Abstract
Background
Respiratory syncytial virus (RSV) lower respiratory tract infection (LRTI) is associated with high mortality in patients with hematologic malignancies (HM). We sought to determine whether allogeneic hematopoietic cell transplant (allo‐HCT) recipients would be at higher risk for 60‐day mortality.
Methods
We examined a retrospective cohort of adults with HM with or without HCT treated for RSV LRTI (n = 154) at our institution from 1996‐2013. We defined possible RSV LRTI as RSV detected only in the upper respiratory tract with new radiologic infiltrates and proven RSV LRTI as RSV detected in BAL fluid with new radiologic infiltrates. Immunodeficiency Scoring Index (ISI) and Severe Immunodeficiency (SID) criteria were calculated for HCT recipients. Multivariable logistic regression analyses were performed to identify independent risk factors associated with 60‐day all‐cause mortality.
Results
Mortality was high in HM patients (25%), but there was no difference between those without HCT, autologous or allo‐HCT recipients in logistic regression models. Separate multivariate models showed that at RSV diagnosis, neutropenia (OR 8.3, 95% CI 2.8‐24.2, P = 0.005) and lymphopenia (OR 3.7, 95% CI 1.7‐8.2, P = 0.001) were associated with 60‐day mortality. Proven LRTI was associated with higher 60‐day mortality (neutropenia model: OR 4.7, 95%CI 1.7‐13.5; lymphopenia model: OR 3.3, 95% CI 1.2‐8.8), and higher ICU admission. In HCT recipients, high ISI and very severe immunodeficiency by SID criteria were associated with higher 60‐day all‐cause mortality.
Conclusions
Mortality is similarly high among HM patients without HCT and HCT recipients. High‐grade immunodeficiency and detection of RSV from BAL fluid are associated with higher 60‐day mortality.
Keywords: hematologic malignancy, hematopoietic cell transplantation, lower respiratory tract infection, mortality, respiratory syncytial virus
Abbreviations
- Allo‐HCT
allogeneic hematopoietic cell transplant
- Auto‐HCT
autologous hematopoietic cell transplant
- BAL
bronchoalveolar lavage
- CT
computed tomography
- CXR
chest X‐ray
- HCT
hematopoietic cell transplant
- HM
hematologic malignancy
- hMPV
human metapneumovirus
- ICU
intensive care unit
- ISI
Immunodeficiency Scoring Index
- LRTI
lower respiratory tract infection
- PIV
parainfluenza virus
- RSV
respiratory syncytial virus
- SID
Severe Immunodeficiency
- URTI
upper respiratory tract infection
1. INTRODUCTION
Infection with respiratory syncytial virus (RSV) is a major cause of death in in immunocompromised patients with hematologic malignancies (HM) with or without hematopoietic cell transplantation (HCT).1, 2 The incidence of infection is estimated between 2%‐17% in HCT recipients,3 and mortality rates after lower respiratory tract infection (LRTI) can reach up to 80%.4, 5, 6 New therapies and strategies are needed to improve outcomes after RSV LRTI in immunocompromised hosts.
Once an RSV diagnosis is confirmed, risk stratification in HM patients is critical given the high cost of therapy with aerosolized ribavirin7 and high mortality rates after LRTI.3, 5, 8, 9, 10, 11 Prior studies have identified lymphopenia, neutropenia, and RSV detection in lower respiratory samples as important risk factors for mortality after LRTI.8, 11, 12, 13 Recognition of individuals at high risk for adverse outcomes could improve the prompt delivery of ribavirin treatment, which may reduce mortality and morbidity.9
We previously developed an immunodeficiency scoring index based on clinical characteristics and laboratory parameters to standardize the risk stratification practices of allogeneic HCT (allo‐HCT) recipients with RSV upper respiratory tract infection (URTI), a group at high risk for adverse outcomes after RSV Infection.13, 14 We hypothesized that among HM patients without HCT and those who are autologous HCT (auto‐HCT) or allo‐HCT recipients, allo‐HCT recipients would be at the highest risk for mortality. We further hypothesized that isolation of RSV and other respiratory pathogens from the lower respiratory tract among patients who underwent fiberoptic bronchoscopy would be associated with higher mortality, as demonstrated in parainfluenza viral infection.15 We also sought to determine whether more severe immunodeficiency in HCT recipients, as determined by the Immunodeficiency Score Index (ISI)13, 16 and the severe immunodeficiency (SID) criteria,17, 18 predicted mortality after RSV LRTI.
2. MATERIALS AND METHODS
2.1. Study design
We conducted a retrospective review of all adults with any type of leukemia or lymphoma or multiple myeloma (HM), some of whom underwent auto‐ or allo‐HCT, and who were 18 years of age or older with an RSV infection cared for at The University of Texas MD Anderson Cancer Center from 01/96 to 07/13. Patients were identified from a prospectively‐maintained infection control database of viral infections. We collected data on patients’ demographics, underlying malignancy, immune status at diagnosis, antiviral therapy, thoracic imaging and microbiologic data. We also collected data on outcomes including hospital admission, intensive care unit (ICU) admission, mechanical ventilation, and all‐cause mortality at days 30 and 60. This study was approved by our Institutional Review Board (DR09‐0696 and PA12‐0483) in accordance with the Helsinki Declaration of the World Medical Association, and waiver of informed consent was granted.
2.2. Definitions
We defined RSV LRTI as (a) microbiologic confirmation of RSV in nasal wash or swab or BAL fluid and (b) new infiltrates on CXR or chest CT within 5 days of microbiologic confirmation. Microbiologic confirmation was performed using either shell vial culture or direct immunofluorescent antibody testing as per institutional practice at that time. We further stratified those with RSV LRTI by possible or proven RSV LRTI.15 We defined possible RSV LRTI as RSV detection in the upper respiratory tract with new pulmonary infiltrates on chest imaging suggestive of viral etiology and proven RSV LRTI as any patient with RSV detected in the lower respiratory tract (obtained by BAL) and new pulmonary infiltrates on chest imaging suggestive of viral etiology. Because direct immunofluorescent antibody testing is less sensitive than PCR for the detection of RSV,19 it is possible that some patients with no detection of RSV in BAL fluid had proven RSV LRTI. Therefore, patients with no RSV detected in BAL were also considered to have possible RSV LRTI. BAL co‐pathogens were defined as non‐RSV organisms (virus, bacteria or fungus) that are known to cause pneumonia in humans. We defined neutropenia as an absolute neutrophil count < 500 cells/mm3 and lymphopenia as an absolute lymphocyte count < 200 cells/mm3. We considered patients who received ribavirin therapy prior to the diagnosis of LRTI as having received ribavirin at the URTI stage, and patients who received ribavirin therapy at the time of diagnosis of LRTI as having received ribavirin at the LRTI stage.
