Abstract
Leptomeningeal carcinomatosis (LM) is an infrequent, yet morbid and often fatal complication of non-small cell lung cancer (NSCLC). Management of LM is multimodal, often involving systemic chemotherapy, radiotherapy, and a variety of symptom management maneuvers to address elevated intracranial pressure, pain and mood changes that can accompany the disease. Increasingly it is recognized that tumors with actionable mutations in NSCLC, including epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) translocations, respond well to systemic therapy with tyrosine kinase inhibitors yet often progress in the central nervous system (CNS). Therefore, more information is needed regarding the natural history and optimal management of leptomeningeal disease in specific molecular subtypes of NSCLC. The case below summarizes our institution’s management of a patient with ALK-positive NSCLC who developed leptomeningeal carcinomatosis while on targeted treatment with crizotinib (Xalkori) within the context of current NCCN guidelines and recently published studies.
Keywords: NSCLC, Targeted Therapy, Leptomeningeal Disease, ALK Translocation
Case
The patient is a 55 year-old Asian man with minimal smoking history (1 pack-year in the distant past), but a significant history of coronary artery disease with stent placement requiring maintenance with clopidogrel (Plavix) and aspirin. He presented with subacute cough, scant hemoptysis, worsening dyspnea on exertion and night sweats. Chest X-ray upon return from a trip to Asia revealed a right upper lobe lung mass. Physical exam revealed a palpable right supraclavicular lymph node. Fine need aspiration biopsy of the supraclavicular lymph node showed metastatic adenocarcinoma of lung primary (CK7+, CK20-, TTF1+). The patient’s tumor was negative for both KRAS and an EGFR activating mutations. PET-CT showed the right upper lobe lung mass as well as bone, liver and multiple lymph node metastases. MRI of the brain was negative for intracranial disease.
He was initially treated with carboplatin and pemetrexed for 6 cycles followed by continuation maintenance pemetrexed for 17 cycles until systemic progression was noted on CT scan with development of a new thoracic spine bone lesion, as well as growth in lung, lymph node and liver lesions. MRI of the brain at that time revealed an asymptomatic 9 mm brain metastasis that was treated with stereotactic radiotherapy.
He was then treated with erlotinib for 2 cycles but developed rapid disease progression. The patient was then placed briefly on docetaxel, but mucositis and neutropenia were dose limiting. ALK fluorescent in situ hybridization (FISH) testing became commercially available at this time and the patient’s tumor was positive for an ALK translocation by break-apart FISH analysis. Crizotinib was initiated at 250 mg orally twice daily. He had a rapid partial response and symptomatic improvement, working full-time and traveling. This continued for 10 months but he then developed headache, confusion, nausea and vomiting. A MRI of the brain revealed enhancement of the leptomeninges consistent with leptomeningeal carcinomatosis, progressive brain metastases and ventriculomegaly (Figure 1). An elevated opening pressure of 32 mm H2O was noted on lumbar puncture and CSF cytology was positive for metastatic adenocarcinoma. The patient’s headache, nausea and vomiting resolved shortly after lumbar puncture. He completed palliative whole brain radiation to 30 Gy and received a ventriculoperitoneal (VP) shunt for increased intracranial pressure. Over the next several weeks he had recurrence of neurologic symptoms, so he was transitioned to hospice care.
Figure 1:
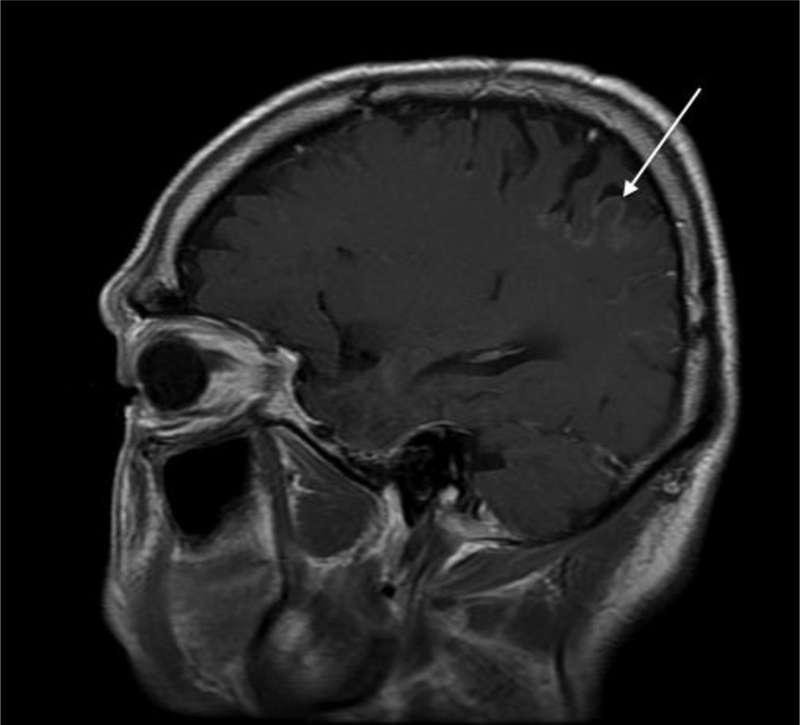
The patient’s MRI with gadolinium contrast showing abnormal enhancement of a cortical gyrus consistent with leptomeningeal disease (arrow).
