Abstract
Background:
Current data suggest that opioid misuse or opioid use disorder (OUD) may be over represented among tobacco users. However, this association remains understudied in primary care settings. A better understanding of the extent of heterogeneity in opioid misuse among primary care patients who use tobacco may have implications for improved primary care-based screening, prevention, and intervention approaches.
Methods:
Data were derived from a sample of 2,000 adult (aged ≥18) primary care patients across 5 distinct clinics. Among past-year tobacco users (n=882), we assessed the prevalence of opioid misuse and OUD by sociodemographic characteristics and past-year polysubstance use. Latent class analysis (LCA) was used to identify heterogeneous subgroups of tobacco users according to past-year polysubstance use patterns. Multinomial logistic regression was used to examine variables associated with LCA-defined class membership.
Results:
Past-year tobacco use was reported by >84% of participants who reported past-year opioid misuse or OUD. Among those reporting past-year tobacco use, the prevalence of past-year opioid misuse and OUD was 14.0% and 9.5%, respectively. The prevalence of opioid misuse or OUD was highest among tobacco users who were male or unemployed. Three LCA-defined classes among tobacco users were identified including a tobacco-minimal drug use group (78.0%), a tobacco-cannabis use group (10.1%), and a tobacco-opioid/polydrug use group (11.9%). Class membership differed by sociodemographic characteristics.
Conclusions:
Results from this study support the benefit of more comprehensive assessment of and/or monitoring for opioid misuse among primary care patients who use tobacco, particularly for those who are male, unemployed, or polydrug users.
Keywords: Primary care, opioid use disorder, tobacco use, polysubstance, latent class analysis
1. Introduction
Despite declining rates in tobacco use in the U.S. (Giovino et al., 1994; Jamal et al., 2016), disparities persist among vulnerable population subgroups. Available data suggest that adults who report opioid misuse (i.e., prescription opioids or heroin) or have opioid use disorder (OUD) are particularly over-represented among tobacco users in the U.S. For example, data derived from the National Survey on Drug Use and Health (NSDUH) show that current smokers are over 3 times more likely than never smokers to endorse past-month or past-year opioid misuse or OUD (Moeller et al., 2018; Zale et al., 2015). Additionally, the prevalence of past-month opioid misuse has increased from 2002 to 2014 among current smokers but not never-smokers (Moeller et al., 2018). Potential mechanisms governing the association between tobacco and opioid misuse may include mutual reinforcement between pain and tobacco use or the pharmacological interaction between nicotine and opioids (e.g., sensitization/tolerance to reinforcing effects of opioids as a function of tobacco exposure) (Ditre et al., 2011; Shi et al., 2010; Vihavainen et al., 2008). Taken together, these data suggest that tobacco use may be an important predictive variable of opioid misuse or disorder, which has implications for prevention, screening, and intervention strategies. Thus, a better understanding of the patterns of opioid misuse or disorder among tobacco users is warranted to inform such efforts in the face of the substantial and growing rate of opioid-related morbidity and mortality in the .S. (CBHSQ, 2014; Ronan and Herzig, 2016; Seth et al., 2018; Vivolo-Kantor et al., 2018).
Existing data on tobacco and opioid misuse have largely been drawn from survey samples of the general population. While these data have been critical to providing initial evidence of the association between tobacco and opioid misuse, there is also a need to extend this research to general medical settings in order to more accurately inform clinical practice. Primary care settings, in particular, are an important contact point within the healthcare system well suited to identify unmet treatment need for substance use and provide early intervention or referral to specialty treatment (Edelman et al., 2018). Prior studies suggest that the prevalence of substance use disorders is disproportionally higher in primary care settings compared to the general population (Pilowsky and Wu, 2012; Wu et al., 2017). This over-representation may be due to consequential medical problems and other negative sequelae of substance use for which primary care treatment is sought (Cherpitel and Ye, 2008). Indeed, research suggests there may be medical issues that underlie the presence of tobacco and opioid misuse for which treatment in primary care is received including chronic pain, hepatitis C, HIV, depression, or other psychiatric disorders (LaRowe et al., 2018; Parker et al., 2018; Sehgal et al., 2012; Zale et al., 2015). Furthermore, routine screening for tobacco use should be standard practice in many primary care settings based on recommendations from the U.S. Preventative Services Taskforce (Siu,2015).Hence, this practice may be leveraged to identify opioid misuse and provide early intervention given the strong association between tobacco use and opioid misuse. We are not aware, however, of any prior studies that have examined the association between tobacco and opioid misuse among primary care patients specifically in order to inform such efforts.
Additional research is also needed to examine the extent of opioid misuse among different subgroups of tobacco users. Support for more detailed analyses is drawn from research showing that prescription opioid misuse and/or prescription OUD may vary as a function of level of cigarette consumption and sex (Zale et al., 2015). However, data is lacking on the extent to which other potentially predisposing factors or prognostic indicators among tobacco users may be differentially associated with opioid misuse or OUD. Knowledge of these factors among primary care patients may be useful to providers in order to inform efforts aimed at preventing opioid misuse or diversion of prescribed opioids. Polysubstance use among tobacco users is one factor in particular that warrants attention given its association with worse drug-related outcomes and health problems. For example, research shows that individuals reporting polysubstance use or disorder have increased odds of mental health problems, HIV/HCV risk behaviors, or overdose (Connor et al., 2014). Further information is needed, however, on the concurrent use of other drugs with opioids in the context of tobacco use in a primary care setting. Existing data are limited to studies among general population samples using traditional, variable-centered approaches, which assume sample homogeneity and examine opioid misuse and other substance use variables individually among tobacco users. In contrast, person-centered approaches assume sample heterogeneity to identify subgroups with shared characteristics, which are otherwise not directly observable (Muthen and Muthen, 2000). Latent class analysis (LCA) is one such approach commonly applied in healthcare research to empirically define unobservable subgroups of patients according to certain health behaviors (Wu et al., 2011a; Wu et al., 2010; Wu et al., 2011b). This information is important because it may inform the development of screening and intervention strategies with a broader impact as opposed to disorder-specific information. No prior study, however, has used a LCA approach to examine polysubstance patterns among past-year tobacco users in primary care.