2.3. Clinical evaluation of RSV infection
Radiographic images were independently reviewed by two pulmonologists (E.V. and A.S.) and discrepancies were resolved by mutual agreement. We excluded patients who had resolving infiltrates from a known prior LRTI and those with clinical volume overload as assessed by echocardiography, right heart catheterization, or response to diuretics. Prior to 2001, 19 patients did not have images available for independent review. In those cases, the radiologist's interpretation, as documented in the medical record, was used. Bronchoscopy was performed in a dedicated endoscopy suite under the supervision of an experienced pulmonologist.20 The results from bronchial washing were excluded to minimize contamination from the upper respiratory tract. For patients with possible RSV LRTI who did not undergo bronchoscopy (n = 93), the reason for not performing bronchoscopy was recorded when available (Table S1).
2.4. Grading of immunodeficiency in HCT recipients
All HCT recipients were scored using ISI and SID criteria at the time of RSV LRTI. ISI ranges from 0‐12, and ISI scores were calculated as previously described with 3 points given each for ANC < 500 cells/mm3 and ALC < 200 cells/mm3, 2 points for age ≥40 years, and 1 point given each for myeloablative conditioning regimen, graft‐versus‐host disease, corticosteroids within 30 days of RSV infection, and recent or pre‐engraftment allo‐HCT.13 HCT recipients are then stratified into low‐risk (ISI 0‐2), moderate risk (ISI 3‐6) and high‐risk (ISI 7‐12) strata. SID criteria classify HCT recipients into three strata: moderate immunodeficiency (MID), severe immunodeficiency (SID), and very severe immunodeficiency (vSID).17, 18 MID was defined as HCT > 6 months prior to LRTI, leukocyte count > 2000 cells/mm3 and ALC > 100 cells/mm3, while SID was defined as HCT ≤ 6 months prior to LRTI, T‐cell depletion ≤ 3 months prior to LRTI, B‐cell depletion ≤ 3 months prior to LRTI, graft‐versus‐host disease ≥ grade 2, leukocyte count 2000 cells/mm3, ALC ≤ 100 cells/mm3, or immunoglobulin count ≤ 6.5 g/L. vSID was defined as ≥ 2 SID risk factors being present at the time of LRTI.
2.5. Statistical analysis
Descriptive statistics were calculated for demographic, clinical, and therapeutic data. Categorical variables were compared using chi‐square or Fisher's exact test and continuous variables were compared using one‐way analysis of variance. Multivariable logistic regression analyses were performed to identify independent risk factors associated with 60‐day all‐cause mortality. All patients were followed for the entire 60 days, and outcomes were right‐censored after 60‐days. All variables were entered into the multivariable model, and backward elimination was used to only include variables with P < 0.1 in the final model. Univariate logistic regression analyses were performed determine whether ISI and SID criteria were associated with 60‐day all‐cause mortality. Kaplan‐Meier survival curves and log‐rank tests were performed to compare the probability of survival between patients with different types of LRTI (possible vs proven), between patients with different microbiological results in BAL (BAL not performed, no growth, RSV, RSV and co‐pathogens, and other pathogens only), and between patients in different immunodeficiency risk strata (MID/SID/vSID and low/moderate/high ISI). All statistical analyses were performed using R version 3.3 (R Foundation for Statistical Computing, Vienna, Austria). All tests were two‐sided with a significance level of 0.05.
3. RESULTS
3.1. Clinical characteristics and outcomes of patients with possible and proven RSV LRTI
We identified 154 patients who met our inclusion and exclusion criteria, 61 (40%) of whom underwent bronchoscopy with BAL (Table 1). Median age was 54 years (range 18‐79 years) and 65 (42%) were female. There were 69 (45%) patients without HCT, 28 (18%) with auto‐HCT and 58 (38%) with allo‐HCT. Possible RSV LRTI was diagnosed in 131 (85%) patients and proven RSV LRTI in 23 (15%) patients. Allo‐HCT recipients were younger, and auto‐HCT recipients were more likely to have multiple myeloma as an underlying malignancy and to be on corticosteroids. Among HCT recipients, the median time between HCT and LRTI was 215 days (interquartile range: 58‐693 days). Patients without HCT were more likely to receive chemotherapy within 1 month of RSV LRTI and have neutropenia or lymphopenia, but were less likely to receive ribavirin therapy. RSV alone was detected in 18 (30%) of the 63 BAL samples, RSV with concurrent non‐RSV respiratory pathogens was detected in 5 (8%), non‐RSV pathogens alone were detected in 12 (20%), and no pathogens were detected in 26 (43%). The majority of patients who did not undergo bronchoscopy (82 of 93) were not referred for the procedure at the discretion of their primary oncologist or transplant physician (Table S1).
Table 1.
