Abstract
The intermingling of genomes that characterizes sexual reproduction requires haploid gametes in which parental homologs have recombined. For this, homologs must pair during meiosis. In a crowded nucleus where sequence homology is obscured by the enormous scale and packaging of the genome, partner alignment is no small task. Here we review the early stages of this process. Chromosomes first establish an initial docking site, usually at telomeres or centromeres. The acquisition of chromosome-specific patterns of binding factors facilitates homolog recognition. Chromosomes are then tethered to the nuclear envelope and subjected to nuclear movements that ‘shake off’ inappropriate contacts while consolidating homolog associations. Thereafter, homolog connections are stabilized by building the synaptonemal complex or its equivalent and creating genetic crossovers. Recent perspectives on the roles of these stages will be discussed.
Introduction
A vital requirement for sexual reproduction is the creation of haploid gametes from diploid progenitors. This task, meiosis, is achieved by a highly ordered process comprising two consecutive nuclear divisions after one round of DNA replication. The key to meiosis lies in the first division (meiosis I, MI), in which pairs of homologous chromosomes, one derived from each parent, segregate from each other after having undergone homologous recombination (HR). In the subsequent division, meiosis II (MII), the sister chromatids within each homologous chromosome separate, leading to genome haploidization. During a lengthy prophase stage before MI, the respective homologs need to find each other and establish an intimate physical connection, referred to as homolog pairing, that provides the context for HR. Just as mitotic sister chromatid cohesion ensures that sisters remain connected until anaphase in order to correctly distribute chromatids to each new cell, homologs are held together in MI via HR-mediated physical contact. Hence, meiotic HR needs to accurately connect true homologs. Not only does HR provide this accuracy of segregation, but it also serves to create new combinations of maternal and paternal DNA within chromosomes.
The processes described in this review are aimed at reaching a specific outcome - changing the basic chromosome structure from that of single or entwined sister chromatids (depending on cell cycle stage) to a structure in which chromatid pairs are further held in a fully aligned configuration. This configuration is widely thought to comprise a central axis in which close associations between homologs occur, separated by chromatin loops that project from the central axis. This paired structure provides an environment in which DNA double strand breaks (DSBs) can be formed and processed in a controlled manner that leads to HR between homologs rather than sisters. Such ‘programmed’ DSBs, a cornerstone of meiotic prophase, are formed by the meiosis-specific endonuclease Spo11 along with a host of regulatory factors. The establishment of this regulatory environment requires intricately choreographed chromosome movements.
In concert with the crucial role of pairing and HR in promoting meiotic chromosome segregation, defective meiotic pairing leads to severe problems. These are often manifest in the co-segregation of homologs to one pole during MI (non-dysjunction) or the precocious separation of sister chromatids at MI. These events lead to aneuploid gametes and are the major causes of infertility, miscarriage, and genetic abnormalities such as Down’s syndrome. Moreover, much of the food we eat comprises meiotic products (such as fruits and grains). Therefore, understanding meiotic efficiency and the formation of viable gametes will enhance our ability to boost fertility and global food production. In the next sections we introduce the steps that chromosomes take on the meiotic prophase dance floor.
Ensuring correct pairing
While different organisms have developed a range of strategies to manage the meiotic pairing process, these strategies tend to follow a similar pattern:
Tentative coupling - Contacts are often first established between specific regions that are common to every chromosome (centromeres and/or telomeres). These contacts are often independent of homology in other regions of the chromosome.
Tethering of the genome - Using a conserved mechanism, chromosomes are tethered to a defined volume at the nuclear envelope (NE), often near the centrosome. The widely conserved ‘telomere bouquet’ is the most prominent example of this tethering.
Nuclear movement - Dramatic chromosome movements, such that they often traverse the cell, constitute the next conserved stage of meiosis. This chromosomal motion promotes pairing between homologs and the elimination of ectopic interactions with non-homologous chromosomes.
Stabilization of the contacts commonly involves construction of a proteinaceous connection between homologs called the synaptonemal complex (SC, reviewed in [1]and [2]). The SC is composed of a transverse filament zipper-like structure connecting the two chromosome axes. Not only does the SC keep homologs together, but also it assists in HR, eventually creating genetic crossovers (CO) that physically connect the homologs (reviewed by [3]). Structural components of the central axis and transverse filament, as well as general DNA repair factors, participate in the CO process. While most organisms create COs in the context of SC, some organisms use only one of these stabilization mechanisms; for example, Drosophila melanogaster males lack COs, and fission yeast lack transverse filaments. The order of DSB and SC formation, as well as their level of interdependence, are also variable. As SC and CO formation have been reviewed extensively, we focus on the first three stages.
