Abstract
With the limited capacity for self‐repair in the adult CNS, efforts to stimulate quiescent stem cell populations within discrete brain regions, as well as harness the potential of stem cell transplants, offer significant hope for neural repair. These new cells are capable of providing trophic cues to support residual host populations and/or replace those cells lost to the primary insult. However, issues with low‐level adult neurogenesis, cell survival, directed differentiation and inadequate reinnervation of host tissue have impeded the full potential of these therapeutic approaches and their clinical advancement. Biomaterials offer novel approaches to stimulate endogenous neurogenesis, as well as for the delivery and support of neural progenitor transplants, providing a tissue‐appropriate physical and trophic milieu for the newly integrating cells. In this review, we will discuss the various approaches by which bioengineered scaffolds may improve stem cell‐based therapies for repair of the CNS.
Abbreviations
- BDNF
brain‐derived neurotrophic factor
- ECM
extracellular matrix
- EPO
erythropoietin
- ESCs
embryonic stem cells
- GDNF
glial cell line‐derived neurotrophic factor
- IPSCs
induced pluripotent stem cells
- NGF
nerve growth factor
- PD
Parkinson's disease
- PSCs
pluripotent stem cells
- SAPs
self‐assembling peptides
- TEM
transmission electron microscopy
- VM
ventral midbrain
Introduction
With an increasingly ageing population, the incidence of chronic neurodegenerative disorders, such as Parkinson's disease (PD), Huntington's disease, Alzheimer's disease and amyotrophic lateral sclerosis, as well as acute insults including ischaemic and haemorrhagic stroke is on the rise. Each of these is characterized by cognitive, sensory and/or motor impairments underpinned by loss of neuronal subpopulations (Lindvall and Kokaia, 2010). In both instances, chronic and acute injuries, approaches are needed to manage the neurological deficits induced by this cell loss. However, current therapeutic options predominantly focus on managing symptoms using drugs, physical therapy and deep brain stimulation (Lindvall and Kokaia, 2010). Consequently, the development of new and novel therapies is in critical demand.
Unlike many tissues, the CNS has limited capacity for repair. Until the 1960s, it was thought that we were born with our complete complement of neurons; since then, discrete pockets of new neurons have been identified in the adult brain (Altman, 1962). These new cells, however, are seemingly few in number and, despite increased numbers following injury (within these discrete locations in the brain) are unable to restore neuronal numbers lost to disease or injury. Persistent research strives to understand the mechanisms that underpin adult neurogenesis, with the hope of exploiting these processes to enhance repair (Lindvall and Kokaia, 2011, 2015).
Recognizing the limited capacity for self‐repair in the adult brain, an alternative experimental approach has been the replacement of lost neurons through transplantation. Most effectively demonstrated using embryonic tissue, preclinical and clinical trials have provided proof of principle that newly implanted neurons can survive, structurally integrate and alleviate disease‐associated symptoms (Kirkeby et al., 2017b). Since the discovery of pluripotent stem cells (PSCs), and the significant advancement in the directed differentiation of these cells into restricted neuronal fates, the feasibility of cell replacement therapies is becoming increasingly recognized (Kokaia and Lindvall, 2012; Barker et al., 2017).
Despite the therapeutic potential of these two approaches targeted at brain repair (i.e. stimulation of endogenous neurogenesis and cell transplantation), both present ongoing challenges for the field including adequate cell numbers/tissue availability, survival, directed differentiation and appropriate integration into the host tissue (Castilho et al., 2000; Lindvall and Kokaia, 2010; Kokaia and Lindvall, 2012; Barker et al., 2017). Research has shown that exposure of stem cells to the appropriate physical and chemical environment improves fate specification and cellular integration; however, it can be difficult to provide these cues in a temporally and spatially appropriate manner in vivo (Kriks et al., 2011; Kirkeby et al., 2012; Shi et al., 2012; Niclis et al., 2017a).
In an effort to improve cell‐based therapies for neural repair, bioengineered scaffolds have been proposed. Biomaterials are being investigated for their potential to restore tissue architecture, enhance cell survival and differentiation, and promote plasticity and integration of both endogenous and transplanted stem cells (Rodriguez et al., 2012; Jendelova et al., 2016; Moriarty et al., 2018b). In this review, we will discuss the progress (and challenges) in stimulating endogenous repair and cell transplantation from the perspective of two common neural injuries; one that is chronic yet discrete in nature (PD) and an acute injury that is notably more diffuse and complex in its requirements for repair (stroke). We will describe the structural and functional benefits bioengineered scaffolds may have in advancing cell‐based therapies for these, and other, neural injuries.
Cell replacement therapy for Parkinson's disease
PD is a movement disorder caused by progressive death of ventral midbrain (VM) dopaminergic neurons and subsequent degeneration of the nigrostriatal pathway (Winkler et al., 2005). Pharmacotherapy, using dopamine‐modulating drugs, is capable of restoring motor functions yet has no effect on disease progression, is limited by side effects and shows waning efficacy over time. Yet it was this evidence, restoring motor deficits with dopamine, that inspired cell replacement therapy for the disease. Dopamine progenitors, isolated from the developing fetal VM, were shown to survive, achieve synaptic integration and restore dopaminergic transmission following implantation into Parkinsonian rodents and non‐human primates (Figure 1) (Thompson and Parish, 2013; Kirkeby et al., 2017b). These findings led to a series of clinical trials through the 1990s that similarly demonstrated the capacity for these newly implanted dopamine progenitors to restore deficits for up to 24 years, despite ongoing disease progression (Winkler et al., 2005). However, the outcomes were highly variable – largely attributed to the source of the donor tissue. Fetal tissue, procured under consent from elective abortions, was unreliable (Winkler et al., 2005). Considerable variability in donor tissue age, tissue integrity, dissection procedures and microbiological status of the isolated tissue rendered it incompatible with the standardization, quality control and safety measures necessary for clinical application. Added to this were the ethical and bioavailability constraints of fetal tissue. Combined, this highlighted the need for an alternative, standardized donor source.
Figure 1.
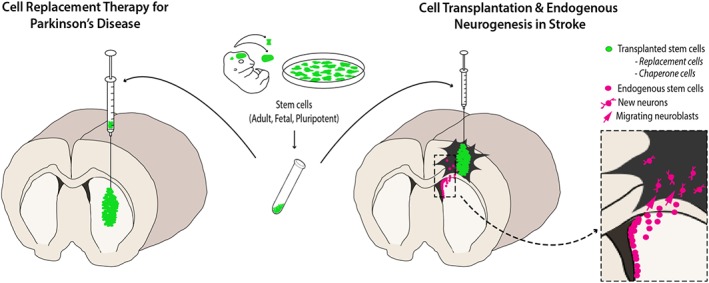
Stem cell‐based therapies for neural repair. Stem cells/neural progenitors (green), isolated from fetal tissue, can be transplanted directly into the injured host tissue, illustrated here in a model of Parkinson's disease and stroke. As an alternative, more standardized and sustainable cell source, pluripotent stem cells (PSCs; requiring in vitro differentiation prior to in vivo delivery) are increasingly being studied in tissue repair. Studies have demonstrated the capacity for these transplanted cells to survive and functionally integrate, replacing neurons lost to the primary injury. Transplanted cells can also act as chaperone cells to support surrounding tissue. Alternatively, quiescent stem cells, present within discrete locations within the host brain (magenta), can be mobilized to replace neurons (endogenous neurogenesis) and/or deliver trophic cues, targeted at reducing injury and promoting repair.
The discovery of human embryonic stem cells (ESCs) in 1998 (Thomson et al., 1998), and subsequently of induced pluripotent stem cells (iPSCs) in 2007 (Takahashi et al., 2007), proclaimed new beginnings in regenerative medicine. Human ESCs, derived from the inner cell mass of blastocysts procured from excess in vitro fertilization embryos, and iPSCs, generated by genetic reprogramming of somatic cells, provide a sustainable cell source with the capacity to differentiate into restricted lineages – thereby providing an attractive cell source for cell‐based therapies. Surprisingly, despite monumental efforts, only in recent years have protocols emerged that generate bona fide VM dopaminergic neurons (Kriks et al., 2011; Kirkeby et al., 2012). The key discovery that underpinned this advancement was the recognition that VM dopaminergic neurons originated from floorplate progenitor pools (and not the neuroectoderm, as once thought). Consequently, early ventralization of the PSCs in culture (via modulation of sonic hedgehog signalling) results in appropriate patterning and generation of developmentally relevant cells that progressively expressed markers such as FOXA2 and LMX1A (early dopamine progenitors), PITX3 (late dopamine progenitors), NURR1 (post‐mitotic dopamine precursors) and TH (differentiated dopamine neuron) (Kriks et al., 2011; Kirkeby et al., 2012, 2017b). Similar to fetal tissue studies, these PSC‐derived dopamine progenitors are capable of surviving and functionally integrating into PD models, with the anticipation of clinical trials commencing by late 2018 (Barker et al., 2017).
While in vitro differentiation routinely shows high proportions of correctly specified VM progenitors, at the time amenable to transplantation, the ability to predict their capacity to give rise to grafts rich in dopaminergic neurons remains a black box. Most evidently demonstrated by the group of Malin Pamar (Kirkeby et al., 2017a, 2017b), >30 independent human PSC differentiations of seemingly similar VM progenitor fate prior to grafting (>80% LMX1A + FOXA2 + OTX2+) showed vastly different outcomes in vivo – with some grafts showing no or few TH+ dopaminergic neurons, while others contained high dopamine yields, capable of functional impacts. Such outcomes suggest that a greater understanding and control of the differentiation of human PSCs remains to be achieved.