Characteristics of the study cohort
| No HCT | Allogeneic‐HCT | Autologous HCT | Total | P‐value | |
|---|---|---|---|---|---|
| Number | 69 | 57 | 28 | 154 | |
| Median age (range) | 60 (18‐79) | 51 (23‐70) | 60 (22‐77) | 54 (18‐79) | 0.03 |
| Sex, n (%) | |||||
| Female | 28 (41) | 22 (39) | 15 (54) | 65 (42) | 0.39 |
| Male | 41 (59) | 35 (61) | 13 (46) | 89 (58) | |
| Malignancy, n (%) | |||||
| Leukemia | 43 62) | 34 (60) | 2 (7) | 79 (51) | <0.001* |
| Lymphoma | 13 (19) | 20 (35) | 12 (43) | 45 (29) | |
| Myeloma | 13 (19) | 3 (5) | 14 (50) | 30 (20) | |
| Recent chemotherapy (within 1 mo prior to RSV), n (%) | 52 (75) | 17 (30) | 13 (46) | 82 (53) | <0.001* |
| Corticosteroid (within 1 mo prior to RSV diagnosis), n (%) | 43 (62) | 29 (51) | 22 (79) | 94 (61) | 0.046 |
| Neutropenia at RSV diagnosis (ANC < 500 cells/mm3) n (%) | 35 (51) | 6 (11) | 6 (21) | 47 (31) | <0.001 |
| Lymphocytopenia at RSV diagnosis (ALC < 200 cells/mm3), n (%) | 33 (48) | 15 (26) | 6 (21) | 54 (35) | 0.01 |
| Ribavirin therapy, n (%) | |||||
| None | 19 (28) | 3 (5) | 1 (4) | 23 (15) | <0.001 |
| URTI stage | 3 (4) | 6 (11) | 5 (18) | 14 (9) | |
| LRTI stage | 47 (68) | 48 (84) | 22 (78) | 117 (76) | |
| RSV LRTI | |||||
| Possible | 60 (87) | 48 (84) | 23 (82) | 131 (85) | 0.84 |
| Proven | 9 (13) | 9 (16) | 5 (18) | 23 (15) | |
| Organisms detected in BAL | |||||
| None | 12 | 8 | 6 | 26 | 0.540 |
| RSV | 6 | 7 | 5 | 18 | |
| RSV + co‐pathogens | 3 | 2 | 0 | 5 | |
| Co‐pathogens | 2 | 6 | 4 | 12 | |
ALC, absolute lymphocyte count; ANC, absolute neutrophil count; BAL, bronchoalveolar lavage; HCT, hematopoietic cell transplant; HM, hematologic malignancy; LRTI, lower respiratory tract infection; RSV, respiratory syncytial virus; URTI, upper respiratory tract infection. Allo‐HCT recipients were younger, and auto‐HCT recipients were more likely to have multiple myeloma as an underlying malignancy and to be on corticosteroids. Patients without HCT were more likely to receive chemotherapy within 1 month of RSV LRTI and have neutropenia or lymphopenia, but were less likely to receive ribavirin therapy (in bold).
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.2. Outcomes after RSV LRTI
Hospitalization at the time of RSV was common among patients in our cohort (61%), although patients without HCT were less likely to be hospitalized (Table 2). There were no differences in rates of ICU admission, mechanical ventilation, 30‐day mortality, or 60‐day mortality between patients without HCT, allo‐HCT recipients, and auto‐HCT recipients, and 60‐day mortality was high across all groups (39/154, 25%). Patients with proven LRTI were more likely to be admitted to the ICU (48% vs 31%, P = 0.03), and to die at 30 days (36% vs 14%, P = 0.03) and 60 days (52% vs 21%, P = 0.003) after LRTI onset (Table 3).
Table 2.
Health care utilization and mortality rates by type of hematopoietic cell transplant
| Health care use and mortality rates | No HCT (n = 69) | Allogeneic‐HCT (n = 57) | Autologous HCT (n = 28) | Total (n = 154) | P‐value |
|---|---|---|---|---|---|
| Hospitalization, n (%) | 30 (43) | 44 (77) | 20 (71) | 94 (61) | <0.001* |
| ICU admission, n (%) | 21 (30) | 15 (26) | 6 (21) | 42 (27) | 0.65 |
| Mechanical ventilation, n (%) | 12 (17) | 12 (21) | 6 (21) | 30 (19) | 0.83 |
| 30 d mortality (from LRTI diagnosis to death), n (%) | 11 (16) | 11 (19) | 4 (14) | 26 (17) | 0.85 |
| 60 d mortality (from LRTI diagnosis to death), n (%) | 18 (26) | 16 (28) | 5 (18) | 39 (25) | 0.59 |
ICU, intensive care unit; LRTI, lower respiratory tract infection; RSV, respiratory syncytial virus. Patients without HCT were less likely to be hospitalized (in bold).
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 3.
Health care use and mortality rates in patients with possible and proven RSV‐LRTI
| Health care use and mortality rates | Possible RSV LRTI (n = 131) | Proven RSV LRTI (n = 23) | Total (n = 154) | P‐value |
|---|---|---|---|---|
| Hospitalization, n (%) | 83 (63) | 11 (52) | 94 (61) | 0.24 |
| ICU admission, n (%) | 31 (24) | 11 (48) | 42 (27) | 0.03 |
| Mechanical ventilation, n (%) | 22 (17) | 8 (36) | 30 (19) | 0.08 |
| 30 d mortality (from LRTI diagnosis to death), n (%) | 18 (14) | 8 (36) | 26 (17) | 0.03 |
| 60 d mortality (from LRTI diagnosis to death), n (%) | 27 (21) | 12 (52) | 39 (25) | 0.003 |
ICU, intensive care unit; LRTI, lower respiratory tract infection; RSV, respiratory syncytial virus. Patients with proven LRTI were more likely to be admitted to the ICU, and to die at 30 days and 60 days after LRTI onset (in bold).