To address these gaps in knowledge, here we conducted a secondary data analysis of a large and diverse sample of primary care patients obtained for a multisite clinical trial study of the National Drug Abuse Treatment Clinical Trials Network (NIDA CTN) (McNeely et al., 2016). Using this sample, the goals of this study were 1) to examine the extent of tobacco use by opioid misuse status, 2) to examine the prevalence of opioid misuse and OUD among tobacco users by sociodemographics and concurrent substance use, and 3) to use LCA to identify heterogeneous subgroups of tobacco users according to polysubstance use patterns. Considering any level of tobacco use, regardless of severity, elicits advice or intervention from primary care clinicians, all analyses were focused on past-year tobacco use in order to capture the full extent of opioid misuse or OUD. We hypothesized that the majority of primary care patients reporting opioid misuse or OUD would also endorse tobacco use. We further hypothesized that the prevalence of opioid misuse and OUD among primary patients reporting tobacco use would be distinguished by demographic and other substance use characteristics.
2. Methods
2.1. Study sample
Data were derived as a secondary analysis of the NIDA CTN Tobacco, Alcohol, Prescription medications, and other Substance (TAPS) Tool Study (CTN-0059), which assessed the performance of a novel brief substance screening and assessment tool (McNeely et al., 2016). The total sample included 2000 participants who were recruited across 5 primary care clinics in the eastern U.S. from August 2014 to April 2015. The sites included a Federal Qualified Health Center in Baltimore, MD (n=589), a public hospital-based clinic in New York, NY (n=534), a university-based health center in Richmond, VA (n=211), and two non-academic community-based primary care practices in Kannapolis, NC (n=287 and n=379). Patients were eligible for the study if they were 18 years or older, able to provide informed consent, able to comprehend spoken English, and physically able to complete the screening and assessment measures.
Methods of the TAPS Tool Study have been reported in detail previously (Wu et al., 2016). Briefly, patients were recruited by trained research assistants from the waiting area of each clinic. Patients who were interested were brought to a private room in the clinic where they were assessed for eligibility and verbal consent was obtained. Eligible participants were informed that their participation was confidential and their responses would not be shared with primary care staff. They completed a 2-stage screening and brief assessment tool as part of the parent trial procedures. Additionally, participants completed a battery of standard reference measures of substance use and substance use-related problems, which was administered by the research assistants. Participants received $20 for the completion of these study assessments. This use of the TAPS Tool data for this analysis was approved by the Duke University Health System Institutional Review Board.
2.2. Study variables
Sociodemographic variables included self-reported age, sex, race, ethnicity, education, marital status, and employment status. ast-year substance use/misuse and past-year substance use disorder (SUD) were assessed using the modified World Health Organization World Mental Health Composite International Diagnostic Interview (WMH-CIDI) (McNeely et al., 2016; WHO, 2015). The modified WMH-CIDI assessed past-year use/misuse and SUD of the following classes of substances: tobacco, alcohol, cannabis, cocaine/crack, prescription opioids, heroin, prescription stimulants, prescription sedatives, methamphetamine, hallucinogens, inhalants, and other nonspecific drugs. Because the WMH-CIDI does not include all of the DSM-5 tobacco use disorder criteria, tobacco use disorder was assessed by adapting the items from the WMH-CIDI drug section. We operationalized past-year opioid misuse or OUD to include prescription opioids or heroin. Using the modified CIDI, participants who responded affirmatively to any past-year use of a substance were administered additional questions to assess whether DSM-5 criteria for SUD was met in the past 12 months for that substance. DSM-5 SUD criteria was assessed using the modified CIDI such that existing CIDI items were mapped onto the DSM-5 classifications by omitting the item on legal problems and including the one on craving. All substance-specific criteria for SUD were assessed (i.e., no skip pattern was used during assessments). Based on the DSM-5, SUD was defined as meeting ≥2 DSM criteria for a given substance. Furthermore, lifetime and past 3-month substance use was assessed using the World Health Organization Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) (Humeniuk et al., 2008).
2.3. Data analysis
The prevalence of past-year tobacco use was examined in the total sample by demographic characteristics and opioid use status (i.e., any misuse in lifetime, past-year, and/or past 3 months; past-year OUD). We also examined the prevalence of past-year opioid misuse and OUD among past-year tobacco users by sociodemographic characteristics and other past-year substance use or SUD status. Next, LCA was conducted to identify distinct classes of past-year tobacco users sharing similar drug use behaviors. Specifically, LCA was applied to five dichotomous past-year substance use variables including opioids, cannabis, cocaine, prescription stimulants/sedatives, and other drugs (i.e., methamphetamine, hallucinogens, inhalants, or other nonspecific drugs). LCA models were fit using maximum likelihood estimation. Results of the LCA provided estimates of class membership probabilities and within-class probabilities of the past-year substance use variables. We estimated models with one to four classes and selected the best fitting model based on a combination of criteria including the likelihood-ratio (G2) test, Akaike’s Information Criterion (AIC), Bayesian Information Criterion (BIC) statistic, sample size-adjusted BIC (ABIC), and entropy (Kaplan, 2004). The BIC statistic was given the most weight compared to the other goodness of fit measures based on its performance in simulation studies, with lower BIC values suggesting a better model fit (Nylund et al., 2007). We also considered the class size in selecting a model that would be applicable to subsequent analysis of associated sociodemographic variables to improve interpretability. After the final model was selected, multinomial logistic regression was used to estimate differences in sociodemographics between LCA-defined subgroups. Preliminary analyses indicated that convergence of the LCA model was not allowed when the past-year alcohol use variable was included in the model, potentially because of its high prevalence among tobacco users. Thus, past-year alcohol use was included as a separate independent variable in the multinomial logistic regression model of the LCA-defined subgroups.
3. Results
3.1. Prevalence of tobacco use among primary care patients
Among the total sample (n=2000), past-year tobacco use was reported by 44.1% (n=882) of participants (Table 1). The prevalence of past-year tobacco use was similar across study sites except for one (Maryland site; Table S1). The prevalence of past-year tobacco use was highest among males (54.4%), whites (46.5%), those with less than high school education (54.8%), and those who were unemployed (52.5%), disabled (51.3%), or never married (50.6%). Past-year tobacco use was reported by 84.8% of participants who reported past-year opioid misuse and by 89.4% who met criteria for past-year OUD.