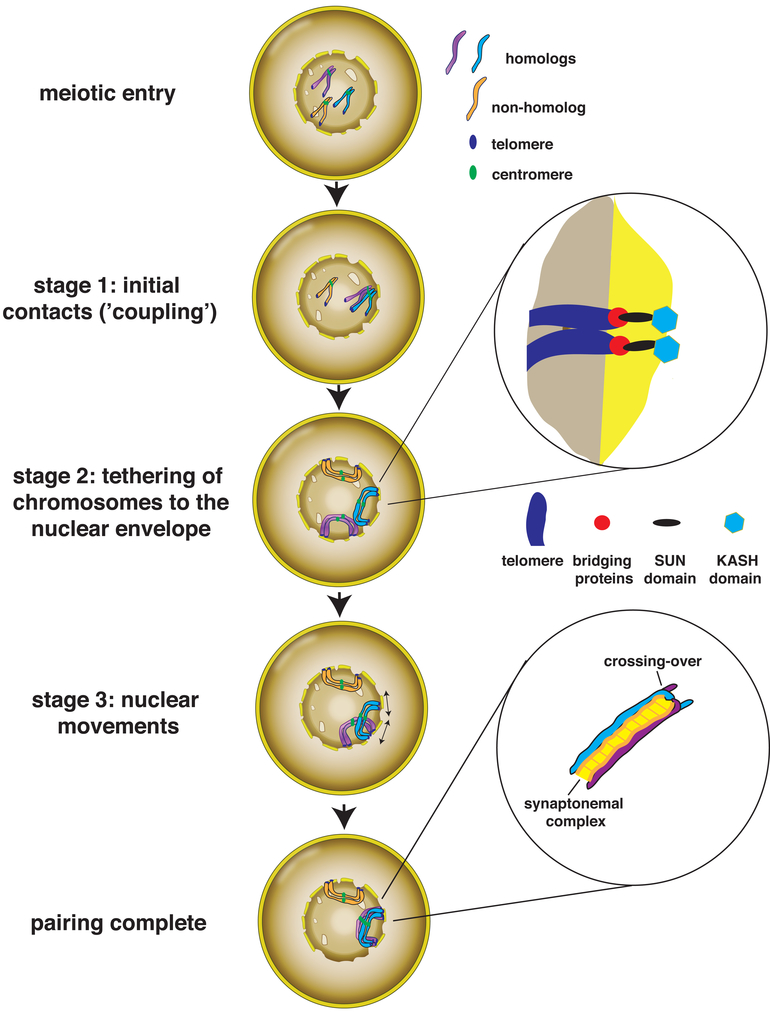
Fig. 1 schematizes these stages, while Table 1 catalogs the details of the pairing process in several well-studied organisms.
Figure 1. Schematic of cellular events leading to homolog pairing.
Stage 1: Initial contacts (‘coupling’): Many organisms use centromeres (budding yeast) or telomeres (mouse) as the initial point of contact (see text). The contacts can occur either between homologs or between non-homologs.
Stage 2: Tethering of chromosomes to the nuclear envelope: Using the conserved SUN-KASH proteins (see blow up section on right), telomeres (as in fission yeast) centromeres (as in Drosophila oocytes) or other specialized regions (as in C. elegans) are tethered to the nuclear envelope.
Stage 3: Nuclear movements: Dramatic movements specific to meiotic prophase promote homolog alignment and ‘shake off’ unwanted interactions with non-homologous sequences.
Table 1:
Summary of early meiotic pairing behavior in well-studied organisms:
| Initial contacts and coupling |
Factors involved |
Tethering to the NE |
Factors involved |
Nuclear movements |
Factors involved |
Sample References | |
|---|---|---|---|---|---|---|---|
| S. cerevisiae | Non-homologous centromeres | Zip1 Rec8 Spo11 |
Telomeres tether to the NE, near SPB. Loose bouquet | Ndj1 Csm4 Mps3 |
Telomere led burst movements in 3 axes | Actin | [62] [4] [53] |
| S. pombe | Co-localization of telomeres and centromeres | Taz1/Rap1 Bqt1/2 Nuf2 Sad1 |
Telomeres tether to the NE, at SPB. Tight bouquet | sad1 Kms1 Bqt1/2 Taz1/Rap1 |
Telomere and SPB led continuous movement in mainly 1 axis | Dynein Tubulin Hrs1 | [51,72] [27,54] |
| Z. mays♂ | Predominantly homologous centromeres | AFD1 SMC6 Active centromeres |
Telomeres gather at nuclear periphery | Pam1 | Mostly telomere led movement in 3 axes | Actin tubulin | [73,74] [9] |
| C. elegans♀ | ? | - | Tethering of pairing-centers to the NE | HIM-8 ZIM1-3 ZYG-12 SUN-1 PLK-2 |
Pairing-center led burst movement in 3 axes | Tubulin Dyenin | [37] [39] [65] |
| D. melanogaster♀ | Homologous centromeres | C (3) G Smc1/3 ORD |
Tethering of centromeres to the NE | C(3)G Smc1/3 ORD |
? | - | [42,75] |
| M. musculus♂ | Homologous telomeres | Sun1 Spo11 |
Tethering of telomeres to the NE | Sun1 Kash5 |
Telomere led movement in 3 axes | - | [76] [11] |
‘The speed dating session’- initial, relatively disorganized chromosome contacts and their potential role
When two large bodies such as chromosomes start to sample each other, where should they begin? Identification of a shared initial anchorage site has potential to facilitate the process. Most organisms utilize centromeres or telomeres, the two landmarks shared among all chromosomes, as large-scale anchors. Accumulation of specific components at these sites, as well as interstitial chromosome sites, allows homologs to mirror each other. Here we will adhere to the term ‘coupling’ (coined by [4]) to distinguish these events from the later, homology dependent interactions.
In Saccharomyces cerevisiae, centromere coupling precedes other pairing events and occurs independently of DSB formation [4]. This early coupling depends on Zip1 [4], a conserved coiled-coil protein that connects the cores of apposed homologs, forming a major component of the transverse filament [5]. Zip1 is distinct from other SC components as it localizes to centromeres independently of other SC proteins (Zip2/3 and Red1), even though its participation in SC depends on them. Zip1 may play a structural role in centromere coupling through its ability to oligomerize and form filaments [6].
Another factor on which centromere coupling depends is Rec8 [7], the meiosis-specific kleisin subunit of the cohesin ring that holds sister chromatids together. Rec8-containing cohesin rings localize to the meiotic centromere where they are modified by the shugoshin complex to prevent the cleavage of Rec8 in MI so it persists on the centromere, holding the sister chromatids together, until MII [8]. Localization of Zip1 to the centromere depends upon Rec8 [7]. Therefore, centromere coupling is mediated by proteins that later play key roles in meiosis; indeed, coupling may help to concentrate these proteins at sites from which true pairing is initiated, an idea supported by the observation that coupling between non-homologous centromeres is gradually replaced by associations between homologous centromeres [4].
