Abstract
Study Objectives:
The demand for continuous positive airway pressure (CPAP) therapy outpaces available resources in most health care settings. We sought to evaluate predictors of nonroutine CPAP follow-up visits to improve resource utilization.
Methods:
We randomly analyzed 1,141 of the 2,446 patients who had received at least 1 year of CPAP therapy. Reasons for contacts, type (routine = R, nonroutine = NR), and mode (face-to-face or not, physician, nurse) were collected.
Results:
A total of 771 patients were classified R, and 370 NR. Age, profession, and sex did not affect the NR frequency. Symptoms increased the odds ratio for NR 12.1-fold, somnolence 34.8-fold, and suffocation at night 10.4-fold. Patients with nonroutine reasons abandoned CPAP therapy significantly (7.6-fold) more frequently than patients with routine reasons.
Conclusions:
Symptoms during CPAP therapy predicted the nonroutine contacts well. In line with this, patients with symptoms have become a priority follow-up group, and could constitute the only follow-up policy when dealing with insufficient medical resources.
Citation:
Avellan-Hietanen H, Brander P, Bachour A. Symptoms during CPAP therapy are the major reason for contacting the sleep unit between two routine contacts. J Clin Sleep Med. 2019;15(1):47–53.
Keywords: APAP, CPAP, follow-up, health care policy, outpatient, sleep apnea
BRIEF SUMMARY
Current Knowledge/Study Rationale: There is no consensus for the frequency or the way CPAP therapy follow-up should be performed. The number of patients undergoing CPAP therapy is increasing and medical resources are restricted. An alternative follow-up program could be focused on the patients who benefit the most from follow-up.
Study Impact: We showed that patients with symptoms who are undergoing CPAP therapy contact our sleep clinic significantly more often than those without symptoms. Following only patients with symptoms on demand would be an alternative follow-up method.
INTRODUCTION
Continuous positive airway pressure (CPAP) therapy is the standard treatment for obstructive sleep apnea (OSA).1 It lowers the apnea-hypopnea index (AHI), reduces nocturnal hypoxemia2 and daytime somnolence, and improves sleep regulation.3 However, patients' adherence to the treatment is often suboptimal.4–6 In order to improve patients' long-term adherence to the treatment, and to continue to troubleshoot and manage problems that may arise with the use of CPAP devices, patients are often routinely seen by trained health care personnel at regular intervals.7 In addition, extra follow-up may be needed; for instance, when the patient's weight changes or when symptoms of sleep apnea are present. The CPAP device itself requires regular checks, as do all other electrical devices. However, there is no international consensus regarding how the long-term follow-up of CPAP treatment should be organized.
In the United States, the Centers for Medicare and Medicaid Services (CMS) and third-party payers require an initial face-to-face follow-up visit with a physician between 31 and 91 days after the start of CPAP therapy in order to assess the patients' adherence to, and benefits and side effects related to, CPAP treatment.8 For individuals with OSA who drive, there are more specific regulations in many countries. The recent European Standards and Guidelines for Drivers with Obstructive Sleep Apnea,9 recommend that the need for, and compliance with, CPAP treatment should be subject to medical review at regular intervals not exceeding 1 year for professional drivers, and 3 years for other drivers.
Our sleep unit is responsible for organizing CPAP treatment and follow-up for patients with OSA in the Helsinki area (population of 650,000 inhabitants). The CPAP devices and interfaces are purchased by the hospital following competitive tendering as stipulated in public procurement legislation. Therefore, the public hospitals own the CPAP devices, which are then offered to patients free of charge. If the patient forgoes treatment, he or she must return the device to the clinic.
All patients are followed up at our clinic to ensure good therapy response and adherence. Beyond 1 year of therapy, contacts with the patients are organized routinely every 12 to 15 months. However, recently we have noticed that, in most patients, no major changes to CPAP therapy are made during these routine follow-up visits.
The purpose of this study was to: (1) analyze the reasons for nonroutine contacts; (2) to study their timing related to the previous routine contact; and (3) find predictors for these non-routine contacts.