Added to this, and similarly observed in preclinical and clinical fetal tissue grafts, is the notably low proportion of dopaminergic neurons within the grafts, with most studies reporting between 3 and 8% of the total graft (Kriks et al., 2011; Kirkeby et al., 2012, 2017b; Doi et al., 2014; Samata et al., 2016; Niclis et al., 2017b). While functional assessments have revealed sufficient dopaminergic neurons for restoration of motor functions in rodent and non‐human primate models, questions remain regarding the impact of the non‐dopaminergic cells to graft function. Only recently, using reporter stem cell lines to specifically track the contribution of dopaminergic neurons to the graft, has the level of non‐dopamine influence been recognized. While the assessment of young grafts (<6 weeks after implantation) reveals high proportions of dopamine progenitors, reflective on their in vitro identity, at more protracted time periods (>6 months), TH+ dopaminergic neurons only contribute to a fraction of the graft, suggesting failure of progenitor maturation and/or expansion of the incorrectly specified (yet remaining FOXA2+) cells from culture (Niclis et al., 2017b).
Obviously, the survival of dopaminergic neurons is of critical importance, being the most decisive factor in governing whether a transplant is successful in ameliorating symptoms yet is challenging at many levels. There are a number of key events that impact on their survival including (i) handling of the donor cells prior to implantation (inclusive of their dissociation, preparation medias and storage); (ii) the physical process of implantation (noting the shear forces exerted on the cells during injection); and (iii) integration into the host tissue, recognizing that the adult brain into which transplants are placed lacks many neurotrophic cues that are normally present when dopamine progenitors/neurons and their axons navigate their way in situ during development. Studies report dopamine cell survival rates post implantation within the adult brain of <20% for fetal tissue grafts compared to <10% for human PSC‐derived dopamine progenitor grafts (Castilho et al., 2000; Niclis et al., 2017b).
A further challenge for the field has been graft integration, with numerous studies observing that human PSC‐derived dopamine transplants show notably poorer striatal innervation than their human VM fetal counterparts, with a recent direct comparative study reporting a fourfold reduction within the target dorsolateral striatum (Grealish et al., 2014). While a variety of trophic cues, namely, glial cell line‐derived neurotrophic factor (GDNF), have been demonstrated to improve the survival and plasticity of fetal tissue grafts, their potential for PSC‐derived grafts are only just beginning to be realized (Moriarty et al., 2018a). Moving forwards, in addition to the identification of trophic cues capable of supporting survival and plasticity of human PSC‐derived grafts, will be the need for safe and efficient delivery approaches that are both spatially and temporally relevant to the duration of graft integration. While the past 40 years of research have made significant advancements in stem cell differentiation and transplantation for PD, a new wave of interest into the potential of bioengineered scaffolds to overcome some of the existing/persistent challenges has begun.
Cell‐based therapy for stroke
Stroke, caused by disruption of blood flow to the brain, results in a mortally injured necrotic core (primary injury) that is surrounded by the penumbra–vulnerable tissue that can also die (i.e. the secondary injury) but is potentially salvageable (Lindvall and Kokaia, 2011). With the exception of thrombolytic drugs and surgical clot removal, both targeted at restoring blood flow and protection of the penumbra, no treatments are currently available to improve functional recovery in patients. In this regard, cell transplantation, to replace and/or protect circuitry, offers new and realistic hope (Lindvall and Kokaia, 2011).
However, unlike PD, the challenges of cell‐based repair following stroke are notably greater. Here, the injury can affect many brain areas and involve a variety of cell populations. Furthermore, the task is notably greater than replacing a single discrete cell population, requiring all or many of the following: (i) restoration of gross tissue architecture; (ii) deployment of replacement cells; (iii) establishment of a suitable micro‐environment for graft cells survival and integration, while also supporting endogenous cells in the penumbra; and (iv) ensuring vascularization of the new tissue.
Evidently, a key hurdle for cell transplantation targeted at stroke is how best to get the implanted cells to survive and ‘knit’ into the host tissue when implanted into the necrotic core. The alternative is to transplant the cells into the penumbra/peri‐infarct tissue; however, this may risk damaging vulnerable and potentially salvageable tissue. Despite this, studies have demonstrated with varying success the capacity of adult or fetal‐derived neural progenitors to survive, differentiate and functionally integrate into the stroke‐injured brain. These studies have also demonstrated the capacity of the grafted stem cells to migrate into the injury site and show electrophysical properties suggestive of mature neurons (Figure 1) (Bacigaluppi et al., 2008; Lindvall and Kokaia, 2010).
Much like the limitations of fetal (and adult) neural stem cell (NSC) sources in PD, the availability of pluripotent stem cell lines has enabled a new wave of research into cell‐based therapies for repair of the acutely injured CNS. With relatively little effort, human iPSCs and ESCs can be differentiated with high efficiency into neural progenitors, by dual Smad inhibition (inhibition of TGFβ and BMP pathways) (Chambers et al., 2009; Shi et al., 2012). These PAX6+ dorsal forebrain progenitors present a neural population that conveniently aligns with the common stroke models that induce infarcts within the cortex. Following transplantation, these PSC‐derived neurons exhibit a remarkable capacity for long‐distance anatomical integration and establishment of appropriate electrophysiological properties (Denham et al., 2012; Espuny‐Camacho et al., 2013; Niclis et al., 2017c) and, similar to fetal and adult NSC grafts, provide varying degrees of functional recovery (Chan‐Ling et al., 2008; Jiang et al., 2011; Gomi et al., 2012; Oki et al., 2012; Jensen et al., 2013; Qin et al., 2013; Tornero et al., 2013; Tatarishvili et al., 2014). Behavioural recovery, however, has commonly been observed over timeframes inconsistent with the maturity of implanted human neurons, thereby making it difficult to assess the direct contribution of these cells to neuronal replacement.
Research groups have also explored the capacity of immortalized NSC lines, as well as non‐NSC sources (inclusive of olfactory ensheathing cells) to promote repair (Lindvall and Kokaia, 2011; Jendelova et al., 2016). These alternative stem cell sources have the advantage of accessibility and availability. While some degree of neuronal differentiation and functional integration into host neural circuitry has been reported, the general consensus from studies transplanting these immortalized NSC lines and non‐NSCs into the stroke‐injured brain (and similarly in traumatic brain and spinal cord injuries) is that the implanted cells act in a paracrine manner – promoting varying degrees of functional recovery through mechanisms other than cell replacement (see reviews – Lindvall and Kokaia, 2011; Jendelova et al., 2016). The implanted cells have been reported to secrete growth and trophic factors, stimulate endogenous neurogenesis (inclusive of the migration and differentiation of these neural progenitors in the injury site), promote angiogenesis and reduce inflammation. Each of these directly impacts on the penumbra to reduce infarct volume.
In more recent years, collective efforts have been made to improve cell transplantation for acute neural injuries by the inclusion of biomaterials to restore tissue architecture, and support transplanted and residual host neurons, while also delivering trophic cues to influence inflammation, angiogenesis, and neurogenesis. These will be described in greater detail below.
Stimulating endogenous neurogenesis in stroke
In addition to cell transplantation, targeted at replacing lost neurons and/or providing paracrine effects to protect penumbral tissue, is the possibility of stimulating endogenous repair. Within the adult brain, two discrete niches, the subventricular zone of the lateral ventricle and the dentate gyrus of the hippocampus, provide a pool of slowly dividing stem cells. Following stroke, these niche sites up‐regulate their production of stem cells, with new cells migrating into the injured tissue (Figure 1). Reports are conflicting as to whether these new progenitors are capable of differentiating into functionally integrated neurons, appropriate to the tissue in which they reside, or whether they act as trophic support cells (Arvidsson et al., 2002; Kokaia and Lindvall, 2012; Parent et al., 2002; Wright et al., 2016; Zhang et al., 2010). Nevertheless, it is recognized that the rate of turnover of these new cells is insufficient to promote repair. Consequently, strategies to augment the neurogenic response to a level that may be therapeutically relevant, inclusive of promoting migration of neuroblasts into the injured tissue and their neuronal differentiation, could see self‐repair strategies for brain repair. A number of studies have improved the level of injury‐induced neurogenesis by delivery of growth factors, morphogens and inflammatory‐modulating molecules (see reviews – Lindvall and Kokaia, 2011, 2015). However, delivery approaches are not trivial in the context of necrotic injuries. The lack of substratum makes efforts to deliver and maintain protein expression at the primary injury site and adjacent penumbra a challenge. Added to this, many recombinant proteins rapidly diffuse away from the site of delivery and are often rapidly degraded, thereby requiring continual infusion (such as via cumbersome pumps and catheters that risk blockage). Viral vectors require delivery into the already vulnerable penumbra, risking further damage, as well as risks of off‐target gene expression and the associated concerns of persistent and/or elevated delivery, beyond the necessary therapeutic window, that could impart negative effects on regeneration. Hence, while various proteins can enhance neurogenesis, new strategies to control temporal and spatial delivery could significantly increase the potential of endogenous neurogenesis‐based repair.
In the following sections, we present an overview of the many types of biomaterials and how they may be employed to promote neural repair by rebuilding tissue architecture, supporting implanted and residual host cells (inclusive of promoting stem cell proliferation, differentiation, migration and plasticity), stimulating neurogenesis and delivering functional proteins.