Table 1.
Prevalence of past-year tobacco use by demographic and substance use characteristics among adult primary care patients (n=2000).
| Past-year tobacco use, yes |
|||
|---|---|---|---|
| n | Row % | 95% CI | |
| Total | 882 | 44.10 | 41.94–46.29 |
| Sex | |||
| Male | 475 | 54.35 | 51.03–57.62 |
| Female | 406 | 36.12 | 33.36–38.97 |
| Age in years | |||
| 18–34 | 247 | 46.96 | 42.73–51.23 |
| 35–49 | 243 | 46.02 | 41.82–50.29 |
| 50+ | 392 | 41.44 | 38.34–44.61 |
| Race/ethnicity | |||
| White, non-Hispanic | 268 | 46.45 | 42.41–50.53 |
| Black/African American, non-Hispanic | 481 | 45.46 | 42.48–48.47 |
| Hispanic | 93 | 39.91 | 33.84–46.32 |
| Other/unknown | 40 | 30.30 | 23.11–38.61 |
| Education | |||
| Less than high school | 210 | 54.83 | 49.82–59.74 |
| High school/GED | 277 | 47.92 | 43.88–52.00 |
| Some college/associate/bachelor/graduate degree |
395 | 38.05 | 35.15–41.05 |
| Employment | |||
| Employed | 272 | 38.20 | 34.71–41.83 |
| Unemployed | 220 | 52.51 | 47.72–57.24 |
| Disabled | 242 | 51.27 | 46.77–55.75 |
| Other | 148 | 37.28 | 32.67–42.14 |
| Marital Status | |||
| Married/cohabited | 181 | 34.54 | 30.60–38.71 |
| Separated/divorced/widowed | 238 | 42.58 | 38.54–46.71 |
| Never married | 463 | 50.55 | 47.31–53.77 |
| Lifetime opioid misuse | |||
| No | 601 | 37.73 | 35.38–40.13 |
| Yes | 281 | 69.04 | 64.39–73.34 |
| Past-year opioid misuse | |||
| No | 755 | 40.81 | 38.59–43.07 |
| Yes | 123 | 84.83 | 78.10–89.76 |
| Past 3-month opioid misuse | |||
| No | 795 | 41.97 | 39.77–44.21 |
| Yes | 87 | 82.08 | 73.69–88.21 |
| Past-year opioid use disorder | |||
| No | 794 | 41.77 | 39.57–44.00 |
| Yes | 84 | 89.36 | 81.51–94.12 |
| Past-year non-opioid drug use among tobacco users†, yes |
|||
| Cannabis | 299 | 33.90 | 30.85–37.09 |
| Cocaine | 121 | 13.72 | 11.61–16.15 |
| Rx stimulants/Rx sedatives | 75 | 8.50 | 6.84–10.53 |
| Other drugs‡ | 58 | 6.58 | 5.12–8.41 |
Prevalence of past-year drug use among tobacco users (n=882) rather than the total sample.
Other drugs included methamphetamine, hallucinogens, inhalant, and other nonspecific drugs.
Missing values were not reported. CI: confidence interval; GED: general education diploma.
3.2. Prevalence of opioid misuse and OUD among tobacco users in primary care
Among the 882 participants reporting past-year tobacco use, approximately one in seven (14.0%) reported past-year opioid misuse (Table 2). In contrast, the prevalence of past-year opioid misuse among non-tobacco users was 2.0%. The prevalence of past-year opioid misuse was higher among tobacco users who were male (17.7%) or unemployed (21.4%). Approximately one in ten (9.5%) tobacco users reported past-year OUD. The prevalence of past-year OUD was higher among tobacco users who were unemployed than those who were employed (17.3% vs. 5.2%). The prevalence of past-year opioid misuse and OUD, respectively among those with past-year tobacco use, was highest among those reporting past-year nonmedical prescription stimulant/sedative use (61.3%; 42.7%), followed by those reporting past-year cocaine use (44.6%; 35.5%). Similar patterns of results were found among those with past-year tobacco use who had other past-year SUDs.
Table 2.
Prevalence of past-year opioid misuse and opioid use disorder among primary care patients reporting past-year tobacco use (n=882).
| Past-year opioid misuse, yes |
Past-year opioid use disorder, yes |
|||
|---|---|---|---|---|
| Past-year tobacco users | Row % | 95% CI | Row % | 95% CI |
| Total | 13.95 | 11.82–16.39 | 9.52 | 7.76–11.64 |
| Sex | ||||
| Male | 17.68 | 14.52–21.37 | 12.00 | 9.38–15.23 |
| Female | 9.61 | 7.11–12.86 | 6.65 | 4.61–9.50 |
| Age in years | ||||
| 18–34 | 12.15 | 8.64–16.81 | 7.69 | 4.98–11.70 |
| 35–49 | 18.11 | 13.77–23.43 | 12.35 | 8.79–17.08 |
| 50+ | 12.50 | 9.59–16.14 | 8.93 | 6.49–12.16 |
| Race/ethnicity | ||||
| White, non-Hispanic | 13.43 | 9.86–18.04 | 8.96 | 6.09–12.98 |
| Black/African American, non-Hispanic | 12.27 | 9.63–15.50 | 8.52 | 6.35–11.36 |
| Hispanic | 24.73 | 17.08–34.38 | 16.13 | 10.03–24.92 |
| Other/unknown | 12.50 | 5.46–26.11 | 10.00 | 3.96–23.05 |
| Education | ||||
| Less than high school | 15.24 | 11.01–20.72 | 11.43 | 7.80–16.44 |
| High school/GED | 18.77 | 14.61–23.79 | 14.44 | 10.79–19.07 |
| Some college/associate /bachelor/graduate degree |
9.87 | 7.31–13.21 | 5.06 | 3.30–7.69 |
| Employment | ||||
| Employed | 8.82 | 6.00–12.79 | 5.15 | 3.09–8.45 |
| Unemployed | 21.36 | 16.46–27.25 | 17.27 | 12.85–22.82 |
| Disabled | 14.46 | 10.59–19.45 | 9.92 | 6.76–14.33 |
| Other | 11.49 | 7.30–17.63 | 5.41 | 2.76–10.30 |
| Marital Status | ||||
| Married/cohabited | 10.50 | 6.82–15.81 | 5.52 | 3.03–9.87 |
| Separated/divorced/widowed | 12.61 | 8.97–17.42 | 8.82 | 5.84–13.11 |
| Never married | 15.98 | 12.93–19.60 | 11.45 | 8.86–14.67 |
| Past-year substance use, yes | ||||
| Alcohol | 16.77 | 14.05–19.90 | 11.18 | 8.95–13.89 |
| Cannabis | 22.41 | 18.05–27.47 | 14.38 | 10.86–18.81 |
| Cocaine | 44.63 | 36.07–53.51 | 35.54 | 27.57–44.39 |
| Rx stimulants/Rx sedatives | 61.33 | 50.02–71.54 | 42.67 | 32.10–53.95 |
| Other drugs† | 37.93 | 26.56–50.80 | 22.41 | 13.59–34.66 |
| Past-year substance use disorder, yes | ||||
| Alcohol | 23.86 | 18.44–30.27 | 16.24 | 11.75–22.03 |
| Cannabis | 23.93 | 17.11–32.41 | 14.53 | 9.27–22.04 |
| Cocaine | 51.14 | 40.87–61.31 | 44.32 | 34.39–54.72 |
| Rx stimulants/Rx sedatives | 61.54 | 42.53–77.57 | 53.85 | 35.46–71.24 |
| Other drugs† | 58.33 | 38.83–75.53 | 41.67 | 24.47–61.17 |
Other drugs included methamphetamine, hallucinogens, inhalant, and other nonspecific drugs.