METHODS
Study Population
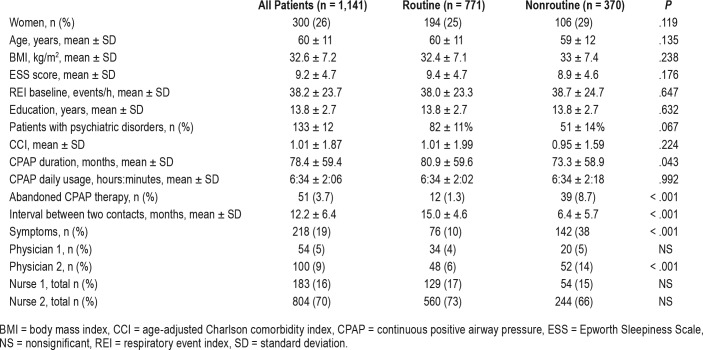
We reviewed all our patients treated with CPAP for OSA. We selected all patients with CPAP duration of more than 1 year. Of these 2,466 patients using CPAP, we randomly selected 1,225 patients (50%) for further analyses. Randomization was performed with IBM SPSS Statistics software (version 22.0.0.1) (IBM Corp, Armonk, New York, United States). The selected population did not differ from the rest regarding age and body mass index (BMI). Patients with obesity hypoventilation syndrome or central sleep apnea, and patients using bilevel CPAP therapy (in total 84 patients) were excluded. Thus, the final study population consisted of 1,141 patients (Table 1).
Table 1.
Study groups.
This study received approval from the institutional review board at Helsinki University Hospital on January 30, 2013, code 69/2013.
The indication for initiating CPAP therapy in OSA was a respiratory event index (REI) ≥ 15 events/h, or REI ≥ 5 events/h if the patient had daytime hypersomnolence or significant comorbidities. Patients starting CPAP underwent a 1-hour familiarization session at the sleep clinic with the CPAP device and masks, as described previously.10 Two to three months after CPAP initiation, patients were also checked to ensure good therapy response. Thereafter, follow-up contacts were routinely planned annually.
Organization of CPAP Follow-Up
The annual follow-up control was performed in four different ways. The patients were categorized into the following groups according the follow-up method used: physician 1, physician 2, nurse 1, nurse 2 (Figure 1).
Figure 1. Flowchart of the organization of CPAP follow-up after 1 year from CPAP initiation.
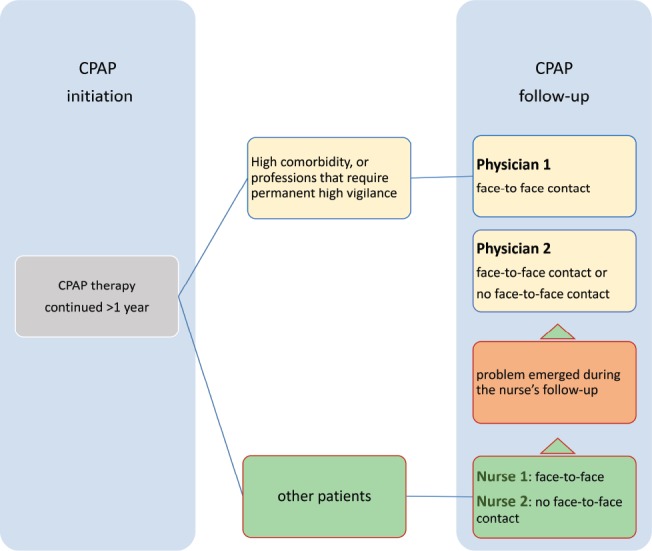
CPAP = continuous positive airway pressure.
Physician 1
Patients in this group were professional drivers or belonged to other professions that required permanent high vigilance and patients with high comorbidities. They were seen each time by a pulmonary physician and by a nurse (both trained in sleep medicine). First, the nurse downloaded the CPAP data and checked the device and interfaces, and thereafter the patient was seen by the physician who examined the patient, and assessed the response, potential side effects, and problems related to CPAP therapy, as well as evaluated the patient's working and driving capacity.
Physician 2
Patients in this group were first seen by a nurse trained in sleep medicine, but these patients had CPAP-related problems that bypassed the sleep nurse's competence, such as those who sought alternative sleep apnea therapy, needed medical certification related to sleep apnea or work capacity, or had emerging sleep apnea symptoms that the nurse could not resolve. These problems were resolved by the physician, but not necessarily in a face-to-face meeting.