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.3. Predictors of mortality after RSV LRTI
In univariate logistic regression models, neutropenia at RSV diagnosis (odds ratio [OR] 4.0, 95% confidence interval [CI] 1.9‐8.6), lymphopenia at RSV diagnosis (OR 3.9, 95% CI 1.8‐8.3), and proven LRTI (OR 4.2, 95% CI 1.7‐10.6) were predictive of mortality (Table 4). Because lymphopenia and neutropenia exhibited a high degree of collinearity, we created two multivariable models; Model 1 included neutropenia but not lymphopenia as a candidate variable for the final model, while Model 2 included lymphopenia but not neutropenia as a candidate variable for the final model. In Model 1, after adjustment for neutropenia, proven LRTI, and ribavirin therapy, allo‐HCT recipients were at a higher risk for mortality than patients without HCT or auto‐HCT recipients (adjusted OR 3.7, 95%CI 1.2‐11.9, P = 0.03). Neutropenia (adjusted OR 8.3, 95% CI 2.8‐24.2, P = 0.005) was the strongest predictor of 60‐day mortality. Proven LRTI remained a strong predictor of mortality after adjustment (adjusted OR 4.7, 95%CI 1.7‐13.5). Similarly, in model 2, lymphopenia (adjusted OR 3.7, 95% CI 1.7‐8.2) and proven LRTI (adjusted OR 3.3, 95%CI 1.2‐8.8) were associated with higher mortality. However, allo‐HCT was not associated with higher mortality in model 2. Ribavirin therapy remained in both models but was not significantly associated with lower mortality (Model 1: URTI stage: adjusted OR 0.1, 95% 0.01‐1.3; P = 0.08; LRTI stage: adjusted OR 0.6, 95% CI 0.2‐1.9, P = 0.43; Model 2: URTI stage: adjusted OR 0.2, 95% 0.02‐1.5, P = 0.10; LRTI stage: adjusted OR 0.7, 95% CI 0.2‐1.8, P = 0.43). There was no significant difference in mortality among patients with RSV LRTI during the first half (1996‐2004, 30% mortality) or the second half of the study period (2005‐2013, 25% mortality) (OR 0.7 for second half, 95% CI 0.3‐1.6, P = 0.4).
Table 4.
Clinical predictors of 60 d all‐cause mortality
| Survivors | Non‐survivors | Unadjusted OR | P‐value | Model 1 Adjusted OR | P‐value | Model 2 Adjusted OR | P‐value | |
|---|---|---|---|---|---|---|---|---|
| Number | 115 | 39 | ||||||
| Median age (range) | 54 (18‐79) | 55 (22‐78) | 1.0 (0.99‐1.04) | 0.31 | ||||
| Sex | ||||||||
| Female | 53 (46) | 12 (31) | 1.0 | 0.10 | ||||
| Male | 62 (54) | 27 (69) | 1.9 (0.9‐4.2) | |||||
| Type of HCT | ||||||||
| HM without HCT | 51 (44) | 18 (46) | 1.0 | 1.0 | ||||
| Allogeneic‐HCT | 41 (36) | 16 (41) | 1.1 (0.5‐2.4) | 0.80 | 3.7 (1.2‐11.9) | 0.03 | ||
| Autologous HCT | 23 (20) | 5 (13) | 0.6 (0.2‐1.9) | 0.39 | 1.6 (0.4‐6.4) | 0.54 | ||
| Corticosteroids within 30 d | ||||||||
| No | 49 (43) | 11 (28) | 1.0 | 0.11 | ||||
| Yes | 66 (57) | 28 (72) | 1.7 (0.8‐3.8) | |||||
| Neutropenia at RSV diagnosis | ||||||||
| No | 89 (77) | 18 (46) | 1.0 | 1.0 | ||||
| Yes | 26 (23) | 21 (54) | 4.0 (1.9‐8.6) | <0.001 | 8.3 (2.8‐24.2) | <0.001 | ||
| Lymphopenia at RSV diagnosis | ||||||||
| No | 84 (73) | 16 (41) | 1.0 | 1.0 | ||||
| Yes | 31 (27) | 23 (59) | 3.9 (1.8‐8.3) | <0.001 | 3.7 (1.7‐8.2) | 0.001 | ||
| Type of LRTI | ||||||||
| Possible | 104 (90) | 27 (69) | 1.0 | 1.0 | 1.0 | |||
| Proven | 11 (10) | 12 (31) | 4.2 (1.7‐10.6) | 0.002 | 4.7 (1.7‐13.5) | 0.004 | 3.3 (1.2‐8.8) | 0.02 |
| Ribavirin therapy | ||||||||
| None | 15 (13) | 8 (20) | 1.0 | 1.0 | 1.0 | |||
| URTI stage | 13 (11) | 1 (3) | 0.1 (0.01‐1.1) | 0.09 | 0.1 (0.01‐1.3) | 0.08 | 0.2 (0.02, 1.5) | 0.10 |
| LRTI stage | 87 (76) | 30 (77) | 0.7 (0.3‐1.7) | 0.37 | 0.6 (0.2‐1.9) | 0.43 | 0.7 (0.2‐1.8) | 0.43 |
HCT, hematopoietic stem cell transplant; HM, hematologic malignancy; LRTI, lower respiratory tract infection; OR, odds ratio; RSV, respiratory syncytial virus; URTI, upper respiratory tract infection.
Model 1 included neutropenia, but not lymphopenia, as a candidate variable for the final multivariate model, while Model 2 included lymphopenia, but not neutropenia. In model 1, allo‐HCT, neutropenia, and proven LRTI were associated with higher 60‐day mortality. In model 2, lymphopenia and proven LRTI were associated with higher 60‐day mortality (in bold).
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Kaplan‐Meier survival curves are shown for patients with HM but no HCT, allo‐HCT recipients, and auto‐HCT recipients in Figure 1. There was no significant difference in survival between those groups (P = 0.63). Kaplan‐Meier curves demonstrated that patients with proven LRTI had lower survival rates than those with possible LRTI (P < 0.001, Figure 2). Sub‐group analyses revealed that there were significant differences between the survival curves of patient who did not undergo bronchoscopy, those with a negative BAL, those with only non‐RSV pathogens detected in BAL, those with RSV detected in BAL, and those with RSV and additional pathogens detected in BAL (P = 0.001, Figure 3). Patients who did not undergo bronchoscopy had similar survival to those with a negative BAL and those with non‐RSV pathogens detected on BAL. Patients with RSV and additional pathogens detected in BAL fluid had the lowest survival, though, likely due to the small sample size, there was no significant difference between these patients and those with RSV alone detected in BAL fluid (P = 0.10, Figure S1). Survival rates were not significantly different in patients who started ribavirin therapy in the URTI stage of infection, ribavirin therapy in the LRTI stage of infection, or no ribavirin therapy (P = 0.16, Figure 4).
Figure 1.