Biomaterials as tissue engineering scaffolds
Tissue engineering scaffolds have been fabricated from both organic and inorganic materials that have been engineered to interface with specific biological systems (application dependent) to advance regenerative outcomes. In the context of cell‐based therapies for neural repair, the hope is that biomaterials will overcome or reduce many of the challenges currently impeding the field. These bioengineered scaffolds are rationally designed to restore tissue architecture where required, provide physical support for cells (during differentiation and/or implantation), as well as deliver relevant trophic cues to new and residual host cells with spatial and temporal control (Figure 2). To achieve such goals, it will be important to identify materials that are stable, biocompatible/minimally immunogenic and easy to deliver in vivo. Neural tissue engineering exploits scaffold design, material selection and scaffold morphology to synergistically direct and control cell survival, proliferation, differentiation and/or integration of neural cells – whether they are host or graft‐derived.
Figure 2.
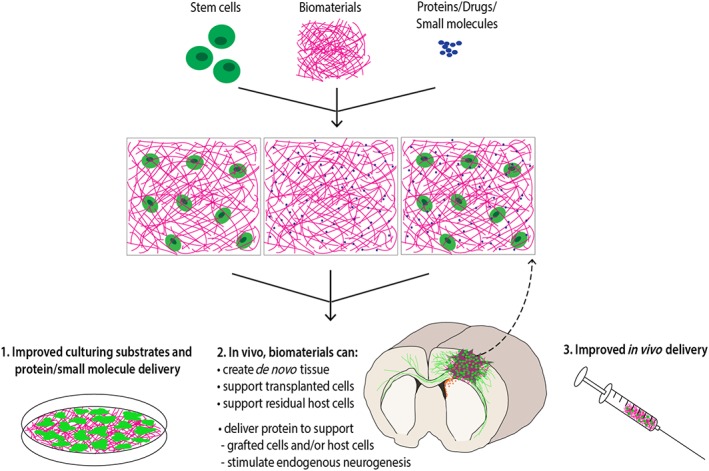
Applications for biomaterials in neural repair. Following disease or injury, biomaterials can be used to rebuild tissue architecture; they can be blended together with cells and/or incorporate functional proteins, small molecules and drugs. By providing physical and chemical biomimetics of the neural environment, these biomaterials can enhance the survival, differentiation and plasticity of neural cells in vitro, as well as following transplantation. These attributes can also contribute to enhanced endogenous neurogenesis. Hydrogel scaffolds can provide additional benefits, reducing shear forces exerted on cells during injection into the host tissue.
While there is a wide array of biomaterials used as tissue engineering scaffolds, electrospun and hydrogel materials are the most common. Electrospinning is performed by dissolving the intended polymer in an aqueous solution, subsequently applying an electric charge as the solution is slowly dispensed out of a needle. The electrostatic repulsion that occurs as the charge accumulates on the solution droplet at the end of the needle is enough to overcome surface tension and causes a thin stream of solution to jet out towards a grounded collector. The solvent evaporates during flight, and a sheet of polymer nanofibres is formed (Figure 3Ai). Electrospun nanofibrous scaffolds provide an ideal simulation of neural tissue as fibre alignment (Figure 3Aii–iii), diameter and interfibre distance can be regulated to generate a surface permissive for neural cell adhesion and axon support (Nisbet et al., 2007, 2009b, 2008). By way of example, we have shown, with poly‐ε‐caprolactone (PCL), that these nanofibre scaffolds can enhance cell survival, differentiation and/or axon growth in vitro, as well as influence both host‐ and graft‐derived neurons in the intact brain (Nisbet et al., 2009b; Horne et al., 2010; Wang et al., 2012). However, the bulkiness of these scaffold sheets makes them difficult to deliver in vivo and consequently more popular for use in vitro or for application in nerve repair. In the peripheral nervous system and spinal cord injury, the bandaging/wrapping potential of electrospun materials can be used to form a cylindrical nerve conduit (Schaub et al., 2016). Also, aligned fibres (formed using a rotating or oscillating collector during electrospinning) can provide directional guidance to cells. When poly‐l‐lactic acid (PLLA) nerve conduits were grafted into a 3 mm thoracic spinal cord gap in rats, electrospun conduits allowed infiltration of endogenous cells, and the gaps were closed, noting that the aligned PLLA material produced the longest axonal regeneration compared to randomly oriented electrospun fibres and a non‐electrospun film (Hurtado et al., 2011).
Figure 3.
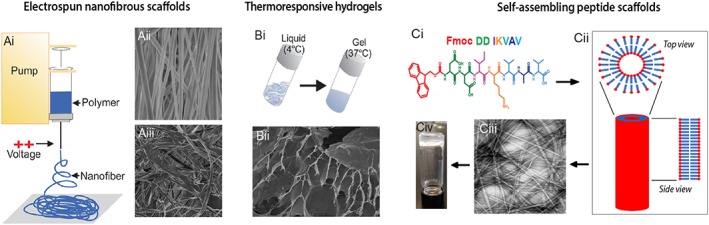
Biomaterials employed in neural repair. (Ai) Electrospun scaffolds are fine nanofibrous meshes formed by the axial stretching of a viscoelastic polymer solution under an applied voltage. Modifications in synthesis can result in scaffold sheets with (Aii) aligned or (Aiii) random fibres that can influence cellular differentiation and neurite growth. (Bi) Hydrogels are microporous scaffolds that consist of hydrophilic polymer chains interspersed in water. Thermoresponsive hydrogels can be biologically advantageous for in vivo delivery; being liquid at one temperature (e.g. 4°C) yet gelling at another (37°C). (Bii) TEM image of a xyloglucan hydrogel. (C) Self‐assembling peptide (SAP) scaffolds result from non‐covalent intermolecular forces that result in the formation of organized scaffolds. (Ci) Example peptide sequence for a laminin epitope IKVAV. (Cii) Schematic of the organized assembly of peptides. (Ciii) TEM of an IKVAV‐SAP and (Civ) demonstration of its gel properties, under defined pH and shear‐force conditions.
Hydrogels are three‐dimensional (3D) networks of hydrated polymers. As the two main components of the extracellular matrix (ECM) are nanofibrous proteins and proteoglycan hydrogels (Frantz et al., 2010), these two biomaterials are each well suited to mimic the extracellular environment. Numerous groups have fabricated a variety of hydrogel‐based biomaterials targeted at modelling the native 3D neural environment, repeatedly demonstrating that neural progenitors/neurons cultured on these scaffolds adopt more in vivo‐like morphologies, differentiate and survive longer than those cultured on conventionally coated culture‐ware. Many of these biocompatible scaffolds have also been shown to enhance survival and integration of transplanted neural progenitors (Rodriguez et al., 2012; Moriarty et al., 2018a).
Hydrogels are often a more attractive option for use in the brain than electrospun nanofibres. Their fluid‐like nature and 3D structure render them ideal for filling irregular voids and interfacing with surrounding tissue, and they can provide an aqueous environment in which cells can interact, without disrupting the local paranchyma (Figure 3B). Studies have demonstrated that the co‐implantation of fetal and human ESC‐derived neural progenitors together with matrigel or collagen‐based hydrogels can promote the survival and neuronal differentiation of transplanted cells while additionally reducing host inflammation and overall infarct volume in the stroke‐injured brain (Jin et al., 2010; Yu et al., 2010; Zhong et al., 2010). Thinning hydrogels, either thermoresponsive or mechanically shear thinning, are particularly attractive. Thermoresponsive hydrogels change mechanical properties with temperature. For example, xyloglucan is a plant‐derived thermoresponsive polysacharide hydrogel that flows readily at 4°C, enabling injection into the paranchyma, but stiffens into a gel at 37°C (Figure 3Bi). Studies have demonstrated that this biocompatible hydrogel is capable of supressing the host inflammatory responses (Nisbet et al., 2009a, 2010), and enhancing the integration of fetal neural transplants in rodent models of PD, by influencing neural progenitor survival, differentiation and axonal plasticity (Wang et al., 2016). Similarly, collagen, another thermoresponsive hydrogel, has also been reported to dampen the host immune response to encapsulated cells (Hoban et al., 2013; Moriarty et al., 2017) while also enhancing their survival and function after transplantation (Moriarty et al., 2018a, 2017). Physical hydrogels (those with non‐covalent crosslinks) can undergo shear thinning, in which the application of shear stress (such as that applied during injection via needle) causes the hydrogel to flow readily, and stiffen into a gel once the stress is removed. These mechanisms are appealing for neural implantation because cells can be mixed into the liquid hydrogel just prior to injection. On injection, the fluid flows to fill extracellular spaces in the brain, filling irregular voids (where required) and interfacing fully with surrounding tissue, then promptly self‐assembling within minutes into a stiffer gel that can be tailored to match the mechnical properties of the brain, and support surrounding host, or newly implanted cells. Such materials [including hyaluronic acid (HA) and collagen gels] have been shown to support both fetal and human PSC‐derived progenitors in vitro and cell engraftment in PD and stroke models (Jin et al., 2010; Yu et al., 2010; Zhong et al., 2010; Adil et al., 2017; Moriarty et al., 2018a, 2017; see also reviews – Jendelova et al., 2016; Moriarty and Dowd, 2018c; Moriarty et al., 2018b).