Missing values were not reported. CI: confidence interval; GED: general education diploma.
3.3. Patterns of polysubstance use among patients who reported past-year tobacco use
Based on fit indices, the final LCA model selected was one consisting of three classes (Table S2). That is, the 3-class model had the highest entropy estimate (0.78) compared to the 2- and 4-class model and a lower BIC value (3264) compared to the 4-class model (3270). Class 1 (tobacco-minimal drug use group; 78.0% of sample) was characterized by low probability of any past-year drug use (Figure 1; Table S3). Class 2 (tobacco-cannabis use group; 10.1% of sample) was characterized by high probability of past-year cannabis use (1.00) and moderate probability of other past-year drug use (0.30). Class 3 (tobacco-opioid/polydrug use group; 11.9% of sample) was characterized by high probability of opioid misuse (0.83), and moderate probability of past-year cannabis (0.63), cocaine (0.58), and prescription stimulant/sedative use (0.51).
Figure 1.
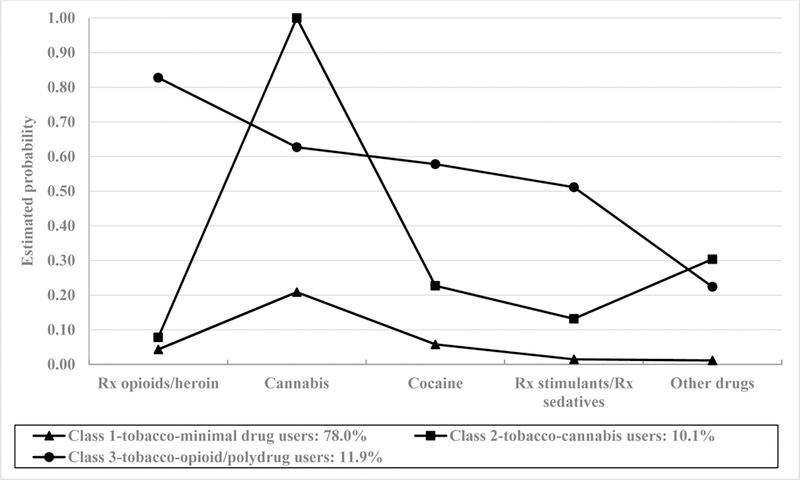
Estimated probabilities of drug use for the 3-class latent class analysis model (n=878).
3.4. Correlates of LCA-defined classes
Younger age (vs. ages 35–49; 50+) and past-year alcohol use were associated with increased odds of being in the tobacco-cannabis user class relative to the tobacco-minimal drug use class (Table 3). Being male, white (vs. black), less educated (vs. some college or post-secondary degree), unemployed (vs. employed), disabled (vs. employed), and past-year alcohol use were associated with increased odds of being in the tobacco-opioid/polydrug use class relative to the tobacco-minimal drug use class. There were no significant differences in the study variables between the tobacco-opioid/polydrug use group and the tobacco-cannabis use group.
Table 3.
Adjusted multinomial logistic regression of LCA-defined drug use groups among adult primary care adults reporting past-year tobacco use (n=877†).
