Abstract
Background and Purpose
Glucagon‐like peptide‐1 (GLP‐1) is an incretin hormone that regulates insulin biosynthesis and secretion in a glucose‐dependent manner and has been reported to induce vasodilatation. Here, we examined the possible vasorelaxant effect of GLP‐1 and its underlying mechanisms.
Experimental Approach
Rat mesenteric arteries (diameter ≈ 200–400 μm) and human s.c. arteries were mounted in microvascular myographs for isometric tension recordings. The effect of GLP‐1 on vascular responses was examined under normoglycaemic conditions and at high glucose concentrations.
Key Results
In rat mesenteric arteries and human s.c. arteries without branches, physiological concentrations (1–100 nM) of GLP‐1(7‐36) and liraglutide failed to cause relaxation or affect contractions evoked by electrical field stimulation. In contrast to GLP‐1(7‐36), liraglutide induced relaxations antagonized by the GLP‐1 receptor antagonist, exendin‐(9‐39), in branched mesenteric arteries. In contrast to liraglutide, GLP‐1 leftward shifted the concentration relaxation curves for bradykinin in s.c. arteries from patients with peripheral arterial disease, an effect resistant to exendin‐(9‐39). Under normoglycaemic conditions, neither GLP‐1 nor liraglutide affected ACh relaxation in rat mesenteric arteries. In arteries exposed to 40 mM glucose, GLP‐1, in contrast to liraglutide, potentiated ACh‐induced relaxation by a mechanism that was not antagonized by exendin‐(9‐39). GLP‐1 decreased superoxide levels measured with dihydroethidium in rat mesenteric arteries exposed to 40 mM glucose.
Conclusions and Implications
GLP‐1 receptors are involved in the liraglutide‐induced relaxation of branched arteries, under normoglycaemic conditions, while GLP‐1 inhibition of vascular superoxide levels contributes to GLP‐1 receptor‐independent potentiation of endothelium‐dependent vasodilatation in hyperglycaemia.
Abbreviations
- AUC
area under curve
- DPP‐4
dipeptidyl peptidase 4
- EFS
electrical field stimulation
- GLP‐1
glucagon‐like peptide‐1
- GLP‐1R
glucagon‐like peptide‐1 receptor
- PEG‐SOD
polyethylene glycol SOD
- PSS
physiological salt solution
What is Already Known
GLP‐1 is an incretin hormone reported to induce vasodilatation and lower BP.
What this Study Adds
GLP‐1 and the GLP‐1 analogue induce vasodilatation by GLP‐1 receptor‐independent and GLP‐1 receptor‐dependent mechanisms.
What is the Clinical Significance
Different GLP‐1 analogue effects may help in optimizing prevention of cardiovascular complications in Type 2 diabetes.
Introduction
Glucagon‐like peptide‐1 (GLP‐1) is a gut‐derived incretin hormone secreted in response to nutrient ingestion. GLP‐1 has a blood glucose‐lowering effect by stimulating an increase in insulin biosynthesis and secretion and by inhibiting glucagon secretion in a glucose‐dependent manner. Moreover, GLP‐1 inhibits gastric emptying, augments satiety and promotes weight loss (Holst, 2004; Davies et al., 2015). Growing evidence suggests that it may also play a role in the regulation of cardiovascular function (Drucker, 2016). The GLP‐1 analogue liraglutide is approved for the treatment of Type 2 diabetes mellitus (T2D) and obesity (Ussher and Drucker, 2014; EMA, 2017) and decreased BP and the risk of cardiovascular events (Fonseca et al., 2014; Gejl et al., 2015; Marso et al., 2016; Starup‐Linde et al., 2014). GLP‐1 and liraglutide bind to the GLP‐1 receptor, a GPCR (Ussher and Drucker, 2014; Alexander et al., 2017a). In the cardiovascular system, the GLP‐1 receptor is expressed in smooth muscle cells, the sinoatrial node (Pyke et al., 2014), the myocardium and microvascular endothelium (Ban et al., 2008; Bhashyam et al., 2010). However, some papers have questioned data obtained using standard antisera to detect GLP‐1 receptors (Pyke and Knudsen, 2013; Panjwani et al., 2012).
Agonists of GLP‐1 receptors induce vasodilatation, increase blood flow and improve vascular endothelial function (Drucker, 2016; Gejl et al., 2012), but the results of studies in isolated arteries are conflicting. Generally, it has been assumed that the vasodilatory actions of GLP‐1 are dependent on GLP‐1 receptors, but some studies proposed vascular effects independent of these receptors (Ban et al., 2008). It has also been questioned whether the cleaved metabolite, GLP‐1(9‐36), of native GLP‐1(7‐36) plays a role (Bayram et al., 2014; Ban et al., 2008). Several pathways have been suggested to mediate the vascular effects of GLP‐1 receptor agonists. These include direct effect on the smooth muscle cells, endothelium‐dependent release of NO (Nystrom et al., 2005) and pathways involving an increase in cGMP or cAMP as well as protection against endothelial dysfunction (Ban et al., 2008; Green et al., 2008; Bayram et al., 2014; Koska et al., 2015; Salheen et al., 2015). However, an indirect vasoconstricting effect of GLP‐1 causing inhibition of relaxations induced by VEGF has also been described in mesenteric arteries and was associated with a decrease in endothelial cell calcium (Egholm et al., 2016). Therefore, the effect on vascular tone of GLP‐1 is unclear, and a possible effect of GLP‐1 on the peripheral autonomic nervous system has not been examined despite the fact that several other gut‐derived peptides regulate the release of noradrenaline in the vasculature (Gradin et al., 2006). Moreover, hyperglycaemia generates ROS which have been proposed to impair endothelium‐dependent relaxation, and growing evidence reveals that GLP‐1 receptor stimulation may reduce ROS (Salheen et al., 2015; Steven et al., 2017) and hence improve endothelial function (Salheen et al., 2015).
In the present study, we aimed to examine the pre‐junctional and post‐junctional vasorelaxant effects of native GLP‐1(7‐36), liraglutide and GLP‐1(9‐36) in rat and human resistance vessels, in order to elucidate the signalling pathways involved in GLP‐1‐mediated regulation of vascular tone. We hypothesized that stimulation of GLP‐1 receptors regulates vascular tone by direct effects on signalling pathways determining the contractile level of the smooth muscle cells, by an endothelium‐dependent pathway or by inhibition of noradrenaline release from nerves in the vascular wall during conditions with increased sympathetic nerve activity. Moreover, we aimed to investigate whether GLP‐1, by inhibition of superoxide production, may improve ACh relaxations in rat mesenteric arteries exposed to hyperglycaemic conditions.
Methods
Tissue preparations
All animal care and experimental procedures were conducted in accordance with the Danish legislation of animal use for scientific procedures and approved by the Danish Experimental Animal Inspectorate (permission 2014‐15‐2934‐01059), according to the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). Adult male Wistar rats (10–12 weeks old) of the Hannover strain (RGD Cat# 1566433, RRID:RGD_1566433) and weighing 300–350 g were obtained from either Taconic, Ry, Denmark, or Janvier Laboratories, France. The rats were housed in cages (Techniplast, Buguggiate, Italy, 800 cm2) filled with aspen woodchips (Tapvei, Datesand, Manchester, UK) and nesting material (Soft Paper Wool/LBS, UK) as bedding and under constant climatic conditions (20°C, 60% humidity, 12:12 h light–dark cycle). The animals had access to food (Altromin 1324, Brogaarden ApS, Denmark) and water ad libitum.
The animals were selected randomly, and, wherever possible, observations were made without knowledge of the treatments administered. The rats were killed by a blow to the head followed by exsanguination. The mesenteric bed was removed, and it was immediately placed in cold physiological salt solution (PSS) (4°C) of the following composition (mM): NaCl 119, KCl 4.7, KH2PO4 1.18, MgSO4 1.17, NaHCO3 25, CaCl2 1.6, EDTA 0.026 and glucose 5.5. The solution was gassed with 5% CO2 in air to maintain the pH at 7.4. Third‐order mesenteric arteries (internal diameter ≈ 200–300 μm) were dissected without branches (straight segments) or with at least one major branch (branched vessels).