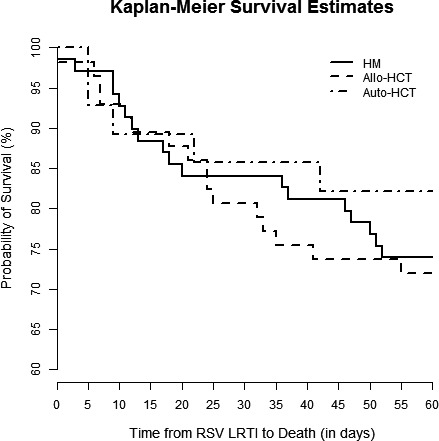
Kaplan‐Meier survival cures for patients with HM but no HCT (solid line), allo‐HCT recipients (dashed line), and auto‐HCT recipients (alternating dots and dashes). There was no difference among survival rates between these groups (log‐rank test, P = 0.63)
Figure 2.
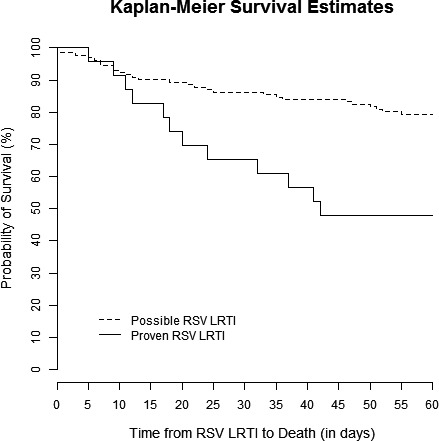
Kaplan‐Meier survival curves for patients with possible (dashed line) and proven (solid line) RSV LRTI. Patients with proven RSV LRTI had significantly lower survival rates (log‐rank test, P < 0.001)
Figure 3.
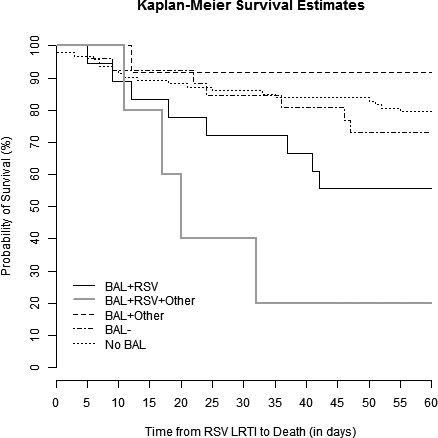
Kaplan‐Meier survival curves for patients who underwent bronchoscopy, stratified by microbiological results, and patients who did not undergo bronchoscopy (short dashed line). Patients who underwent bronchoscopy were further divided into BAL positive for RSV (solid black line), BAL positive for RSV and another respiratory pathogen (solid grey line), BAL positive for only a non‐RSV respiratory pathogen (long dashed line) and a BAL without growth (alternate short and long dashed lines). A log‐rank test showed significant differences in survival rates between groups (log‐rank test, P = 0.001)
Figure 4.
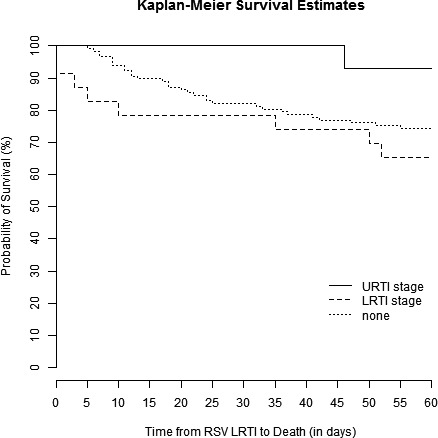
Kaplan‐Meier survival curves for patients who started ribavirin therapy at the URTI stage of infection (solid line), ribavirin therapy at the LRTI stage of infection (long dashed line), or no ribavirin therapy (short dashed line). There was no significant difference in survival rates between these groups (log‐rank test, P = 0.16)
3.4. Effect of immunodeficiency on mortality in HCT recipients with RSV LRTI
We calculated ISI grade and SID criteria for 85 HCT recipients and measured association with mortality. High‐risk individuals as identified by ISI had a higher risk for mortality than low‐risk individuals (OR 10.5, 95% CI 1.9‐56.6, P = 0.01, Table 5). Similarly, individuals with vSID had a higher risk for mortality than those with MID (OR 11.1, 95% CI 2.8‐45.1, P < 0.001). Both ISI grade and SID criteria predicted time to mortality, as demonstrated by Kaplan‐Meier survival curves (Figure 5). When analyzing ISI as a continuous variable, each 1‐point increase in ISI increased the odds of 60‐day mortality by 40% (OR 1.4, 95% CI 1.15‐1.8, P = 0.001). ISI predicted mortality in allo‐HCT recipients, but not in auto‐HCT recipients; however, this analysis of subgroups is limited by sample size (allo‐HCT: OR 1.7, 95% CI 1.2‐2.4, P = 0.001; auto‐HCT: OR 1.1, 95% CI 0.8‐1.6, P = 0.46). Agreement between ISI and SID criteria was modest (weight Cohen's kappa coefficient: 0.44, 95% CI 0.27‐0.61).
Table 5.
Impact of immunodeficiency on 60‐d mortality after RSV LRTI in HCT recipients
| Immunodeficiency Grade | Mortality (n = 85) | OR (95% CI) | P‐value |
|---|---|---|---|
| ISI | |||
| Low‐risk | 2/21 (10%) | 1.0 | – |
| Moderate‐risk | 8/43 (19%) | 2.2 (0.4‐11.3) | 0.36 |
| High‐risk | 10/21 (48%) | 10.5 (1.9‐56.6) | 0.01 |
| SID criteria | |||
| MID | 3/39 (8%) | 1.0 | – |
| SID | 5/19 (26%) | 4.3 (0.9‐20.4) | 0.07 |
| vSID | 14/27 (52%) | 11.1 (2.8‐45.1) | <0.001 |
ISI, Immunodeficiency Scoring Index; LRTI, lower respiratory tract infection; MID, moderate immunodeficiency; RSV, respiratory syncytial virus; SID, severe immunodeficiency; vSID, very severe immunodeficiency. Patients with high‐risk ISI or vSID were more likely to die at 60 days after LRTI (in bold).
