Abstract
Introduction
Approximately, 20% of patients with pancreatic ductal adenocarcinoma have resectable disease at diagnosis. Given improvements in locoregional and systemic therapies, some patients with borderline resectable pancreatic cancer (BRPC) can now undergo successful resection. The outcomes of patients with BRPC after neoadjuvant therapy remain unclear.
Methods
A prospectively maintained single-institution database was utilized to identify patients with BRPC who were managed at the Johns Hopkins Pancreas Multidisciplinary Clinic (PMDC) between 2013 and 2016. BRPC was defined as any tumor that presented with radiographic evidence of the involvement of the portal vein (PV) or superior mesenteric vein (SMV) that was deemed to be technically resectable (with or without the need for reconstruction), or the abutment (< 180° involvement) of the common hepatic artery (CHA) or superior mesenteric artery (SMA), in the absence of involvement of the celiac axis (CA). We collected data on treatment, the course of the disease, resection rate, and survival.
Results
Of the 866 patients evaluated at the PMDC during the study period, 151 (17.5%) were staged as BRPC. Ninety-six patients (63.6%) underwent resection. Neoadjuvant chemotherapy was administered to 142 patients (94.0%), while 78 patients (51.7%) received radiation therapy in the neoadjuvant setting. The median overall survival from the date of diagnosis, of resected BRPC patients, was 28.8 months compared to 14.5 months in those who did not (p < 0.001). Factors associated with increased chance of surgical resection included lower ECOG performance status (p = 0.011) and neck location of the tumor (p = 0.001). Forty-seven patients with BRPC (31.1%) demonstrated progression of disease; surgical resection was attempted and aborted in 12 patients (7.9%). Eight patients (5.3%) were unable to tolerate chemotherapy; six had disease progression and two did not want to pursue surgery. Lastly, four patients (3.3%) were conditionally unresectable due to medical comorbidities at the time of diagnosis due to comorbidities and failed to improve their status and subsequently had progression of the disease.
Conclusion
After initial management, 31.1% of patients with BRPC have progression of disease, while 63.6% of all patients successfully undergo resection, which was associated with improved survival. Factors associated with increased likelihood of surgical resection include lower ECOG performance status and tumor location in the neck.
Keywords: Borderline pancreatic cancer, Neoadjuvant therapy, Rate of resectability (Denominator), Survival
Introduction
Pancreatic ductal adenocarcinoma (PDAC) accounts for 3% of all cancers in the USA and is the third leading cause of all cancer-related deaths in the USA.1 Despite improvements in surgical techniques and systemic therapies, the survival of these patients remains poor with the current 5-year survival being a dismal 9.1%.2 These poor outcomes are associated with the asymptomatic nature of the disease resulting in diagnosis at later stages of disease and a high likelihood of early systemic spread.2,3
Surgical resection remains the only curative form of therapy; however, at the time of diagnosis, only 20% of the patients are deemed to have disease initially amenable to surgical intervention. An additional 20% of patients present with borderline resectable pancreatic cancer (BRPC) as defined by the National Comprehensive Cancer Network (NCCN) guidelines based on radiographic evidence of surroundi ng vasculatur e involvement.4 Patients with BRPC comprise a group of patients with PDAC who are at a high risk of having positive margins if upfront resection is pursued.5 In addition, multiple studies have reported these patients have increased probability of early recurrence of local and systemic disease resulting in poorer outcomes.6–13 Although some patients are treated with an aggressive surgery-first approach and adjuvant systemic therapies, a preoperative chemotherapy and/or chemoradiation approach is becoming more common for all patients with BRPC.14 The pre-operative treatment approach for the treatment of patients with BRPC has many potential benefits including a higher likelihood of achieving an R0 resection and early treatment of systemic disease for undetected micrometastasis.15–18 Furthermore, it allows evaluation of the aggressiveness of the disease resulting in a better selection of surgical candidates.15 After neoadjuvant chemoradiation treatment, patients with BRPC disease may achieve similar overall survival rates to those with resectable pancreatic cancer following a successful R0 resection.18,19 However, despite these theoretical advantages, there are some possible disadvantages to using this approach. In particular, there is a perceived risk of disease progression due to the lack of effective systemic therapies, which makes curative resection an impossibility for some patients.15
A majority of the literature available on outcomes of patients with BRPC is limited to those who undergo re-section of disease, and thus, the outcomes of all BRPC patients remain poorly understood. The aim of this study was to evaluate and report the outcomes of all patients with BRPC who are being managed in this contemporary era of neoadjuvant therapy.
Methods
Study Design and Data Collection
A retrospective study was conducted on consecutive patients who were managed for BRPC at the Johns Hopkins Pancreas Multidisciplinary Clinic (PMDC) from January 2013 through April 2016. All patients were identified from a prospectively maintained institutional database. The primary inclusion criterion was a diagnosis of BRPC at the time of initial evaluation at the PMDC.