An additional promising subclass of tissue engineering scaffolds are self‐assembling peptide (SAP) hydrogel scaffolds that have the advantageous physical and morphological attributes of both electrospun scaffolds and hydrogels (Rodriguez et al., 2013) (Figure 3C). These materials are formed from specifically engineered peptide molecules that spontaneously self‐assemble into supramolecular nanofibrous structures. This forms a hydrogel network that offers physical support mimetic of the natural ECM. The design of SAPs initially focused on promoting self‐assembly, for instance, the use of repeating small and alternating hydrophobic/hydrophilic amino acid sequences in RADA16 to encourage β‐sheet interactions, to achieve the physically supportive nanofibre structures (Cormier et al., 2013). These physical hydrogels are also shear thinning, meaning they readily flow as liquids when shear stress is applied, and return to a stiffer gel when the force is removed (Rodriguez et al., 2013). More recently, SAPs have been further engineered to provide biological support as well, by including relevant protein epitopes within the SAP sequence (Rodriguez et al., 2013). The importance of specific biological sequences in SAPs was confirmed by comparing the fibronectin epitope arginyl‐glycyl‐aspartyl (RGD) to a biologically inert but structurally equivalent scrambled sequence (DGR), which resulted in a reduction by 60% of cell viability when employed as a substrate in vitro (Modepalli et al., 2014). In addition, these semi‐synthetic materials are xenogeneic‐free, inherently less batch‐variable (compared to many in vitro and in vivo used products such as matrigel and geltrex) and degrade into predictable metabolites.
The potential of these SAP scaffolds is only now becoming fully appreciated by neural regenerative researchers. While biologists have invested significant amounts of effort to understand the proteins and genes that underpin direct differentiation and graft integration, notably less time has been spent understanding the function of the ECM molecules. By way of example, laminins, the major ECM proteins in the brain and once thought of as merely cell adhesion molecules, are now receiving increasing attention due to their influences on stem cell maintenance, survival, differentiation and plasticity (Theocharidis et al., 2014). Of more specific relevance here, laminin has been shown to promote survival of dopaminergic neurons during murine development (by suppressing the cell death associated protein, PTEN), and dopamine differentiation (by increasing the expression of genes critical for VM dopamine identity, including LMX1A and PITX3) (Zhang et al., 2017). Added to this, recent protocols for the specification of human PSCs into restricted neuronal fates (including cortical and dopaminergic) have highlighted the benefit of culturing on laminin substrates (Lancaster et al., 2013; Kirkeby et al., 2017a, 2017b; Niclis et al., 2017a).
In the light of this, we, and others, have explored fabricating SAPs that present epitopes for the major components of the brain's ECM, namely, fibronectin and laminin. These studies have demonstrated that the laminin‐based amino acid sequences isoleucine‐lysine‐valine‐alanine‐valine (IKVAV; Figure 3Ci) and tyrosine‐isoleucine‐glycine‐serine‐arginine (YIGRS) as well as the RGD fibronectin epitope are capable of positively influencing the survival, proliferation and differentiation of both fetal and/or human PSC‐derived dopaminergic and cortical progenitors in vitro as well as following transplantation (Silva et al., 2004; Rodriguez et al., 2014, 2018; Somaa et al., 2017). Interestingly, the presentation of these short epitopes has been shown to enhance neuronal adhesion, differentiation and axonal growth of neural progenitors with greater efficiency than the full‐length proteins themselves, due to the high density of signals that can be presented (Silva et al., 2004). Most recently, implantation of this IKVAV SAP together with human ESC‐derived cortical progenitors has been shown to induce functional recovery in stroke models, superior to cell grafts alone, by mechanisms that include increased graft survival/size and neuronal differentiation. These new neurons, in the presence of the laminin‐based SAP, displayed more maturity, reflected in their morphology and electrophysiological function. Interestingly, not only did this tissue‐specific biomaterial support the implanted cells, but greater angiogenesis and reduced secondary degeneration were also observed (Somaa et al., 2017), contributing to enhanced motor function recovery.
Hydrogels and electrospun materials can also be used together in composite biomaterials to achieve the benefits of both material classes. However, the composites inherently combine the material drawbacks as well, which often renders them unsuitable for injection and, more common, in spinal cord injury. A composite of aligned poly ε‐caprolactone‐co‐ethyl ethylene phosphate electrospun and collagen hydrogel materials was used to treat spinal cord injury in rats. The materials also incorporated controlled neurotrophin delivery, resulting in aligned axon regeneration from endogenous cells (Nguyen et al., 2017). To maintain the desirable injectability of hydrogels within a hydrogel/electrospun composite, the electrospun scaffold can be cut into loose, short fibres that can then be mixed within hydrogels. This technique has recently been used to create a composite of electrospun PCL fibres within a thermoresponsive xyloglucan hydrogel for use in a rodent model of PD, where it was shown to improve the integration of fetal tissue grafts (Wang et al., 2016). A similar composite of PLA fibres within an IKVAV SAP hydrogel has also been developed and tested in vitro (Bruggeman et al., 2017). Both of these short fibre composite materials were also used to provide controlled growth factor delivery and are discussed further in the relevant section below.
Scaffold biodegradation
In addition to considerations surrounding the biocompatibility of biomaterials employed in tissue repair are issues pertaining to the longevity of presentation requirements and/or impact following scaffold degradation (Maclean et al., 2016). It is often assumed that biodegradable scaffolds will be most advantageous, given the perception that the long‐term presence of a foreign body in the CNS will be invasive and may cause additional tissue damage (Maclean et al., 2016). However, the employment of biodegradable scaffolds requires specific material engineering design to ensure that cytotoxic molecules are not released during scaffold degradation. In these instances, biomaterials such as SAPs may be most optimal, as they degrade into non‐toxic amino acids, that can provide the added benefit of being metabolically utilized by adjacent cells (Ellis‐Behnke et al., 2006).
It is also critical that the rate of degradation will allow the structural integrity to be maintained, such that the scaffold supports the host tissue and/or implanted cells for a sufficient duration to avoid collapse and loss of functional capacity. The question of whether a biodegradable or non‐biodegradable material should be used in repair will depend on the type of neural injury. There have been many preclinical spinal cord injury studies where filling the injury cavity alone has resulted in improved outcomes (see review – Nomura et al., 2006). In this context, a non‐degradable scaffold that provides a bridging network for the support of axonal regeneration may be more optimal. The requirements for stroke are similar – where decreasing the lesion volume can result in significant functional improvement through the support of both endogenous and grafted cells (Somaa et al., 2017; Nisbet et al., 2018; Tuladhar et al., 2018). In the context of more discrete injuries such as PD and Huntington's disease, a scaffold that provides physical and chemical support for the survival and differentiation of cells upon implantation, but then slowly degrades once the cells have appropriately integrated within the host circuitry, may be a desirable alternative. As such, it is clear that the development of scaffolds for cell therapy should be tailored to meet the specific needs and requirements for each given neural injury.
Support during injection
In addition to the physical support offered by biomaterial scaffolds in situ, they can also provide support and protection during delivery. Specifically, hydrogels have been used to reduce damaging shear forces experienced by cells during injection (Figure 4). The laminar flow dynamics of Newtonian liquids [which includes saline solutions and media, typically used in current clinical procedures (Foster et al., 2017)] during injection result in varying flow rates, with the highest flow rate in the middle of the needle (Amer et al., 2017). This results in cell stretching as the cells experience different velocities (Chisti, 2001). These shear forces are believed to cause rupture in cell membranes, which, along with shear stress and high pressure, causes cell death post‐injection (Aguado et al., 2012; Foster et al., 2017). Hydrogels, and in particular shear‐thinning hydrogels, can ameliorate these effects by providing a more consistent flow profile throughout the needle during injection (Amer et al., 2017). By way of example, a chitosan‐based shear‐thinning hydrogel was shown to significantly increase survival of rat NSCs 1, 3 and 5 days post‐injection through a 21 guage needle (Wei et al., 2016). This is particularly applicable to the transplantation of vulnerable cell types, such as dopaminergic progenitors, which experience significant cell death after implantation (Boonman and Isacson, 1999; Zawada et al., 1988).
Figure 4.
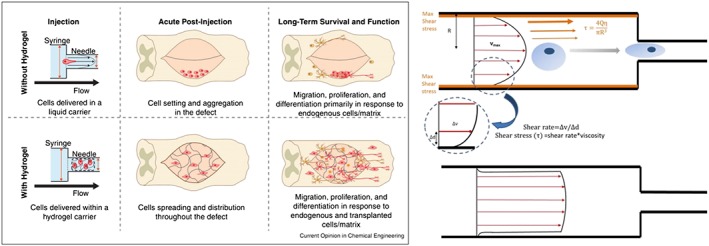
Shear forces on cell during injection. Left: cells injected using a hydrogel (Foster et al., 2017). Right: forces/flow in a shear‐thinning hydrogel (Amer et al., 2017).
Utilizing biomaterials to deliver trophic support in vitro and in vivo
In vitro, a cocktail of small molecules and proteins are supplemented into the culture media to promote neural induction and regional specification into lineage‐restricted neuronal subpopulations such as midbrain dopaminergic or cortical progenitors. In vivo, numerous proteins such as neurotrophins, morphogens, chemokines and axon guidance molecules have been demonstrated to influence survival, differentiation and plasticity of new and residual stem cells (Chilton, 2006; Tayalia and Mooney, 2009; Moshayedi et al., 2016; Rodriguez et al., 2018; Bruggeman et al., 2018). However, even under optimal conditions, differentiations result in heterogeneous populations inclusive of proliferating progenitors, immature and mature neurons of the desired phenotype, as well as off‐target populations. In vivo, survival, differentiation and plasticity also remain below optimal levels. Underpinning these outcomes is likely the lack of stability of many of these proteins – with basic FGF (FGF‐2) having an elimination t 1/2 of 40 min (Edelman et al., 1993; Lazarous et al., 1997), while the t 1/2 of nerve growth factor (NGF) and brain‐derived neurotrophic factor (BDNF) in vivo is similarly short, at just 45 min and 2 h respectively (Krewson and Saltzman, 1996; Bruggeman et al., 2016). Consequently, despite frequent (commonly daily) media and protein changes, differentiating stem cell cultures are exposed to extreme dose fluctuations that likely underpin the asynchronistic and heterogenic differentiations.