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 5.
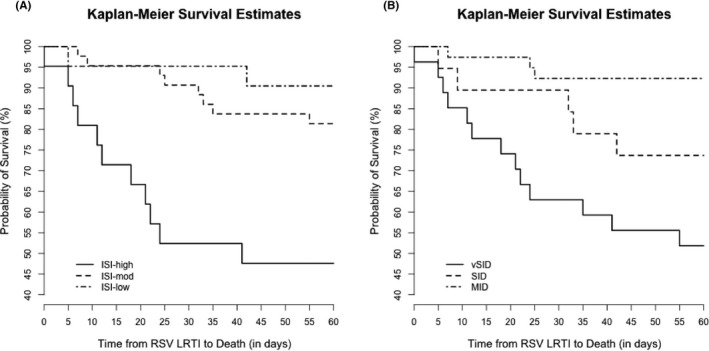
Kaplan‐Meier survival curves for HCT recipients with a) low, moderate, or high ISI and b) with MID, SID, or vSID. Both ISI and SID criteria predicted time to death (P < 0.001 for both scores)
4. DISCUSSION
We observed high mortality after RSV LRTI in HM patients, with or without HCT. However, there was no difference in 60‐day mortality between HM patients without HCT, allo‐HCT and auto‐HCT recipients. Lymphopenia and neutropenia were independent predictors of 60‐day mortality. Furthermore, detection of RSV from BAL fluid, defined as proven RSV LRTI was associated with higher all‐cause mortality at 30 and 60 days and a higher rate of ICU admission. High‐grade immunodeficiency, as defined by ISI grade and SID criteria, was associated with higher rates of 60‐day all‐cause mortality.
Consistent with other studies of viral LRTI, we found that mortality in HM patients, with or without HCT, was high. Our mortality rate of 25% across groups is similar to other studies of HM patients with RSV LRTI. In patients with acute myeloid leukemia at our institution, 5/27 (19%) patients with RSV LRTI died.9 In contemporary cohorts of HCT recipients with RSV LRTI, the overall mortality ranged from 11%‐30%.3, 5, 8, 10, 11 Allo‐HCT recipients often have high‐risk underlying malignancies and require immunosuppression as compared to auto‐HCT recipients.14 However, we found that mortality was high in all HM patients regardless of HCT status. This is similar to other groups who have found no difference in the risk for mortality between allo‐HCT and auto‐HCT recipients. Seo et al21found no difference in the risk for mortality between allo‐HCT and auto‐HCT recipients who developed LRTI with RSV, parainfluenza (PIV), influenza, or rhinovirus. Similarly, Renaud et al22 found that in a mixed cohort of patients with human metapneumovirus (hMPV) LRTI and RSV LRTI, overall mortality after RSV infection was high (35%), but there was no significant difference in mortality between allo‐HCT and auto‐HCT recipients, though the HR approached statistical significance (HR 5.2, 95% CI 0.7‐39, P = 0.1). We found higher mortality among allo‐HCT recipients in one multivariate model that included neutropenia in lieu of lymphopenia as a marker of immune impairment, but this result was not reproducible in the other model that included lymphopenia. Furthermore, we found no difference in survival as assessed by Kaplan‐Meier analyses or by logistic regression. While it is possible that allo‐HCT recipients are at a higher risk for death after adjusting for the presence of neutropenia, our study cannot definitively prove this.
Previous studies showed that neutropenia8, 13 and lymphopenia8, 12, 13 are associated with a higher risk of death in HCT recipients with RSV LRTI. In separate multivariate models, we found that lymphopenia and neutropenia were associated with 60‐day mortality. Lymphopenia is associated with increased progression from URTI to LRTI,13, 23 a well‐known marker of mortality.17, 24 Similarly, neutropenia is independently associated with progression to RSV LRTI.13 Conversely, normal lymphocyte counts are associated with low rates of progression to LRTI.23 Furthermore, we found that high‐grade immunodeficiency, as defined by ISI and SID criteria, was highly‐associated with mortality. Whether using ISI or SID criteria, high‐grade immunodeficiency was associated with a 60‐day all‐cause mortality around 50% after RSV LRTI, which is similar to prior studies. The initial development cohort for ISI consisted of 237 allo‐HCT recipients with RSV LRTI or URTI13 and mortality was high among those who did not receive ribavirin at the URTI stage and progressed to LRTI (63% at 90 days) and among those who presented with an LRTI and received ribavirin at diagnosis (50% at 90 days). Importantly, the ISI was developed in a cohort of allo‐HCT recipients and was designed to predict progression from RSV URTI to LRTI. As our current study shows, ISI is less useful in auto‐HCT recipients, particularly since certain criteria such as graft‐versus‐host disease may not be applicable. Similarly, a study of 45 patients with RSV LRTI found that high‐risk patients, as identified by ISI, had a RSV‐associated mortality of 50%.16 High ISI is also associated with high mortality after influenza infection.25 SID criteria were first studied in 34 patients with RSV infection, of whom 18% died.17 Risk factors for mortality included LRTI, pre‐engraftment status and vSID at RSV diagnosis. A recent study of SID criteria in 66 patients with RSV, PIV, and hMPV infection showed a mortality of 32% in patients with vSID, and no deaths in patients with MID or SID. Our findings are consistent with prior studies that show that immunocompromised patients are at high risk for mortality after RSV infection.8, 16, 17, 18
In agreement with other studies,8, 26 we did not find an association between corticosteroid use at the time of LRTI and mortality. Corticosteroid use was used as part of the Immunodeficiency Scoring Index, a tool to predict the risk of progression from RSV URTI to LRTI in allo‐HCT recipients, but was not independently associated with progression to RSV LRTI.13 However, in a mixed cohort of patients with hMPV and RSV LRTI, corticosteroid use and hypoxemia were associated with increased mortality.22 Similarly, corticosteroid use at the diagnosis of RSV infection is associated with a higher incidence of hypoxemia in a dose‐dependent fashion.11 Future prospective studies are necessary to investigate the timing and dosage of corticosteroid administration and its relationship with mortality after RSV LRTI.
