiness.22
Our study correlated ESS and results of subsequent in-laboratory PSG of men and women. Among men, increases in ESS carried an association with increased odds of presence and severity of SDB. In women, an association between ESS and AHI was only seen in severe SDB, and the relationship was much less robust than in men. In addition, ESS scores for women without SDB were higher than for men. Therefore, increased false-positive results may be noted for women if the ESS is used as part of the clinical screening process for SDB. Alternative explanations may be present to account for increased ESS values observed in women without SDB.
Physiologic parameters also appear different for men versus women with SDB. Sleep efficiency was greater for women. Overall arousal indices in persons with and without SDB were higher in men compared with women. Within the SDB population, the arousal indices between men and women with moderate and severe SDB were not significantly different. We found that arousal index had a significant effect on ESS severity. The results may explain why ESS only correlated with presence of severe SDB in women. Our observations may indicate that higher levels of SDB severity are more physiologically similar between men and women. From a clinical perspective, screening tools that assess for daytime sleepiness may be helpful in identifying women at risk for severe SDB but may miss those with mild to moderate severity.
In this cohort, BMI was higher in women than in men. This may indicate that women with higher BMI are referred more commonly for SDB evaluation. However, our data also revealed that the association between BMI and AHI was less robust in women than men. Sleep state differences were also observed between the sexes. Although overall AHI values were higher in men with mild to moderate SDB, women with mild to moderate SDB had higher AHI values during REM sleep than their male counterparts. In persons with severe SDB, women had slightly higher mean total AHI values as well as REM-related AHI values compared with men; however, the differences were not significant. These results also suggest that at severe levels of SDB, men and women share similar physiologic traits. It is possible that the differences in REM-related AHI values in women with mild to moderate SDB may relate to symptomatic differences between the sexes.
Further work is needed to examine the differences in SDB between men and women and to correlate these physiologic parameters with clinical symptomatology. Menopausal status could also be a factor in some of the differences noted in women, given that most women in this cohort were middle-aged. Such investigation may lead to development of sex-specific screening tools that more accurately assess for SDB in the female population.
The strengths of our study include its systematic evaluation of a large number of patients for whom ESS score was obtained before testing for SDB. Only the patients who underwent attended in-laboratory PSG were included, thus providing data on parameters including RERA indices and sleep efficiency. Limitations include: the retrospective nature of the study, the study was performed in a clinical population, and the indication for PSG was not known. In addition, given the large number of participants, we have combined the presence of either obstructive or central sleep apnea as SDB. The clinical features of each disease process may be different. However, less than 10% of the patients with SDB had a central apnea index of 5 or more per hour; most patients with SDB had primarily or solely obstructive sleep apnea.
CONCLUSIONS
In our study, ESS did not correlate with presence of SDB in women. A relationship between ESS and SDB in women was observed only in those with severe disease. There was also a lesser association between BMI and SDB in women than in men. Our results demonstrate that common clinical screening tools and physical markers for SDB may not be sufficient to use in the female population. Clinicians may wish to use alternative assessments to assess risk of SDB in female patients that do not rely on measurements of daytime sleepiness or BMI. Multiple physiologic and sleep-state differences were observed in women with SDB at mild to moderate severity levels. Men and women with severe SDB shared similar data characteristics. Further work regarding the reasons for sex-specific differences in mild and moderate SDB and the associated effect on SDB symptoms is needed and may help in the eventual generation of sex-specific screening tools.
DISCLOSURE STATEMENT
This work was completed at the Mayo Clinic Center for Sleep Medicine, Rochester, Minnesota. All authors have seen and approved the manuscript. Portions of this research were presented in abstract form at the 23rd Congress of the European Sleep Research Society, September 13–16, 2016, Bologna, Italy. The authors report no conflicts of interest. This report was supported by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- ESS
Epworth Sleepiness Scale
- OR
odds ratio
- PSG
polysomnography
- REM
rapid eye movement
- RERA
respiratory effort-related arousal
- SDB
sleep-disordered breathing
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T. Rationale, design and findings from the Wisconsin Sleep Cohort Study: Toward understanding the total societal burden of sleep disordered breathing. Sleep Med Clin. 2009;4(1):37–46. doi: 10.1016/j.jsmc.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3 Pt 1):608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 4.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14(6):486–495. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1453. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 6.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182(2):269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward KL, Hillman DR, James A, et al. Excessive daytime sleepiness increases the risk of motor vehicle crash in obstructive sleep apnea. J Clin Sleep Med. 2013;9(10):1013–1021. doi: 10.5664/jcsm.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 9.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20(9):705–706. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 10.Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5th ed. St. Louis, MO: Elsevier; 2011. [Google Scholar]
- 11.Sampaio R, Pereira MG, Winck JC. Psychological morbidity, illness representations, and quality of life in female and male patients with obstructive sleep apnea syndrome. Psychol Health Med. 2012;17(2):136–149. doi: 10.1080/13548506.2011.579986. [DOI] [PubMed] [Google Scholar]
- 12.Subramanian S, Guntupalli B, Murugan T, et al. Gender and ethnic differences in prevalence of self-reported insomnia among patients with obstructive sleep apnea. Sleep Breath. 2011;15(4):711–715. doi: 10.1007/s11325-010-0426-4. [DOI] [PubMed] [Google Scholar]
- 13.Uyar M, Vrt O, Bayram N, et al. Gender differences with respect to psychiatric comorbidity in obstructive sleep apnea syndrome. South Med J. 2011;104(7):495–498. doi: 10.1097/SMJ.0b013e31821fbfb0. [DOI] [PubMed] [Google Scholar]
- 14.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 15.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 16.McDaid C, Griffin S, Weatherly H, et al. Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea-hypopnoea syndrome: a systematic review and economic analysis. Health Technol Assess. 2009;13(4):iii–iv. xi–xiv, 1–119, 43–274. doi: 10.3310/hta13040. [DOI] [PubMed] [Google Scholar]
- 17.Berry RB, Brooks R, Gamaldo C, et al. AASM Scoring Manual updates for 2017 (Version 2.4) J Clin Sleep Med. 2017;13(5):665–666. doi: 10.5664/jcsm.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baldwin CM, Kapur VK, Holberg CJ, Rosen C, Nieto FJ Sleep Heart Health Study Group. Associations between gender and measures of daytime somnolence in the Sleep Heart Health Study. Sleep. 2004;27(2):305–311. doi: 10.1093/sleep/27.2.305. [DOI] [PubMed] [Google Scholar]
- 19.Drakatos P, Ghiassi R, Jarrold I, et al. The use of an online pictorial Epworth Sleepiness Scale in the assessment of age and gender specific differences in excessive daytime sleepiness. J Thorac Dis. 2015;7(5):897–902. doi: 10.3978/j.issn.2072-1439.2014.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev. 2008;12(6):481–496. doi: 10.1016/j.smrv.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valipour A, Lothaller H, Rauscher H, Zwick H, Burghuber OC, Lavie P. Gender-related differences in symptoms of patients with suspected breathing disorders in sleep: a clinical population study using the sleep disorders questionnaire. Sleep. 2007;30(3):312–319. doi: 10.1093/sleep/30.3.312. [DOI] [PubMed] [Google Scholar]
- 22.Young T, Hutton R, Finn L, Badr S, Palta M. The gender bias in sleep apnea diagnosis. Are women missed because they have different symptoms? Arch Intern Med. 1996;156(21):2445–2451. [PubMed] [Google Scholar]