A majority of the patients underwent an evaluation by a computed tomography (CT) scan performed according to the Johns Hopkins pancreas protocol. The subsequent decision regarding management and resectability was determined in the multidisciplinary setting (including surgeons, medical oncologists, radiation oncologists, pathologists, and radiologists). Recommendations for treatment followed NCCN guidelines for pancreatic adenocarcinoma.4 As per the NCCN guidelines, BRPC was defined as any tumor that presented with radiographic evidence of the involvement of the portal vein (PV) or superior mesenteric vein (SMV) that was deemed to be technically resectable (with or without the need for reconstruction), or the abutment (< 180° involvement) of the common hepatic artery (CHA) or superior mesenteric artery (SMA), in the absence of involvement of the celiac axis (CA). Patients with resectable, locally advanced, or metastatic disease at the time of their initial evaluation were excluded. Furthermore, patients with a diagnosis of pancreatic tumors other than PDAC were also excluded from further analysis.
All the clinicopathological details were collected and analyzed, including age, gender, comorbidities, Eastern Cooperative Oncology Group (ECOG) performance status, the location of the tumor, type of involved vessel, type and duration of neoadjuvant chemotherapy, receipt of neoadjuvant radiation therapy, and cancer antigen 19–9 (CA19–9) serum level. For patients who underwent surgical resection, we collected the data of the type of pancreatectomy, need for vascular resection, tumor size, number of harvested and positive lymph nodes, grade of tumor differentiation, response to therapy, margin status, lymphovascular and perineural invasion, and receipt of adjuvant chemotherapy and/or chemoradiation therapy.
The study was approved by the Institutional Review Board for Human Research and complied with all Health Insurance Portability and Accountability Act regulations.
Primary and Secondary Outcomes
The primary outcome was the rate of surgical resection of BRPC. The decision to take the patients to the operating room was made based on patient’s performance status, the technical feasibility of resection, and the absence of disease progression or metastasis.
The endpoint for the survival analysis was overall survival (OS), which was defined as the time interval between the date of diagnosis and the date of death. Time was censored at the date of the last follow-up assessment for patients who were alive. For patients who underwent surgical resection, recurrence-free survival (RFS) was also assessed. RFS was defined as the time interval between the date of surgical resection and the date of cancer recurrence based on imaging evidence or the date of last follow-up for patients who did not have recurrence of disease.
Statistical Analysis
Continuous variables were reported as a mean and standard deviation or median and interquartile range (IQR), as deemed appropriate. Categorical variables were summarized as frequencies and percentages. The unpaired Student’s t test or Wilcoxon test was used to analyze continuous variables, while the chi-square or Fisher’s exact test was used to analyze the categorical variables. Odds ratio and 95% confidence intervals were calculated for the outcomes. Logistic regression was used for detailed analysis on factors associated with surgical resection.
Survival curves were estimated using the Kaplan-Meier method, and differences between curves were investigated using the log-rank test. A two-sided p value of < 0.05 was considered to be statistically significant. All statistical analyses were performed using Stata v. 14.2 (TX, USA).
Results
Patient Characteristics
During the study period, 151 (17.5%) of the 866 consecutive patients managed at the Johns Hopkins PMDC were staged as having BRPC. The detailed characteristics of these patients are presented in Table 1. Neoadjuvant chemotherapy was administered in 142 patients (94.0%).
Table 1.
Baseline characteristics of all patients with BRPC
| Variables | N (%) |
|---|---|
| Patients, n (%) | 151 (100) |
| Age (years), mean ± SD | 65.0 ± 10.6 |
| Sex, n (%) | |
| Male | 72 (47.7) |
| Female | 79 (52.3) |
| Race, n (%) | |
| White | 133 (88.0) |
| African-American | 9 (6.0) |
| Other | 9 (6.0) |
| History of hypertension, n (%) | 83 (55.0) |
| History of diabetes mellitus, n (%) | 43 (28.5) |
| ECOG performance status, n (%) | |
| 0 | 33 (21.9) |
| 1 | 93 (61.6) |
| 2 | 19 (12.6) |
| 3 | 6 (3.9) |
| History of prior abdominal surgery, n (%) | 63 (41.7) |
| History of prior malignancy, n (%) | 51 (33.8) |
| History of smoking, n (%) | 60 (39.7) |
| History of alcohol abuse, n (%) | 48 (31.8) |
| Jaundice (present), n (%) | 66 (43.7) |
| Weight loss (present), n (%) | 56 (37.1) |
| Abdominal pain (present), n (%) | 82 (54.3) |
| CA19–9 at diagnosis (U/mL), median (IQR) | 107.7 (38.0–295.3) |
| Tumor location, n (%) | |
| Head | 65 (43.1) |
| Uncinate | 13 (8.6) |
| Neck | 52 (34.4) |
| Body/tail | 21 (13.9) |
| Radiological evidence of involvement of the portal vein/superior mesenteric vein, n (%) | 133 (88.1) |
| Radiological evidence of involvement of the superior mesenteric artery, n (%) | 36 (23.8) |
| Radiological evidence of involvement of the common hepatic artery, n (%) | 39 (25.8) |
| Type of neoadjuvant chemotherapy, n (%) | |
| Fluorouracil-based | 66 (43.7) |
| Fluorouracil and gemcitabine-based | 14 (9.3) |
| Gemcitabine-based | 60 (39.7) |
| Others | 2 (1.3) |
| Chemotherapy duration (months), median (IQR) | 2.76 (1.68–4.00) |
| Neoadjuvant radiotherapy (performed), n (%) | 78 (51.7) |
Plus-minus values are means ± standard deviation
ECOG Eastern Cooperative Oncology Group
Clinicopathological Features of Patients with Resected BRPC
A total of 96 patients (63.6%) underwent surgical resection of disease. The clinicopathological features of these patients are presented in Table 2. The median time from diagnosis to surgery was 6.2 months (IQR, 4.3–7.7), and the median duration of neoadjuvant therapy was 3.00 months (IQR, 2.00–4.00). Vascular resection was required in 47 patients (48.9%).