Nurse 1
Patients in this group had face-to-face contact with a nurse trained in sleep medicine who downloaded the CPAP data and checked the device, evaluated response, potential side effects, and problems related to CPAP therapy, and replaced masks or CPAP tubes and, if considered necessary, referred the patient later to a physician. This group consisted of patients who fulfilled at least one of the following criteria:
Patients who had limited physical mobility and needed help when handling the devices
Patients who had communication difficulties
Patients who wanted to have a personal face-to-face contact with a professional but did not fulfil the physician 1 or 2 group criteria
Nurse 2
This group included all patients who failed to fulfil the criteria of the other groups. A nurse trained in sleep medicine analyzed therapy responses without face-to-face contact. Every year the patient was sent a letter with a questionnaire including questions on their current weight and assessment of Epworth Sleepiness Scale (ESS) score, and an invitation to bring the CPAP device to the hospital, where the receptionist (a nonmedical person) collected the questionnaire and downloaded the CPAP data (at the time of the study, follow-up by telemedicine was unavailable). The patient was allowed to leave a written message for the nurse about his/her CPAP treatment (problems, side effects, masks, etc.). Within a 2-week period, the nurse checked and analyzed the downloaded data and wrote a short note about the therapy response that was then sent to the patient. The nurse was permitted to plan face-to-face contact with a nurse or physician when necessary.
Routine and Nonroutine Contacts
Study patients were divided into two groups—routine (R) and nonroutine (NR)—based on their contacts with the sleep unit. A routine contact was defined as a preplanned and prescheduled contact with a time interval of at least 1 year, and a non-routine contact included all other contacts with the sleep unit. All the patients who had at least one nonroutine contact with the sleep unit were included in the NR group; and the rest of the patients were assigned to the R group. Patients with multiple nonroutine contacts were also analyzed separately.
Data Collection
For all study patients, the baseline (ie, before CPAP treatment) data on patient characteristics, comorbidity, and diagnostic sleep study, and data related to CPAP treatment (device, interfaces, and treatment results, ie, daily usage of CPAP, treatment duration, time elapsed since last contact, and cessation of CPAP treatment) were collected. Duration of CPAP treatment, counted in months, was defined as the time between CPAP initiation and the last contact with the sleep unit.
Comorbidity was evaluated using the age-adjusted Charlson comorbidity index (CCI). This index encompasses 19 medical conditions weighted from 1 to 6 with total scores ranging from 0 to 37 (0 = no comorbidity).11
All contacts with the sleep unit were recorded, and the type of contacts (routine or nonroutine) were collected. The main reasons for the nonroutine contacts were determined; the patients' symptoms, side effects related to CPAP, and need for administrative procedures (ie, need to obtain medical certificates for insurance or driving authorities) were recorded. The patients were classified as symptomatic if any symptom was present at the contact, or nonsymptomatic if no symptom was present.
Statistical Analysis
The physician 1, physician 2, nurse 1, nurse 2, R, and NR groups were compared for both baseline and treatment data. The analysis of variance, chi-square test, and the t test were used when appropriate. A value of P < .05 was considered to be statistically significant. Both stepwise and forced models of logistic regression analysis were applied to define factors that may predict the patient having a nonroutine contact by using the variable nonroutine as the dependent factor, and the patients' age, sex, CCI, REI baseline, CPAP-AHI, education level, existence of psychiatric disorder, or belonging to a specific follow-up group as the independent factors. IBM SPSS Statistics software was used to compute differences in demographic, clinical, and measured variables.
RESULTS
The study group consisted of 1,141 patients in total; 771 were assigned to the R group and 370 to the NR group (Table 1).