The study using human arterial tissue was approved by the Regional Ethics Committee, Central Denmark (permission number: 1‐10‐72‐208‐15) and conducted in accordance with the principles of the Helsinki Declaration II for medical research. All participants gave informed consent prior to participation. To be included in the study, the patients had to be (i) adult competent persons, (ii) Caucasians and (iii) diagnosed with peripheral arterial disease or ischaemic heart disease. Both diabetic and non‐diabetic patients were included. The patients were excluded if they were currently treated with GLP‐1 analogues. Human arteries were obtained from the Department of Vascular Surgery, Aalborg University Hospital. Subcutaneous tissue samples were obtained from 23 patients undergoing vascular surgery, and they were placed in cold PSS immediately after extraction. Subcutaneous arteries with internal diameters ≈ 200–400 μm were dissected.
Isometric tension recordings
Arterial segments (approximately 2 mm long) were mounted on two wires (40 μm in diameter) in microvascular myographs (Danish Myotechnology, Aarhus, Denmark) for isometric tension recordings. The vessels were equilibrated in oxygenated (5% CO2 in air) PSS (pH 7.4) heated to 37°C. To determine the lumen diameter that the artery would have had in vivo when relaxed and under a transmural pressure of 100 mmHg, the vessels were stretched following a standardized procedure. Arteries were set to 90% of this diameter, which is the optimal vessel circumference for maximal force development (Mulvany and Halpern, 1977). To test tissue viability, arterial segments were subjected to an isotonic PSS containing a high concentration of potassium (123 mM) or noradrenaline (1–10 μM) resulting in maximal contraction of the vessels. To test endothelial function, the vessels were contracted with phenylephrine or noradrenaline and then stimulated with ACh (10 μM) in rat arteries and with bradykinin (30 nM) in human arteries. Only rat mesenteric arteries relaxing more than 75% to ACh and human s.c. arteries relaxing more than 50% to bradykinin were included in the study.
The possible direct effect of GLP‐1 and analogues in rat and human resistance arteries was examined by adding increasing cumulative concentrations of GLP‐1(7‐36), liraglutide or GLP‐1(9‐36) (0.1 pM–100 nM) to vessels contracted with phenylephrine (5–10 μM). To address the potential development of tachyphylaxis, a single physiological concentration of GLP‐1(7‐36) (1 nM) was added in a series of experiments. To investigate whether GLP‐1 was degraded, preparations were incubated with a dipeptidyl peptidase 4 (DPP‐4) inhibitor, linagliptin (10−6 M), before adding GLP‐1. All treated vessels were compared with control vessels contracted with phenylephrine alone and assessed at the same time.
To consider whether the vasoregulatory effect of GLP‐1 is endothelium dependent, we measured the effects of incubation with therapeutic concentrations (10 nM) of GLP‐1(7‐36), liraglutide and GLP‐1(9‐36) for 20 min before adding increasing cumulative concentrations of ACh (0.1 nM–10 μM) to rat mesenteric arteries contracted with phenylephrine (10 μM). The experiments were performed at a low glucose concentration of 5.5 mM and at a very high glucose concentration, as a model of hyperglycaemia. In the hyperglycaemic condition, the vessels were incubated for at least 2 h with 40 mM of glucose before running the protocol, as described by Salheen et al. (2015). The very high concentration of glucose was based on findings in previous studies, demonstrating that incubation with a similar high glucose concentration (44 mM) for a short time (4–6 h) impaired endothelium‐dependent relaxation in isolated vessels (Tesfamariam et al., 1991; Qian et al., 2006). The responses were examined during co‐incubation with the GLP‐1 receptor antagonist exendin‐(9‐39) (100 nM). Similar experiments were performed in human s.c. arteries. Concentration–response curves for another endothelium‐dependent relaxant, bradykinin (0.1 pM–100 nM), was obtained in human arteries contracted with phenylephrine (5 μM) and preincubated with therapeutic concentrations (10 nM) of GLP‐1(7‐36) and liraglutide. Furthermore, human arteries were incubated with a combination of GLP‐1(7‐36) and exendin‐(9‐39) (100 nM) to examine whether the effect of GLP‐1 is mediated through GLP‐1 receptors. In all the experiments, equivalent solvent concentrations (vehicle) were added to a control vessel assayed in parallel, as a time control.
To investigate the role of superoxide in the inhibition of ACh relaxations in high glucose, mesenteric arteries exposed to 40 mM for 2 h of glucose were incubated with vehicle or the SOD scavenger, tempol (10−4 M), and a concentration–response curve was constructed for ACh. To clarify whether the GLP‐1‐receptor‐independent effect of GLP‐1 on ACh relaxation in high glucose is due to an antioxidant effect of GLP‐1, rat mesenteric arteries were incubated with indomethacin (10−6 M) and the superoxide generator pyrogallol (30 μM) for 30 min, phenylephrine was added and the preparations were incubated with vehicle, GLP‐1 (10−8 M), liraglutide (10−8 M), tempol (10−4 M) or linagliptin (10−6 M). Finally, concentration–response curves were constructed for ACh.
Detection of vascular superoxide anions
The levels of vascular superoxide anions were measured using the oxidative fluorescent dye dihydroethidium (Cat# D1137, Invitrogen, Thermo Fisher Scientific, Denmark) (Symons et al., 2006; Christensen et al., 2007). Freshly dissected mesenteric arteries were placed in PSS (see composition above) in Eppendorf vials at 37°C. They were kept in normoglycaemic conditions (5.5 mM glucose) or in high glucose (40 mM) for 2.5 h, and those with high glucose during the last 30 min were incubated with vehicle, polyethylene glycol superoxide dismutase (PEG‐SOD, 900 U·mL−1), GLP‐1 (10−8 M), liraglutide (10−8 M) or linagliptin (10−6 M). All segments were incubated with dihydroethidium (4 μM) or with PBS (vehicle/time control) at 37°C in light‐protected vials, rinsed once with 400 μL to PBS to remove non‐oxidized dihydroethidium and mounted on slides with the mounting medium Vectashield (Vector Laboratories, Inc., Burlingame, CA, USA). O2 − production was estimated using fluorescence microscopy (Leica, Mannheim, Germany) with an ethidium bromide excitation, 488 nm; ethidium bromide emission, 580 to 630 nm band‐pass filter. Fluorescence measurements were performed, and the vascular area measured was calculated on the basis of white light pictures. Identical settings were used for all vascular segments and pictures. Relative fluorescence intensity was measured using the image analysis software (Fiji, RRID:SCR_002285) (Schindelin et al., 2012). The data are reported as PEG‐SOD sensitive fluorescence.
Electrical field stimulation
Electrical field stimulation (EFS) was performed as previously described (Simonsen et al., 2008). Platinum electrodes (2 × 2 mm) secured in plastic mounting heads were placed on either side of the vessel approximately 1 mm from the vessel wall. The electrodes were connected to an electrical stimulator with a constant current output (CS200; Danish Myotechnology). EFS (16 Hz, 0.3 ms pulses, 10 s trains, 40 mA) was applied every 5 min. Increasing cumulative concentrations of GLP‐1(7‐36) and liraglutide (0.1–300 nM) were added 3 min before each stimulation. The contractile responses were expressed relative to an average of two initial control stimulations and compared with a parallel running time control. To obtain reproducible contractions, propranolol (1 μM), a β‐adrenoceptor antagonist, and cocaine (1 μM), an inhibitor of neuronal noradrenaline reuptake, were added. The vessels were also stimulated in the presence of tetrodotoxin, a blocker of the voltage‐gated sodium channels in nerve cell membranes.
Data calculations and statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). In view of previous experience, each group had to contain preparations from a minimum of 5–6 different subjects. Relaxation and contractile responses in the arteries were measured as the force ΔF (mN) developed by the artery and illustrated the changes in active wall tension. Relaxation in contracted arteries was presented as percentage of the contraction level just before adding the relaxing agents. Differences in concentration–response relationships between treatments were analysed using two‐way ANOVA followed by Bonferroni corrections. The fluorescence results for vascular superoxide were compared using one‐way ANOVA followed by Tukey's multiple comparisons test. Comparison of −logEC50 values was analysed with a t‐test. Results were considered statistically significant at P < 0.05. GraphPad Prism 7 (GraphPad Prism, RRID:SCR_002798, Inc., San Diego, CA, USA) was used for the statistical analysis.