| Tobacco-cannabis use group vs. tobacco-minimal drug use group |
Tobacco- opioid/polydrug use group vs. tobacco- minimal drug use group |
Tobacco-opioid- polydrug use group vs. tobacco-cannabis use group |
||||
|---|---|---|---|---|---|---|
| Adjusted odds ratio (AOR) | AOR | 95%CI | AOR | 95%CI | AOR | 95%CI |
| Sex | ||||||
| Male | 1.00 | 1.00 | 1.00 | |||
| Female | 0.61 | 0.33–1.12 | 0.46 | 0.28–0.76 | 0.76 | 0.37–1.56 |
| Age in years | ||||||
| 18–34 | 1.00 | 1.00 | 1.00 | |||
| 35–49 | 0.45 | 0.21–0.97 | 0.82 | 0.43–1.55 | 1.80 | 0.74–4.39 |
| 50+ | 0.25 | 0.11–0.59 | 0.64 | 0.33–1.23 | 2.52 | 0.95–6.68 |
| Race/ethnicity | ||||||
| White, non-Hispanic | 1.00 | 1.00 | 1.00 | |||
| Black/African American, non- Hispanic |
0.84 | 0.42–1.66 | 0.57 | 0.33–0.99 | 0.68 | 0.30–1.52 |
| Hispanic/other/unknown | 1.06 | 0.45–2.52 | 0.84 | 0.41–1.71 | 0.79 | 0.29–2.16 |
| Education | ||||||
| Less than high school | 1.00 | 1.00 | 1.00 | |||
| High school/GED | 1.09 | 0.48–2.47 | 0.84 | 0.47–1.49 | 0.77 | 0.31–1.93 |
| Some | 1.06 | 0.48–2.34 | 0.52 | 0.29–0.95 | 0.49 | 0.20–1.23 |
| college/associate/bachelor/graduate degree |
||||||
| Employment | ||||||
| Employed | 1.00 | 1.00 | 1.00 | |||
| Unemployed | 1.98 | 0. 94–4.19 | 2.63 | 1.40–4.96 | 1.33 | 0.54–3.25 |
| Disabled | 1.51 | 0. 60–3.80 | 2.34 | 1.18–4.66 | 1.55 | 0.53–4.53 |
| Other | 1.69 | 0. 73–3.93 | 2.04 | 0.95–4.37 | 1.20 | 0.42–3.42 |
| Marital Status | ||||||
| Married/cohabited | 1.00 | 1.00 | 1.00 | |||
| Separated/divorced/widowed | 0.81 | 0.28–2.32 | 0.70 | 0.35–1.41 | 0.87 | 0.26–2.86 |
| Never married | 1.40 | 0.61–3.22 | 0.82 | 0.45–1.51 | 0.58 | 0.22–1.54 |
| Past-year alcohol use | ||||||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 4.63 | 1.77–12.07 | 4.73 | 2.46–9.09 | 1.02 | 0.33–3.15 |
| Study site (state) | ||||||
| New York | 1.00 | 1.00 | 1.00 | |||
| Maryland | 1.17 | 0.55–2.52 | 1.67 | 0.95–2.94 | 1.42 | 0.60–3.38 |
| Virginia/North Carolina | 0.35 | 0.14–0.86 | 0.27 | 0.13–0.57 | 0.77 | 0.26–2.31 |
4 patients were excluded due to missing substance use data; 1 patient was excluded due to missing data on sex.
AOR: adjusted odds ratio; CI: confidence interval; GED: general education diploma; LCA: latent class analysis. Boldface: p<0.05.
4. Discussion
The present study, to our knowledge, is the first to examine the association between tobacco and opioid misuse and to apply LCA to identify distinct polysubstance use patterns with tobacco use among a large sample of primary care patients. Key results from our study included 1) the large majority (>84%) of primary care patients reporting past-year opioid misuse or OUD were also past-year tobacco users, 2) the prevalence of opioid misuse and disorder among primary patients who used tobacco varied by sociodemographic and polysubstance use variables, and 3) three heterogeneous subgroups of primary care patients who used tobacco were defined by LCA according to substance use patterns, which included a tobacco-minimal drug use group (78.0% of sample), a tobacco-cannabis use group (10.1% of sample), and a tobacco-opioid/polydrug use group (11.9% of sample). We further found that each subgroup was distinguished by sociodemographic characteristics, which is relevant for public health and clinical intervention.
Previous research has only focused on samples from the general population or specialty treatment settings, which also showed elevated prevalence of tobacco use among persons with opioid misuse or OUD. For instance, data derived from the NSDUH from 2006–2014 showed that approximately 80% of adults who were opioid dependent smokers met criteria for nicotine dependence (Parker et al., 2018). Among addiction treatment samples, the prevalence of smoking ranged from 73.5% to 94.0% among patients who were treated primarily for opioid misuse (Guydish et al., 2011). In comparison, the prevalence of cigarette smoking among the general adult .S. population during the same time frame of these studies was 15.0% – 26.0% (Giovino et al., 1994; Jamal et al., 2016). Our study extends these findings by providing primary care-specific data, which is important given the critical opportunity that primary care settings have in addressing opioid misuse and tobacco use.
The disproportionate rate of tobacco use among adults who misuse opioids or have OUD bears implications for clinical interventions in primary care. Research has shown that the mortality rate of persons with OUD who smoke is four times greater than those with OUD who do not smoke (Hser et al., 1994; Hurt et al., 1996). Moreover, it has been reported that cigarette smokers with opioid dependence had greater rates of and more severe nicotine dependence than smokers without opioid dependence (Parker et al., 2018). Although most patients with OUD reported a desire to quit cigarette smoking (Hall et al., 2018; Nahvi et al., 2006), smoking cessation interventions generally considered effective among other populations (e.g., nicotine replacement therapy, varenicline, and bupropion) have limited effectiveness among OUD-smokers (Miller and Sigmon, 2015). More research is needed to elucidate potential factors mediating pharmacotherapy and behavioral treatment efficacy among this subgroup, including the timing of the quit attempt, role of nicotine withdrawal, and other biological, social, and environmental factors (Miller and Sigmon, 2015).
Another implication from these findings is that the high prevalence of tobacco use among patients reporting opioid misuse may be leveraged to identify unmet treatment need for opioid misuse/OUD. Indeed, many primary care settings should already be screening for tobacco use based on recommendations from the U.S. Preventive Services Task Force (Siu, 2015). Congruent with this practice, research shows that tobacco users are most often identified in a primary care setting (Pine-Abata et al., 2013). Overall, we found that the prevalence of opioid misuse and OUD among tobacco users was 14.0% and 9.5%, respectively. These prevalence rates are relatively higher than what has been found among general population samples of tobacco users. For instance, two studies utilizing data from the NSDUH estimated that the prevalence of DS −4 OUD among daily smokers was approximately 2% (Parker et al., 2018; Zale et al., 2015), which is more than four times lower than the rate from this study’s sample of tobacco users. The prevalence of past-year opioid misuse among daily cigarette smokers from the NSDUH was 10.5% (Zale et al., 2015), which is also relatively lower than what was found in our study. Taken together, these findings further underscore the critical role that primary care settings may play in identifying unmet treatment need for OUD and initiating treatment.
The present study also revealed differences in the prevalence of opioid misuse and OUD among tobacco users by sociodemographic characteristics and polysubstance use. Regarding sociodemographic differences, we found higher prevalence of opioid misuse or OUD among males and those who were unemployed. Similar patterns of associations have been found among the overall general population (Han et al., 2017), although at relatively lower prevalence. Primary care providers may prioritize these subgroups of patients who use tobacco for targeted prevention, closer monitoring, and screening for opioid misuse/OUD. Additionally, a better understanding of the mechanisms governing the association between tobacco use and opioid misuse is needed to inform such efforts. It is possible that tobacco use may covary with other factors associated with opioid misuse/OUD such as chronic pain and/or depression (Volkow and McLellan, 2016). In particular, researchers have proposed a reciprocal model of pain and cigarette smoking such that each may exacerbate one another via a positive feedback loop and lead to increased opioid use (Ditre et al., 2011).