Maize meiosis presents a coupling scenario with instructive similarities to budding yeast [9]. Maize centromeres couple in early leptotene, before other pairing events, but these associations are predominantly (~65%) between homologous centromeres. These interactions depend on the homolog of budding yeast Rec8 but do not depend on the maize Zip1 ortholog. Rather, the SMC5/6 complex, previously known as a DNA repair factor and member of the Structural Maintenance of Chromosomes family that includes cohesins and condensins [10], promotes centromere coupling in maize and later participates in the SC [9].
During mouse spermatogenesis, early (pre-leptotene) DSB-independent homolog interactions are observed [11]. While these interactions share some features of the coupling discussed above and nonhomologous interactions among both centromeres and telomeres are observed, a significant proportion of these interactions appear to occur between homologous telomeres. These early telomeric interactions depend on the mouse SUN-domain protein Sun1 ([12], see below). SUN (after Sad1 and Unc-84) domain proteins are NE-bound and have conserved roles in linking chromosomes with the NE.
What is the role of coupling? This subject has been under much debate. Telomeres or centromeres, being shared among all chromosomes, are ideal docking sites for holding chromosomes together while they search for homology. One compelling hypothesis is that coupling promotes the achiasmate (or ‘distributive’) segregation of chromosomes [4,13]. This mechanism elevates the rate of correct segregation of homologs lacking COs and is widely conserved [14] [15,16]. Achiasmate segregation is precipitated by mutation of CO pathway components but can also occur naturally, especially when a chromosome is very small and lacks sufficient DSB initiation sites. In both budding yeast and in Drosophila oocytes [17,18], this pathway requires centromere coupling [13], presumably because the coupled centromeres can nucleate more lasting achiasmate associations.
Additional ideas regarding the functions of coupling come from a consideration of the factors involved, as these factors also accumulate at interstitial sites where they promote the ability of homologous chromosomes to recognize their mirror images. Mouse telomere coupling depends on Spo11, but not on its catalytic activity [11]. In budding yeast, Spo11 is particularly concentrated at centromeres in early prophase, although DSBs are not created at centromeres [19]. Like Zip1, Spo11 accumulation at centromeres is Rec8 dependent [20]. This recalls earlier budding yeast work demonstrating that Spo11 acts in a non-catalytic capacity to promote multiple interstitial homolog interactions concomitant with passage of the pre-meiotic replication fork; this activity of Spo11 is proposed to couple pre-meiotic S-phase and the attendant establishment of sister chromatid cohesion with later homolog recognition events [21,22]. Hence, Spo11 appears at least in some chromosome regions to take on a structural or regulatory role in addition to its catalytic role, both in mouse and yeast. This scenario may conceptually recall the functions of Rec8, which also loads during meiotic replication and not only confers sister chromatid cohesion but also coordinates meiotic events like CO formation and monopolar spindle attachment at MI. Indeed, the replication-coupled association of factors like Spo11, Rec8 and heterochromatin elements has been suggested to establish a pattern of marks that align homologous regions in the proper register [17,18,22,23]; the spacing patterns between such elements on individual chromosomes may provide a ‘barcode’ that facilitates the eventual close apposition of homologs [23]. In addition, early interactions of heterochromatic regions (like centromeres and telomeres) with regulatory meiotic proteins may also contribute to the protection of these regions from CO events. Failure to execute this protection leads to CO events at centromeres or telomeres, in turn causing homolog nondisjunction at MI and perhaps genetic disorders such as Down’s syndrome [24].
Gathering at the periphery of the dance floor – the bouquet stage
After first contacts between the chromosomes have been established, a widely conserved prelude to extensive pairing occurs: the tethering of the chromosomes to a defined volume at the NE. In many organisms, the telomeres gather at a specific NE location, often close to the centrosome. This telomere-gathering event, the ‘telomere bouquet’, has been known for more than a century [25], but only recently have its roles started to emerge. Tethering assists in bringing the homologs closer together [26] [27], Nonetheless, the importance of the bouquet in promoting pairing itself has been debated. Some studies (especially in budding yeast: [28], [29]) suggest that the bouquet configuration itself has a minor role in pairing, but that by connecting chromosomes to the NE and cytoskeleton, it links chromosomes to the movement-generating apparatus (see below).
Fission yeast has an especially lengthy bouquet stage with known determinants, allowing disruption of the bouquet and a wealth of information on its function. Upon meiotic induction, the meiotic prophase-specific Bqt1/2 proteins bind the telomeric Rap1/Taz1 complex, and recruit the SUN-domain protein Sad1 [30]. SUN domain proteins are generally bound within the NE by KASH-domain (Klarsicht, ANC-1 and Syne-1 homology [31]) outer NE proteins. The SUN-KASH complex thereby bridges chromatin with the outer NE and in turn, the forces of the cytoskeleton. The meiotic expression of Bqt1/2 and consequent formation of a bridge from telomeres to the cytoplasmic microtubule network results in the movement of telomeres to the yeast centrosome (the spindle pole body or SPB) where they remain throughout prophase [32]. Deletion of genes encoding bouquet formation factors (Bqt1/2 or Taz1/Rap1) dismantles the bouquet, disrupts meiotic pairing and therefore reduces CO [33,34] [27].