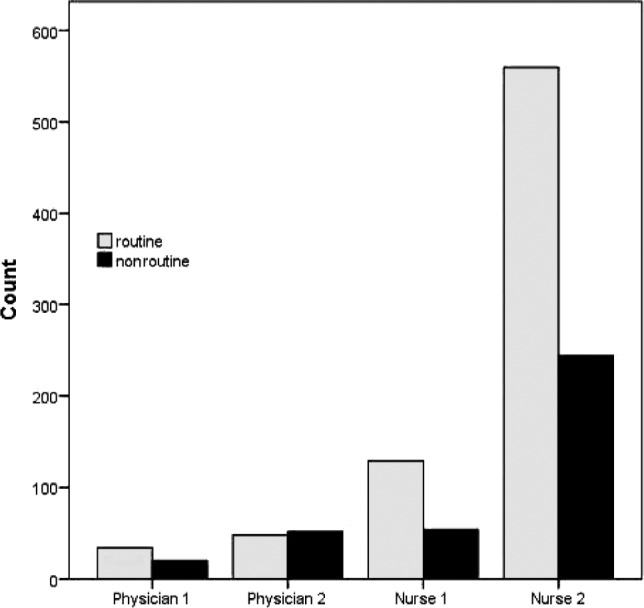
The physician 1 group contained 54 patients; physician 2, 100 patients; nurse 1, 183 patients; and nurse 2, 804 patients (Table 1).
A total of 36 patients failed to show up for their routine appointment but showed up later to their new appointment. Five patients died during the study period.
The NR contacts were significantly more present in group physician 2 (52 out of 100, P < .001) compared to the rest of groups (Figure 2).
Figure 2. Distribution of routine and nonroutine contacts among different follow-up groups.
Routine Versus Nonroutine Contacts
The mean CPAP duration (73.3 ± 58.9 versus 80.9 ± 59.6 months, t1139 = 2.029, P = .043) and the time after the last routine contact (6.4 ± 5.7 versus 15.0 ± 4.6 months, t1139 = 227.046, P < .001), was significantly shorter in the NR group when compared to the R group, respectively (Table 1). Patients in the NR group were more often followed up by the doctor (ie, belonged to physician 2 group).
No significant differences were found between the patients in the R and NR groups regarding their age, sex, baseline BMI, ESS and REI, education years, psychiatric disorders, or comorbidity index (Table 1). The daily CPAP use was similar in both groups (NR: 6:34 ± 2:18 versus R: 6:34 ± 2:02 hours:minutes, t581 = −0.009, P = .992).
In total, 695 patients (90%) s in the R group and 228 patients (62%) in the NR group showed no disturbing symptoms (χ21, n = 1141 = 131.587, P < .001).
Time Interval Between Two Consecutive Contacts
The mean time interval ± standard deviation between two routine contacts was 15 ± 5 months, and between the last routine contact and the first nonroutine contact was 6 ± 6 months (Table 1). However, 80% of NR contacts occurred within 1 month from the last routine contact.
Reasons for Nonroutine Contacts
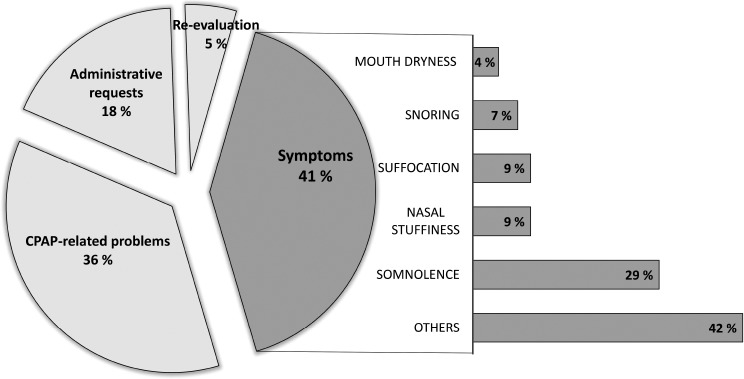
Disturbing symptoms were by far the first reason for a nonroutine contact (41%), followed by problems related to the CPAP treatment (36%). The third major reason was related to administrative requests (18%), such as a need for a medical certificate. Five percent of patients requested a new evaluation or an alternative to their CPAP therapy (Figure 3).
Figure 3. Reasons and description of symptoms for nonroutine contacts.
CPAP = continuous positive airway pressure.
Among symptoms, somnolence was by far the most frequent (29%), followed by nasal stuffiness (9%), suffocation (9%), snoring (7%), and mouth dryness (4%). Patients also complained of various other symptoms (42%), such as headache, pain, insomnia, cramps, dizziness, or tinnitus.
Regarding problems related to CPAP therapy, 34% concerned interfaces and 64% the CPAP device, of which more than half were related to a broken device, and one-fifth to the memory card.