High‐risk HM patients with RSV infection, particularly HCT recipients, should be considered as candidates for ribavirin therapy at the URTI stage, as early treatment may prevent LRTI.9 We did not find a significant difference in mortality in patients who started ribavirin therapy when it was given at the LRTI stage, but our study was not designed to investigate the efficacy of ribavirin. Furthermore, ribavirin given at the LRTI stage can still be beneficial in some instances, as demonstrated in few studies.8, 10, 27, 28 Despite the lack of statistical significance, there was a trend toward a protective effect of ribavirin for mortality in our cohort when given at the URTI stage in patients who eventually developed RSV LRTI. Future prospective studies are necessary to validate the optimal route (oral or aerosolized) and timing (URTI or LRTI) of ribavirin therapy.
Some groups define patients as having LRTI when RSV is detected in the upper respiratory tract and there are lower respiratory tract symptoms or new pulmonary infiltrates.5, 9, 17, 24, 29, 30 However, we found that detection of RSV in BAL is predictive of worse outcomes. This suggests that the subset of patients with RSV detected in BAL (ie, proven LRTI) should be distinguished from those without (ie, possible LRTI) as they may represent a distinct group with higher mortality where prompt and aggressive management may be desirable. Similar to our findings, Waghmare et al11 found that patients who had RSV detected in BAL fluid had higher mortality rates and a greater requirement for supplemental oxygen than those who did not. In addition, HCT recipients with PIV detected in BAL fluid had higher mortality than those with PIV detected in nasal wash alone (47% vs 3%).15 High mortality rates have also been demonstrated in immunocompromised patients with HM, with or without HCT, and detection of human metapneumovirus (43% in HCT recipients at 100 days),22 human rhinovirus (41% at 90 days in HCT recipients),21 and human coronavirus (35% at 90 days in HCT recipients and 14% at 90 days in HM patients without HCT)31 in BAL fluid.
There are several limitations to our findings. This is a retrospective cohort analysis, and our findings require further confirmation in prospective studies. Not all patients were referred for bronchoscopy, so those with possible RSV LRTI may have been less sick, resulting in a referral bias. However, if patients were not referred because they were too sick to undergo bronchoscopy, this would bias our results towards the null hypothesis. We used viral culture and direct immunofluorescent antibody testing to detect RSV and other respiratory viral pathogens as this was the institutional practice at the time. Caution is necessary when extrapolating our findings to patients in whom respiratory viruses are detected using polymerase chain reaction assays. Though patients with no RSV isolation from BAL fluid were considered to have possible RSV LRTI, our Kaplan‐Meier survival analyses suggest that there is no meaningful difference in survival between those patients and those who did not have BAL, which supports the consolidation of those two groups of patients. It is also possible that we included patients with RSV infection and volume overload or other non‐infectious etiologies for pulmonary infiltrates. Misclassification of such patients would likely bias our results towards the null hypothesis if patients with volume overload were classified as having possible LRTI. Associations of ISI with mortality in auto‐HCT recipients should be interpreted with caution, since ISI was not developed to quantify immunodeficiency in auto‐HCT recipients. Because of the small size of the cohort and the small number of deaths (n = 39), we could not measure the interaction of proven RSV LRTI with immunodeficiency scoring, and our findings need to be replicated in a larger, prospective cohort.
In conclusion, we found that mortality was high in HM patients, with or without HCT, who developed RSV LRTI. Neutropenia and lymphopenia were predictive of 60‐day mortality. In addition, high‐risk ISI or vSID at RSV diagnosis were associated with higher 60‐day mortality. Furthermore, detection of RSV in BAL fluid was associated with higher 60‐day all‐cause mortality than in those without RSV detected in BAL. Routine calculation of immunodeficiency scores may improve risk stratification in HCT recipients. Data from BAL may complement these immunodeficiency scores13, 16, 17, 18, 25 and be useful for identifying patients with RSV infection or other respiratory viruses who are at the greatest risk for death. Such patients would be ideal candidates for early ribavirin therapy or for clinical trials for new investigational drugs.
CONFLICT OF INTEREST
RFC receives research grants from Gilead and Consulting/Advisory Boards honoraria from Ablynx, Janssen, and ADMA Biologics. All other authors report no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
EV, AS, SAF, DPS, YZ, LL, LB, EAH and RFC contributed to the research design. EV, AS, and RFC contributed to the writing of the paper. EV, AS, SAF, JK, JA, AB, and RFC contributed to the conduct of the study. EV, AS, DPS, YZ, LL, and RFC contributed to data analysis.
Supporting information
ACKNOWLEDGEMENTS
The authors would like to thank Ann Sutton in the Department of Scientific Publications at the University of Texas MD Anderson Cancer Center for her assistance in editing this manuscript.
Vakil E, Sheshadri A, Faiz SA, et al. Risk factors for mortality after respiratory syncytial virus lower respiratory tract infection in adults with hematologic malignancies. Transpl Infect Dis. 2018;20:e12994 10.1111/tid.12994
Funding information
This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (K23 AI117024 to A.S.), the American Cancer Society (Mentored Research Scholar Grants in Applied and Clinical Research, MRSG‐16‐152‐01‐CCE to D.P.S.), and the National Cancer Institute at the National Institutes of Health (Cancer Center Support Grant, P30 CA016672, Biostatistics Resource Group).
Vakil and Sheshadri contributed equally to the manuscript and should both be considered first authors.