Materials
ACh, bradykinin, linagliptin, noradrenaline hydrochloride, phenylephrine, propranolol, cocaine, tempol and GLP‐1 amide fragment 7‐36 (human) were all purchased from Sigma‐Aldrich (St Louis, MO, USA). GLP‐1(7‐36) amide (human and rat), GLP‐1(9‐36) amide and exendin‐3(9‐39) amide were from Tocris Bioscience (Bristol, UK). Tetrodotoxin from BioNordika (Herlev, Denmark). All chemicals were dissolved in distilled water except for GLP‐1(7‐36) human (1% acetic acid). Victoza injection liquid (liraglutide) from Novo Nordisk (Copenhagen, Denmark) was diluted in bubbled PSS, without Ca++, at 37°C.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a, 2017b).
Results
Direct effect of GLP‐1 receptor agonists on smooth muscle cell relaxation
In straight rat and human resistance arteries contracted with phenylephrine, native GLP‐1 failed to cause relaxation. When adding a single physiological concentration of GLP‐1 (1 nM) to the contracted vessels, the observed response was similar to the loss of tone in the parallel running time control (Figure 1A,B and Supporting Information Figure S1). Concentration–response curves in both rat mesenteric arteries and human s.c. arteries showed no or only a very modest effect, as the observed GLP‐1‐mediated relaxation was close to 0% (Figure 1C,D). Incubation with a DPP‐4 inhibitor, linagliptin (10−6 M), did not cause relaxation or increase the response to GLP‐1 (Supporting Information Figure S1).
Figure 1.
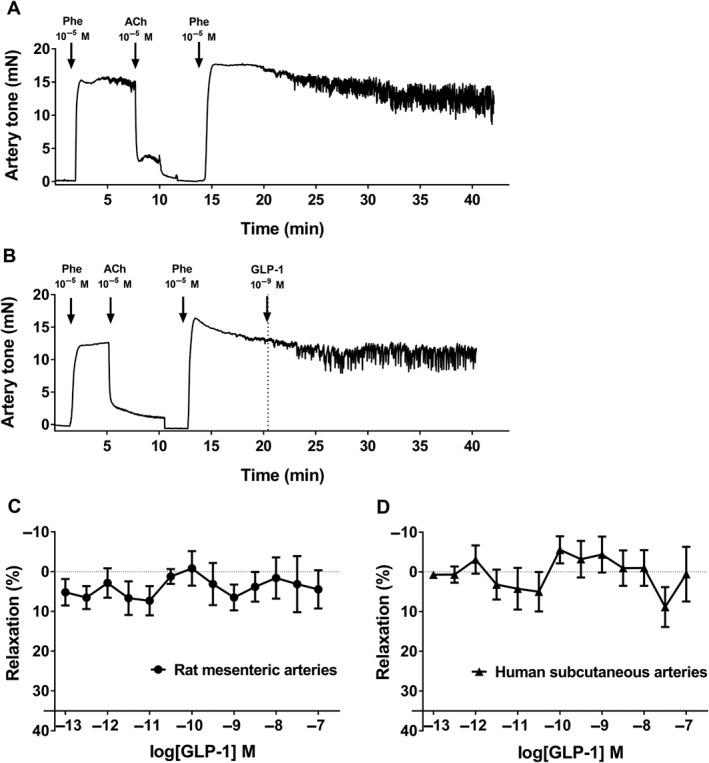
Lack of vasodilatation to native GLP‐1 in rat mesenteric and human s.c. arteries without branches. (A, B) Representative original traces from the myograph experiments in rat mesenteric arterial segments. Both vessels relax to a sufficient level when adding ACh. (A) Time control contracted with phenylephrine (Phe). (B) Vessel contracted with phenylephrine and exposed to a single physiological concentration of GLP‐1. Concentration–response curves for GLP‐1 in (C) rat mesenteric arteries (n = 7) and (D) human s.c. arteries (n = 9). The results are means ± SEM.
Likewise, no vasorelaxant effect was found for native GLP‐1 or GLP‐1(9‐36) in branched mesenteric arteries (Figure 2A,B). Whereas liraglutide induced significant relaxation compared with vehicle, to approximately 40% of maximal relaxation, as demonstrated on the concentration–response curve in branched mesenteric arteries (Figure 2C). This effect was antagonized by adding of the GLP‐1 receptor antagonist exendin(9‐39) (Figure 2D).
Figure 2.
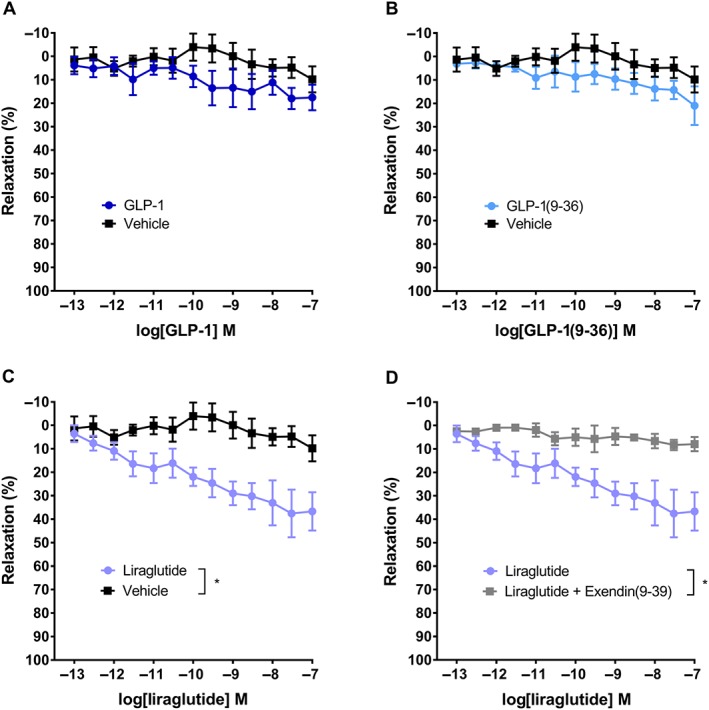
Vasodilatation induced by liraglutide in rat mesenteric arteries with branches. Concentration–response curves for (A) native GLP‐1 (n = 6) and vehicle (n = 6), (B) GLP‐1(9‐36) (n = 6) and vehicle (n = 6), (C) liraglutide (n = 6) and vehicle (n = 6) and (D) liraglutide (n = 6) and liraglutide plus exendin(9‐39) (n = 6). The results are means ± SEM. *P < 0.05, significantly different as indicated; two‐way ANOVA.
Effect of GLP‐1 receptor agonists on the EFS‐evoked contractions
To examine the potential influence of GLP‐1 on the sympathetic nerves in the vascular wall, the vessels were contracted by EFS. Representative traces from the EFS experiments illustrate the lack of inhibitory effect of GLP‐1 and liraglutide on the responses to EFS (Figure 3A and Supporting Information Figure S2), indicating that GLP‐1 and liraglutide have no effect on the neurotransmitter release from the nerves in the vascular wall. GLP‐1 and liraglutide did not have any significant effect on either the amplitude of the contractions or the area under curve (AUC, Figure 3B,C). Tetrodotoxin, a blocker of the voltage‐gated sodium channels in nerve cell membranes, inhibited the EFS‐evoked contractions. This ensured that the contractions examined here were due to stimulation of the nerves and not to a direct effect on the smooth muscle cells.
Figure 3.