Predictors for Nonroutine Contacts
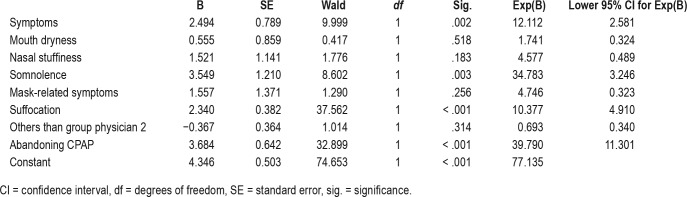
The stepwise and forced models of logistic regression showed that the only independent factor for a patient having a nonroutine contact was the follow-up group (physician 1, physician 2, nurse 1, or nurse 2) into which the patient belonged. The probability for a patient in the physician 2 group to contact the sleep unit in a nonroutine way between the two routine controls was 1.44 times higher than patients in other follow-up groups (odds ratio = 1.44). More than half of the patients (52%) belonging to the physician 2 group contacted in a nonroutine way, whereas this percentage (34%) was significantly lower in the other groups (χ21, n = 1141 = 19.162, P < .001) (Figure 2 and Table 2).
Table 2.
Stepwise and forced models of logistic regression analysis to define factors that predict the patient having a nonroutine contact by using the variable nonroutine as the dependent factor.
Moreover, patients with symptoms had significantly higher risk for nonroutine visits with an odds ratio of 12.1 compared to patients without symptoms. Of these symptoms, somnolence complaint was the most important predictor of the nonroutine contact with an odds ratio of 34.8, followed by feelings of suffocation at night (odds ratio = 10.4) (Table 2).
Abandoning CPAP Therapy
In the NR group, 39 patients had abandoned CPAP therapy, or 7.6 times more often than in the R group (12 patients) (χ21, n = 1111 = 48.183, P < .001). The annual abandoning rate was 3.7% for all patients, 1.3% for the R group, and 8.7% for the NR group.
Characteristics of Patients With Multiple Nonroutine Contacts
Ninety percent of the patients in the NR group contacted the sleep clinic only once during the study period (n = 334) and 10% (n = 34) more than once. A difference was noticed regarding comorbidity. Those who contacted the sleep clinic twice or more in the NR group had significantly higher CCI than those with one contact (F1, 364 = 12.403, P < .001).
DISCUSSION
We routinely follow up our patients who are prescribed CPAP at 12- to 15-month intervals after their first year of therapy. Despite this routine follow-up system, one-third of our patients contacted us nonroutinely.
Symptoms were the main cause for a nonroutine visit, followed by problems related to CPAP devices. Somnolence has been shown to increase motor vehicle crashes and near-misses.12 Our patients were instructed to consult the sleep unit if they become somnolent, or if they believed that their driving capacity decreased. We found that patients with somnolence during CPAP were 35 times more likely to contact our sleep unit than those without somnolence. The second major symptom was a feeling of suffocation at night. This may indicate insufficient therapeutic pressures, emergence of extra air leak from the mask, or other causes. Their management usually required evaluation by a CPAP therapy specialist.
The second major reason for nonroutine contacts were problems related to CPAP devices or interfaces. Replacing the CPAP device resolves most of the device-related problems. CPAP devices become relatively outdated over 5 years. Replacing outdated devices increases the need for sleep unit contacts and should be planned in advance. Problems related to CPAP interfaces have been reported to be challenging to resolve.6 We have shown previously that mask switching resolved mask-related problems in only 61% of cases.
Adherence to CPAP therapy improves with the severity of sleep apnea.10,13 During our follow-up period, the severity of sleep apnea showed no effect on the frequency of visits. After the sleep apnea is treated, the baseline severity did not influence the frequency of follow-up.
The daily adherence to CPAP therapy in our patients was good, with a daily use of over 6 hours. Recently, Walter et al.14 reported linear correlation between hours of CPAP use and reduction in health care utilization. However, we found no differences in hours of CPAP use between the R and NR groups. The yearly abandoning rate was 3.7% and in agreement with previous reports.15 The risk for treatment cessation was considerable in patients in the NR group. The yearly abandoning rate was 8.7% for patients in the NR group, and only 1.3% in the R group. These results agree with our previous study,6 in which we reported a high abandoning CPAP therapy rate of 12.7% in patients who switched their CPAP interface within 1 year, and also in agreement with Kreivi et al.,16 who reported that nasal stuffiness during CPAP may lead to abandoning CPAP therapy.