Table 2.
Clinicopathological characteristics of 96 patients with resected BRPC
| Variables | N (%) |
|---|---|
| Time from diagnosis to surgery (months), median (IQR) | 6.2 (4.3–7.7) |
| Type of pancreatectomy, n (%) | |
| Pancreaticoduodenectomy | 79 (82.3) |
| Distal pancreatectomy | 14 (14.6) |
| Total pancreatectomy | 3 (3.1) |
| Need for vascular resection, n (%) | 47 (48.9) |
| Tumor size (cm), median (IQR) | 2.8 (1.8–3.9) |
| Grade of tumor differentiation, n (%) | |
| Well differentiated | 1 (1.2) |
| Moderately differentiated | 54 (65.9) |
| Poorly differentiated/undifferentiated | 27 (32.9) |
| Perineural invasion (present), n (%) | 68 (71.6) |
| Lymphovascular invasion (present), n (%) | 47 (48.9) |
| Nodal disease (present), n (%) | 41 (42.7) |
| Margin status (negative), n (%) | 74 (77.1) |
| Treatment effect | |
| Complete, n (%) | 14 (16.7) |
| Extensive, n (%) | 21 (25.0) |
| Moderate, n (%) | 24 (28.6) |
| Poor, n (%) | 25 (29.7) |
| Adjuvant therapy (performed), n (%) | 58 (60.4) |
| Recurrence of disease at last follow-up (present), n (%) | 58 (60.4) |
| Type of recurrence, n (%) | |
| Local only | 28 (48.3) |
| Liver only | 8 (13.8) |
| Distant other than the liver | 8 (13.8) |
| Multiple sites | 14 (24.1) |
On histopathological examination, the median size of the tumor was 2.8 cm (IQR, 1.8–3.9). Nodal disease was present in 41 patients (42.7%), and 22 patients (22.9%) had positive resection margins. The presence of nodal disease was not associated with the receipt of neoadjuvant chemoradiation (p =0.885) or need for vascular resection (p = 0.227). Similarly, positive margin status was not associated with the receipt of neoadjuvant chemoradiation (p = 0.599) or need for vascular resection (p = 0.550). In terms of response to neoadjuvant therapy, data were available on 84 patients (87.5%); 14 patients (16.7%) had complete response, while 21 (25.0%) demonstrated extensive response, 24 (28.6%) demonstrated moderate response, and the remaining 25 patients (29.8%) had a poor response to therapy. Adjuvant therapy was administered in 58 (60.4%) of the 96 resected patients.
Impact of Surgical Resection on Outcomes of Patients with BRPC
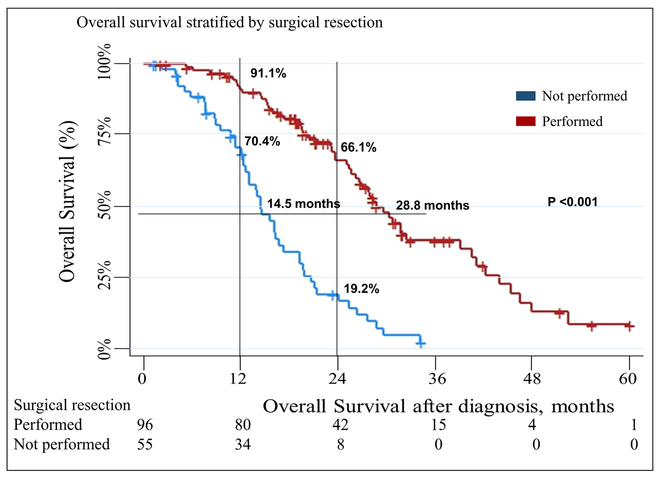
The median follow-up of all patients was 19.1 months (IQR,12.0–27.8). The median survival, from the time of diagnosis, of all patients included in the study was 23.7 months (IQR,14.5–34.1). Patients who underwent surgical resection had a median survival of 28.8 months (IQR, 20.3–43.9) as compared to that of 14.5 months (IQR, 10.7–20.7) in patients who could not be resected (p < 0.001) (Fig. 1). The 1-year and 2-year survival rates of patients with resected disease were91.1% and 66.1% as compared to 70.4% and 19.2%, respectively, in the unresected cohort.