The specific disruption of the bouquet has enabled identification of its additional roles, seemingly unrelated to pairing per se (but see below; [35]). The connection between the bouquet and SPB was shown to be crucial for meiotic spindle formation [35]. Moreover, zygotes lacking the bouquet sustain chromosomes that fail to assemble proper centromeres, suggesting that the bouquet forms a nuclear microenvironment that promotes centromere assembly (MK, A. Fennell and JPC, submitted).
Thus, bouquet function encompasses not only the gathering of chromosomes and their coupling to nuclear movement-generating machinery (see below), but also the formation of a region containing a high concentration of telomere factors (including general heterochromatin factors and noncoding RNAs) that promote other crucial meiotic processes, like spindle and centromere assembly. Perhaps the utility of the bouquet for pairing preserved its existence, allowing other properties of the clustered telomeres to be exploited by the cell to coordinate multiple meiotic events.
An eye-opening variation on the theme of telomere tethering occurs in Caenorhabditis elegans. In this worm, specialized regions called pairing centers (PCs) are found on each chromosome. PCs are distinct from telomeres although they are near chromosome ends, and are bound by proteins specific for each individual PC (or two in some cases). PC-binding proteins tether PCs to the NE via the conserved SUN-KASH connection (SUN-1 and ZYG-12) [36,37]. Intriguingly, this interaction creates a signaling hub that integrates several meiotic events. PCs recruit the Polo kinase PLK-2 in a CHK-2 kinase-dependent manner [38,39]. This results in SUN-1 phosphorylation in the context of PC/SUN-1 interactions, which in turn stimulates SUN/KASH-mediated communication between PCs and the cytoskeleton, resulting in dynein motor-driven chromosome movements (see below) [40]. Moreover, modifications within the PC/SUN-1 complex set up a signaling array used later in meiosis. For instance, only upon full homolog synapsis is PLK-2 released from PCs, allowing further meiotic progression; conversely, the persistence of DSBs, the absence of COs or asynapsis of a homolog pair lead to the persistence of SUN-1 phosphorylation [41] and delayed PC disassembly. Thus, the PC system maintains (and has been instrumental in defining) the principles of chromosome tethering: restricting chromosomes to constrained nuclear volumes, promoting chromosome movements and creating regulatory hubs. Whether PCs play roles in promoting spindle formation analogous to the fission yeast bouquet remains to be determined. [37].
The situation in Drosophila melanogaster female meiosis follows similar principles with intriguing differences. In this organism, homologous centromeres are paired throughout meiotic prophase [18]. This pairing occurs earlier than pairing in the rest of the genome, persists later, and eventually leads to the formation of a ‘centromere bouquet’ [42] which depends on SC formation factors and cohesins [42] and appears to assume the roles of both coupling and tethering to the NE. Drosophila centromeres thus fulfill functions usually performed by telomeres during meiotic prophase. It is interesting to note that centromeres and telomeres also share certain roles in fission yeast meiosis (A. Fennell, A. Fernandez-Alvarez and JPC, submitted), as both can interchangeably promote proper spindle formation. This underscores the concept of interchangeability between telomeres and centromeres not only between evolutionary families but also within species.
Telomere tethering at the NE is also observed in mouse spermatocytes through most of meiotic prophase. Telomere clustering in the bouquet configuration occurs transiently at the zygotene stage [43] [44] and requires Sun1 [12], Sun2 [45] and the cohesion subunit SMC 1β, which has genome-wide roles in SC formation [46]. Despite these similarities with other organisms, murine telomere tethering and bouquet formation seem to be independent of Rap1 [47]. Nonetheless, tantalizing data consistent with potentially wide-ranging roles of the mouse bouquet, reminiscent of those of fission yeast, have emerged. In oocytes from fourth-generation telomerase knockout mice, defective meiotic spindles, as well as chromosomes which fail to attach to those spindles that do form, have been observed [48].
A model for the roles of chromosome tethering emerges from the foregoing studies and others. Chromosomes are tethered to the NE by a conserved mechanism, facilitating their movement (see below) and pairing. In addition, this specialized chromosome configuration is exploited as a platform from which to integrate a myriad of additional meiotic processes. Initial choreography is thus set up, and the dance may begin.
The “mix and match” dance - Prophase nuclear movements
Oscillatory movements within meiotic nuclei had been noticed in rat spermatocytes in the 1970s [49]. The appearance of elongated horsetail-shaped nuclei in fission yeast zygotes also suggested extensive prophase movements [50]. With the advent of facile live observation of meiosis two decades later [51] [52] [53], such movements have been confirmed as a conserved phenomenon.
In fission yeast, the nucleus oscillates back and forth during prophase with the clustered telomeres in tow, pulling the nucleus into the above-mentioned horsetail shape. Elaborate work has deciphered the mechanisms responsible for this movement, which follows the oscillatory movement of the SPB [51] and depends on astral (cytoplasmic) microtubules (MTs) [54]and cortically anchored dynein [55]. Motors redistribute from the trailing to the leading MTs in response to changes in load force that occur behind the nucleus as it approaches the distal end of the cell [56]. The machinery required to orchestrate this movement includes meiosis-specific components like Hrs1, which nucleates a specialized prophase MT organizing center that stabilizes the connection between the SPB and the MT minus ends and promotes SPB movement [57,58].
The identification of components of this nuclear movement machinery whose disruption does not affect SPB integrity has enabled study of the roles of meiotic nuclear movement. Loss of the dynein heavy chain or Hrs1 leads to reduced movement and consequently reduced homolog pairing, along with increased ectopic recombination. [27] [57,59]. Nonetheless, spore viability is only mildly affected by these mutations, perhaps because only three COs are sufficient to support viable meiosis (as fission yeast has a haploid chromosome number of three). Mutations affecting bouquet formation and mutations affecting nuclear movement have an additive effect on chromosome segregation error rates (MK, Alex Fennell and JPC, submitted) [60,61]. These observations underline the different roles assumed by the bouquet and nuclear movement.