Seventy percent of our CPAP follow-ups could be organized without face-to-face contact. This is in accordance with a previous report in which an annual follow-up was more likely to lead to administrative rather than to face-to-face clinical intervention.17
Shortening the interval between the routine contacts would reduce nonroutine follow-ups, but may also increase health care costs. It is claimed that the proliferation of information on the Internet and consumerism leads patients to demand more expensive tests and treatments.18 Nonetheless, our patients showed justified reasons for their contacts, as these were related to symptoms or to the CPAP device.
There were limitations in our study. Public health care in Finland provides CPAP devices free of charge. After CPAP therapy is approved, no more administrative justification is needed to continue therapy. This system considerably reduces administrative work regarding reimbursement and associated health care contacts. Thus, our results cannot be directly compared with those countries with different health care and health insurance systems. No data were collected on the intervention caused by the nonroutine visits nor the consequences of our interventions. We only studied patients who had been treated for at least 1 year in order to exclude early dropouts from our study. Percentage of, and reasons for, nonroutine contacts during the first year of treatment might be different.
We agree with Zarhin and Oksenberg19 that implementing a follow-up mechanism is not an easy feat, as time and resources are limited and often scarce in many health care systems. When medical resources are restricted, CPAP follow-up should be intensified during the first year of CPAP therapy.15 Nonetheless, the optimal follow-up interval is still to be determined. Shortening the interval between the routine contacts would reduce the nonroutine ones, meanwhile it may increase the health care cost.
Our results showed that a 12- to 15-month follow-up interval was associated with 30% extra contact, which seems considerable. Reducing the interval to 6 months would not reduce these extra contacts as they occurred within the first months after the previous visit. An alternative policy could include on-demand follow-up for those patients manifesting symptoms. Our results suggest that after the first year, CPAP follow-up could be selectively targeted to patients with symptoms. Further studies should investigate this approach, such as a randomized study to evaluate two different modes of CPAP follow-up—the routine with a regular yearly follow-up versus the follow-up on demand—and also a multicenter study from different countries to take into consideration different insurance coverage and CPAP follow-up practice.
In conclusion, extra visits in between the routine appointments were not prevented by the yearly follow-up. The major reason was symptoms during CPAP. An alternative method of follow-up could be on-demand visits for those who manifest the need for it.
DISCLOSURE STATEMENT
Work for this study was performed at the Sleep Unit, Heart and Lung Centre, Helsinki University Hospital, Finland. IRB approval number 69/2013 date January 30th. All authors have seen and approved the manuscript. Heidi Avellan-Hietanen reports financial support offered by the Helsinki University Hospital Research Fund for the year 2017 number Y-koodi Y2017SK002, VY 1169006 and for the year 2018 number Y-koodi Y2018SK002, VY 1169006. Adel Bachour reports financial support offered by the Helsinki University Hospital Research Fund for the year 2017 number Y-koodi Y2017SK002, VY 1169006 and for the year 2018 number Y-koodi Y2018SK002, VY 1169006. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: Dr. Avellan-Hietanen participated in the design of the study, data collection, analysis and wrote the manuscript; Dr. Brander approved the design of the study, participated in the analyses of data and in writing the manuscript; Dr. Bachour participated in the design of the study, data collection, analysis, and in writing the manuscript.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CCI
Charlson comorbidity index
- CPAP
continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- NR
nonroutine group
- OSA
obstructive sleep apnea
- R
routine group
- REI
respiratory event index
REFERENCES