A particularly high prevalence of opioid misuse/OUD was also found among tobacco users in primary care who reported concurrent illicit substance use. These findings are consistent with other studies of non-primary care settings (Parker et al., 2018). We extended the literature, however, by using LCA to empirically specify unobserved subgroups of tobacco users in primary care by their substance use patterns. This method stands in contrast to most previous research on patterns of the use of multiple substances among tobacco users, which have focused on bivariate analyses or more generalized approaches. Our analysis identified three latent classes among tobacco users. Notably, the class characterized by high probability of opioid misuse also had relatively high probability of other illicit substance use including cannabis (0.63), cocaine (0.58), and nonmedical prescription stimulants/sedatives (0.51). Hence, primary care providers should be aware of these substance use patterns, which could lead to initiating conversations with patients to further assess the motives, perceived risks/benefits, and frequency/intensity of other drug use among those who use tobacco. In particular, the misuse of other prescription medications or illicit drugs with opioids has been associated with increased odds of overdose, psychiatric disorders, and poorer quality of life (Kandel et al., 2017; McCabe et al., 2006; McCall Jones et al., 2017; Wu et al., 2010).
Our analysis also indicated a latent class of primary care tobacco users characterized by the high probability of concurrent past-year cannabis use. This subgroup of patients warrants concern considering some evidence suggests the dual use of cannabis and tobacco may be associated with greater problems than use of either cannabis or tobacco alone. For example, current research shows that dual tobacco and cannabis use is associated with greater likelihood of cannabis use disorder, poorer cannabis cessation outcomes, more alcohol and other drug use, and greater psychosocial problems (Montgomery, 2015; Peters et al., 2012; Pulvers et al., 2018; Ramo et al., 2013). Taken together, these findings indicate the value in screening for and assessing problem cannabis use in primary care among patients who use tobacco.
Consistent with our hypothesis, class membership differed by sociodemographic characteristics. However, we found that membership in the tobacco-opioid/polydrug class was distinguished by more sociodemographic correlates than the tobacco-cannabis class when compared to the tobacco-minimal drug user class. That is, male sex, white race, lower education, unemployment, being disabled, and alcohol use were associated with the tobacco-opioid/polydrug class. These findings suggest the need for increased prevention efforts for opioid misuse among these subgroups of primary care patients who use tobacco, especially when prescribed opioids for pain. In contrast, only younger age and alcohol use were associated with the tobacco-cannabis class.
Limitations of this study include its cross sectional nature, which did not allow for causal inferences or determination of temporal relationships. Our results also relied on self-reported data of participants, which may have been subject to recall bias or social-desirability bias. As a result, underreporting is expected and prevalence estimates of substance use should be considered conservative. The prevalence of opioid and other prescription drug misuse may be underestimated in our study, given its basis in primary care, due to concern that reported use may affect the likelihood of obtaining the medication from a primary care provider. However, participants were informed that their responses were anonymous and confidential and would not be shared with the primary care team. Another limitation was that our sample included primary care patients from urban and suburban clinics in the eastern region of the U.S., which may limit generalizability to other regions. In particular, two of the practices (NY and MD) were urban clinics serving high proportions of low-income or publicly insured patients, which contributed to 56% of the sample. Urban populations with lower socioeconomic status tend to have a high prevalence of tobacco use (Jamal et al., 2016), which may have contributed to the relatively high proportion of past-year tobacco use in our sample (44%). More than one fifth of our study sample were adults who were unemployed (21%) or were disabled (23.6%). Frequent medical care may be likely among these subgroups thereby resulting in disproportionate representation in our sample. Finally, tobacco use in our study was limited to use of any tobacco product in the past-year. While this variable may be most practical for routine screening in primary care, future studies may consider the association of opioid misuse and other dimensions of tobacco use (e.g., frequency and patterns of use, type of tobacco product, etc.). One study utilizing national survey data, however, did not find differences between frequency of tobacco use within the past year and odds of opioid misuse or OUD (Zale et al., 2015). Nonetheless, additional research is needed given the reported differences between heavy and light/intermittent smokers (Fagan and Rigotti, 2009; Shiffman et al., 2012), the association of broader tobacco product use with increased risk behaviors (Bombard et al., 2009; Kristjansson et al., 2015; Pulvers et al., 2018; Wills et al., 2015), and the emerging trend of new tobacco products (Harrell et al., 2017). Despite these limitations, our analysis represents an important step to better understand the association between tobacco use and opioid misuse in primary care.
In conclusion, the present study supports the benefit of more comprehensive assessment of opioid misuse among primary care patients who use tobacco. Our study also highlights the heterogeneity in regards to the extent of opioid misuse or OUD by patient sociodemographic and substance use characteristics. Thus, primary care providers should not only recognize the association between tobacco use and opioid misuse but also the differential liability of misuse among some patient subgroups, which may be used to inform prevention or early intervention efforts. Going forward, future research should continue to investigate the mechanisms governing concurrent tobacco use and opioid misuse in order to inform strategies of more effectively treating this unique patient subgroup. It also remains critical to continue promoting the adoption of substance use disorder treatment in primary care and supporting providers to ensure they have the training, education, and resources to diagnose and treat patients with substance use disorder.
Supplementary Material
Highlights.
We analyzed substance use in a large and diverse sample of primary care patients.
Almost 9 of 10 primary care patients with opioid use disorder were tobacco users.
More than 1 of 10 primary care patients who used tobacco reported opioid misuse.
Opioid misuse was highest among tobacco users who were male and unemployed
We identified 3 latent classes of tobacco users according to polysubstance use.
Acknowledgements:
Geetha A. Subramaniam is an employee of the National Institute on Drug Abuse (NIDA) Center for the Clinical Trials Network (CCTN), the funding agency for this project. Her participation in this publication arises from her role as a project scientist on a cooperative agreement for this study (CTN-0059; ClinicalTrials.Gov number: NCT02110693). The opinions expressed in this paper are solely those of the authors and do not represent the official position of the NIH or the U.S. government.