Added to this, in the broader field of drug delivery, systemic administration (e.g. i.v.) is the norm, and targeted delivery from the bloodstream is one of the most significant areas of research focus – but using biomaterial scaffolds as localized drug reservoirs bypasses this issue. In the CNS, this localization also means bypassing the blood–brain barrier, which can inhibit the delivery of large or hydrophilic molecules from the bloodstream (Stockwell et al., 2014). Currently, direct and ongoing protein/drug delivery to the brain is achieved by i.c.v. (Paul et al., 2015) or intraparenchymal catheters (Tuladhar et al., 2018), which are invasive and ongoing, and cause iatrogenic injury. Delivery of viral vectors, to sustain protein delivery from transduced cells, has also been explored; however, it presents further challenges in controlling the temporal and spatial delivery, as well as dose. Consistency in delivered dose is an especially important factor to consider because actual fluctuations are not always clear from drug delivery data as conventionally displayed. Cumulative release profiles inherently fail to show the large natural variability of the in situ delivery rate. In this regard, the incorporation of drugs/proteins/small molecules into biomaterials, targeted for in vitro and in vivo use, provides a means for more tightly controlled (sustained and consistent) release. Methods of protein incorporation within biomaterials and examples of their application, in the context of neural repair, are described below.
Encapsulation of proteins within biomaterials
Hydrogel materials are very well suited to providing diffusion‐based protein delivery, with the exact release profile dependent on the hydrogel mesh size, degree of swelling and electrostatic properties, as well as the properties of the protein being delivered. Proteins can also be mixed directly into electrospun materials through solution or emulstion electrospinning, when the protein is soluble or insoluble respectively in the original polymer solution (Faccendini et al., 2017). Surprisingly, neither the organic solvent conditions of the polymer solution nor the electric current applied during electrospinning generally affect protein bioactivity. Insoluble proteins remain in the aqueous phase regions of the emulsion when electrospinning (Faccendini et al., 2017) and in the resulting fibres (Liu et al., 2018b), and the actual current involved in electrospinning is so low that electrospinning directly onto living tissue is a safe practice (Yan et al., 2016). NGF and GDNF have been emulsion electrospun within several polymers [poly‐l‐lactide‐co‐caprolactone, poly‐lacto‐co‐glycolic acid, poly‐dl‐lactic acid (PDLLA)], with bioactivity confirmed by increased neurite outgrowth and neural differentiation of PC12 cells in vitro (Li et al., 2010; Liu et al., 2018b). Aligned PLA fibres were emulsion electrospun with 6‐aminonicotinamide (6AN); the material demonstrated a 2 week sustained release and concomitantly reduced astrocyte activity (effect of 6AN) and directed dorsal root ganglia axonal outgrowth (effect of fibre alignment) (Schaub and Gilbert, 2011). A drawback of this technique is that emulsion electrospinning can affect the material properties of the fibre scaffold, including fibre diameter and alignment (Johnson et al., 2016).
Simple mixing of proteins into hydrogels generally provides a more sustained delivery than an injection of protein in solution. Loading soluble BDNF or EGF into HA hydrogels was shown to improve iPSC‐derived NPC survival in vitro (Moshayedi et al., 2016) and promote endogenous NSC proliferation in an animal model of stroke (Cooke et al., 2011; Wang et al., 2013). GDNF mixed directly into a collagen hydrogel with primary dopaminergic neurons resulted in dramatic increases in dopaminergic cell survival and host tissue reinnervation (Moriarty et al., 2018a, 2017). We recently demonstrated the benefit of delivering BDNF within an IKVAV SAP hydrogel to promote the survival and differentiation of human ESC‐derived cortical progenitors in a stroke model, also demonstrating increased vascularization of the graft and protection of the host tissue compared to BDNF delivery in the absence of the scaffold (Nisbet et al., 2018). A limitation of this approach, however, is that the exact duration and level of delivery are dependent on the interactions between the specific protein and the specific material – and are not inherently optimized. As such, more involved methods are required to tightly control delivery profiles. Several of the most prevalent designed protein delivery systems from biomaterials are discussed below.
Covalent and affinity immobilizations of proteins onto biomaterials
Immobilization delivery systems primarily focus on providing sustained and/or localized delivery. In these systems, proteins are attached to the biomaterial scaffold permanently or semi‐permanently as their diffusional release is significantly slowed. The attachment can be achieved via covalent bonding, chemical crosslinkers and/or strong affinities between biomolecules. Covalent bonding, with a chemical crosslinker, has been used to immobilize proteins such as BDNF and GDNF onto the nanofibres of electrospun scaffolds, resulting in sustained presentation over several months (Wang et al., 2014a, 2016, 2012). These functionalized scaffolds enhanced the survival, proliferation, differentiation and/or plasticity of rodent neural progenitors in vitro and following in vivo implantation (Wang et al., 2016, 2012; Rodriguez et al., 2018; Somaa et al., 2017). This is an effective method to provide long‐term presentation of extracellular signalling molecules that do not need to be internalized by cells to function. In this context, the duration of presentation becomes dependent on the material degradation timeframe.
Immobilization via affinity interactions is more common, as it requires minimal or no chemical modification of the protein of interest and can allow a controlled level of diffusion out of the material. Heparin binding is commonly used to achieve this, whereby a biomaterial is functionalized with heparin in order to semi‐immobilize any of the heparin‐binding growth factors – including PDGF, vascular endothelial growth factor, FGF, TGFβ and the neurotrophin families (Martino et al., 2013). In a rodent stroke model, such a heparin‐modified scaffold (an HA hydrogel) was used to retain BDNF, in an effort to support the survival and differentiation of transplantated human iPSC‐derived neural progenitors (Moshayedi et al., 2016). While the heparin system is very versatile, with so many heparin‐binding growth factors, its versatility is also one of the main drawbacks of the system. While the approach can be used for many proteins, it cannot be used to independently tune the release profiles of multiple proteins since each would have a release profile dictated by the heparin in the biomaterial. In the aforementioned stroke study, the relative concentrations of two growth factors were optimized before in vivo testing, but there was no attempt to independently control their release (Moshayedi et al., 2016). The prevelance of heparin binding among natural ECM proteins can also present complications in vivo, when host ECM proteins are abundant (Tuladhar et al., 2018). Consequently, newer stategies are being developed to provide more specific heparin binding, including the use of heparin fractions with protein‐specific affinity (Wang et al., 2014b, 2014a). Other affinity pairs (e.g. streptavidin‐biotin and barnase‐barstar) can also be used. A methacrylate‐chitosan hydrogel with covalently immobilized streptavidin mixed with biotinylated growth factors IFN‐γ and PDGF was tested in a hemisection spinal cord injury in rats and showed increased neuronal and oligodendrocyte differentiation respectively of implanted NSPCs (Li et al., 2016). Using multiple affinity immobilization pairs within a single material can allow spatial and dose control of multiple proteins (Wylie et al., 2011), but the temporal control is still limited. Immobilization systems all work by stopping or slowing diffusion but cannot provide sequential release.
Protein delivery vehicles
Using distinct protein delivery vehicles within tissue engineering scaffolds presents another protein delivery option. Nanoparticles are the most common delivery vehicle. An advantage of nanoparticle systems is the ease of their incorporation into both hydrogel and electrospun materials; they are mixed in directly like proteins but with added layers of control to delivery. Layers, specifically, are the most common method of controlling the delivery profile from nanoparticles. Particles are formed with different proteins in different layers, or with the addition of shielding layers, to provide additional diffusional barriers and delay release. A dual particle system, consisting of (i) PEG particles containing EGF and (ii) layered particles containing erythropoietin (EPO) in the core, shielded by an outer particle layer, were mixed into a hydrogel and used in a mouse stroke model to enhance endogenous neurogenesis. Here they demonstrated the capacity of EGF to promote stem cell proliferation, with subsequent, rather than simultaneous, EPO increasing neuroprotection – effects that were superior to direct protein infusion (Wang et al., 2013). The layered control mechanism can also be used with electrospun materials, using layered sheets of electrospun fibres containing different proteins. Layers of NGF‐ and GDNF‐loaded PDLLA were used to provide sequential delivery of growth factors in vitro (Liu et al., 2018a). A similar layered electrospun material has been used to delivery the neurotrophin NT‐3, BDNF and PDGF in a rodent model of crushed sciatic nerve. It was found that fast delivery of NT‐3 and BDNF followed by slow delivery of PDGF yielded the greatest nerve recovery (Hong et al., 2018). Interesting in this study was the use of layering to independently optimize material properties and protein delivery, with an aligned fibre PCL layer used as the outermost cell‐interfacing layer, and random PLGA fibres used in underlying protein reservoir layers (Hong et al., 2018).
Nanoparticles can also be used in encapsulation‐free protein delivery systems. Here, electrostatic surface interactions were sufficient to bind growth factors to the surface of polymer nanoparticles, allowing the particles to act as delivery vehicles, while protecting the protein growth factors from the harsh chemical conditions involved in encapsulation (Pakulska et al., 2016). This system requires complimentary surface charges between the polymer and the protein being delivered. While many proteins have been encapsulated and delivered without harm from the chemical process, this encapsulation‐free study is particularly interesting as it highlights how easily proteins can be immobilized by physical electrostatic forces. These interactions must be considered in other protein delivery and biomaterial scaffold systems, as they may affect the in situ delivery profiles.