In budding yeast, the telomeres of the bouquet associate loosely in the vicinity of the SPB [62]. Nuclear movements termed ‘rapid prophase movements’ are observed during this period and are led by telomeres along several axes at different speeds [53]. Telomeres become transiently associated with a network of cytoplasmic nucleus-hugging actin cables that are continuous with the actin cytoskeleton; within these associations, the telomeres are passive, moving due to the dynamic nature of the actin cables [63]. Impairment of this movement correlates with defective pairing, while telomere clustering per se plays a more minor role in pairing that is postulated to stem from the more efficient movement of chromosomes in the bouquet configuration [28,29]. Mutations that compromise nuclear movement also confer altered patterns of CO, non-dysjunction at MI and increased interactions between non-homologous sequences [53,64].
Prophase movements are also well-characterized in C. elegans. PCs move in bursts along multiple axes ([65], reviewed in [66]), in a tubulin/dynein-dependent manner as in fission yeast [67]. The chromosome end proximal to the PC moves more than the other chromosome end, resulting in chromosome stretching and force exertion. This movement allows the PC to explore a much greater volume of the nucleoplasm; indeed, knockdown of dynein motors results in delayed pairing and synapsis [65,67], again demonstrating a role for movements in meiotic pairing. Movement is also required for elimination of ectopic interactions between non-homologous chromosomes.
While extensive information has been gathered, a complete consensus as to the function of prophase chromosome movements is still elusive. Compelling evidence for roles in facilitating local homolog searches, assisting global alignment, disrupting non-homologous interactions, and resolving chromosome interlocks [68] [52,64] [69] suggests a constellation of dance moves that ‘shake, rattle and roll’ the chromosomes into refining their partner choice and heightening their interactions.
Help along the way - RNA as an anchor point between homologs
How is alignment between homologs achieved at the local level? Cohesin and/or Spo11 binding patterns are likely organizers, as discussed above. Another appealing model invokes the interaction of transcripts of homologous loci. Support for a role of non-coding RNAs (ncRNAs) comes from analysis of pairing of the fission yeast sme2 locus [70]. A ncRNA from this locus contributes to the robust and early pairing of the region harboring this locus. Abrogation of the sme2 transcript abolishes robust pairing in that region while its transfer to an ectopic region confers ectopic early and robust pairing. Whether ncRNAs promote pairing via RNA-RNA, RNA-protein or more diffuse regional interactions is thus far unknown, but a role for ncRNAs in homolog pairing could be consistent with several phenomena such as cycles of interaction and disengagement of paired loci and the different levels of pairing seen in different regions of the genome [27,70]. Whether this is a general phenomenon in fission yeast pairing is still unclear. If found to be widespread, such ncRNAs could provide a level of specificity that would assist in establishing the proper register between aligning homologs.
Finale of the act - chromosome spooning
The stages depicted above initiate the pairing process. With SC formation and the CO process, the initial contacts are stabilized and the homologs can begin to act as a paired unit. By following tagged repeat arrays on numerous locations on fission yeast chromosomes, Hiraoka and colleagues [27] have followed the dynamics of pairing of different chromosome loci. Not only are the dynamics complex, with numerous cycles of association and dissociation, but also the timing of stable association differs along the chromosome and correlates with distance from the tethering point (pairing occurs more quickly closer to the telomeres). Points closer to the tethering point may experience greater force exertion leading to rapid zippering. In budding yeast [71], multiple factors such as cohesins and nuclear movements independently affect pairing at specific homologous sites.
Conclusions
The vast challenge of maneuvering chromosomes into homologous alignment follows intuitive principles whose details are gradually emerging. Common chromosomal features such as centromeres and telomeres serve as gathering sites to initiate the process; similarly, the deposition of chromosome-specific patterns of marks during meiotic S-phase may facilitate accurate zippering. Crucially, the attachment of chromosomes to the NE triggers extensive motions that allow homologous marks to encounter each other. Hence, a combination of local gluing interactions and global force-generation helps homologs in the complex task of finding the optimal partner and engaging in intimate steps on the meiotic dancing floor.
Acknowledgments
We thank the Cooper lab for fruitful discussions, Alex Fennell for comments on the manuscript and Hani Ebrahimi for help with figure preparation. MK and JPC have been funded by Cancer Research UK, the European Research Council, a Marie Curie postdoctoral fellowship to MK, and the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kleckner N: Chiasma formation: chromatin/axis interplay and the role(s) of the synaptonemal complex. Chromosoma 2006, 115:175–194. [DOI] [PubMed] [Google Scholar]
- 2.de Boer E, Heyting C: The diverse roles of transverse filaments of synaptonemal complexes in meiosis. Chromosoma 2006, 115:220–234. [DOI] [PubMed] [Google Scholar]
- 3.Youds JL, Boulton SJ: The choice in meiosis - defining the factors that influence crossover or non-crossover formation. J Cell Sci 2011, 124:501–513. [DOI] [PubMed] [Google Scholar]
- 4.Tsubouchi T, Roeder GS: A synaptonemal complex protein promotes homology-independent centromere coupling. Science 2005, 308:870–873. [DOI] [PubMed] [Google Scholar]
- 5.Sym M, Engebrecht JA, Roeder GS: ZIP1 is a synaptonemal complex protein required for meiotic chromosome synapsis. Cell 1993, 72:365–378. [DOI] [PubMed] [Google Scholar]
- 6.Sym M, Roeder GS: Zip1-induced changes in synaptonemal complex structure and polycomplex assembly. J Cell Biol 1995, 128:455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bardhan A, Chuong H, Dawson DS: Meiotic cohesin promotes pairing of nonhomologous centromeres in early meiotic prophase. Mol Biol Cell 2010, 21:1799–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitajima TS, Kawashima SA, Watanabe Y: The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature 2004, 427:510–517. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Pawlowski WP, Han F: Centromere pairing in early meiotic prophase requires active centromeres and precedes installation of the synaptonemal complex in maize. Plant Cell 2013, 25:3900–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study characterizes early chromosome pairing in maize meiocytes. The authors show that centromeres pair as early as the leptotene stage, with a tendency towards homologous centromere pairing; functional centromeres (not just centromere sequences) are required. Additional experiments reveal several factors that are required for this early pairing, including a SMC6 family member.