Fig. 1.
Overall survival stratified by surgical resection
Upon further evaluation of patients who underwent surgical resection, the median RFS was found to be 13.4 months (IQR, 7.6–31.4) (Fig. 2). Fifty-seven patients (59.4%) had no evidence of recurrence at 12 months postoperatively, as compared to 42 patients (43.8%) at 24 months after resection. The median follow-up of these patients was 21.8 months (15.7–30.8). At the time of last follow-up, 58 patients (60.4%) had developed recurrence, of whom 48.3% (N = 28) had a local recurrence. The pattern of recurrence was not associated with the receipt of chemoradiation (p = 0.658), receipt of adjuvant therapy (p = 0.387), or need for vascular resection (p = 0.340). None of the established prognostic factors of outcomes in patients with PDAC including nodal involvement, grade of tumor differentiation, perineural and lymphovascular invasion, and margin status were significantly associated with RFS (all p > 0.05). Similarly, none of the aforementioned factors were associated with postsurgical overall survival (all p > 0.05).
Fig. 2.
Recurrence-free survival in patients with resected BRPC
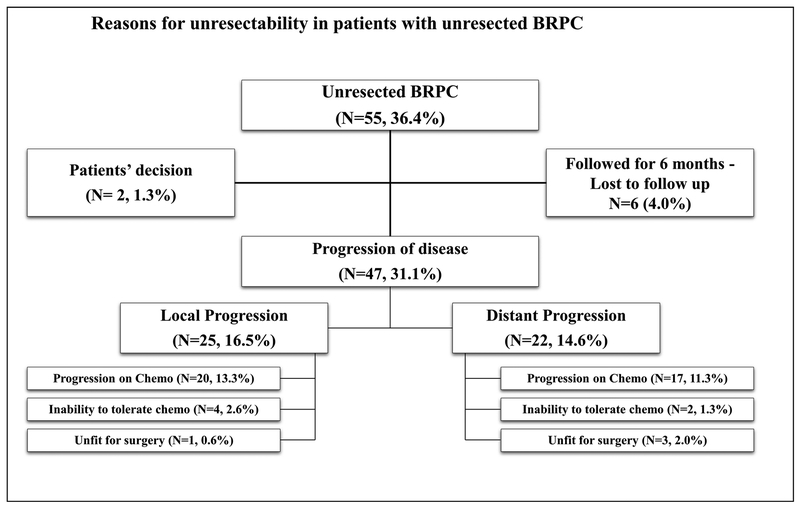
Reasons for Unresectability
Fifty-five patients (36.4%) with BRPC did not undergo resection. Of all BRPC patients included in this study, 47 patients (31.1%) had progression of disease. Three distinct reasons were identified for unresectability which included (i) radiological progression of disease during receipt of neoadjuvant chemo-therapy, (ii) intolerance to chemotherapy with subsequent progression of disease, and (iii) conditionally unresectable disease where medical comorbidities precluded resection (Fig. 3).
Fig. 3.
Reasons for unresectability in patients with unresected BRPC
Radiological progression during receipt of neoadjuvant chemotherapy was the cause of unresectability in 37 patients (24.6%). Of these, 20 patients (13.3%) progressed locally, while 17 (11.3%) had distant progression of disease.
Intolerance to neoadjuvant chemotherapy was identified as the driver of unresectability in six patients (3.9%). These patients subsequently developed local (N = 4, 2.6%) or distant (N = 2, 1.3%) progression of disease.
Conditionally unresectable disease where medical comorbidities precluded resection resulted in unresectability in four patients (2.6%); one patient (0.6%) progressed locally, and three patients (2.0%) had distant progression of disease.
Additionally, two (1.3%) patients could not tolerate chemo-therapy and decided not to pursue any subsequent care including surgical resection. Six patients (4.0%) were followed for 6 months during the initial treatment and, subsequently, were lost to follow-up.
Factors Associated with Surgical Resection of Disease
The characteristics of patients stratified by surgical resection are presented in Table 3. Factors associated with increased likelihood of surgical resection identified in the univariate analysis included younger age at diagnosis, the absence of hypertension or diabetes mellitus, lower ECOG performance status, negative history of alcohol use, lower CA19–9 level at presentation, location of the tumor at the pancreatic neck, and lack of radiographic evidence of involvement of the common hepatic artery (all p < 0.05).
Table 3.