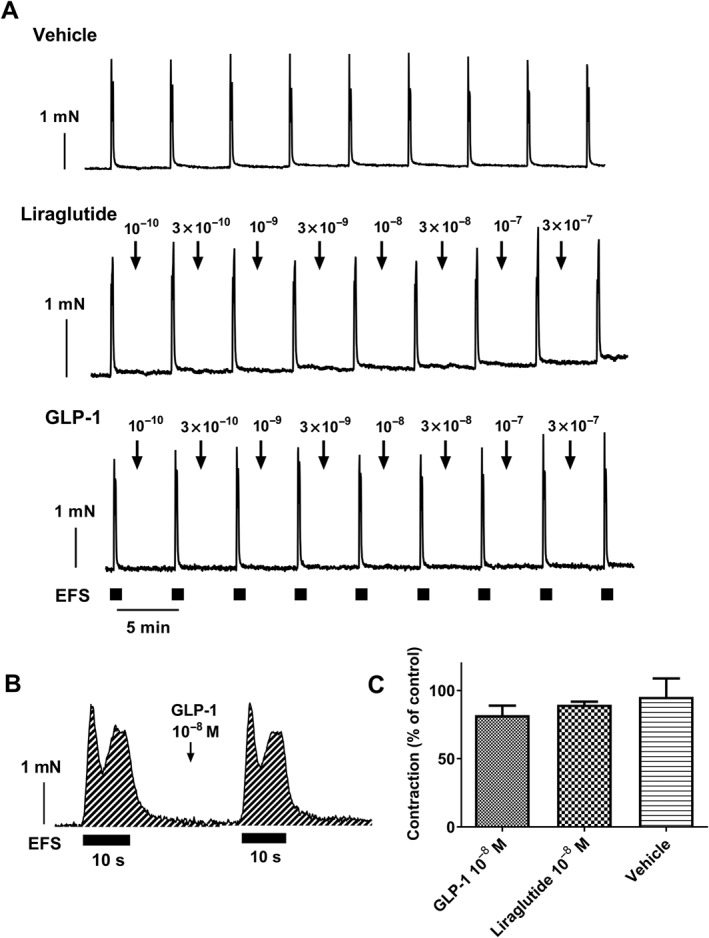
Lack of effect of native GLP‐1 and liraglutide on neurogenic contractions in rat mesenteric arteries. (A) Representative original traces showing contractions induced by EFS in the absence and in the presence of increasing concentrations (M) of native GLP‐1 and liraglutide. Squares mark stimulation intervals (10 s). The traces are each representative of 5–6 different experiments. (B) Enlargement of the responses showing area under the curve (AUC) at one control stimulation and after adding native GLP‐1 (10−8 M) and (C) average AUC for the contractions in experiments where vehicle (n = 6), GLP‐1 (n = 6) or liraglutide (n = 5) was added. AUC of the contractions is presented as percentage of an initial control stimulation. Data are presented as means ± SEM. Unpaired t‐test showed no significant difference between treatments.
Effect of GLP‐1 and liraglutide on endothelium‐dependent relaxation in human subcutaneous arteries
In human s.c. vessels from patients with peripheral arterial disease (Table 1), GLP‐1 (10−8 M) leftward‐shifted concentration relaxation curves for the endothelium‐dependent vasodilator, bradykinin (Figure 4A). Incubation with liraglutide (10−8 M) failed to change the concentration–response curves for bradykinin in human s.c. arteries (Figure 4B). Vessels were obtained from patients diagnosed without diabetes (non‐diabetics; n = 6) and with diabetes (diabetic patients; n = 4). GLP‐1 also leftward‐shifted the concentration–response curves for bradykinin in s.c. vessels from non‐diabetic patients (n = 6), while the effect was not apparent in s.c. arteries from patients with diabetes (n = 4; Supporting Information Figure S3). In a pooled sample of arteries from patients with and without diabetes, GLP‐1 still leftward‐shifted the concentration–response curves for bradykinin in the presence of the GLP‐1 receptor antagonist exendin(9‐39) (n = 5, Figure 4C).
Table 1.
Clinical characteristics of included patients
| Variables | Study population (N = 23) |
|---|---|
| Age (years) | 72.5 ± (8.8) |
| Male gender | 14 (61%) |
| Blood glucose (mM) | 7.1 ± (3.3) |
| Systolic BP (mmHg) | 145.7 ± (16) |
| Diastolic BP (mmHg) | 77.3 ± (13.6) |
| Cholesterol (mM) | 4.3 ± (1.1) |
| Smokers | 12 (52%) |
| Previous smokers | 23 (100%) |
| T2D | 9 (39%) |
| PAD | 15 (65%) |
| Hypertension | 13 (57%) |
| Lipid‐lowering therapy | 21 (91%) |
| DPP‐4 inhibitor treatment | 0 (0%) |
Data are means±SD or n (%). PAD, peripheral artery disease.
Figure 4.
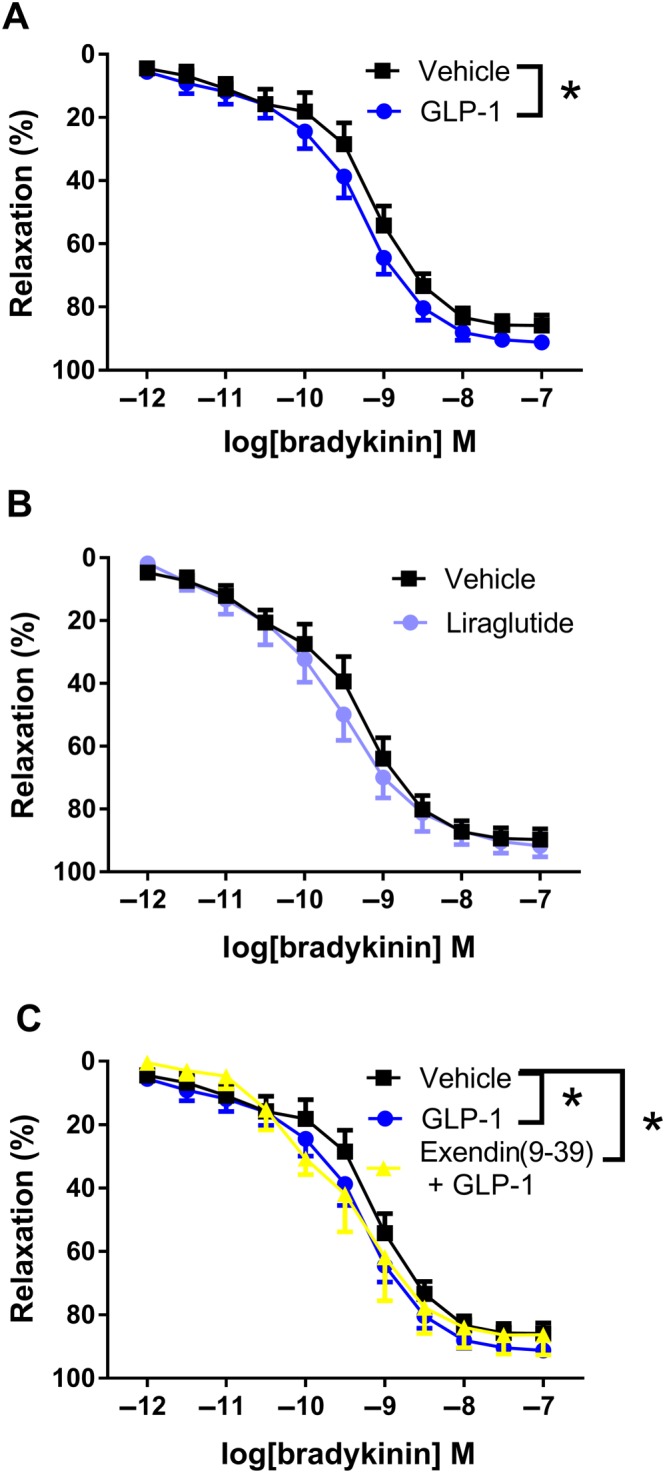
Bradykinin‐induced relaxation in human s.c. arteries. (A) Average concentration–response curves for bradykinin in the absence (n = 10) and in the presence of GLP‐1 (10−8 M, n = 10). (B) Concentration–response curves for bradykinin in the absence (n = 10) and in the presence of liraglutide (10−8 M, n = 10). (C) Average concentration–response curves for bradykinin in the absence and in the presence of GLP‐1 (10−8 M) and GLP‐1 plus the GLP‐1 receptor antagonist, exendin(9‐39) (n = 5). The results are means ± SEM. *P < 0.05, significantly different as indicated; two‐way ANOVA, followed by Bonferroni post test.