- 10.Nasmyth K, Haering CH: The structure and function of SMC and kleisin complexes. Annu Rev Biochem 2005, 74:595–648. [DOI] [PubMed] [Google Scholar]
- 11.Boateng KA, Bellani MA, Gregoretti IV, Pratto F, Camerini-Otero RD: Homologous pairing preceding SPO11-mediated double-strand breaks in mice. Dev Cell 2013, 24:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study shows that in early leptotene mouse spermatocytes, contacts between homologous telomeres are frequent and depend on SUN1 and a non-catalytic function of Spo11. This work establishes that DSB-independent early homolog interactions are conserved.
- 12.Ding X, Xu R, Yu J, Xu T, Zhuang Y, Han M: SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev Cell 2007, 12:863–872. [DOI] [PubMed] [Google Scholar]
- 13.Kemp B, Boumil RM, Stewart MN, Dawson DS: A role for centromere pairing in meiotic chromosome segregation. Genes Dev 2004, 18:1946–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koehler KE, Hassold TJ: Human aneuploidy: lessons from achiasmate segregation in Drosophila melanogaster. Ann Hum Genet 1998, 62:467–479. [DOI] [PubMed] [Google Scholar]
- 15.Wolf KW: How meiotic cells deal with non-exchange chromosomes. Bioessays 1994, 16:107–114. [DOI] [PubMed] [Google Scholar]
- 16.Hawley RS, Theurkauf WE: Requiem for distributive segregation: achiasmate segregation in Drosophila females. Trends Genet 1993, 9:310–317. [DOI] [PubMed] [Google Scholar]
- 17.Karpen GH, Le MH, Le H: Centric heterochromatin and the efficiency of achiasmate disjunction in Drosophila female meiosis. Science 1996, 273:118–122. [DOI] [PubMed] [Google Scholar]
- 18.Dernburg AF, Sedat JW, Hawley RS: Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell 1996, 86:135–146. [DOI] [PubMed] [Google Scholar]
- 19.Robine N, Uematsu N, Amiot F, Gidrol X, Barillot E, Nicolas A, Borde V: Genome-wide redistribution of meiotic double-strand breaks in Saccharomyces cerevisiae. Mol Cell Biol 2007, 27:1868–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kugou K, Fukuda T, Yamada S, Ito M, Sasanuma H, Mori S, Katou Y, Itoh T, Matsumoto K, Shibata T, et al. : Rec8 guides canonical Spo11 distribution along yeast meiotic chromosomes. Mol Biol Cell 2009, 20:3064–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cha RS, Weiner BM, Keeney S, Dekker J, Kleckner N: Progression of meiotic DNA replication is modulated by interchromosomal interaction proteins, negatively by Spo11p and positively by Rec8p. Genes Dev 2000, 14:493–503. [PMC free article] [PubMed] [Google Scholar]
- 22.Weiner BM, Kleckner N: Chromosome pairing via multiple interstitial interactions before and during meiosis in yeast. Cell 1994, 77:977–991. [DOI] [PubMed] [Google Scholar]
- 23.Ishiguro K, Kim J, Fujiyama-Nakamura S, Kato S, Watanabe Y: A new meiosis-specific cohesin complex implicated in the cohesin code for homologous pairing. EMBO Rep 2011, 12:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study identifies a new mammalian meiosis specific cohesion subunit, Rad21L. Rad21L and Rec8 show a mutually exclusive localization pattern prior to synapsis and are removed in a Polo kinase-dependent manner. The study suggests patterns of cohesin binding as potential barcodes that align homologs.
- 24.Stewart MN, Dawson DS: Changing partners: moving from non-homologous to homologous centromere pairing in meiosis. Trends Genet 2008, 24:564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scherthan H: A bouquet makes ends meet. Nat Rev Mol Cell Biol 2001, 2:621–627. [DOI] [PubMed] [Google Scholar]
- 26.Harper L, Golubovskaya I, Cande WZ: A bouquet of chromosomes. J Cell Sci 2004, 117:4025–4032. [DOI] [PubMed] [Google Scholar]
- 27.Ding DQ, Yamamoto A, Haraguchi T, Hiraoka Y: Dynamics of homologous chromosome pairing during meiotic prophase in fission yeast. Dev Cell 2004, 6:329–341. [DOI] [PubMed] [Google Scholar]
- 28.Lee CY, Conrad MN, Dresser ME: Meiotic chromosome pairing is promoted by telomere-led chromosome movements independent of bouquet formation. PLoS Genet 2012, 8:e1002730. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study aims at looking at the relationship between bouquet formation, nuclear movement and pairing. The authors show that nuclear movement is more important for normal pairing than bouquet formation per se, at least in S. cerevisiae.
- 29.Sonntag Brown M, Zanders S, Alani E: Sustained and rapid chromosome movements are critical for chromosome pairing and meiotic progression in budding yeast. Genetics 2011, 188:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study shows that prophase nuclear movement is important for sustaining normal levels of meiotic pairing and recombination. The authors analyze different mutations in the gene encoding Csm4, which participates in bouquet formation and prophase movements.