Factors associated with resection in patients with BRPC
| Variables | Not resected (N = 55) | Resected (N= 96) | Univariate analysis | Multivariate analysis | |
|---|---|---|---|---|---|
| p value | OR (95% CI) | p value | |||
| Age (years), median (IQR) | 68.5 (62.0–75.8) | 64.5 (57.1–69.7) | < 0.001 | 0.969 (0.926–1.015) | 0.187 |
| Sex, n (%) | 0.939 | ||||
| Male | 26 (36.1) | 46 (63.9) | |||
| Female | 29 (36.7) | 50 (63.3) | |||
| Race, n (%) | 0.638 | ||||
| White | 50 (37.6) | 83 (62.4) | |||
| African-American | 2 (22.2) | 7 (77.8) | |||
| Other | 3 (33.3) | 6 (66.7) | |||
| Hypertension, n (%) | 40 (48.2) | 43 (51.8) | 0.001 | 0.569 (0.236–1.375) | 0.211 |
| Diabetes mellitus, n (%) | 22 (51.2) | 21 (48.8) | 0.018 | 0.681 (0.266–1.746) | 0.424 |
| ECOG performance status, n (%) | < 0.001 | 0.429 (0.222–0.827) | 0.011 | ||
| 0 | 8 (24.2) | 25 (75.8) | |||
| 1 | 27 (29.0) | 66 (71.0) | |||
| 2 | 14 (73.7) | 5 (26.3) | |||
| 3 | 6 (100.0) | 0 (0.0) | |||
| Prior abdominal surgery, n (%) | 24 (38.1) | 39 (61.9) | 0.718 | ||
| Prior malignancy, n (%) | 18 (35.3) | 33 (64.7) | 0.837 | ||
| History of smoking, n (%) | 22 (36.7) | 38 (63.3) | 0.960 | ||
| History of alcohol use, n (%) | 11 (22.9) | 37 (77.1) | 0.019 | 1.528 (0.615–3.797) | 0.361 |
| Jaundice (present), n (%) | 26 (39.4) | 40 (60.6) | 0.504 | ||
| Weight loss (present), n (%) | 26 (46.4) | 30 (53.6) | 0.056 | ||
| Abdominal pain (present), n (%) | 35 (42.7) | 47 (57.3) | 0.081 | ||
| CA19–9 at presentation (U/mL), median (IQR) | 223.0 (85.6–563.0) | 74.8 (31.4–195.5) | 0.005 | 0.999 (0.998–1.001) | 0.243 |
| Tumor location, n (%) | < 0.001 | 1.714 (1.232–2.383) | 0.001* | ||
| Head | 35 (53.9) | 30 (46.1) | |||
| Uncinate | 6 (46.2) | 7 (53.8) | |||
| Neck | 5 (9.6) | 47 (90.4) | |||
| Body/tail | 9 (42.9) | 12 (57.1) | |||
| Radiological evidence of involvement of PV/SMV, n | 48 (63.1) | 85 (63.9) | 0.817 | ||
| (%) | |||||
| Radiological evidence of involvement of SMA, n (%) | 9 (25.0) | 27 (75.0) | 0.103 | ||
| Radiological evidence of involvement of CHA, n (%) | 8 (20.5) | 31 (79.5) | 0.017 | 2.01 (0.708–5.723) | 0.189 |
| Neoadjuvant chemotherapy | 0.149 | ||||
| Fluorouracil-based, n (%) | 21 (31.8) | 45 (68.2) | |||
| Fluorouracil and gemcitabine-based, n (%) | 9 (64.3) | 5 (35.7) | |||
| Gemcitabine-based, n (%) | 24 (40.0) | 36 (60.0) | |||
| Others, n (%) | 1 (50.0) | 1 (50.0) | |||
| Neoadjuvant chemoiherapy (months), mean ± SD | 2.0 (1.5–4.0) | 3.0 (2.0–4.0) | 0.692 | ||
| Neoadjuvant radiation | 21 (26.9) | 57 (73.1) | 0.012 | 1.247 (0.531–2.931) | 0.612 |
Values in bold are considered to be significant and represent p-value of < 0.05
ECOG Eastern Cooperative Oncology Group, PV/SMV, portal vein/superior mesenteric vein, SMA superior mesenteric artery, CHA common hepatic artery, SD standard deviation, IQR interquartile range
Post hoc analysis (resected vs. non-resected): head tumors and neck tumors, p = 0.001; head tumors and uncinate tumors, p = 0.656; and head tumors and body/tail tumors, p = 0.695
On the multivariable analysis, factors that were found to be independently associated with increased likelihood of resection included lower ECOG performance status and location of the tumor (Table 3). A vast majority of patients who underwent resection had an ECOG performance status of 0 or 1 (N = 91,94.8%) as compared to 63.6% (N = 35) of patients in the unresected cohort. The rate of resectability in patients with tumors located in the head was similar to that of patients with tumors in the uncinate (OR 1.407; 95% CI 0.313–6.334, p =0.656) and the body/tail of the pancreas (OR 1.278; 95% CI0.375–4.360, p = 0.695). As compared to tumors located in the head, those located in the neck were more likely to be resected (OR 6.748; 95% CI 2.176–20.926, p = 0.001).