Discussion
The mainstay of first-line treatment in patients with metastatic non-small cell lung adenocarcinoma whose tumors do not harbor an EGFR activating mutation or an ALK translocation is platinum based doublet chemotherapy with or without the VEGF inhibitor bevacizumab. NCCN guidelines for NSCLC currently list docetaxel, paclitaxel, pemetrexed, vinorelbine, vinblastine, gemcitabine and etoposide as proven effective agents when combined with carboplatin or cisplatin. In patients with non-squamous histology, a first line pemetrexed-based platinum doublet is frequently employed, with or without bevacizumab, based on phase III data showing on overall survival benefit of cisplatin/pemetrexed compared with cisplatin/gemcitabine in first line treatment of metastatic non-squamous histology lung cancer patients1. Pemetrexed containing first line regimens are well tolerated by most patients and have a much lower incidence of alopecia, neuropathy, and cytopenias than many other regimens. However, the additional benefit of adding bevacizumab to a platinum/pemetrexed backbone has been called into question by the recently presented Point Break Trial, which showed that overall survival was not increased with carboplatin/pemetrexed/bevacizumab followed by pemetrexed/bevacizumab maintenance compared to carboplatin/paclitaxel and bevacizumab followed by bevacizumab maintenance2. These results have lowered enthusiasm for a pemetrexed backbone when bevacizumab is utilized, though this is still a reasonable first-line treatment regimen.
Several retrospective analyses have shown a progression-free survival (PFS) benefit with pemetrexed compared to other chemotherapy agents in patients with ALK+ tumors, although prospective data is lacking3,4. This benefit may be due to lower levels of thymidylate synthase (TS) seen in ALK+ NSCLC tumors5. However, recently published data indicates that the preferential activity of pemetrexed may be associated with patients who have a never or light smoker history rather than the presence of an ALK translocation, which in itself is also associated with a light/never smoking history6. In the absence of significant toxicity or progression, we often continue pemetrexed after 4–6 cycles of platinum/pemetrexed doublet chemotherapy, which is presently a NCCN Category 2A recommendation. A statistically significant 2.9 month median improvement in overall survival (OS) following continuation maintenance with pemetrexed was observed in the phase III PARAMOUNT trial, as presented at the 2012 ASCO Annual Meeting7.
FDA approval of bevacizumab in NSCLC is based on the phase III ECOG 4599 clinical trial, in which the addition of bevacizumab to a carboplatin and paclitaxel backbone led to a statistically significant 2-month improvement in median overall survival in patients with non-squamous NSCLC8. One of the trial exclusion criteria was anti-coagulation, including patients with regular use of 325 mg of ASA or other inhibitors of platelet function. There is also a modest increased risk of coronary thrombosis in patients on bevacizumab9. In another large phase III randomized trial adding bevacizumab to cisplatin and gemcitabine, patients were allowed to stay on bevacizumab after occurrence of venous thrombosis on trial. In total, 9% of patients on this study were on anticoagulation with low molecular weight heparin or warfarin and none experienced pulmonary hemorrhage10. In a large phase IV trial including bevacizumab, the incidence of grade 3 or greater bleeding on anti-coagulation was 4%11. Several ongoing cooperative group clinical trials with bevacizumab and current NCCN guidelines do not prohibit patients on anticoagulation from receiving bevacizumab.
Though this patient had only scant hemoptysis, he had active coronary artery disease (CAD) with two recent stent placements and was on ASA 325 mg and clopidogrel, so the decision was made to withhold bevacizumab. Our institution is comfortable treating patients with anti-coagulation and bevacizumab based on the aforementioned data, but there remains concern in the setting of hemoptysis, active CAD or other arterial thrombotic disease and with clopidogrel and other very potent direct platelet inhibitors (ticlopidine, cilostazol).