- 30.Chikashige Y, Tsutsumi C, Yamane M, Okamasa K, Haraguchi T, Hiraoka Y: Meiotic proteins bqt1 and bqt2 tether telomeres to form the bouquet arrangement of chromosomes. Cell 2006, 125:59–69. [DOI] [PubMed] [Google Scholar]
- 31.Kracklauer MP, Link J, Alsheimer M: LINCing the nuclear envelope to gametogenesis. Curr Top Dev Biol 2013, 102:127–157. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi A, Asakawa H, Haraguchi T, Hiraoka Y: Reconstruction of the kinetochore during meiosis in fission yeast Schizosaccharomyces pombe. Mol Biol Cell 2006, 17:5173–5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper JP, Watanabe Y, Nurse P: Fission yeast Taz1 protein is required for meiotic telomere clustering and recombination. Nature 1998, 392:828–831. [DOI] [PubMed] [Google Scholar]
- 34.Nimmo ER, Pidoux AL, Perry PE, Allshire RC: Defective meiosis in telomere-silencing mutants of Schizosaccharomyces pombe. Nature 1998, 392:825–828. [DOI] [PubMed] [Google Scholar]
- 35.Tomita K, Cooper JP: The telomere bouquet controls the meiotic spindle. Cell 2007, 130:113–126. [DOI] [PubMed] [Google Scholar]
- 36.MacQueen AJ, Colaiacovo MP, McDonald K, Villeneuve AM: Synapsis-dependent and -independent mechanisms stabilize homolog pairing during meiotic prophase in C. elegans. Genes Dev 2002, 16:2428–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacQueen AJ, Phillips CM, Bhalla N, Weiser P, Villeneuve AM, Dernburg AF: Chromosome sites play dual roles to establish homologous synapsis during meiosis in C. elegans. Cell 2005, 123:1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harper NC, Rillo R, Jover-Gil S, Assaf ZJ, Bhalla N, Dernburg AF: Pairing centers recruit a Polo-like kinase to orchestrate meiotic chromosome dynamics in C. elegans. Dev Cell 2011, 21:934–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Penkner AM, Fridkin A, Gloggnitzer J, Baudrimont A, Machacek T, Woglar A, Csaszar E, Pasierbek P, Ammerer G, Gruenbaum Y, et al. : Meiotic chromosome homology search involves modifications of the nuclear envelope protein Matefin/SUN-1. Cell 2009, 139:920–933. [DOI] [PubMed] [Google Scholar]
- 40.Labella S, Woglar A, Jantsch V, Zetka M: Polo kinases establish links between meiotic chromosomes and cytoskeletal forces essential for homolog pairing. Dev Cell 2011, 21:948–958. [DOI] [PubMed] [Google Scholar]
- 41.Woglar A, Daryabeigi A, Adamo A, Habacher C, Machacek T, La Volpe A, Jantsch V: Matefin/SUN-1 phosphorylation is part of a surveillance mechanism to coordinate chromosome synapsis and recombination with meiotic progression and chromosome movement. PLoS Genet 2013, 9:e1003335. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study addresses the role of the SUN-1 phosphorylation events that occur during meiosis in C. elegans. The pairing center/SUN-1 complex acts as a checkpoint hub, integrating phosphorylation and dephosphorylation signals, for several meiotic events. De-phosphorylation occurs only when the meiotic events are completed, allowing further meiotic progression.
- 42.Takeo S, Lake CM, Morais-de-Sa E, Sunkel CE, Hawley RS: Synaptonemal complex-dependent centromeric clustering and the initiation of synapsis in Drosophila oocytes. Curr Biol 2011, 21:1845–1851. [DOI] [PubMed] [Google Scholar]; ** This study shows that chromosome pairing and synapsis in Drosophila oocytes initiate at centromeres. This pairing precedes pairing in other regions of the genome and leads to a cluster of centromeres at the NE. Hence, flies form a centromere bouquet.