Role of the funding source: This study was made possible by the U.S. National Institutes of Health (grant numbers UG1DA040317, UG1DA013034, UG1DA013035, U10DA013727, and R01MD007658). The data analysis and preparation of this manuscript was supported primarily by National Institute on Drug Abuse (grant number UG1DA040317). The sponsoring agency had no further role in the study design and analysis, the writing of the report, or the decision to submit the paper for publication. Geetha Subramaniam was a scientific protocol coordinator and collaborator under the cooperative agreement for the study data used for this manuscript (CTN-0059 TAPS Tool). Geetha Subramaniam has had no programmatic responsibilities for R01MD007658. The opinions expressed in this paper are solely those of the authors and do not represent the official position of the U.S. government.
Li-Tzy Wu also has received research funding from Patient-Centered Outcomes Research Institute and Alkermes Inc. William S. John also has received research funding from Patient-Centered Outcomes Research Institute. Paolo Mannelli has received consultation fees from Guidepoint Global and research funding from The Laura and John Arnold Foundation, Orexo, and Alkermes Inc., and served on Scientific Advisory Boards for Alkermes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The other authors have no conflicts of interest to disclose.
References
- Bombard JM, Pederson LL, Koval JJ, O’Hegarty M, 2009. How are lifetime polytobacco users different than current cigarette-only users? Results from a Canadian young adult population. Addict. Behav 34, 1069–1072. [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics Quality (CBHSQ), 2014. Treatment Episode Data Set (TEDS): 2002–2012. National Admissions to Substance buse Treatment Services, BHSIS Series−71, (SMA) 14–4850. Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Cherpitel CJ, Ye Y, 2008. Drug use and problem drinking associated with primary care and emergency room utilization in the US general population: data from the 2005 national alcohol survey. Drug Alcohol Depend 97, 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JP, Gullo MJ, White A, Kelly AB, 2014. Polysubstance use: diagnostic challenges, patterns of use and health. Curr Opin Psychiatry 27, 269–275. [DOI] [PubMed] [Google Scholar]
- Ditre JW, Brandon TH, Zale L, Meagher MM, 2011. Pain, nicotine, and smoking: research findings and mechanistic considerations. Psychol. Bull 137, 1065–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman EJ, Oldfield BJ,etrault JM, 2018. Office-Based Addiction Treatment in Primary Care: Approaches That Work. Med. Clin. North Am 102, 635–652. [DOI] [PubMed] [Google Scholar]
- Fagan P, Rigotti NA, 2009. Light and intermittent smoking: the road less traveled. Nicotine Tob Res 11, 107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovino GA, Schooley MW, Zhu BP, Chrismon JH, Tomar SL, Peddicord JP, Merritt RK, Husten G, Eriksen MP, 1994. Surveillance for selected tobacco-use behaviors--United States, 1900–1994. MMWR CDC Surveill. Summ 43, 1–43. [PubMed] [Google Scholar]
- Guydish J, Passalacqua E, Tajima B, Chan M, Chun J, Bostrom A, 2011. Smoking prevalence in addiction treatment: a review. Nicotine Tob Res 13, 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Humfleet GL, Gasper JJ, Delucchi KL, Hersh DF, Guydish JR, 2018. Cigarette Smoking Cessation Intervention for Buprenorphine Treatment Patients. Nicotine Tob Res 20, 628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM, 2017. Prescription Opioid Use, Misuse, and Use Disorders in U.S. Adults: 2015 National Survey on Drug Use and Health. Ann. Intern. Med 167, 293–301. [DOI] [PubMed] [Google Scholar]
- Harrell PT, Naqvi SMH, Plunk AD, Ji M, Martins SS, 2017. Patterns of youth tobacco and polytobacco usage: The shift to alternative tobacco products. Am. J. Drug Alcohol Abuse 43, 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, McCarthy WJ, Anglin MD, 1994. Tobacco use as a distal predictor of mortality among long-term narcotics addicts. Prev. Med 23, 61–69. [DOI] [PubMed] [Google Scholar]
- Humeniuk R, Ali R, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, De Lacerda RB, Ling W, Marsden J, Monteiro M, 2008. Validation of the alcohol, smoking and substance involvement screening test (ASSIST). Addiction 103, 1039–1047. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton LJ 3rd, 1996. Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA 275, 1097–1103. [DOI] [PubMed] [Google Scholar]
- Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM, 2016. Current Cigarette Smoking Among Adults - United States, 2005–2015. MMWR Morb. Mortal. Wkly. Rep 65, 1205–1211. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Hu MC, Griesler P, Wall M, 2017. Increases from 2002 to 2015 in prescription opioid overdose deaths in combination with other substances. Drug Alcohol Depend 178, 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D, 2004. The Sage handbook of quantitative methodology for the social sciences Sage. [Google Scholar]
- Kristjansson AL, Mann MJ, Sigfusdottir ID, 2015. Licit and Illicit ubstance Use by Adolescent E-Cigarette Users Compared with Conventional Cigarette Smokers, Dual Users, and Nonusers. J. Adolesc. Health 57, 562–564. [DOI] [PubMed] [Google Scholar]
- LaRowe LR, Chilcott LN, Zvolensky MJ, Vanable PA, Flood K, Ditre JW, 2018. Associations between Pain-Related Anxiety, Gender, and Prescription Opioid Misuse among Tobacco Smokers Living with HIV/AIDS. Subst. Use Misuse, 1–10. [DOI] [PMC free article] [PubMed]
- McCabe SE, Cranford JA, Morales M, Young A, 2006. Simultaneous and concurrent polydrug use of alcohol and prescription drugs: prevalence, correlates, and consequences. J. Stud. Alcohol 67, 529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall Jones C, Baldwin GT, Compton WM, 2017. Recent Increases in Cocaine-Related Overdose Deaths and the Role of Opioids. Am. J. Public Health 107, 430–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeely J, Wu LT, Subramaniam G, Sharma G, Cathers LA, Svikis D, Sleiter L, Russell L, Nordeck C, Sharma A, O’Grady KE, Bouk LB, Cushing C, King J, Wahle A, Schwartz RP, 2016. Performance of the Tobacco, Alcohol, Prescription Medication, and Other Substance Use (TAPS) ool for Substance Use Screening in Primary Care Patients. Ann. Intern. Med 165, 690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ME, Sigmon SC, 2015. Are Pharmacotherapies Ineffective in Opioid-Dependent Smokers? Reflections on the Scientific Literature and Future Directions. Nicotine Tob Res 17, 955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Fink DS, Gbedemah M, Hasin DS, Galea S, Zvolensky MJ, Goodwin RD, 2018. Trends in Illicit Drug Use Among Smokers and Nonsmokers in the United States, 2002–2014. J. Clin. Psychiatry 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery L, 2015. Marijuana and tobacco use and co-use among African Americans: results from the 2013, National Survey on Drug Use and Health. Addict. Behav 51, 18–23. [DOI] [PubMed] [Google Scholar]