Recently, short fibres cut from electrospun scaffolds have been prepared as protein delivery vehicles in electrospun‐hydrogel composite materials. PCL fibres with covalently immobilized GDNF in a xyloglucan hydrogel showed enhanced integration of cell grafts in a rodent model of PD (Wang et al., 2016). Emulsion electrospun PLA fibres loaded with GDNF achieved a 1 week delay in delivery in vitro when mixed into an IKVAV SAP hydrogel, while not interfering with sustained release from the hydrogel component of the composite (thus allowing temporally distinct delivery of multiple growth factors from a single material) (Bruggeman et al., 2017). These short fibre systems have potential benefits over nanoparticle systems in that they also provide the electrospun nanofibre structure that has been shown to support neural cells.
Conclusion
Recent research into the use of stem cells for neural repair has branched out in several directions: optimizing the cell populations used in the graft, providing appropriate support to the graft during and after implantation and more sophisticated protein delivery systems to provide trophic support. These are very diverse fields, with topics ranging from nanotechnology in biomaterial scaffold development to cell biology in the investigation of stem cell differentiation, and to fluid dynamics in the assessment of shear forces experienced during injection. While significant progress has been made in all of these directions, there are still gaps in the efficacy of stem cell treatments. Truly effective stem cell strategies will require a combined approach from all of these avenues of advancement, which is not a trivial matter. It has been noted in this review that many of the protein delivery strategies discussed affect the properties of the biomaterial scaffold used. This presents an obstacle to the concomitant optimization of physical and trophic support. Similarly, we have recently discovered (unpublished) that scaffolds providing optimal physical support during injection via a needle do not necessarily provide the best support post‐transplantation and vice versa. Protein delivery systems are predominantly at the stage of single‐protein delivery, and when multiple proteins are delivered, the dose and timing is not optimized. Results are often described in terms of significant improvements rather than optimization or achieving ideal conditions.
Part of this is a paucity of knowledge as to what defines the optimal conditions. There is a significant gap between in vitro and in vivo results and a limited understanding of how the varying aspects of a biomaterial or stem cell therapy methods affect each other. There has been some work using the design of experiments approach to more systematically address this problem and assess/optimize many variables at once (Moshayedi et al., 2016), and more work like this will certainly advance the field. Stem cell therapy is a multi‐faceted topic, and the vastness of the future directions is itself a step forward. The field has progressed significantly in identifying more areas that impact on stem cell therapies, which will ultimately allow for a significant improvement in these methods. There are many active avenues of research in the field and exciting advancements being made.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
Conflict of interest
The authors declare no competing interests.
Acknowledgement
C.L.P. was supported by a Viertel Charitable foundation senior research fellowship, Australia.
Bruggeman, K. F. , Moriarty, N. , Dowd, E. , Nisbet, D. R. , and Parish, C. L. (2019) Harnessing stem cells and biomaterials to promote neural repair. British Journal of Pharmacology, 176: 355–368. 10.1111/bph.14545.
References
- Adil MM, Vazin T, Ananthanarayanan B, Rodrigues GMC, Rao AT, Kulkarni RU et al (2017). Engineered hydrogels increase the post‐transplantation survival of encapsulated hESC‐derived midbrain dopaminergic neurons. Biomaterials 136: 1–11. [DOI] [PubMed] [Google Scholar]
- Aguado BA, Mulyasasmita W, Su J, Lampe KJ, Heilshorn SC (2012). Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A 18: 806–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J (1962). Are new neurons formed in the brains of adult mammals? Science 135: 1127–1128. [DOI] [PubMed] [Google Scholar]
- Amer MH, Rose FRAJ, Shakesheff KM, Modo M, White LJ (2017). Translational considerations in injectable cell‐based therapeutics for neurological applications: concepts, progress and challenges. npj Regenerative Medicine 2: 23 10.1038/s41536-017-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O (2002). Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8: 963–970. [DOI] [PubMed] [Google Scholar]
- Bacigaluppi M, Pluchino S, Martino G, Kilic E, Hermann DM (2008). Neural stem/precursor cells for the treatment of ischemic stroke. J Neurol Sci 265: 73–33. [DOI] [PubMed] [Google Scholar]
- Barker RA, Parmar M, Studer L, Takahashi J (2017). Human trials of stem cell‐derived dopamine neurons for Parkinson's disease: dawn of a new era. Cell Stem Cell 21: 569–573. [DOI] [PubMed] [Google Scholar]
- Boonman Z, Isacson O (1999). Apoptosis in neuronal development and transplantation: role of caspases and trophic factors. Exp Neurol 156: 1–5. [DOI] [PubMed] [Google Scholar]
- Bruggeman KF, Rodriguez AL, Parish CL, Williams RJ, Nisbet DR (2016). Temporally controlled release of multiple growth factors from a self‐assembling peptide hydrogel. Nanotechnology 27: 385102. [DOI] [PubMed] [Google Scholar]
- Bruggeman KF, Wang Y, Maclean FL, Parish CL, Williams RJ, Nisbet DR (2017). Temporally controlled growth factor delivery from a self‐assembling peptide hydrogel and electrospun nanofibre composite scaffold. Nanoscale 9: 13661–13669. [DOI] [PubMed] [Google Scholar]
- Bruggeman KF, Williams RJ, Nisbet DR (2018). Dynamic and responsive growth factor delivery from electrospun and hydrogel tissue engineering materials. Adv Healthc Mater 7 10.1002/adhm.201700836. [DOI] [PubMed] [Google Scholar]
- Castilho RF, Hansson O, Brundin P (2000). Improving the survival of grafted embryonic dopamine neurons in rodent models of Parkinson's disease. Prog Brain Res 127: 203–231. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L (2009). Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 27: 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan‐Ling T, Daadi MM, Maag A‐L, Steinberg GK (2008). Adherent self‐renewable human embryonic stem cell‐derived neural stem cell line: functional engraftment in experimental stroke model. PLoS ONE 3: e1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton JK (2006). Molecular mechanisms of axon guidance. Dev Biol 292: 13–24. [DOI] [PubMed] [Google Scholar]
- Chisti Y (2001). Hydrodynamic damage to animal cells. Crit Rev Biotechnol 21: 67–110. [DOI] [PubMed] [Google Scholar]
- Cooke MJ, Wang Y, Morshead CM, Shoichet MS (2011). Controlled epi‐cortical delivery of epidermal growth factor for the stimulation of endogenous neural stem cell proliferation in stroke‐injured brain. Biomaterials 32: 5688–5697. [DOI] [PubMed] [Google Scholar]
- Cormier ARP, Xiaodong, Zimmerman MI, Zhou H‐X, Paravastu AK (2013). Molecular structure of RAdopamine16‐I designer self‐assembling peptide nanofibers. ACS Nano 7: 7562–7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham M, Parish CL, Leaw B, Wright J, Reid CA, Petrou S et al (2012). Neurons derived from human embryonic stem cells extend long‐distance axonal projections through growth along host white matter tracts after intra‐cerebral transplantation. Front Cell Neurosci 6: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi D, Samata B, Katsukawa M, Kikuchi T, Morizane A, Ono Y et al (2014). Isolation of human induced pluripotent stem cell‐derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Reports 2: 337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman ER, Nugent MA, Karnovsky MJ (1993). Perivascular and intravenous administration of basic fibroblast growth factor: vascular and solid organ deposition. Proc Natl Acad Sci U S A 90: 1513–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis‐Behnke RG, Liang YX, You SW, Tay DK, Zhang S, So KF et al (2006). Nano neuro knitting: peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision. Proc Natl Acad Sci U S A 103: 5054–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espuny‐Camacho I, Michelsen KA, Gall D, Linaro D, Hasche A, Bonnefont J et al (2013). Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo . Neuron 77: 440–456. [DOI] [PubMed] [Google Scholar]
- Faccendini A, Vigani B, Rossi S, Sandri G, Bonferoni MC, Caramella CM et al (2017). Nanofiber scaffolds as drug delivery systems to bridge spinal cord injury. Pharmaceuticals (Basel) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster AA, Marquardt LM, Heilshorn SC (2017). The diverse roles of hydrogel mechanics in injectable stem cell transplantation. Curr Opin Chem Eng 15: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz C, Stewart KM, Weaver VM (2010). The extracellular matrix at a glance. J Cell Sci 123: 4195–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomi M, Takagi Y, Morizane A, Doi D, Nishimura M, Miyamoto S et al (2012). Functional recovery of the murine brain ischemia model using human induced pluripotent stem cell‐derived telencephalic progenitors. Brain Res 1459: 52–60. [DOI] [PubMed] [Google Scholar]