Effect of GLP‐1 and liraglutide on endothelium‐dependent relaxation in rat mesenteric small arteries
At a normoglycaemic condition of 5.5 mM glucose, GLP‐1 did not change the relaxation to ACh in rat mesenteric arteries (Supporting Information Figure S4). In the first set of experiments with the model of hyperglycaemia, incubation of rat mesenteric vessels with 40 mM of glucose significantly impaired the relaxant response to ACh (Figure 5A). Based on these findings and to ensure that only vessels that had actually been affected by the hyperglycaemic condition were included in the GLP‐1 study, experiment with a 40 mM glucose control vessel that relaxed more than 40% at 30 nM ACh was excluded (n = 6). GLP‐1 significantly improved the relaxation to increasing concentrations of ACh at a high glucose concentration (Figure 5B). The response to ACh at high glucose in the presence of GLP‐1 was similar to the response to ACh at a low glucose concentration. Exendin(9‐39) did not block this effect (Figure 5C). The GLP‐1 potentiation of ACh‐induced relaxation was examined at GLP‐1 concentrations of 0.1–10 nM, though only 10 nM GLP‐1 significantly improved ACh potency (Supporting Information Figure S4). The GLP‐1 analogue, liraglutide, and the metabolite of GLP‐1, GLP‐1(9‐36), failed to change ACh‐induced relaxations (Figure 5D,E). Incubation with the superoxide scavenger, tempol (10−4 M), leftward‐shifted the concentration–response curves for ACh in mesenteric arteries exposed to high glucose (Figure 5F).
Figure 5.
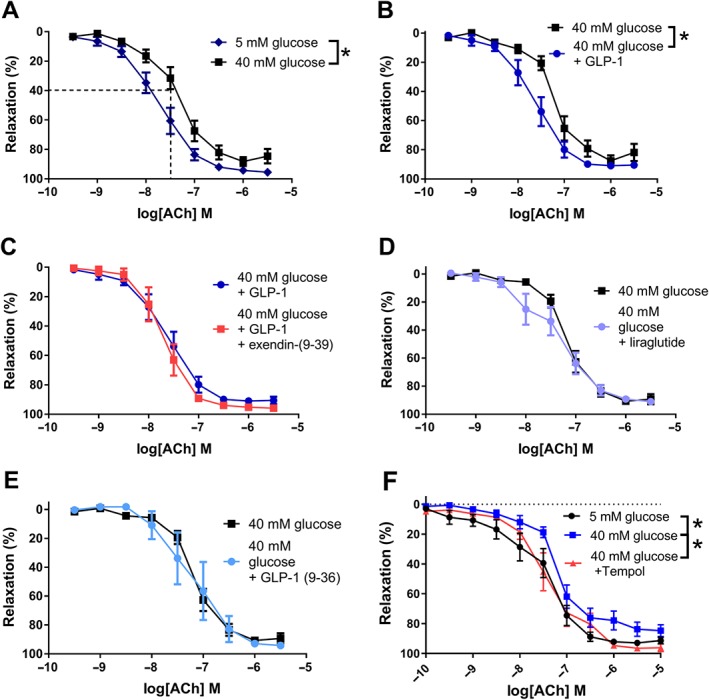
GLP‐1 potentiates ACh‐induced relaxation in rat mesenteric arteries kept in high glucose. Concentration–response curves for ACh in rat meseneric arteries without branches kept (A) at 5.5 mM (n = 13) and 40 mM (n = 15) glucose, (B) at 40 mM glucose in the absence (n = 11) and in the presence of GLP‐1 (10−8 M, n = 12), (C) at 40 mM glucose in the presence of GLP‐1 (10−8 M) without (n = 11) and with (n = 4) exendin‐(9‐39), (D) at 40 mM glucose in the absence (n = 9) and in the presence of liraglutide (10−8 M, n = 10), (E) at 40 mM glucose in the absence (n = 10) and in the presence of GLP‐1(9‐36) (10−8 M, n = 5) and (F) at 40 mM glucose in the absence (n = 10) and in the presence of the SOD mimetic, tempol (10−4 M, n = 10), and at 5.5 mM glucose (n = 10). Results are means ± SEM. *P < 0.05, significantly different as indicated; two‐way ANOVA, followed by Bonferroni post test.
Effect of GLP‐1 and liraglutide on vascular superoxide levels
To investigate the role of superoxide in the effects of GLP‐1, rat mesenteric arteries were exposed to the superoxide generator pyrogallol in the absence and the presence of GLP‐1 and liraglutide. In the presence of pyrogallol, concentration–response curves for ACh were leftward‐shifted after incubation with GLP‐1 (Figure 6A), while the presence of liraglutide failed to change the curves for ACh in arteries exposed to pyrogallol (Figure 6B).
Figure 6.
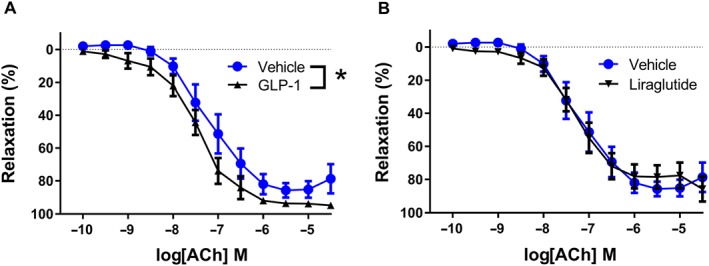
GLP‐1 potentiates ACh‐induced relaxation in rat mesenteric arteries exposed to the superoxide generator, pyrogallol. Concentration–response curves for ACh in rat mesenteric arteries without branches exposed to the superoxide generator, pyrogallol (3 × 10−5 M) (A) in the absence (n = 10) and in the presence of GLP‐1 (10−8 M, n = 10) or (B) in the absence (n = 10) and in the presence of liraglutide (10−8 M, n = 10). The experiments were performed in the presence of indomethacin (10−6 M). Results are means ± SEM. *P < 0.05, significantly different as indicated; two‐way ANOVA followed by Bonferroni post test.
To directly measure vascular superoxide levels, the preparations were stained with dihydroethidium (Figure 7A,F). The changes in fluorescence were expressed relative to the effect of the superoxide scavenger, PEG‐SOD (Figure 7G). Incubation with high glucose (40 mM) markedly increased the vascular superoxide levels compared with vessels kept at 5 mM glucose. GLP‐1 decreased the superoxide levels in vascular preparations exposed to high glucose, while the effect was less pronounced for liraglutide and linagliptin (Figure 7G).
Figure 7.
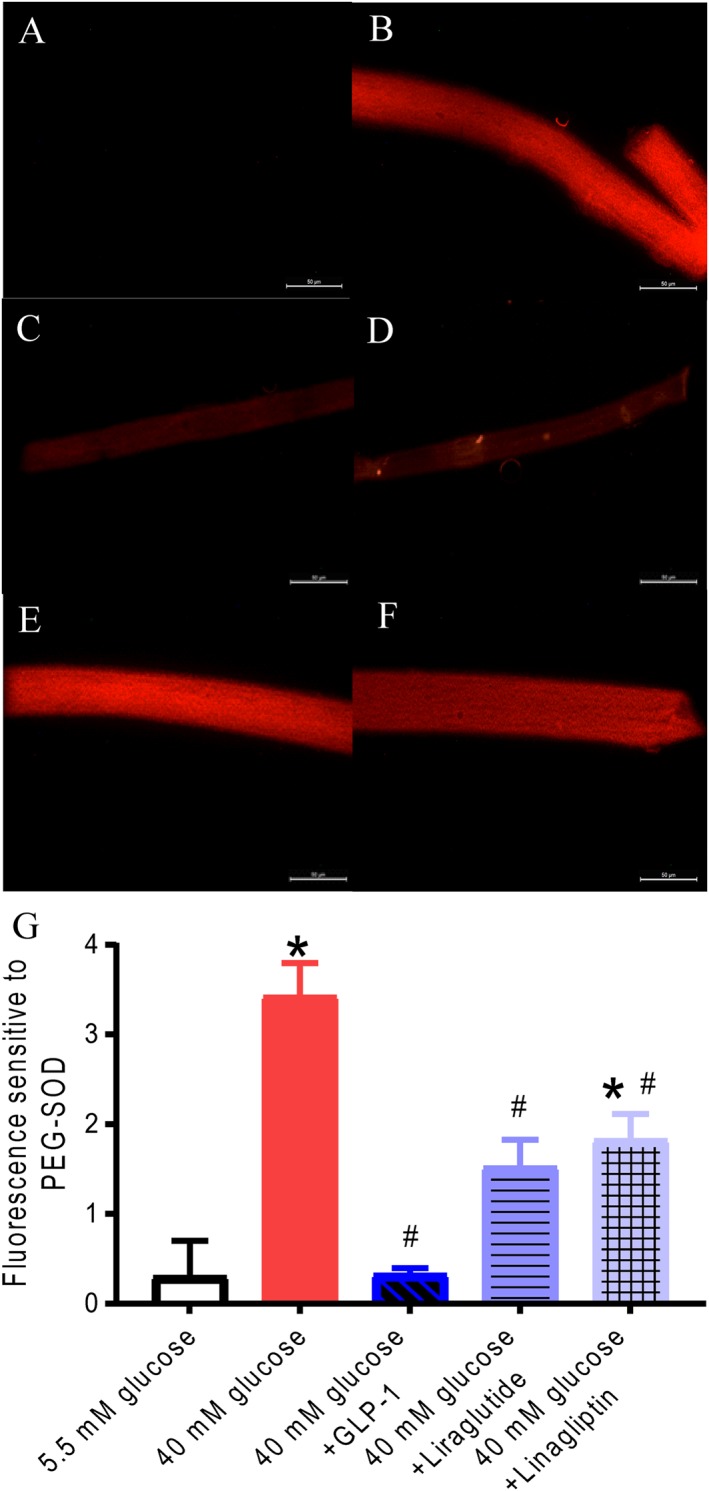
Dihydroethidium staining of rat mesenteric arteries is increased by high glucose and decreased by GLP‐1. Representative fluorescent photomicrographs of mesenteric arteries incubated in (A) normoglycaemic conditions (5.5 mM glucose) and (B) 40 mM glucose, (C) 40 mM glucose and PEG‐SOD (500 U·L−1), (D) 40 mM glucose and GLP‐1 (10−8 M), (E) 40 mM glucose and liraglutide (10−8 M) or (F) 40 mM glucose and linagliptin (10−6 M). White bars correspond to 50 μm. (G) Average results for dihydroethidium staining obtained in arteries from nine animals. Columns are means (n = 9) ± SEM. *P < 0.05, significantly different from 5 mM glucose; # P < 0.05, significantly different from 40 mM glucose‐exposed vessels; one‐way ANOVA followed by Tukey's multiple comparisons test.