- 1.Loube DI, Gay PC, Strohl KP, Pack AI, White DP, Collop NA. Indications for positive airway pressure treatment of adult obstructive sleep apnea patients: a consensus statement. Chest. 1999;115(3):863–866. doi: 10.1378/chest.115.3.863. [DOI] [PubMed] [Google Scholar]
- 2.Kushida CA, Littner MR, Hirshkowitz M, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29(3):375–380. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 3.Mysliwiec V, Gill J, Matsangas P, Baxter T, Barr T, Roth BJ. IGF-1: a potential biomarker for efficacy of sleep improvement with automatic airway pressure therapy for obstructive sleep apnea? Sleep Breath. 2015;19(4):1221–1228. doi: 10.1007/s11325-015-1142-x. [DOI] [PubMed] [Google Scholar]
- 4.Bachour A, Maasilta P. Mouth breathing compromises adherence to nasal continuous positive airway pressure therapy. Chest. 2004;126(4):1248–1254. doi: 10.1378/chest.126.4.1248. [DOI] [PubMed] [Google Scholar]
- 5.Balakrishnan K, James KT, Weaver EM. Predicting CPAP use and treatment outcomes using composite indices of sleep apnea severity. J Clin Sleep Med. 2016;12(6):849–854. doi: 10.5664/jcsm.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachour A, Vitikainen P, Maasilta P. Rates of initial acceptance of PAP masks and outcomes of mask switching. Sleep Breath. 2016;20(2):733–738. doi: 10.1007/s11325-015-1292-x. [DOI] [PubMed] [Google Scholar]
- 7.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 8.Positive Airway Pressure (PAP) Device for OSA: Face to Face (F2F) Encounter Template Guidance. Centers for Medicare & Medicaid Services website. [Accessed December 4, 2018]. https://www.cms.gov/Research-Statistics-Data-and-Systems/Computer-Data-and-Systems/Electronic-Clinical-Templates/DMEPOS-Templates/DMEPOS-Positive-Airway-Pressure-Devices.html. PAP Device for OSA F2F Encounter Template Draft R1.0c 4/12/2018.
- 9.McNicholas W, editor. The Obstructive Sleep Apnea Working Group. New Standards and Guidelines for Drivers with Obstructive Sleep Apnoea Syndrome: Report of the Obstructive Sleep Apnoea Working Group. Brussels, Belgium: European Commission; 2013. [Google Scholar]
- 10.Kreivi HR, Maasilta P, Bachour A. Willingness score obtained after a short CPAP trial predicts CPAP use at 1 year. Sleep Breath. 2014;18(1):207–213. doi: 10.1007/s11325-013-0872-x. [DOI] [PubMed] [Google Scholar]
- 11.Hall WH, Ramachandran R, Narayan S, Jani AB, Vijayakumar S. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer. 2004;4:94. doi: 10.1186/1471-2407-4-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward KL, Hillman DR, James A, et al. Excessive daytime sleepiness increases the risk of motor vehicle crash in obstructive sleep apnea. J Clin Sleep Med. 2013;9(10):1013–1021. doi: 10.5664/jcsm.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yetkin O, Kunter E, Gunen H. CPAP compliance in patients with obstructive sleep apnea syndrome. Sleep Breath. 2008;12(4):365–367. doi: 10.1007/s11325-008-0188-4. [DOI] [PubMed] [Google Scholar]
- 14.Walter RJ, Hagedorn SI, Lettieri CJ. Impact of diagnosing and treating obstructive sleep apnea on healthcare utilization. Sleep Med. 2017;38:73–77. doi: 10.1016/j.sleep.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Van Zeller M, Severo M, Santos AC, Drummond M. 5-years APAP adherence in OSA patients--do first impressions matter? Respir Med. 2013;107(12):2046–2052. doi: 10.1016/j.rmed.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Kreivi HR, Maasilta P, Bachour A. Persistence of upper-airway symptoms during CPAP compromises adherence at 1 year. Respir Care. 2016;61(5):652–657. doi: 10.4187/respcare.04113. [DOI] [PubMed] [Google Scholar]
- 17.Nannapaneni S, Morgenthaler TI, Ramar K. Assessing and predicting the likelihood of interventions during routine annual follow-up visits for management of obstructive sleep. J Clin Sleep Med. 2014;10(8):919–924. doi: 10.5664/jcsm.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell EG, Pham-Kanter G, Vogeli C, Iezzoni LI. Physician acquiescence to patient demands for brand-name drugs: results of a national survey of physicians. JAMA Intern Med. 2013;173(3):237–239. doi: 10.1001/jamainternmed.2013.1539. [DOI] [PubMed] [Google Scholar]
- 19.Zarhin D, Oksenberg A. Ambivalent adherence and nonadherence to continuous positive airway pressure devices: a qualitative study. J Clin Sleep Med. 2017;13(12):1375–1384. doi: 10.5664/jcsm.6828. [DOI] [PMC free article] [PubMed] [Google Scholar]