Crizotinib (Xalkori), an oral ALK and MET small molecule tyrosine kinase inhibitor, has recently been given accelerated approval by the FDA for metastatic NSCLC patients harboring an ALK translocation as established by the companion FISH diagnostic test. Approval is expected based on the recently presented results of the phase III PROFILE 1007 (A Phase III Trial of Crizotinib Versus Standard of Care in Patients With Advanced Non– Small-Cell Lung Cancer With a Specific Alteration of the Anaplastic Lymphoma Kinase Gene) trial of crizotinib versus physician’s choice of pemetrexed or docetaxel in the second-line setting and beyond. A PFS benefit (7.7 vs. 3 months) of crizotinib was noted, though no OS benefit was seen in an interim analysis most likely due to the high degree of crossover (62%)12.
FDA approval of crizotinib did not restrict its indication to the relapsed/refractory setting, so crizotinib can be given in the first-line even though the clinical trials presented to date have looked at it in patients who have progressed after first line chemotherapy. The current NCCN guidelines list crizotinib as a default category 2A recommendation, rather than conventional platinum based chemotherapy regimens, as first line therapy in metastatic NSCLC patients with FISH ALK+ tumors. The use of first-line crizotinib compared to cytotoxic chemotherapy is based on extrapolated data from the results of several large, randomized phase III trials showing improvement in progression-free survival, but not overall survival with EGFR targeted TKIs compared to platinum doublet chemotherapy in patients with tumors harboring EGFR activating mutations13–15. A randomized, phase III trial investigating first line crizotinib versus carboplatin or cisplatin and pemetrexed in patients with FISH ALK+ tumors is ongoing (NCT01639001).
This patient was diagnosed and started on first line chemotherapy before ALK FISH testing was routinely available. He developed leptomeningeal disease while on crizotinib. The CNS is a known site of progression with this agent16. In patients with ALK+ tumors who have active CNS disease we consider a pemetrexed-based regimen, preferably with platinum in a patient who is not heavily pretreated, and bevacizumab if they do not otherwise have a contraindication, as p pemetrexed appears to have activity in CNS disease17,18. Though bevacizumab administration is contraindicated in untreated parenchymal brain metastases, there is sufficient evidence at this time to support its use in patients with treated stable CNS disease19,20. There is also phase III data to support the use of bevacizumab in patients who develop radiation necrosis after brain irradiation for metastases21. We have given bevacizumab in patients with active leptomeningeal disease with some success and without significant toxicity, but it is not clear what role bevacizumab will ultimately play in the treatment of LM.
The mean cerebrospinal fluid (CSF) penetration of erlotinib is about 5–10% of the systemic concentration 22. Pulsed high-dose erlotinib has been used as a strategy to treat LM in patients with tumors harboring EGFR activating mutations23,24. Crizotinib has some documented CSF penetration, though lower penetration compared to erlotinib17. However, leptomeningeal involvement of cancer may lead to compromise of the blood brain barrier with potentially increased CNS penetration. In a recent phase II study of crizotinib in patients with ALK+ tumors, 22% of patients with asymptomatic, non-irradiated brain metastases had a radiographic brain response25.
After diagnosis of leptomeningeal disease, this patient received whole brain radiotherapy to 30 Gy and VP shunt placement with limited improvement in neurologic function. Analysis of LM outcomes is mainly from retrospective studies as prospective trials are limited for this uncommon complication. As in other malignancies, LM disease is a poor prognostic indicator in NSCLC26. Though whole brain radiotherapy has not shown an overall survival benefit in a retrospective analysis, we consider palliative brain and possibly spinal radiation in our LM patients for symptomatic relief26. If a patient with an ALK+ tumor develops isolated CNS or other metastases while on crizotinib, radiating the site of progression and restarting crizotinib may delay further progression based on a retrospective analysis16. However, patients with LM disease were excluded from this analysis. There is no published data yet regarding the safety and efficacy of concurrent crizotinib and radiotherapy. Novel ALK inhibitors with potentially better CNS penetration are in development and will hopefully provide additional therapeutic options for patients like the one described here27.
In a patient with hydrocephalus and symptomatic improvement following large volume lumbar puncture, as in this patient, we consider a VP shunt for palliative relief. Intrathecal chemotherapy with methotrexate or cytarabine or liposomal cytarabine or topotecan is also a treatment option that we use infrequently. A recent single institution, retrospective case-series noted prolonged survival in the small number of patients who received intrathecal chemotherapy, but this may be due to selection bias26.