Discussion
The present study demonstrates that liraglutide induces vasorelaxation in branched rat mesenteric arteries, whereas no direct vasodilating effect and no effect on the sympathetic nerves in the vascular wall of native GLP‐1, GLP‐1(9‐36) or liraglutide, was found in resistance arteries without branches. In contrast to liraglutide, GLP‐1 leftward‐shifted the concentration relaxation curves for bradykinin in s.c. arteries from patients with peripheral arterial disease, an effect resistant to exendin(9‐39). This study also suggests that, at a high glucose concentration, native GLP‐1, by a GLP‐1 receptor‐independent mechanism, may potentiate ACh relaxation in small rat mesenteric arteries by decreasing vascular superoxide levels.
The direct GLP‐1 effect
Previous studies have suggested a direct vasodilating effect of GLP‐1 in isolated mesenteric arteries from rodents and of GLP‐1 receptor agonists in human s.c. arteries, as an explanation of the BP‐lowering effect observed in patients treated with GLP‐1 receptor agonists (Ban et al., 2008; Bayram et al., 2014; Koska et al., 2015). These studies show an effect at GLP‐1 receptors of agonist concentrations varying from 3 pM to 3 nM. We did not find any direct vasodilating effect in human or rat resistance arteries without branches at similar GLP‐1 concentrations. In our experiments, GLP‐1 failed to cause a relaxing response that was any different from the changes in artery tone in our time controls. Neither did we find any vasoconstricting effect of GLP‐1 in mesenteric arteries, as has been suggested in a previous in vivo study in rats (Gardiner et al., 2006). None of the studies that found a direct vasodilating effect of GLP‐1 describe or show a time‐control response. Therefore, it is unclear if they have actually considered the changes in artery tone due to time, as an explanation of the observed GLP‐1‐induced vasodilatation.
Contrary to the findings in vessels without branches, we did observe a direct vasorelaxant effect of liraglutide in branched rat mesenteric arteries that was inhibited by the GLP‐1 receptor antagonist, exendin(9‐39). This finding suggests a GLP‐1 receptor‐dependent and consequently exendin(9‐39)‐sensitive mechanism, consistent with the fact that liraglutide is a selective GLP‐1 receptor agonist. A more pronounced expression of GLP‐1 receptors at branching points in the vascular bed may explain this effect. GLP‐1 receptors are expressed in small arteries of the intestine, co‐localized with smooth muscle α‐actin (Richards et al., 2014), supporting the findings of a direct effect of liraglutide in mesenteric branched resistance arteries. Whether the expression of GLP‐1 receptors is concentrated around branching points remains to be examined in future studies. Both native GLP‐1(7‐36) and the metabolite GLP‐1(9‐36) did not evoke a similar response in branched rat mesenteric arteries. The concentrations of GLP‐1 and its analogues used in these studies (single addition: 1 nM; concentration–response: 0.1 pM–100 nM) are comparable with plasma concentrations measured after infusion of therapeutic doses of GLP‐1 in patients (Toft‐Nielsen et al., 1999; Edwards et al., 1998), ensuring translational results. We therefore suggest that liraglutide induces vasorelaxation in branched mesenteric arteries, whereas GLP‐1 and analogues have no direct vasodilating effect in isolated rat and human small resistance arteries without branches.
GLP‐1 effects on the vascular nerves
Agonists at GLP‐1 receptors have been suggested to increase heart rate by activation of the sympathetic nervous system (Smits et al., 2016), and therefore, GLP‐1 receptor agonists may also regulate vascular tone by interaction with the nerve endings in the vascular wall similar to suggested effects of other gut‐derived peptides (Gradin et al., 2006). This has to our knowledge, not been examined before. EFS is a method that allowed us to activate vascular sympathetic nerve endings in a single isolated vessel. In our experiments, GLP‐1 and liraglutide did not reduce the vascular contractions due to EFS and evoked by neurotransmitter release from vascular sympathetic nerves in rat mesenteric arteries. Neither did they increase the responses, suggesting that GLP‐1 and liraglutide have no effects on the vascular sympathetic nervous activity in rat mesenteric arteries.
GLP‐1 effect on endothelium‐dependent relaxation
A possible GLP‐1 effect on endothelium‐dependent relaxation at normoglycaemic conditions was examined in both rat mesenteric and s.c. arteries from patients with arterial disease. Previous studies in patients have shown apparently conflicting results about a possible GLP‐1 effect on vascular endothelial function. In contrast to healthy subjects, infusion of GLP‐1 increased forearm blood flow in patients with T2D (Nystrom et al., 2004), and the GLP‐1 receptor agonist exenatide potentiated ACh vasodilatation in adipose tissue arterioles (Koska et al., 2010). Others found no effect of the GLP‐1 receptor agonist liraglutide on endothelium‐dependent vasodilatation in T2D patients (Nandy et al., 2014). As the human arteries used in this study were obtained from patients with arterial disease, they may have some degree of endothelial dysfunction. In contrast to liraglutide, GLP‐1 induced a small leftward shift of the concentration–response curves for bradykinin in the human s.c. arteries. Previous studies have shown that exendin‐4 potentiates ACh vasodilatation in human adipose arterioles by a GLP‐1 receptor‐dependent mechanism (Koska et al., 2010). However, in the present study, the effect of GLP‐1 on bradykinin relaxation persisted in the presence of exendin(9‐39) suggesting the effect was independent of GLP‐1 receptors. Taken together, our findings suggest that different mechanisms are involved in the effect of, respectively, GLP‐1 and GLP‐1 analogues on endothelial function in human arteries.