- Grealish S, Diguet E, Kirkeby A, Mattsson B, Heuer A, Bramoulle Y et al (2014). Human ESC‐derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson's disease. Cell Stem Cell 15: 653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoban DB, Newland B, Moloney TC, Howard L, Pandit A, Dowd E (2013). The reduction in immunogenicity of neurotrophin overexpressing stem cells after intra‐striatal transplantation by encapsulation in an in situ gelling collagen hydrogel. Biomaterials 34: 9420–9429. [DOI] [PubMed] [Google Scholar]
- Hong M‐H, Hong HJ, Pang H, Lee H‐J, Yi S, Koh W‐G (2018). Controlled release of growth factors from multilayered fibrous scaffold for functional recoveries in crushed sciatic nerve. ACS Biomater Sci Eng 4: 576–586. [DOI] [PubMed] [Google Scholar]
- Horne MK, Nisbet DR, Forsythe JS, Parish CL (2010). Three‐dimensional nanofibrous scaffolds incorporating immobilized BDNF promote proliferation and differentiation of cortical neural stem cells. Stem Cells Dev 19: 843–852. [DOI] [PubMed] [Google Scholar]
- Hurtado A, Cregg JM, Wang HB, Wendell DF, Oudega M, Gilbert RJ et al (2011). Robust CNS regeneration after complete spinal cord transection using aligned poly‐L‐lactic acid microfibers. Biomaterials 32: 6068–6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendelova P, Kubinova S, Sandvig I, Erceg S, Sandvig A, Sykova E (2016). Current developments in cell: and biomaterial‐based approaches for stroke repair. Expert Opin Biol Ther 16: 43–56. [DOI] [PubMed] [Google Scholar]
- Jensen MB, Yan H, Krishnaney‐Davison R, Al Sawaf A, Zhang SC (2013). Survival and differentiation of transplanted neural stem cells derived from human induced pluripotent stem cells in a rat stroke model. J Stroke Cerebrovasc Dis 22: 304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Lv L, Ji H, Yang X, Zhu W, Cai L et al (2011). Induction of pluripotent stem cells transplantation therapy for ischemic stroke. Mol Cell Biochem 354: 67–75. [DOI] [PubMed] [Google Scholar]
- Jin K, Mao X, Xie L, Galvan V, Lai B, Wang Y et al (2010). Transplantation of human neural precursor cells in Matrigel scaffolding improves outcome from focal cerebral ischemia after delayed postischemic treatment in rats. J Cereb Blood Flow Metab 30: 534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CD, D'Amato AR, Gilbert RJ (2016). Electrospun fibers for drug delivery after spinal cord injury and the effects of drug incorporation on fiber properties. Cells Tissues Organs 202: 116–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M et al (2012). Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep 1: 703–714. [DOI] [PubMed] [Google Scholar]
- Kirkeby A, Nolbrant S, Tiklova K, Heuer A, Kee N, Cardoso T et al (2017a). Predictive markers guide differentiation to improve graft outcome in clinical translation of hESC‐based therapy for Parkinson's disease. Cell Stem Cell 20: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkeby A, Parmar M, Barker RA (2017b). Strategies for bringing stem cell‐derived dopamine neurons to the clinic. Prog Brain Res 230: 165–190. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Lindvall O (2012). Stem cell repair of striatal ischemia. Prog Brain Res 201: 35–53. [DOI] [PubMed] [Google Scholar]
- Krewson CE, Saltzman WM (1996). Transport and elimination of recombinant human NGF during long‐term delivery to the brain. Brain Res 727: 169–181. [DOI] [PubMed] [Google Scholar]
- Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z et al (2011). Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature 480: 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME et al (2013). Cerebral organoids model human brain development and microcephaly. Nature 501: 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarous DF, Shou M, Stiber JA, Dadhania DM, Thirumurti V, Hodge E et al (1997). Pharmacodynamics of basic fibroblast growth factor: route of administration determines myocardial and systemic distribution. Cardiovasc Res 36: 78–85. [DOI] [PubMed] [Google Scholar]
- Li H, Ham TR, Neill N, Farrag M, Mohrman AE, Koenig AM et al (2016). A hydrogel bridge incorporating immobilized growth factors and neural stem/progenitor cells to treat spinal cord injury. Adv Healthc Mater 5: 802–812. [DOI] [PubMed] [Google Scholar]
- Li X, Su Y, Liu S, Tan L, Mo X, Ramakrishna S (2010). Encapsulation of proteins in poly(L‐lactide‐co‐caprolactone) fibers by emulsion electrospinning. Colloids Surf B Biointerfaces 75: 418–424. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z (2010). Stem cells in human neurodegenerative disorders–time for clinical translation? J Clin Invest 120: 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z (2011). Stem cell research in stroke: how far from the clinic? Stroke 42: 2369–2375. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z (2015). Neurogenesis following stroke affecting the adult brain. Cold Spring Harb Perspect Biol 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li X, Xu F, Cong H, Li Z, Song Y et al (2018a). Spatio‐temporal release of NGF and GDNF from multi‐layered nanofibrous bicomponent electrospun scaffolds. J Mater Sci Mater Med 29: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Wang C, Zhao Q, Li X, Xu F, Yao X et al (2018b). Incorporation and release of dual growth factors for nerve tissue engineering using nanofibrous bicomponent scaffolds. Biomed Mater 13: 044107. [DOI] [PubMed] [Google Scholar]
- Maclean FL, Rodriguez AL, Parish CL, Williams RJ, Nisbet DR (2016). Integrating biomaterials and stem cells for neural regeneration. Stem Cells Dev 25: 214–226. [DOI] [PubMed] [Google Scholar]
- Martino MM, Briquez PS, Ranga A, Lutlolf M, Hubbell JA (2013). Heparin‐binding domain of fibrin (ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. PNAS 110: 4563–4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modepalli VN, Rodriguez AL, Li R, Pavuluri S, Nicholas KR, Barrow CJ et al (2014). In vitro response to functionalized self‐assembled peptide scaffolds for three‐dimensional cell culture. Biopolymers 102: 197–205. [DOI] [PubMed] [Google Scholar]
- Moriarty N, Cabré S, Alamilla V, Pandit A, Dowd E (2018a). Encapsulation of young donor age dopaminergic grafts in a GDNF‐loaded collagen hydrogel further increases their survival, re‐innervation and functional efficacy after intra‐striatal transplantation in hemi‐Parkinsonian rats. Eur J Neurosci; 10.1111/ejn.14090. [DOI] [PubMed] [Google Scholar]
- Moriarty N, Dowd E (2018c). Brain repair for Parkinson's disease: is the answer in the matrix? Neural Regen Res 13: 1187–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty N, Pandit A, Dowd E (2017). Encapsulation of primary dopaminergic neurons in a GDNF‐loaded collagen hydrogel increases their survival, re‐innervation and function after intra‐striatal transplantation. Sci Rep 7: 16033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty N, Parish CL, Dowd E (2018b). Primary tissue for cellular brain repair in Parkinson's disease: promise, problems and the potential of biomaterials. Eur J Neurosci; 10.1111/ejn.14051. [DOI] [PubMed] [Google Scholar]
- Moshayedi P, Nih LR, Llorente IL, Berg AR, Cinkornpumin J, Lowry WE et al (2016). Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials 105: 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LH, Gao M, Lin J, Wu W, Wang J, Chew SY (2017). Three‐dimensional aligned nanofibers‐hydrogel scaffold for controlled non‐viral drug/gene delivery to direct axon regeneration in spinal cord injury treatment. Sci Rep 7: 42212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niclis JC, Gantner CW, Alsanie WF, McDougall SJ, Bye CR, Elefanty AG et al (2017a). Efficiently specified ventral midbrain dopamine neurons from human pluripotent stem cells under xeno‐free conditions restore motor deficits in Parkinsonian rodents. Stem Cells Transl Med 6: 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niclis JC, Gantner CW, Hunt CPJ, Kauhausen JA, Durnall JC, Haynes JM et al (2017b). A PITX3‐EGFP reporter line reveals connectivity of dopamine and non‐dopamine neuronal subtypes in grafts generated from human embryonic stem cells. Stem Cell Reports 9: 868–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niclis JC, Turner C, Durnall J, McDougal S, Kauhausen JA, Leaw B et al (2017c). Long‐distance axonal growth and protracted functional maturation of neurons derived from human induced pluripotent stem cells after intracerebral transplantation. Stem Cells Transl Med 6: 1547–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbet DR, Moses D, Gengenbach TR, Forsythe JS, Finkelstein DI, Horne MK (2009a). Enhancing neurite outgrowth from primary neurones and neural stem cells using thermoresponsive hydrogel scaffolds for the repair of spinal cord injury. J Biomed Mater Res A 89A: 24–35. [DOI] [PubMed] [Google Scholar]
- Nisbet DR, Pattanawong S, Ritchie NE, Shen W, Finkelstein DI, Horne MK et al (2007). Interaction of embryonic cortical neurons on nanofibrous scaffolds for neural tissue engineering. J Neural Eng 4: 35–41. [DOI] [PubMed] [Google Scholar]
- Nisbet DR, Rodda AE, Horne MK, Forsythe JS, Finkelstein DI (2009b). Neurite infiltration and cellular response to electrospun polycaprolactone scaffolds implanted into the brain. Biomaterials 30: 4573–4580. [DOI] [PubMed] [Google Scholar]
- Nisbet DR, Rodda AE, Horne MK, Forsythe JS, Finkelstein DI (2010). Implantation of functionalized thermally gelling xyloglucan hydrogel within the brain: associated neurite infiltration and inflammatory response. Tissue Eng Part A 16: 2833–2842. [DOI] [PubMed] [Google Scholar]