Leptomeningeal disease in NSCLC, as in other malignancies remains challenging to treat. However, some limited evidence is mounting that outcomes may be improved in the modern treatment era, though many of the studies are retrospective and thus limited by multiple biases28. A recent retrospective review at our institution also supports improved outcomes with modern systemic chemotherapy and targeted therapies, particularly in patients diagnosed with LM at metastatic presentation and who are typically naïve to systemic treatment29. Patients with NSCLC are living longer and we often seen CNS progression in patients with EGFR+ and ALK+ tumors after progression on targeted therapy that is difficult to treat. Whether this is a feature of the disease itself or the consequence of better systemic therapies and longer life is unclear.
Many patients with leptomeningeal disease have severe neurologic complications and poor functional status. Default category 2A NCCN recommendations for NSCLC patient with an ALK+ tumor and poor functional status (ECOG performance status of 3–4) post crizotinib and other first line chemotherapy treatments include best supportive care. Effective pain control as well as integrated palliative care is always critical in patients with leptomeningeal carcinomatosis who have progressed despite multiple lines of therapy. Hopefully, more effective targeted therapies, including ALK inhibitors with increased CNS activity, will be more successful in treating this devastating complication of NSCLC.
Acknowledgement of Financial Support:
The research described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 5KL2RR025743 (JWR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References:
- 1.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. July 20 2008;26(21):3543–3551. [DOI] [PubMed] [Google Scholar]
- 2.Patel MAS J, Garon EB, Reynolds CH, Spigel DR, Hermann RC, Liu J, Guba SC, Bonomi P, Govindan R. A Randomized, Open-label, Phase 3, Superiority Study Of Pemetrexed (Pem)+Carboplatin (Cb)+Bevacizumab (B) Followed By Maintenance Pem+B Versus Paclitaxel (Pac)+Cb+B Followed By Maintenance B In Patients (pts) With Stage IIIB Or IV Non-squamous Non-small Cell Lung Cancer (NS-NSCLC). Chicago Multidisciplinary Symposium in Thoracic Oncology. 2012;LBPL1. [Google Scholar]
- 3.Camidge DR, Kono SA, Lu X, et al. Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. J Thorac Oncol. April 2011;6(4):774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JO, Kim TM, Lee SH, et al. Anaplastic Lymphoma Kinase Translocation: A Predictive Biomarker of Pemetrexed in Patients with Non-small Cell Lung Cancer. J Thorac Oncol. June 2 2011. [DOI] [PubMed] [Google Scholar]
- 5.David R Gandara EH, Sonal Desai, Mack Philip C., Beckett Laurel, Stephens Craig, Zeger Gary, Danenberg Kathleen D., Maus Martin Karl Herbert, Tianhong Li. Thymidylate synthase (TS) gene expression in patients with ALK positive (+) non-small cell lung cancer (NSCLC): Implications for therapy. J Clin Oncol. 2012;30:suppl; abstr 7582. [Google Scholar]
- 6.Shaw AT, Varghese AM, Solomon BJ, et al. Pemetrexed-based chemotherapy in patients with advanced, ALK-positive non-small cell lung cancer. Ann Oncol. August 10 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luis Paz-Ares FDM, Mircea Dediu, Michael Thomas, Jean-Louis Pujol, Paolo Bidoli, Oliver Molinier, Tarini Prasad Sahoo, Eckart Laack, Martin Reck, Jesus Corral Jaime, Symantha Melemed, William J. John, Nadia Chouaki, Annamaria Zimmermann, Carla Visseren-Grul, Cesare Gridelli. PARAMOUNT: Final overall survival (OS) results of the phase III study of maintenance pemetrexed (pem) plus best supportive care (BSC) versus placebo (plb) plus BSC immediately following induction treatment with pem plus cisplatin (cis) for advanced nonsquamous (NS) non-small cell lung cancer (NSCLC). J Clin Oncol. 2012;30:suppl; abstr LBA7507. [Google Scholar]
- 8.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. December 14 2006;355(24):2542–2550. [DOI] [PubMed] [Google Scholar]
- 9.Ranpura V, Hapani S, Chuang J, Wu S. Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis of randomized controlled trials. Acta Oncol. April 2010;49(3):287–297. [DOI] [PubMed] [Google Scholar]
- 10.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. March 10 2009;27(8):1227–1234. [DOI] [PubMed] [Google Scholar]