Discussion
Treatment of patients with BRPC has evolved considerably over the last decade. Neoadjuvant treatment has the theoretical benefits of higher rates of R0 resection and improved selection of surgical candidates based on their disease biology. However, due to the variable rates of tumor response to available chemotherapy and lack of biomarkers to predict tumor response, disease progression can take place making surgical resection impossible for some patients. Our study provided a detailed insight into the management and outcomes of patients with BRPC. To the best of our knowledge, this is the first study that comprehensively studies a well-defined cohort of patients with BRPC, who were managed by the same multi-disciplinary team since their initial diagnosis, and provides a detailed insight into the course of disease of patients who do not undergo surgical resection. The patterns of progression of disease and the final outcomes of these patients are clearly outlined and discussed in detail.
This study found the rate of resection to be 63.6%, which is comparable to the 50–70% rates reported in the literature.15,20 A recent systematic review and meta-analysis on patients with 935 BRPC reported the rate of exploration in these patients to be 77% and the rate of resectability to be 69%.21 Katz et al. in their initial report of the ALLIANCE trial A021101 reported the rate of resectability in BRPC to be 68% following neoadjuvant treatment (15/22).22 Similar to our findings, the PREOPANC-1 trial reported a rate of resection of 72% in BRPC patients who underwent straight to surgery as compared to 62% in patients who received neoadjuvant therapy prior to resection.
Furthermore, in this study, surgical resection was associated with a significant improvement in the overall survival of patients with BRPC. The median OS of patients with resected disease was 28.8 months, which was similar to that of 28.3 months reported by Quan et al.23 and longer than that of 20 months reported by Kim et al.24 Katz et al. reported the median OS of 15 patients with BRPC who underwent resection to be 21.7 months.22 A recent meta-analysis on BRPC patients reported the OS in patients who received neoadjuvant therapy to be 27.4 months as compared to 12.9 months in patients who did not undergo resection.21
Factors that were significantly associated with increased likelihood of resectability included ECOG performance status and location of the tumor in our study. Previous data have suggested FOLFIRINOX to be associated with increased chance of resection in patients with locally advanced pancreatic cancer (LAPC); however, both type and duration of neoadjuvant were not associated with resectability in this BRPC cohort.25 This could be due to the shorter duration of neoadjuvant therapy in both resected and unresected patients in this study. For patients with LAPC, the length of administration of neoadjuvant therapy is longer than that reported for patients with BRPC. During neoadjuvant treatment, the aggressive tumor behavior declares itself and can explain the duration of neoadjuvant therapy being associated with resectability in LAPC patients. The available studies that report ECOG performance status of resected BRPC patients only report patients with an ECOG performance of 0 or 1.26 In this study, resection was successfully performed in patients with an ECOG performance of ≥ 2. However, these patients had a significantly lower likelihood of surgical resection. Neck lesions that are staged as BRPC tend to abut surrounding arteries with various radio-graphic involvements of the portal vein system. This anatomic relation can explain the higher rate of resectability for these neck lesions after neoadjuvant treatment. Most BRPC lesions located in the neck of the pancreas can be resected without arterial resection.
Another important finding in the study was that imaging response alone was inadequate in determining resect-ability. In this study, resection was attempted and aborted due to the presence of unresectable local disease or the presence of occult metastatic disease in 12 patients. Six patients were found to have occult metastatic disease intraoperatively that was not detected on the immediately preoperative imaging. Similarly, in six patients, the extent of vascular involvement was underappreciated on the imaging, and intraoperatively, the decision to abort the procedure was made predominantly due to the involvement of the SMA. These findings could not be appreciated on the postneoadjuvant, preoperative imaging of these patients. This is similar to the findings of other studies that have evaluated the sensitivity of imaging to determine resectability in these patients.27–29
The rate of margin negative resection in our study was 77.1%, which is similar to that reported in the literature.15,24 In a meta-analysis, the rate of R0 resection in BRPC patients after receipt of neoadjuvant therapy was 54%.21 In another study on 15 BRPC patients who underwent resection, one (7%) underwent an R1 resection. An additional two (14%) patients had tumor within 1 mm of the resection margin.22 Furthermore, in another retrospective study on patients with BRPC and LAPC, the rate of R0 resection was 44.0%.26 We believe that these rates were lower than those reported in our study because the authors also included patients with LAPC. In the PREOPANC-1 trial, the rate of R0 resection was found to be 65% in patients who received neoadjuvant therapy. Despite receipt of neoadjuvant therapy, the rate of nodal disease was 42.7%. The rate of nodal disease reported in the literature varied between 23.5 and 33.3%.15,22,24
While systemic therapies can be effective in approximately one third of resected patients, there still remains a subset of patients who only shows partial or no response to therapy, which reinforces the need for better systemic therapies than those that are currently available to us. Despite resection of disease, the RFS in resected patients was 13.4 months, suggesting that residual systemic disease after resection plays a key role in determining patient outcomes. Interestingly, the well-established predictors of survival reported in the literature on all patients with PDAC were not significantly associated with RFS in resected patients included in this study. These included nodal status, grade of tumor differentiation, margin status, lymphovascular and perineural invasion, and receipt of adjuvant therapy.