As GLP‐1 did not seem to have any effects at normoglycaemic conditions in rat mesenteric arteries, it was interesting to examine if a hyperglycaemic condition comparable with the state of hyperglycaemia in diabetic patients is required for GLP‐1 to mediate its actions. Previous studies found that the GLP‐1 receptor agonist exendin‐4 improved endothelium‐dependent relaxation in vessel exposed to high glucose (Salheen et al., 2015; Koska et al., 2015). We found that exposure of rat mesenteric arteries to high glucose impaired relaxation to ACh and that native GLP‐1 almost reversed this high glucose‐induced damage. As ACh relaxation is dependent on endothelial function, our results suggest that GLP‐1 improves endothelial function in vessels that have been exposed to hyperglycaemia, consistent with the findings by Salheen et al. (2015) and Koska et al. (2015). A GLP‐1 concentration of 10 nM was required to significantly increase the potency of ACh (Supporting Information Figure S4), a concentration similar to the plasma concentrations measured after s.c. injection of therapeutic concentrations of liraglutide (Danne et al., 2017; Damholt et al., 2006). Liraglutide or the cleaved metabolite of GLP‐1, GLP‐1(9‐36), did not reproduce the effect of GLP‐1. This can be due to differences in chemical structure of GLP‐1 and liraglutide (Lund et al., 2014). Nevertheless, we found an effect of GLP‐1 on hyperglycaemia‐induced impairment of endothelium‐dependent relaxation, but as we were not able to show a similar effect of liraglutide, it is uncertain if this is the major mechanism resulting in the lowering of BP in diabetic patients treated with GLP‐1 analogues. Furthermore, it is noteworthy that GLP‐1 plasma levels have been found to be associated with BP in normoglycaemic young healthy adults as well (Krisai et al., 2015), demonstrating that there may be other GLP‐1 effects of importance that affect BP.
Exendin(9‐39) did not attenuate the effect of GLP‐1 suggesting that the effect is independent of GLP‐1 receptors. These data conflict with the results of Salheen et al. (2015), indicating that further studies are needed to clarify the possible independence or dependence on GLP‐1 receptors. However, previous investigations have suggested that exendin‐4 and the DPP‐4 inhibitor, linagliptin, potentiate ACh relaxations in rat mesenteric arteries exposed to high glucose by a mechanism decreasing vascular superoxide levels (Salheen et al., 2015). Also, native GLP‐1 decreased monocyte‐derived oxidative stress (Steven et al., 2017). In the present study, a SOD mimetic, tempol, leftward‐shifted the concentration–response curves for ACh in arteries exposed to high glucose, suggesting vascular superoxide levels played a role in high glucose impairment of ACh relaxation. Moreover, we found that GLP‐1 prevented impairment of ACh relaxation by the superoxide generator pyrogallol, while this was not the case for liraglutide. Finally, measurements of vascular superoxide levels by use of dihydroethium revealed that GLP‐1 markedly decreased the levels. Our results suggest that one vascular action of therapeutic concentrations of native GLP‐1, but not of liraglutide or GLP‐1(9‐36), is that it improves endothelium‐dependent relaxation at a high glucose concentration, possibly by a pathway which is independent of GLP‐1 receptors but involving inhibition of vascular superoxide levels.
In summary, our data suggest that, in normoglycaemic conditions, a mechanism dependent on GLP‐1 receptors was involved in liraglutide relaxation in branched arteries, while GLP‐1 and its analogues, at therapeutic concentrations, have no direct vasodilating effect on the smooth muscle cells in arterial segments without branches and no pre‐junctional effect on the neurotransmitter release from the sympathetic vascular nerves. We also found that inhibition of vascular superoxide levels contributed to the GLP‐1 receptor‐independent potentiation of endothelium‐dependent vasodilatation in hyperglycaemic conditions. The latter mechanism may play a role in GLP‐1‐induced leftward‐shift of concentration relaxation curves for bradykinin in s.c. arteries from patients with peripheral arterial disease. These studies suggest that different mechanisms are involved in GLP‐1‐induced and liraglutide‐induced vasodilatation in small arteries and may contribute to the BP‐lowering effect of GLP‐1 analogues, as previously observed in treatment of patients with Type 2 diabetes.
Author contributions
M.B. contributed to the experimental design, performed and analysed the experiments in rat and human resistance arteries and wrote a first draft of the manuscript. K.D.H. recruited the patients and collected the tissue samples. A.G., J.S.K., A.G.P. and D.D.R.A. performed the additional experiments in rat mesenteric arteries. M.G. and J.R. contributed to the experimental design and the writing. U.S. participated in the design and analysis of the experiments and contributed to the writing.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1 Average effect of single concentrations of GLP‐1 (0.1–1 nM) on phenylephrine contracted vessels and the effect of GLP‐1 (10−8 M) in the absence and the presence of linagliptin (10−6 M) in rat mesenteric arteries.
Figure S2 Lack of effect of native GLP‐1 and liraglutide on maximum peak of neurogenic contractions in rat mesenteric arteries.
Figure S3 Bradykinin induced relaxation in human subcutaneous arteries from patients without (non‐DM) and with diabetes mellitus (DM).
Figure S4 Average effect of single concentrations of GLP‐1 (0.1–10 nM) on phenylephrine contracted vessels and acetylcholine relaxation in arteries kept in physiological saline solution with high glucose (40 mM).
Figure S5 Average effect of single concentration of linagliptin on acetylcholine relaxation in phenylephrine‐contracted vessels exposed to either (A) high glucose or (B) the superoxide generator, pyrogallol.
Acknowledgements
M.B. was supported by a scholarship from the Danish Council for Independent Research and by a grant from the Foundation of Helga and Peter Korning. We thank Heidi Knudsen and Goncalo Esteves for technical help with the experiments.
Bangshaab, M. , Gutierrez, A. , Huynh, K. D. , Knudsen, J. S. , Arcanjo, D. D. R. , Petersen, A. G. , Rungby, J. , Gejl, M. , and Simonsen, U. (2019) Different mechanisms involved in liraglutide and glucagon‐like peptide‐1 vasodilatation in rat mesenteric small arteries. British Journal of Pharmacology, 176: 386–399. 10.1111/bph.14534.
References
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban K, Noyan‐Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M (2008). Cardioprotective and vasodilatory actions of glucagon‐like peptide 1 receptor are mediated through both glucagon‐like peptide 1 receptor‐dependent and ‐independent pathways. Circulation 117: 2340–2350. [DOI] [PubMed] [Google Scholar]
- Bayram Z, Nacitarhan C, Ozdem SS (2014). Effects of glucagon‐like peptide‐1 in diabetic rat small resistance arteries. J Cardiovasc Pharmacol 64: 277–284. [DOI] [PubMed] [Google Scholar]
- Bhashyam S, Fields AV, Patterson B, Testani JM, Chen L, Shen YT et al (2010). Glucagon‐like peptide‐1 increases myocardial glucose uptake via p38α MAP kinase‐mediated, nitric oxide‐dependent mechanisms in conscious dogs with dilated cardiomyopathy. Circ Heart Fail 3: 512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen FH, Hansen T, Stankevicius E, Buus NH, Simonsen U (2007). Elevated pressure selectively blunts flow‐evoked vasodilatation in rat mesenteric small arteries. Br J Pharmacol 150: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SPA, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damholt B, Golor G, Wierich W, Pedersen P, Ekblom M, Zdravkovic M (2006). An open‐label, parallel group study investigating the effects of age and gender on the pharmacokinetics of the once‐daily glucagon‐like peptide‐1 analogue liraglutide. J Clin Pharmacol 46: 635–641. [DOI] [PubMed] [Google Scholar]
- Danne T, Biester T, Kapitzke K, Jacobsen SH, Jacobsen LV, Petri KCC et al (2017). Liraglutide in an adolescent population with obesity: a randomized, double‐blind, placebo‐controlled 5‐week trial to assess safety, tolerability, and pharmacokinetics of liraglutide in adolescents aged 12–17 years. J Pediatr 181: 146–153. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjoth TV et al (2015). Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 314: 687–699. [DOI] [PubMed] [Google Scholar]
- Drucker D (2016). The cardiovascular biology of glucagon‐like peptide‐1. Cell Metab 24: 15–30. [DOI] [PubMed] [Google Scholar]
- Edwards CM, Todd JF, Ghatei MA, Bloom SR (1998). Subcutaneous glucagon‐like peptide‐1 (7‐36) amide is insulinotropic and can cause hypoglycaemia in fasted healthy subjects. Clin Sci 95: 719–724. [DOI] [PubMed] [Google Scholar]
- Egholm C, Khammy MM, Dalsgaard T, Mazur A, Tritsaris K, Hansen AJ et al (2016). GLP‐1 inhibits VEGFA‐mediated signaling in isolated human endothelial cells and VEGFA‐induced dilation of rat mesenteric arteries. Am J Physiol Heart Circ Physiol 311: 1214–1224. [DOI] [PubMed] [Google Scholar]
- EMA . (2017). Victoza: EPAR – product information. 1–26.