- 43.Scherthan H, Weich S, Schwegler H, Heyting C, Harle M, Cremer T: Centromere and telomere movements during early meiotic prophase of mouse and man are associated with the onset of chromosome pairing. J Cell Biol 1996, 134:1109–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scherthan H: Analysis of telomere dynamics in mouse spermatogenesis. Methods Mol Biol 2009, 558:383–399. [DOI] [PubMed] [Google Scholar]
- 45.Schmitt J, Benavente R, Hodzic D, Hoog C, Stewart CL, Alsheimer M: Transmembrane protein Sun2 is involved in tethering mammalian meiotic telomeres to the nuclear envelope. Proc Natl Acad Sci U S A 2007, 104:7426–7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adelfalk C, Janschek J, Revenkova E, Blei C, Liebe B, Gob E, Alsheimer M, Benavente R, de Boer E, Novak I, et al. : Cohesin SMC1beta protects telomeres in meiocytes. J Cell Biol 2009, 187:185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scherthan H, Sfeir A, de Lange T: Rap1-independent telomere attachment and bouquet formation in mammalian meiosis. Chromosoma 2011, 120:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu L, Blasco MA, Keefe DL: Requirement of functional telomeres for metaphase chromosome alignments and integrity of meiotic spindles. EMBO Rep 2002, 3:230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parvinen M, Soderstrom KO: Chromosome rotation and formation of synapsis. Nature 1976, 260:534–535. [DOI] [PubMed] [Google Scholar]
- 50.Robinow CF: The Number of Chromosomes in SCHIZOSACCHAROMYCES POMBE: Light Microscopy of Stained Preparations. Genetics 1977, 87:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chikashige Y, Ding DQ, Funabiki H, Haraguchi T, Mashiko S, Yanagida M, Hiraoka Y: Telomere-led premeiotic chromosome movement in fission yeast. Science 1994, 264:270–273. [DOI] [PubMed] [Google Scholar]
- 52.Koszul R, Kleckner N: Dynamic chromosome movements during meiosis: a way to eliminate unwanted connections? Trends Cell Biol 2009, 19:716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conrad MN, Lee CY, Chao G, Shinohara M, Kosaka H, Shinohara A, Conchello JA, Dresser ME: Rapid telomere movement in meiotic prophase is promoted by NDJ1, MPS3, and CSM4 and is modulated by recombination. Cell 2008, 133:1175–1187. [DOI] [PubMed] [Google Scholar]
- 54.Ding DQ, Chikashige Y, Haraguchi T, Hiraoka Y: Oscillatory nuclear movement in fission yeast meiotic prophase is driven by astral microtubules, as revealed by continuous observation of chromosomes and microtubules in living cells. J Cell Sci 1998, 111 ( Pt 6):701–712. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto A, West RR, McIntosh JR, Hiraoka Y: A cytoplasmic dynein heavy chain is required for oscillatory nuclear movement of meiotic prophase and efficient meiotic recombination in fission yeast. J Cell Biol 1999, 145:1233–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vogel SK, Pavin N, Maghelli N, Julicher F, Tolic-Norrelykke IM: Self-organization of dynein motors generates meiotic nuclear oscillations. PLoS Biol 2009, 7:e1000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanaka K, Kohda T, Yamashita A, Nonaka N, Yamamoto M: Hrs1p/Mcp6p on the meiotic SPB organizes astral microtubule arrays for oscillatory nuclear movement. Curr Biol 2005, 15:1479–1486. [DOI] [PubMed] [Google Scholar]
- 58.Funaya C, Samarasinghe S, Pruggnaller S, Ohta M, Connolly Y, Muller J, Murakami H, Grallert A, Yamamoto M, Smith D, et al. : Transient structure associated with the spindle pole body directs meiotic microtubule reorganization in S. pombe. Curr Biol 2012, 22:562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study characterizes a meiotic prophase-specific microtubule organizing center and its molecular regulation, which is linked with the modification and degradation of Hrs1, a factor crucial for meiotic nuclear movements. Electron tomography reveals a meiosis-specific amorphous structure, reminiscent of the peri-centriolar material of higher eukaryotes, surrounding the meiotic SPB.
- 59.Davis L, Smith GR: The meiotic bouquet promotes homolog interactions and restricts ectopic recombination in Schizosaccharomyces pombe. Genetics 2006, 174:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshida M, Katsuyama S, Tateho K, Nakamura H, Miyoshi J, Ohba T, Matsuhara H, Miki F, Okazaki K, Haraguchi T, et al. : Microtubule-organizing center formation at telomeres induces meiotic telomere clustering. J Cell Biol 2013, 200:385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klutstein MF, A. ; Cooper JP. : submitted.
- 62.Trelles-Sticken E, Dresser ME, Scherthan H: Meiotic telomere protein Ndj1p is required for meiosis-specific telomere distribution, bouquet formation and efficient homologue pairing. J Cell Biol 2000, 151:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koszul R, Kim KP, Prentiss M, Kleckner N, Kameoka S: Meiotic chromosomes move by linkage to dynamic actin cables with transduction of force through the nuclear envelope. Cell 2008, 133:1188–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wanat JJ, Kim KP, Koszul R, Zanders S, Weiner B, Kleckner N, Alani E: Csm4, in collaboration with Ndj1, mediates telomere-led chromosome dynamics and recombination during yeast meiosis. PLoS Genet 2008, 4:e1000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wynne DJ, Rog O, Carlton PM, Dernburg AF: Dynein-dependent processive chromosome motions promote homologous pairing in C. elegans meiosis. J Cell Biol 2012, 196:47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study characterizes the nuclear movement of C. elegans meiocytes. The authors develop methods to film live meiosis in worms and quantify the movements, which are saltatory, dynein dependent, and synapsis-promoting.
- 66.Woglar A, Jantsch V: Chromosome movement in meiosis I prophase of Caenorhabditis elegans. Chromosoma 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sato A, Isaac B, Phillips CM, Rillo R, Carlton PM, Wynne DJ, Kasad RA, Dernburg AF: Cytoskeletal forces span the nuclear envelope to coordinate meiotic chromosome pairing and synapsis. Cell 2009, 139:907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zickler D, Kleckner N: The leptotene-zygotene transition of meiosis. Annu Rev Genet 1998, 32:619–697. [DOI] [PubMed] [Google Scholar]
- 69.Ronceret A, Pawlowski WP: Chromosome dynamics in meiotic prophase I in plants. Cytogenet Genome Res 2010, 129:173–183. [DOI] [PubMed] [Google Scholar]
- 70.Ding DQ, Okamasa K, Yamane M, Tsutsumi C, Haraguchi T, Yamamoto M, Hiraoka Y: Meiosis-specific noncoding RNA mediates robust pairing of homologous chromosomes in meiosis. Science 2012, 336:732–736. [DOI] [PubMed] [Google Scholar]; ** This study shows that in fission yeast, a non-coding RNA contributes to the robust pairing of the locus from which it is transcribed. The authors characterize this RNA in detail and provide an intriguing and plausible molecular explanation for locus specific pairing.
- 71.Lui DY, Cahoon CK, Burgess SM: Multiple opposing constraints govern chromosome interactions during meiosis. PLoS Genet 2013, 9:e1003197. [DOI] [PMC free article] [PubMed] [Google Scholar]