- Muthen B, Muthen LK, 2000. Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcohol. Clin. Exp. Res 24, 882–891. [PubMed] [Google Scholar]
- Nahvi S, Richter K, Li X, Modali L, Arnsten J, 2006. Cigarette smoking and interest in quitting in methadone maintenance patients. Addict. Behav 31, 2127–2134. [DOI] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, Muthén BO, 2007. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural equation modeling 14, 535–569. [Google Scholar]
- Parker MA, Streck JM, Sigmon SC, 2018. Associations between opioid and nicotine dependence in nationally representative samples of United States adult daily smokers. Drug Alcohol Depend 186, 167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters EN, Budney AJ, Carroll KM, 2012. Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addiction 107, 1404–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilowsky DJ, Wu LT, 2012. Screening for alcohol and drug use disorders among adults in primary care: a review. Subst Abuse Rehabil 3, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine-Abata H, McNeill A, Murray R, Bitton A, Rigotti N, Raw M, 2013. A survey of tobacco dependence treatment services in 121 countries. Addiction 108, 1476–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvers K, Ridenour C, Woodcock A, Savin MJ, Holguin G, Hamill S, Romero DR, 2018. Marijuana use among adolescent multiple tobacco product users and unique risks of dual tobacco and marijuana use. Drug Alcohol Depend 189, 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramo DE, Delucchi KL, Hall SM, Liu H, Prochaska JJ, 2013. Marijuana and tobacco co-use in young adults: patterns and thoughts about use. J Stud Alcohol Drugs 74, 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronan MV, Herzig SJ, 2016. Hospitalizations Related To Opioid Abuse/Dependence And Associated Serious Infections Increased Sharply, 2002–12. Health Aff. (Millwood) 35, 832–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal N, Manchikanti L, Smith HS, 2012. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Physician 15, Es67–92. [PubMed] [Google Scholar]
- Seth P, Scholl L, Rudd RA, Bacon S, 2018. Overdose Deaths Involving Opioids, Cocaine, and Psychostimulants - United States, 2015–2016. MMWR Morb. Mortal. Wkly. Rep 67, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Weingarten TN, Mantilla CB, Hooten W, Warner DO, 2010. Smoking and pain: pathophysiology and clinical implications. Anesthesiology 113, 977–992. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Scholl SM, Tindle HA, 2012. Smoking motives of daily and non-daily smokers: a profile analysis. Drug Alcohol Depend 126, 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu AL, 2015. Behavioral and Pharmacotherapy Interventions for Tobacco Smoking Cessation in Adults, Including Pregnant Women: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med 163, 622 – 634. [DOI] [PubMed] [Google Scholar]
- Vihavainen T, Piltonen M, Tuominen RK, Korpi ER, Ahtee L, 2008. Morphine-nicotine interaction in conditioned place preference in mice after chronic nicotine exposure. Eur. J. Pharmacol 587, 169–174. [DOI] [PubMed] [Google Scholar]
- Vivolo-Kantor AM, Seth P, Gladden RM, Mattson CL, Baldwin GT, Kite-Powell A, Coletta MA, 2018. Vital Signs: rends in Emergency Department Visits for Suspected Opioid Overdoses - United States, July 2016-September 2017. MMWR Morb. Mortal. Wkly. Rep 67, 279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, McLellan AT, 2016. Opioid Abuse in Chronic Pain--Misconceptions and Mitigation Strategies. N. ngl. J. Med 374, 1253–1263. [DOI] [PubMed] [Google Scholar]
- Wills TA, Knight R, Williams RJ, Pagano I, Sargent JD, 2015. Risk factors for exclusive e-cigarette use and dual e-cigarette use and tobacco use in adolescents. Pediatrics 135, e43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO), 2015. The World Mental Health Composite International Diagnostic Interview
- Wu LT, Ling W, Burchett B, Blazer DG, Yang C, Pan JJ, Reeve BB, Woody GE, 2011a. Use of item response theory and latent class analysis to link poly-substance use disorders with addiction severity, HIV risk, and quality of life among opioid-dependent patients in the Clinical Trials Network. Drug Alcohol Depend 118, 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, McNeely J, Subramaniam GA, Brady KT, Sharma G, VanVeldhuisen P, Zhu H, Schwartz RP, 2017. DSM-5 substance use disorders among adult primary care patients: Results from a multisite study. Drug Alcohol Depend 179, 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, McNeely J, Subramaniam GA, Sharma G, VanVeldhuisen P, Schwartz RP, 2016. Design of the NIDA clinical trials network validation study of tobacco, alcohol, prescription medications, and substance use/misuse (TAPS) tool. Contemp. Clin. Trials 50, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Woody GE, Yang C, Blazer DG, 2010. Subtypes of nonmedical opioid users: results from the national epidemiologic survey on alcohol and related conditions. Drug Alcohol Depend 112, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Woody GE, Yang C, Pan JJ, Blazer DG, 2011b. Abuse and dependence on prescription opioids in adults: a mixture categorical and dimensional approach to diagnostic classification. Psychol. Med 41, 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zale EL, Dorfman ML, Hooten WM, Warner DO, Zvolensky MJ, Ditre JW, 2015Tobacco Smoking, Nicotine Dependence, and Patterns of Prescription Opioid Misuse: Results From a Nationally Representative Sample. Nicotine Tob Res 17, 1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