The most important finding in this study was that 47 patients (31.1%) could not undergo resection due to progression of disease (both local and distant). This further highlights the importance of disease biology in determining outcomes of these patients. It is evident that the rate of response to therapy in patients remains variable and unpredictable even with improvements in systemic therapies available for PDAC. Future studies including the ALLIANCE trial A021501 will shed more light on the outcomes of these patients.30 There is an ever-increasing need to establish classification systems and identifying subpopulations of patients that respond well to each of the available therapeutics. Additionally, bio-markers of response to therapy need to be identified. In the future, this information may become available to us based on the work on tumor genetics, circulating tumor cells, and tumor-derived organoids. Without personalized therapy, the variability in the rate of response to current therapeutics will persist and a significant number of patients with BRPC will not undergo resection due to the progression of disease.
This study has several limitations. First, it is a retrospective study and suffers from selection bias. The survival difference observed in patients who underwent surgical resection might be confounded by this selection bias. Patients with favorable tumor behavior were potentially selected for resection, while those with aggressive tumor growth declared themselves during their initial management period and never made it to surgery. Since patients in this study were not randomized to surgical resection, the true extent of the benefit of surgical resection might be different from that reported in this study. Second, these patients were selected from a cohort of patients managed at the Johns Hopkins PMDC. There were a small number of patients with BRPC who were managed at our institution by individual providers and were therefore not captured in our study. Third, the micromanagement of the neoadjuvant therapy was not accounted for and should be evaluated in future studies. Fourth, the definition of BRPC was based on tumor radiographic involvement of surrounding veins and arteries. This radiographic involvement may not represent a true pathological vascular involvement. Lastly, the final pathology of patients with unresected disease is not known and these patients could potentially have other periampullary adenocarcinomas.
In conclusion, after initial management, 31.1% of patients with BRPC have progression of disease during neoadjuvant therapy. The remaining 63.6% of patients with BRPC undergo surgical resection, which is associated with improved outcomes. Lower ECOG performance status and tumor location in the neck were associated with increased likelihood of resection. There remains a dire need to better identify the subset of BRPC patients that progress during neoadjuvant therapy in order to maximize treatment effects and enhance survival for this aggressive disease.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a Cancer Journal for Clinicians. 2018;68(1):7–30. 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK et al. Recent progress in pancreatic cancer. CA: a Cancer Journal for Clinicians. 2013;63(5):318–48. 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groot VP, Gemenetzis G, Blair AB, Rivero-Soto RJ, Yu J, Javed AA et al. Defining and Predicting Early Recurrence in 957 Patients With Resected Pancreatic Ductal Adenocarcinoma. Annals of Surgery. 2018. 10.1097/SLA.0000000000002734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network: JNCCN. 2017;15(8): 1028–61. 10.6004/jnccn.2017.0131. [DOI] [PubMed] [Google Scholar]
- 5.Javed AA, Bleich K, Bagante F, He J, Weiss MJ, Wolfgang CL et al. Pancreaticoduodenectomy with venous resection and reconstruction: current surgical techniques and associated postoperative imaging findings Abdominal Radiology (New York: ). 2017. 10.1007/s00261-017-1290-5. [DOI] [PubMed] [Google Scholar]
- 6.Lopez NE, Prendergast C, Lowy AM. Borderline resectable pancreatic cancer: definitions and management. World Journal of Gastroenterology. 2014;20(31):10740–51. 10.3748/wjg.v20.i31.10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper AB, Tzeng CW, Katz MH. Treatment of borderline resectable pancreatic cancer. Current Treatment Options in Oncology. 2013;14(3):293–310. 10.1007/s11864-013-0244-6. [DOI] [PubMed] [Google Scholar]
- 8.McClaine RJ, Lowy AM, Sussman JJ, Schmulewitz N, Grisell DL, Ahmad SA. Neoadjuvant therapy may lead to successful surgical resection and improved survival in patients with borderline resectable pancreatic cancer. HPB: the Official Journal of the International Hepato Pancreato Biliary Association. 2010;12(1): 73–9. 10.1111/j.1477-2574.2009.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blazer M, Wu C, Goldberg RM, Phillips G, Schmidt C, Muscarella P et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Annals of Surgical Oncology. 2015;22(4):1153–9. 10.1245/s10434-014-4225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrams RA, Lowy AM, O’Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Annals of Surgical Oncology. 2009;16(7):1751–6. 10.1245/s10434-009-0413-9. [DOI] [PubMed] [Google Scholar]