References
- 1. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high‐risk adults. N Engl J Med. 2005;352:1749‐1759. [DOI] [PubMed] [Google Scholar]
- 2. Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood. 2011;117:2755‐2763. [DOI] [PubMed] [Google Scholar]
- 3. Chemaly RF, Shah DP, Boeckh MJ. Management of respiratory viral infections in hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 2014;59(Suppl 5):S344‐S351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bowden RA. Respiratory virus infections after marrow transplant: the Fred Hutchinson Cancer Research Center experience. Am J Med. 1997;102:27‐30. [DOI] [PubMed] [Google Scholar]
- 5. Ljungman P, Ward KN, Crooks BN, et al. Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2001;28:479‐484. [DOI] [PubMed] [Google Scholar]
- 6. Ebbert JO, Limper AH. Respiratory syncytial virus pneumonitis in immunocompromised adults: clinical features and outcome. Respiration. 2005;72:263‐269. [DOI] [PubMed] [Google Scholar]
- 7. Chemaly RF, Aitken SL, Wolfe CR, Jain R, Boeckh MJ. Aerosolized ribavirin: the most expensive drug for pneumonia. Transpl Infect Dis. 2016;18:634‐636. [DOI] [PubMed] [Google Scholar]
- 8. Shah DP, Ghantoji SS, Shah JN, et al. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother. 2013;68:1872‐1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Torres HA, Aguilera EA, Mattiuzzi GN, et al. Characteristics and outcome of respiratory syncytial virus infection in patients with leukemia. Haematologica. 2007;92:1216‐1223. [DOI] [PubMed] [Google Scholar]
- 10. Waghmare A, Campbell AP, Xie H, et al. Respiratory syncytial virus lower respiratory disease in hematopoietic cell transplant recipients: viral RNA detection in blood, antiviral treatment, and clinical outcomes. Clin Infect Dis. 2013;57:1731‐1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Waghmare A, Xie H, Kimball L, et al. Supplemental oxygen‐free days in hematopoietic cell transplant recipients with respiratory syncytial virus. J Infect Dis. 2017;216:1235‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chemaly RF, Ghosh S, Bodey GP, et al. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore). 2006;85:278‐287. [DOI] [PubMed] [Google Scholar]
- 13. Shah DP, Ghantoji SS, Ariza‐Heredia EJ, et al. Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood. 2014;123:3263‐3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sureda A, Bader P, Cesaro S, et al. Indications for allo‐ and auto‐SCT for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2015. Bone Marrow Transplant. 2015;50:1037‐1056. [DOI] [PubMed] [Google Scholar]
- 15. Seo S, Xie H, Campbell AP, et al. Parainfluenza virus lower respiratory tract disease after hematopoietic cell transplant: viral detection in the lung predicts outcome. Clin Infect Dis. 2014;58:1357‐1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Damlaj M, Bartoo G, Cartin‐Ceba R, et al. Corticosteroid use as adjunct therapy for respiratory syncytial virus infection in adult allogeneic stem cell transplant recipients. Transpl Infect Dis. 2016;18:216‐226. [DOI] [PubMed] [Google Scholar]
- 17. Khanna N, Widmer AF, Decker M, et al. Respiratory syncytial virus infection in patients with hematological diseases: single‐center study and review of the literature. Clin Infect Dis. 2008;46:402‐412. [DOI] [PubMed] [Google Scholar]
- 18. Spahr Y, Tschudin‐Sutter S, Baettig V, et al. Community‐acquired respiratory paramyxovirus infection after allogeneic hematopoietic cell transplantation: a single‐center experience. Open Forum Infect Dis. 2018;5:ofy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shafik CF, Mohareb EW, Youssef FG. Comparison of direct fluorescence assay and real‐time rt‐PCR as diagnostics for respiratory syncytial virus in young children. J Trop Med. 2011;2011:781919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sampsonas F, Kontoyiannis DP, Dickey BF, Evans SE. Performance of a standardized bronchoalveolar lavage protocol in a comprehensive cancer center: a prospective 2‐year study. Cancer. 2011;117:3424‐3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seo S, Waghmare A, Scott EM, et al. Human rhinovirus detection in the lower respiratory tract of hematopoietic cell transplant recipients: association with mortality. Haematologica. 2017;102:1120‐1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Renaud C, Xie H, Seo S, et al. Mortality rates of human metapneumovirus and respiratory syncytial virus lower respiratory tract infections in hematopoietic cell transplantation recipients. Biol Blood Marrow Transplant. 2013;19:1220‐1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim YJ, Guthrie KA, Waghmare A, et al. Respiratory syncytial virus in hematopoietic cell transplant recipients: factors determining progression to lower respiratory tract disease. J Infect Dis. 2014;209:1195‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Avetisyan G, Mattsson J, Sparrelid E, Ljungman P. Respiratory syncytial virus infection in recipients of allogeneic stem‐cell transplantation: a retrospective study of the incidence, clinical features, and outcome. Transplantation. 2009;88:1222‐1226. [DOI] [PubMed] [Google Scholar]
- 25. Kmeid J, Vanichanan J, Shah DP, et al. Outcomes of influenza infections in hematopoietic cell transplant recipients: application of an immunodeficiency scoring index. Biol Blood Marrow Transplant. 2016;22:542‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seo S, Campbell AP, Xie H, et al. Outcome of respiratory syncytial virus lower respiratory tract disease in hematopoietic cell transplant recipients receiving aerosolized ribavirin: significance of stem cell source and oxygen requirement. Biol Blood Marrow Transplant. 2013;19:589‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghosh S, Champlin RE, Englund J, et al. Respiratory syncytial virus upper respiratory tract illnesses in adult blood and marrow transplant recipients: combination therapy with aerosolized ribavirin and intravenous immunoglobulin. Bone Marrow Transplant. 2000;25:751‐755. [DOI] [PubMed] [Google Scholar]
- 28. Waghmare A, Englund JA, Boeckh M. How I treat respiratory viral infections in the setting of intensive chemotherapy or hematopoietic cell transplantation. Blood. 2016;127:2682‐2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. von Lilienfeld‐Toal M, Berger A, Christopeit M, et al. Community acquired respiratory virus infections in cancer patients‐Guideline on diagnosis and management by the Infectious Diseases Working Party of the German Society for haematology and Medical Oncology. Eur J Cancer. 2016;67:200‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ghosh S, Champlin R, Couch R, et al. Rhinovirus infections in myelosuppressed adult blood and marrow transplant recipients. Clin Infect Dis. 1999;29:528‐532. [DOI] [PubMed] [Google Scholar]
- 31. Ogimi C, Waghmare AA, Kuypers JM, et al. Clinical significance of human coronavirus in bronchoalveolar lavage samples from hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 2017;64:1532‐1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