- 11.Crino L, Dansin E, Garrido P, et al. Safety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): a phase 4 study. Lancet Oncol. August 2010;11(8):733–740. [DOI] [PubMed] [Google Scholar]
- 12.Shaw DWK AT, Nakagawa K, Seto T, Crinò L, Ahn M-J, De Pas T, Besse B, Solomon B, Blackhall FH, Wu Y-L, Thomas M, O’Byrne KJ, Moro-Sibilot D, Camidge R, Hirsh V, Mok TSK, Tassell V, Polli A, Jänne P. Phase III study of crizotinib versus pemetrexed or docetaxel chemotherapy in patients with advanced ALK-positive non-small cell lung cancer (NSCLC) (PROFILE 1007). ESMO. 2012;LBA1_PR. [Google Scholar]
- 13.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. June 24 2010;362(25):2380–2388. [DOI] [PubMed] [Google Scholar]
- 14.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. September 3 2009;361(10):947–957. [DOI] [PubMed] [Google Scholar]
- 15.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. March 2012;13(3):239–246. [DOI] [PubMed] [Google Scholar]
- 16.Andrew James Weickhardt BS, Joseph Malachy Burke, Gregory Gan, Robert Charles Doebele, Bunn Paul A., Gaspar Laurie E., Kavanagh Brian D., D. Ross Camidge. Continuation of EGFR/ALK inhibition after local therapy of oligoprogressive disease in EGFR mutant (Mt) and ALK+ non-small cell lung cancer (NSCLC). J Clin Oncol. 2012;30:suppl; abstr 7526. [Google Scholar]
- 17.Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. May 20 2011;29(15):e443–445. [DOI] [PubMed] [Google Scholar]
- 18.Barlesi F, Gervais R, Lena H, et al. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07–01). Ann Oncol. November 2011;22(11):2466–2470. [DOI] [PubMed] [Google Scholar]
- 19.Socinski MA, Langer CJ, Huang JE, et al. Safety of bevacizumab in patients with non-small-cell lung cancer and brain metastases. J Clin Oncol. November 1 2009;27(31):5255–5261. [DOI] [PubMed] [Google Scholar]
- 20.Gubens WA M, Lynch T, Langer CJ, Socinski MA, Colevas AD, Clement-Duchene C, Wakelee H. A pooled analysis of advanced nonsquamous NSCLC patients with stable treated brain metastases in two phase II trials receiving bevacizumab and pemetrexed as second-line therapy. World Congress on Lung Cancer. 2011:P3093.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. April 1 2011;79(5):1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Togashi Y, Masago K, Fukudo M, et al. Cerebrospinal fluid concentration of erlotinib and its active metabolite OSI-420 in patients with central nervous system metastases of non-small cell lung cancer. J Thorac Oncol. July 2010;5(7):950–955. [DOI] [PubMed] [Google Scholar]
- 23.Clarke JL, Pao W, Wu N, Miller VA, Lassman AB. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol. September 2010;99(2):283–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grommes C, Oxnard GR, Kris MG, et al. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro-oncology. December 2011;13(12):1364–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riely TLE GJ, Salgia R, Ou SI, Gettinger SN, Otterson GA, Lanzalone S, Polli A, Shaw AT. Results of a Global Phase II Study with Crizotinib in Advanced ALK-positive Non-small Cell Lung Cancer (NSCLC) the IASLC Chicago Multidisciplinary Symposium in Thoracic Oncology Sept. 6–8. 2012. [Google Scholar]
- 26.Morris PG, Reiner AS, Szenberg OR, et al. Leptomeningeal Metastasis from Non-small Cell Lung Cancer: Survival and the Impact of Whole Brain Radiotherapy. J Thorac Oncol. November 15 2011. [DOI] [PubMed] [Google Scholar]
- 27.Ranee Mehra DRC, Sunil Sharma, Enriqueta Felip, Daniel Shao-Weng Tan, Vansteenkiste Johan F., Tommaso Martino De Pas, Dong-Wan Kim, Armando Santoro, Geoffrey Liu, Meredith Goldwasser, David Dai, Marietta Radona, Anthony Boral, Alice Tsang Shaw. First-in-human phase I study of the ALK inhibitor LDK378 in advanced solid tumors. J Clin Oncol. 2012;30:suppl; abstr 3007. [Google Scholar]
- 28.Park JH, Kim YJ, Lee JO, et al. Clinical outcomes of leptomeningeal metastasis in patients with non-small cell lung cancer in the modern chemotherapy era. Lung Cancer. June 2012;76(3):387–392. [DOI] [PubMed] [Google Scholar]
- 29.Riess SN JW, Iv M, Zeineh M, Gubens MA, Neal JW, Wakelee HA. Prolonged Survival in Non-Small Cell Lung Cancer (NSCLC) Patients with Leptomeningeal Metastases (LM) in the Modern Treatment Era. Chicago Multidisciplinary Symposium in Thoracic Oncology. 2012;184. [Google Scholar]