- Fonseca VA, DeVries JH, Henry RR, Donsmark M, Thomsen HF, Plutzky J (2014). Reductions in systolic blood pressure with liraglutide in patients with type 2 diabetes: insights from a patient‐level pooled analysis of six randomized clinical trials. J Diabetes Complications 28: 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Bennett T (2006). Mesenteric vasoconstriction and hindquarters vasodilatation accompany the pressor actions of exendin‐4 in conscious rats. J Pharmacol Exp Ther 316: 852–859. [DOI] [PubMed] [Google Scholar]
- Gejl M, Starup‐Linde J, Scheel‐Thomsen J, Gregersen S, Vestergaard P (2015). Risk of cardiovascular disease: the effects of diabetes and anti‐diabetic drugs – a nested case–control study. Int J Cardiol 178: 292–296. [DOI] [PubMed] [Google Scholar]
- Gejl M, Søndergaard HM, Stecher C, Bibby BM, Møller N, Bøtker HE et al (2012). Exenatide alters myocardial glucose transport and uptake depending on insulin resistance and increases myocardial blood flow in patients with type 2 diabetes. J Clin Endocrinol Metab 97: 1165–1169. [DOI] [PubMed] [Google Scholar]
- Gradin KA, Buus CL, Li JY, Frøbert O, Simonsen U (2006). Neuropeptide Y2 receptors are involved in enhanced neurogenic vasoconstriction in spontaneously hypertensive rats. Br J Pharmacol 148: 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BD, Hand KV, Dougan JE, McDonnell BM, Cassidy RS, Grieve DJ (2008). GLP‐1 and related peptides cause concentration‐dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Arch Biochem Biophys 478: 136–142. [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst JJ (2004). On the physiology of GIP and GLP‐1. Horm Metab Res 36: 747–754. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koska J, Sands M, Burciu C, D'Souza KM, Raravikar K, Liu J et al (2015). Exenatide protects against glucose‐ and lipid‐induced endothelial dysfunction: evidence for direct vasodilation effect of GLP‐1 receptor agonists in humans. Diabetes 64: 2624–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koska J, Schwartz EA, Mullin MP, Schwenke DC, Reaven PD (2010). Improvement of postprandial endothelial function after a single dose of exenatide in individuals with impaired glucose tolerance and recent‐onset type 2 diabetes. Diabetes Care 33: 1028–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisai P, Aeschbacher S, Schoen T, Bossard M, van der Stouwe JG, Dorig L et al (2015). Glucagon‐like peptide‐1 and blood pressure in young and healthy adults from the general population. Hypertension 65: 306–312. [DOI] [PubMed] [Google Scholar]
- Lund A, Knop FK, Vilsboll T (2014). Glucagon‐like peptide‐1 receptor agonists for the treatment of type 2 diabetes: differences and similarities. Eur J Intern Med 25: 407–414. [DOI] [PubMed] [Google Scholar]
- Marso SP, Daniels GH, Brown‐Frandsen K, Kristensen P, Mann JFE, Nauck MA et al (2016). Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 375: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany MJ, Halpern W (1977). Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res 41: 19–26. [DOI] [PubMed] [Google Scholar]
- Nandy D, Johnson C, Basu R, Joyner M, Brett J, Svendsen CB et al (2014). The effect of liraglutide on endothelial function in patients with type 2 diabetes. Diab Vasc Dis Res 11: 419–430. [DOI] [PubMed] [Google Scholar]
- Nystrom T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahren B et al (2004). Effects of glucagon‐like peptide‐1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 287: 1209–1215. [DOI] [PubMed] [Google Scholar]
- Nystrom T, Gonon AT, Sjoholm A, Pernow J (2005). Glucagon‐like peptide‐1 relaxes rat conduit arteries via an endothelium‐independent mechanism. Regul Pept 125: 173–177. [DOI] [PubMed] [Google Scholar]
- Panjwani N, Mulvihill EE, Longuet C, Yusta B, Campbell JE, Brown TJ et al (2012). GLP‐1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE −/− mice. Endocrinology 154: 127–139. [DOI] [PubMed] [Google Scholar]
- Pyke C, Heller RS, Kirk RK, Orskov C, Reedtz‐Runge S, Kaastrup P et al (2014). GLP‐1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 155: 1280–1290. [DOI] [PubMed] [Google Scholar]
- Pyke C, Knudsen LB (2013). The glucagon‐like peptide‐1 receptor – or not? Endocrinology 154: 4–8. [DOI] [PubMed] [Google Scholar]
- Qian LB, Wang HP, Qiu WL, Huang H, Bruce IC, Xia Q (2006). Interleukin‐2 protects against endothelial dysfunction induced by high glucose levels in rats. Vascul Pharmacol 45: 374–382. [DOI] [PubMed] [Google Scholar]
- Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S et al (2014). Identification and characterisation of glucagon‐like peptide‐1 receptor‐expressing cells using a new transgenic mouse model. Diabetes 63: 1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salheen SM, Panchapakesan U, Pollock CA, Woodman OL (2015). The DPP‐4 inhibitor linagliptin and the GLP‐1 receptor agonist exendin‐4 improve endothelium‐dependent relaxation of rat mesenteric arteries in the presence of high glucose. Pharmacol Res 94: 26–33. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda‐Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T et al (2012). Fiji: an open‐source platform for biological‐image analysis. Nat Methods 9: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen U, Laursen BE, Petersen JS (2008). ZP120 causes relaxation by pre‐junctional inhibition of noradrenergic neurotransmission in rat mesenteric resistance arteries. Br J Pharmacol 153: 1185–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits MM, Muskiet MHA, Tonneijck L, Hoekstra T, Kramer MHH, Diamant M et al (2016). Exenatide acutely increases heart rate in parallel with augmented sympathetic nervous system activation in healthy overweight males. Br J Clin Pharmacol 81: 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starup‐Linde J, Scheel‐Thomsen J, Gejl M, Vestergaard P, Gregersen S (2014). Liraglutide and insulin are associated with a decreased risk of acute myocardial infarction in type 2 diabetes mellitus patients. J Diabetes Metab 5: 389. [Google Scholar]
- Steven S, Jurk K, Kopp M, Kröller‐Schön S, Mikhed Y, Schwierczek K et al (2017). Glucagon‐like peptide‐1 receptor signalling reduces microvascular thrombosis, nitro‐oxidative stress and platelet activation in endotoxaemic mice. Br J Pharmacol 174: 1620–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons JD, Rutledge JC, Simonsen U, Pattathu RA (2006). Vascular dysfunction produced by hyperhomocysteinemia is more severe in the presence of low folate. Am J Physiol Heart Circ Physiol 290: 181–191. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B, Brown ML, Cohen RA (1991). Elevated glucose impairs endothelium‐dependent relaxation by activating protein kinase C. J Clin Investig 87: 1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft‐Nielsen MB, Madsbad S, Holst JJ (1999). Continuous subcutaneous infusion of glucagon‐like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients. Diabetes Care 22: 1137–1143. [DOI] [PubMed] [Google Scholar]
- Ussher JR, Drucker DJ (2014). Cardiovascular actions of incretin‐based therapies. Circ Res 114: 1788–1803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Average effect of single concentrations of GLP‐1 (0.1–1 nM) on phenylephrine contracted vessels and the effect of GLP‐1 (10−8 M) in the absence and the presence of linagliptin (10−6 M) in rat mesenteric arteries.
Figure S2 Lack of effect of native GLP‐1 and liraglutide on maximum peak of neurogenic contractions in rat mesenteric arteries.
Figure S3 Bradykinin induced relaxation in human subcutaneous arteries from patients without (non‐DM) and with diabetes mellitus (DM).
Figure S4 Average effect of single concentrations of GLP‐1 (0.1–10 nM) on phenylephrine contracted vessels and acetylcholine relaxation in arteries kept in physiological saline solution with high glucose (40 mM).
Figure S5 Average effect of single concentration of linagliptin on acetylcholine relaxation in phenylephrine‐contracted vessels exposed to either (A) high glucose or (B) the superoxide generator, pyrogallol.