- 11.Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Annals of Surgery. 1995;221(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo S, Ammori J, Eads J, Dorth J. The role of neoadjuvant therapy in pancreatic cancer: a review. Future Oncology. 2016;12(5): 669–85. 10.2217/fon.15.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. Journal of the American College of Surgeons. 2008;206(5):833–46; discussion 46–8. 10.1016/j.jamcollsurg.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isaji S, Mizuno S, Windsor JA, Bassi C, Fernandez-Del Castillo C, Hackert T et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology: official journal of the International Association of Pancreatology. 2018;18(1):2–11. 10.1016/j.pan.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Jang JY, Han Y, Lee H, Kim SW, Kwon W, Lee KH et al. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Annals of Surgery. 2018. 10.1097/SLA.0000000000002705. [DOI] [PubMed] [Google Scholar]
- 16.Epstein JD, Kozak G, Fong ZV, He J, Javed AA, Joneja U et al. Microscopic lymphovascular invasion is an independent predictor of survival in resected pancreatic ductal adenocarcinoma. Journal of Surgical Oncology. 2017;116(6):658–64. 10.1002/jso.24723. [DOI] [PubMed] [Google Scholar]
- 17.Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2008;26(21):3496–502. 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 18.Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J . Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Medicine. 2010;7(4):e1000267 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blair AB, Rosati LM, Rezaee N, Gemenetzis G, Zheng L, Hruban RH et al. Postoperative complications after resection of borderline resectable and locally advanced pancreatic cancer: The impact of neoadjuvant chemotherapy with conventional radiation or stereo-tactic body radiation therapy. Surgery. 2018;163(5):1090–6. 10.1016/j.surg.2017.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kommalapati A, Tella SH, Goyal G, Ma WW, Mahipal A. Contemporary Management of Localized Resectable Pancreatic Cancer. Cancers. 2018;10(1). 10.3390/cancers10010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhir M, Malhotra GK, Sohal DPS, Hein NA, Smith LM, O’Reilly EM et al. Neoadjuvant treatment of pancreatic adenocarcinoma: a systematic review and meta-analysis of 5520 patients. World Journal of Surgical Oncology. 2017;15(1):183 10.1186/s12957-017-1240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz MH, Shi Q, Ahmad SA, Herman JM, Marsh Rde W, Collisson E et al. Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surgery. 2016;151(8):e161137 10.1001/jamasurg.2016.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quan K, Sutera P, Xu K, Bernard ME, Burton SA, Wegner RE et al. Results of a prospective phase 2 clinical trial of induction gemcitabine/capecitabine followed by stereotactic ablative radiation therapy in borderline resectable or locally advanced pancreatic adenocarcinoma. Practical Radiation Oncology. 2018;8(2):95–106. 10.1016/j.prro.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Kim HS, Jang JY, Han Y, Lee KB, Joo I, Lee DH et al. Survival outcome and prognostic factors of neoadjuvant treatment followed by resection for borderline resectable pancreatic cancer. Annals of Surgical Treatment and Research. 2017;93(4):186–94. 10.4174/astr.2017.93.4.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gemenetzis G, Groot VP, Blair AB, Laheru DA, Zheng L, Narang AK et al. Survival in Locally Advanced Pancreatic Cancer After Neoadjuvant Therapy and Surgical Resection. Annals of Surgery. 2018. 10.1097/SLA.0000000000002753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosein PJ, Macintyre J, Kawamura C, Maldonado JC, Ernani V, Loaiza-Bonilla A et al. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer. 2012;12:199 10.1186/1471-2407-12-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joo I, Lee JM, Lee ES, Ahn SJ, Lee DH, Kim SWet al. Preoperative MDCT Assessment of Resectability in Borderline Resectable Pancreatic Cancer: Effect of Neoadjuvant Chemoradiation Therapy. AJR: American Journal of Roentgenology. 2018;210(5): 1059–65. 10.2214/AJR.17.18310. [DOI] [PubMed] [Google Scholar]
- 28.Ferrone CR, Marchegiani G, Hong TS, Ryan DP, Deshpande V, McDonnell EI et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Annals of Surgery. 2015;261(1):12–7. 10.1097/SLA.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shrestha B, Sun Y, Faisal F, Kim V, Soares K, Blair A et al. Long-term survival benefit of upfront chemotherapy in patients with newly diagnosed borderline resectable pancreatic cancer. Cancer Medicine. 2017;6(7):1552–62. 10.1002/cam4.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz MHG, Ou FS, Herman JM, Ahmad SA, Wolpin B, Marsh R et al. Alliance for Clinical Trials in Oncology (ALLIANCE) trial A021501: preoperative extended chemotherapy vs. chemotherapy plus hypofractionated radiation therapy for borderline resectable adenocarcinoma of the head of the pancreas. BMC Cancer. 2017;17(1):505 10.1186/s12885-017-3441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]