- Nisbet DR, Wang TY, Bruggeman KF, Niclis JC, Somaa FA, Penna V et al (2018). Shear containment of BDNF within molecular hydrogels promote human stem cell engraftment and post‐infarction remodeling in stroke. Adv Biosyst 2: 1800113. 10.1002/adbi.201800113. [DOI] [Google Scholar]
- Nisbet DR, Yu LM, Zahir T, Forsythe JS, Shoichet MS (2008). Characterization of neural stem cells on electrospun poly (epsilon‐caprolactone) submicron scaffolds: evaluating their potential in neural tissue engineering. J Biomater Sci Polym Ed 19: 623–634. [DOI] [PubMed] [Google Scholar]
- Nomura H, Tator CH, Shoichet MS (2006). Bioengineered strategies for spinal cord repair. J Neurotrauma 23: 496–507. [DOI] [PubMed] [Google Scholar]
- Oki K, Tatarishvili J, Wood J, Koch P, Wattananit S, Mine Y et al (2012). Human‐induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke‐damaged brain. Stem Cells 30: 1120–1133. [DOI] [PubMed] [Google Scholar]
- Pakulska MM, Donaghue IE, Obermeyer JM, Tuladhar A, McLaughlin CK, Shendruk TN et al (2016). Encapsulation‐free controlled release: electrostatic adsorption eliminates the need for protein encapsulation in PLGA nanoparticles. Sci Adv 2: e1600519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM (2002). Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol 52: 802–813. [DOI] [PubMed] [Google Scholar]
- Paul G, Zachrisson O, Varrone A, Almqvist P, Jerling M, Lind G et al (2015). Safety and tolerability of intracerebroventricular PDGF‐BB in Parkinson's disease patients. J Clin Invest 125: 1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Gong G, Sun S, Qi J, Zhang H, Wang Y et al (2013). Functional recovery after transplantation of induced pluripotent stem cells in a rat hemorrhagic stroke model. Neurosci Lett 554: 70–75. [DOI] [PubMed] [Google Scholar]
- Rodriguez AL, Bruggeman KF, Wang Y, Wang TY, Williams RJ, Parish CL et al (2018). Using minimalist self‐assembling peptides as hierarchical scaffolds to stabilise growth factors and promote stem cell integration in the injured brain. J Tissue Eng Regen Med 12: e1571–e1579. [DOI] [PubMed] [Google Scholar]
- Rodriguez AL, Nisbet DR, Parish CL (2012). Stem cells and biomaterials for repair of the damaged central nervous system In: Hayat MA. (ed). Stem cells and Cancer Stem Cells: Therapeutic Applications in Disease and Injury. Springer: Dordrecht, pp. 97–111. [Google Scholar]
- Rodriguez AL, Parish CL, Nisbet DR, Williams RJ (2013). Tuning the amino acid sequence of minimalist peptides to present biological signals via charge neutralised self assembly. Soft Matter 9: 3915–3919. [Google Scholar]
- Rodriguez AL, Wang TY, Bruggeman KF, Horgan CC, Li R, Williams RJ et al (2014). In vivo assessment of grafted cortical neural progenitor cells and host response to functionalized self‐assembling peptide hydrogels and the implications for tissue repair. J Mater Chem B 2: 7771–7778. [DOI] [PubMed] [Google Scholar]
- Samata B, Doi D, Nishimura K, Kikuchi T, Watanabe A, Sakamoto Y et al (2016). Purification of functional human ES and iPSC‐derived midbrain dopaminergic progenitors using LRTM1. Nat Commun 7: 13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub NJ, Gilbert RJ (2011). Controlled release of 6‐aminonicotinamide from aligned, electrospun fibers alters astrocyte metabolism and dorsal root ganglia neurite outgrowth. J Neural Eng 8: 046026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub NJ, Johnson CD, Cooper B, Gilbert RJ (2016). Electrospun fibers for spinal cord injury research and regeneration. J Neurotrauma 33: 1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Kirwan P, Smith J, Robinson HP, Livesey FJ (2012). Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci 15 (477–486): S471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA et al (2004). Selective differentiation of neural progenitor cells by high‐epitope density nanofibers. Science 303: 1352–1355. [DOI] [PubMed] [Google Scholar]
- Somaa FA, Wang TY, Niclis JC, Bruggeman KF, Kauhausen JA, Guo H et al (2017). Peptide‐based scaffolds support human cortical progenitor graft integration to reduce atrophy and promote functional repair in a model of stroke. Cell Rep 20: 1964–1977. [DOI] [PubMed] [Google Scholar]
- Stockwell J, Abdi N, Lu X, Maheshwari O, Taghibiglou C (2014). Novel central nervous system drug delivery systems. Chem Biol Drug Des 83: 507–520. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K et al (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872. [DOI] [PubMed] [Google Scholar]
- Tatarishvili J, Oki K, Monni E, Koch P, Memanishvili T, Buga AM et al (2014). Human induced pluripotent stem cells improve recovery in stroke‐injured aged rats. Restor Neurol Neurosci 32: 547–558. [DOI] [PubMed] [Google Scholar]
- Tayalia P, Mooney DJ (2009). Controlled growth factor delivery for tissue engineering. Adv Mater 21: 3269–3285. [DOI] [PubMed] [Google Scholar]
- Theocharidis U, Long K, ffrench‐Constant C, Faissner A (2014). Regulation of the neural stem cell compartment by extracellular matrix constituents. Prog Brain Res 214: 3–28. [DOI] [PubMed] [Google Scholar]
- Thompson LH, Parish CL (2013). Transplantation of fetal midbrain dopamine progenitors into a rodent model of Parkinson's disease. Methods Mol Biol 1059: 169–180. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz‐Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS et al (1998). Embryonic stem cell lines derived from human blastocysts. Science 282: 1145–1147. [DOI] [PubMed] [Google Scholar]
- Tornero D, Wattananit S, Gronning Madsen M, Koch P, Wood J, Tatarishvili J et al (2013). Human induced pluripotent stem cell‐derived cortical neurons integrate in stroke‐injured cortex and improve functional recovery. Brain 136: 3561–3577. [DOI] [PubMed] [Google Scholar]
- Tuladhar A, Payne SL, Shoichet MS (2018). Harnessing the potential of biomaterials for brain repair after stroke. Frontiers in Materials 5. [Google Scholar]
- Wang C, Poon S, Murali S, Koo CY, Bell TJ, Hinkley SF et al (2014b). Engineering a vascular endothelial growth factor 165‐binding heparan sulfate for vascular therapy. Biomaterials 35: 6776–6786. [DOI] [PubMed] [Google Scholar]
- Wang TY, Bruggeman KA, Sheean RK, Turner BJ, Nisbet DR, Parish CL (2014a). Characterization of the stability and bio‐functionality of tethered proteins on bioengineered scaffolds. J Biol Chem 289: 15044–15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TY, Bruggeman KF, Kauhausen JA, Rodriguez AL, Nisbet DR, Parish CL (2016). Functionalized composite scaffolds improve the engraftment of transplanted dopaminergic progenitors in a mouse model of Parkinson's disease. Biomaterials 74: 89–98. [DOI] [PubMed] [Google Scholar]
- Wang TY, Forsythe JS, Nisbet DR, Parish CL (2012). Promoting engraftment of transplanted neural stem cells/progenitors using biofunctionalised electrospun scaffolds. Biomaterials 33: 9188–9197. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cooke MJ, Sachewsky N, Morshead CM, Shoichet MS (2013). Bioengineered sequential growth factor delivery stimulates brain tissue regeneration after stroke. J Control Release 172: 1–11. [DOI] [PubMed] [Google Scholar]
- Wei Z, Zhao J, Chen YM, Zhang P, Zhang Q (2016). Self‐healing polysaccharide‐based hydrogels as injectable carriers for neural stem cells. Sci Rep 6: 37841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler C, Kirik D, Bjorklund A (2005). Cell transplantation in Parkinson's disease: how can we make it work? Trends Neurosci 28: 86–92. [DOI] [PubMed] [Google Scholar]
- Wright JL, Ermine CM, Jørgensen JR, Parish CL, Thompson LH (2016). Over‐expression of meteorin drives gliogenesis following striatal injury. Front Cell Neurosci 10: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie RG, Ahsan S, Aizawa Y, Maxwell KL, Morshead CM, Stoichet MS (2011). Spatially controlled simultaneous patterning of multiple growth factors in three‐dimensional hydrogels. Nat Mater 10: 799–806. [DOI] [PubMed] [Google Scholar]
- Yan X, Yu M, Zhang LH, Jia XS, Li JT, Duan XP et al (2016). A portable electrospinning apparatus based on a small solar cell and a hand generator: design, performance and application. Nanoscale 8: 209–213. [DOI] [PubMed] [Google Scholar]
- Yu H, Cao B, Feng M, Zhou Q, Sun X, Wu S et al (2010). Combinated transplantation of neural stem cells and collagen type I promote functional recovery after cerebral ischemia in rats. Anat Rec (Hoboken) 293: 911–917. [DOI] [PubMed] [Google Scholar]
- Zawada WM, Zastrow DJ, Clarkson ED, Adams FS, Bell KP, Freed CR (1988). Growth factors improve immediate survival of embryonic dopamine neurons after transplantation into rats. Brain Res 786: 96–103. [DOI] [PubMed] [Google Scholar]
- Zhang D, Yang S, Toledo EM, Gyllborg D, Salto C, Carlos Villaescusa J et al (2017). Niche‐derived laminin‐511 promotes midbrain dopaminergic neuron survival and differentiation through YAP. Sci Signal 10: eaal4165. 10.1126/scisignal.aal4165. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chopp M, Zhang RL, Wang L, Zhang J, Wang Y et al (2010). Erythropoietin amplifies stroke‐induced oligodendrogenesis in the rat. PLoS One 5: e11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Chan A, Morad L, Kornblum HI, Fan G, Carmichael ST (2010). Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabil Neural Repair 24: 636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]


